Abstract
Background
Cell migration is an integral component of intimal hyperplasia development and proteases are pivotal in the process. Understanding the role of urokinase signaling within the cells of vasculature remains poorly defined. The study examines the role of aminoterminal fragment of urokinase (ATF) on a pivotal cross-talk receptor, Epidermal Growth Factor Receptor (EGFR). EGFR is transactivated by both G-protein-coupled receptors and receptor tyrosine kinases and is key to many of their responses. We hypothesize that ADAM allows the transactivation of EGFR by ATF.
Objective
To determine the role of ADAM in EGFR transactivation by ATF in human vascular smooth muscle cells (VSMC) ) during cell migration.
Methods
Human coronary VSMC were cultured in vitro. Assays of EGFR phosphorylation were examined in response to ATF (10nM) in the presence and absence of the matrix metalloprotease (MMP) inhibitor GM6001, the ADAM inhibitors TAPI-0 and TAPI-1, Heparin binding epidermal growth factor (HB-EGF) inhibitor, CRM197, HB-EGF inhibitory antibodies, EGF inhibitory antibodies and the EGFR inhibitor AG1478. siRNA against EGFR and ADAM (9,10,12) and adenoviral delivered Gβγ inhibitor, βARKCT were also used.
Results
ATF produced concentration dependent VSMC migration (by wound assay and boyden chamber), which was inhibited by increasing concentrations of AG1478. ATF was shown to induce time-dependent EGFR phosphorylation, which peaked at 4-fold greater than control. Pre-incubation with the Gβγ inhibitor, βARKCT inhibited EGFR activation by ATF. This migratory and EGFR response was inhibited by AG1478 in a concentration-dependent manner. Incubation with siRNA against EGFR blocked the ATF mediated migratory and EGFR responses.. EGFR phosphorylation by ATF was blocked by inhibition of MMP activity and the ligand HB-EGF. The presence of the ADAM inhibitors, TAPI-0 and TAPI-1 significantly decreased EGFR activation. EGFR phosphorylation by EGF was not interrupted by inhibition of MMP, ADAMs, or HB-EGF. Direct blockade of the EGFR prevented activation by both ATF and EGF. Incubation with siRNA to ADAM-9 and -10 significantly reduced HB-EGF release from VSMC and EGFR activation in response to ATF. siRNA against ADAM-12 had no effect.
Conclusion
ATF can induce transactivation of EGFR by an ADAM-mediated, HB-EGF dependent process. Targeting a pivotal cross-talk receptor such as EGFR is an attractive molecular target to inhibit cell migration
Keywords: Urokinase, epidermal growth factor receptor, transactivation, vascular smooth muscle cell
INTRODUCTION
Vessels remodel during atherogenesis, in response to altered flow and following injury This remodeling has been shown to involve an integrative program of cell proliferation, migration and extracellular matrix modulation (1). The migration of vascular smooth muscle cells (VSMC) involves the complex regulation of proteases, integrins and extracellular molecules leading to the sequence of attachment, detachment and contraction events, which allow a cell to move through the extracellular matrix. Urokinase plasminogen activator (uPA) is a serine protease that is the primary plasminogen activator in tissue remodeling processes and increased serum uPA is associated with development of restenosis after coronary angioplasty (2). In addition to its extracellular proteolytic activity, uPA is also capable of mediating cell signaling. Plasminogen activation is a complex system at the level of the cellular microenvironment (3). uPA is primarily responsible for plasmin generation in tissue remodeling processes, and is localized to the membrane by its receptor, uPAR (4). uPA induces cell migration through uPAR and it appears to be receptor-initiated and G-protein mediated; migration is dependent on ERK1/2 activity. uPAR is involved in a multi-protein complex containing integrins, LRP (LDL related protein), FPRL1 (a G-protein coupled receptor) and EGFR (epidermal growth factor receptor) (5). Interaction with the components of the complex may allow differential cell signaling. Epidermal Growth Factor Receptor (EGFR) is transactivated by both G-protein-coupled receptors and receptor-linked tyrosine kinases and is the key to many of their responses. uPA induces time dependent phosphorylation of the EGFR (6). Inhibition of EGFR reduces ERK1/2 activation and cell migration (6).
uPA is however composed of three domains: aminoterminal (ATF), kringle (K) and carboxyterminal (CTF) fragments, each of which is distinct and biologically active. The aminoterminal (ATF) and kringle fragments mediate VSMC migration (7, 8). With respect to ATF, the response is due to binding and cleavage of uPAR into a cell bound D1 and a soluble D2/D3 fragment. D2/D3 then binds to the low affinity receptor of fMPL, FPRL1, to induce a Gαi-mediated response (9). We have shown that ATF can strongly induce plasmin-independent, VSMC migration in vitro (10), which is both PI3K-ERK1/2 and PI3K-akt dependent (11). This migration can be blocked by inhibition of EGFR. At present, the triple membrane passing signaling (TMPS) mechanism of GPCR induced EGFR activation is a widely accepted model of EGFR transactivation (12–14). In the TMPS model, there is a sequence of three transmembrane signaling events: G-protein coupled receptor activation followed by transmembrane MMP activation, and subsequent activation of the EGFR by release of HB-EGF, a tethered ligand of the EGFR. A potential transmembrane protease systemthat would convey a signal from intracellular domain to the extracellular domain is the A Disintegrin And Metalloproteinase Domains (ADAM) proteases, which span the membrane and can be activated intracellularly to mediate an extracellular MMP action (Fig 1). We hypothesize that ADAM allows the transactivation of EGFR by ATF. This study seeks to determine the role of ADAM in EGFR transactivation by ATF in human vascular smooth muscle cells (VSMC) during cell migration.
Figure 1.
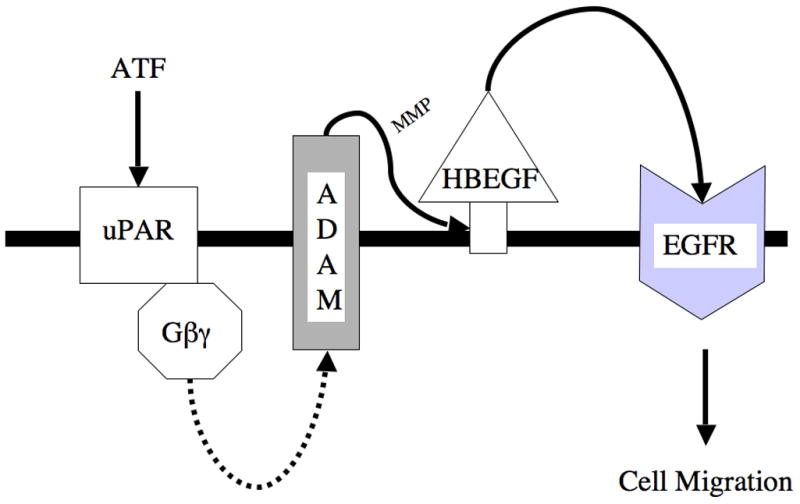
Proposed Triple Membrane Passing System for ATF. ATF binds uPAR and this activates Gβγ and other intracellular molecules to activate A Disintegrin And Metalloproteinase Domains (ADAM) protease. Activation of ADAM associated MMP activity leads to release of the tethered ligand, HB-EGF, which in turn activated the EGFR. Activation of EGFR is necessary for ATF mediated migration,
METHODS
Experimental Design
Human VSMC (passages 3–6, Clontech) were cultured in vitro. Lineage was confirmed by α-actin western blotting. Assays of EGFR phosphorylation were examined in response to ATF in the presence and absence of the matrix metalloprotease (MMP) inhibitor GM6001, the ADAM inhibitors TAPI-0 and TAPI-1, Heparin binding epidermal growth factor (HB-EGF) inhibitor, CRM197, HB-EGF inhibitory antibodies, EGF inhibitory antibodies and the EGFR inhibitor AG1478. Chemical inhibitors and antibodies were incubated for 1 hr before treatment. siRNA against EGFR and ADAM (9,10,12) were also used.
Materials
ATF was purchased from American Diagnostica, Inc. (Greenwich, CT). EGF, heparin were purchased from Sigma Chemical Co (St. Louis, MO). AG1478 and CRM197 were purchased from Calbiochem (La Jolla, CA). GM6001, was purchased from Chemicon International, Inc (Temecula, CA). CRM197 TAPI-0 and TAPI-1 were purchased from Biomol. The Anti–HB-EGF antibody was purchased from R&D Systems, Inc (Minneapolis, MN). The Anti-EGFR antibody (151-IgG) developed by Dr Ann Hubbard was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa (Department of Biological Sciences, Iowa City, IA). siRNA sequences for ADAMs were as follows: ADAM9, AAUCACUGUGGAGACAUUUGCdTdT and AAACUUCC AGUGUGUAGAUGCdTdT; ADAM10, AAUGAAGAGGGACACUUCCCUdTdT and AAGUUGCCUCCUCCUAAACCAdTdT; and ADAM1AACCUCGCUGCAAAGAAUGUGdTdT and AAGACCUUGATACGACUGCUGdTdT. Peroxidase-conjugated antirabbit IgG antibody (raised in goat) and peroxidase-conjugated antimouse IgG antibody (raised in goat) were purchased from Jackson Immuno Research Laboratories, Inc (West Grove, Pa). Phospho- EGFR (Y1068), Total EGFR antibodies were obtained from Cell Signaling Technology, Inc (Beverley, MA). Dulbecco modified Eagle minimal essential medium (DMEM) and Dulbecco phosphate-buffered saline were purchased from Mediatech (Herndon, Va).
Boyden Chamber
Chemotaxis was measured using a 48-well Boyden chamber (Neuroprobe Inc.) and polycarbonate filters (Neuroprobe, Inc., 10 μm pore size, 25 × 80 mm, PVP free) with VSMC as previously described (6, 10). ATF (10nM) was added to the lower wells. Trials included eight or twelve wells per reagent per trial, and were repeated no fewer than three times.
Western Blotting
Cells were allowed to grow to 80% confluence and starved for 48hours. Cells were then stimulated with ATF (10nM) or EGF (10nM) alone and in the presence of pharmacological and peptide inhibitors; cells were harvested at time points from 0 to 30 minutes. Western blotting was performed as previously described (15).
siRNA Transfection
Pre-designed HPCL purified siRNA for gene knockdown for EGFR or control small interference RNA (siRNA) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). and ADAM-9, ADAM-10 and ADAM-12 proteins from Ambion, Inc. (Austin, TX) VSMC of 50% confluence in 60mm plates are starved overnight in 4mL Opti-MEM reduced serum medium (Gibco). siRNA is transfected using Lipofectamine 2000 from Invitrogen, Inc (Carlsbad, CA) following product protocol. Briefly, 22 μl of Lipofectamine 2000 is first incubated in total volume of 250 μl of Opti-MEM for 5 minutes at room temperature. It is then added to 250 μl of Opti-MEM containing 440 pmoles of siRNA. The solution is mixed gently and incubated for 20 minutes at room temperature, after which it is added to the starved plates. The medium can be changed after 4–6 hours of incubation. The cells may now be used between 24–72 hours after transfection for EGFR assays. Scrambled siRNA served as a control. Using the methodologies described, we conducted concentration dependent experiments with siRNA against EGFR, ADAM-9, ADAM-10 and ADAM-12 and demonstrated a concentration dependent decrease in protein expression that was specific for the protein targeted without altering the expression of the other proteins.
Adenoviral Infection
Adenoviral vectors were constructed by Welgen, Inc. (Worcester, MA) using purified plasmid encoding βARKCT, obtained from Guthrie cDNA Resource center. VSMC were plated at 70% confluence in 100mm dishes and allowed to grow overnight. Recombinant adenovirus was then added at the appropriate concentrations (βARKCT; 100 MOI) in a reduced volume of media (1.5 – 2 ml). After 48hr incubation, the media was changed and cells grown for an additional 24h. The cells were then used for EGFR assays. Empty vector served as control.
Measurement of tethered ligand HB-EGF
Cultured VSMC were deprived of serum for 24 h and then cultured in phenol red-free Dulbecco’s modified Eagle’s medium H21. Following exposure to ATF (10nM) alone and in the presence of pharmacological inhibitors, siRNA and adenoviral agents, medium was collected and concentrated using a Centricon centrifugal filter device (YM-3, Millipore, Billerica, MA). Samples were centrifuged at 6500 × g for at least 3 hr until volume was reduced from 4 to 0.5 ml. Concentrates were incubated with 2.5 μg of anti-HB-EGF antibody and protein A-agarose overnight at 4 °C. Immunoprecipitates were washed three times, boiled, and electrophoresed on 15% SDS-polyacrylamide gels. Using conventional Western immunoblotting techniques (HB-EGF levels were assessed using goat polyclonal anti-HB-EGF IgG and donkey anti-goat IgG).
Data and Statistical Analysis
All data are presented as the mean ± standard error of the mean (s.e.m.) and statistical differences between groups were tested with a Kruskal-Wallis nonparametric test with post hoc Dunn’s multiple comparison correction, where appropriate. A p-value less than 0.05 was regarded as significant. Non-significant p-values were expressed as p=ns.
RESULTS
To examine the effect of EGFR on ATF mediated VSMC migration, we employed the Boyden chamber migration assay. ATF (10nM) induced significant smooth muscle cell migration (Fig 2). A similar response was noted if hydroxyurea was added to prevent and control for proliferation. This response to ATF was inhibited by increasing concentrations of the EGFR inhibitor, AG1478 and by application of siRNA against EGFR (Fig 2A and 2B), suggesting that EGFR plays a role in the pathways leading to ATF-mediated cell migration. ATF induced a 4-fold increase in EGFR phosphorylation as determined by activation of Y1068 phosphorylation site; the phosphorylation response was inhibited in a concentration-dependent manner by increasing concentrations of AG1478 (0.1–1000nM) (Fig 3A and 3B). Pre-incubation with siRNA to EGFR inhibited EGFR activation in response to ATF (Fig 3C). ATF is known to mediate its response through Gαi G-proteins (10). Gαi proteins are linked to Gβγ proteins and Gβγ proteins are known to be associated with triple membrane passing system of tyrosine kinase linked receptors such as EGFR. To examine the role of Gβγ, we blocked Gβγ with the inhibitor peptide βARKCT and showed that pre-incubation with βARKCT inhibited EGFR activation by ATF (Fig 3D). No effect was seen with the empty vector or with EGF application. When we pre-incubated VSMC with βARKCT in the Boyden chamber, we saw an 85% decrease in migration in response to ATF (empty vector resulted in a 5% decrease).
Figure 2.
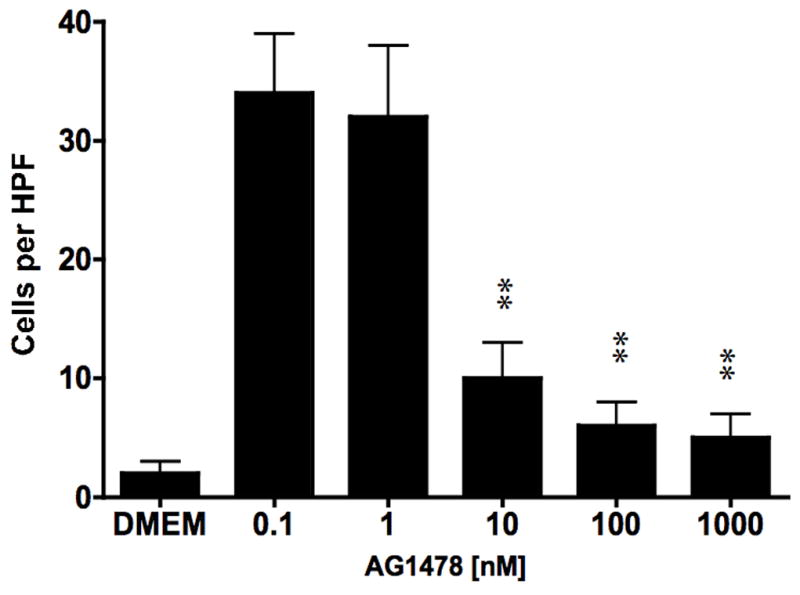
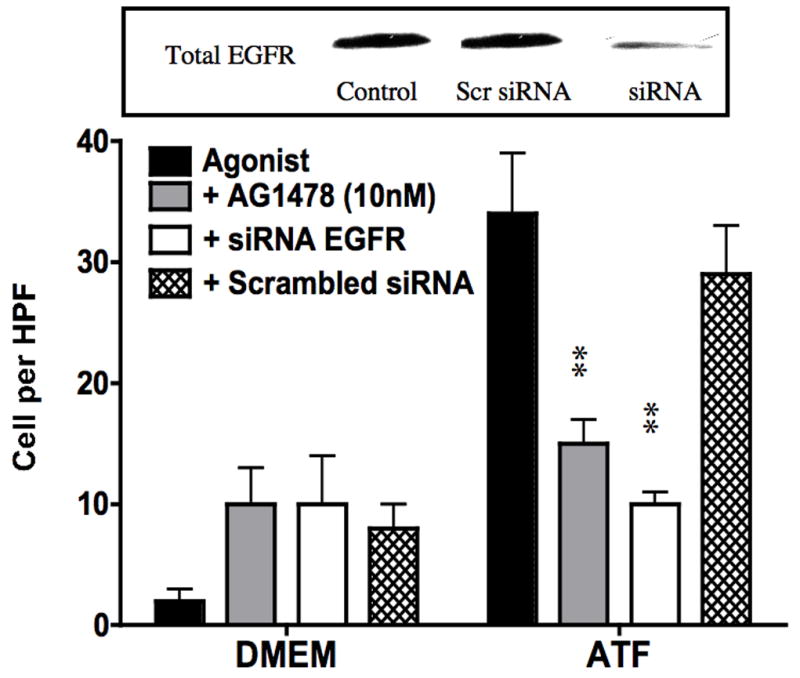
ATF (10nM) induce significant smooth muscle cell migration in the Boyden chamber migration assays. (A) Migration in response to ATF was decreased in a concentration dependent manner by the EGFR inhibitor (AG1478, 1 hr pre stimulation and 12 hrs stimulation); (B) Pre-incubation (4–6 hours) with siRNA to EGFR inhibited the migratory response. Insert shows the efficacy of the siRNA in knocking down the total EGFR protein present. Values are the mean±SEM (n=6, **p<0.01 compared to control).
Figure 3.
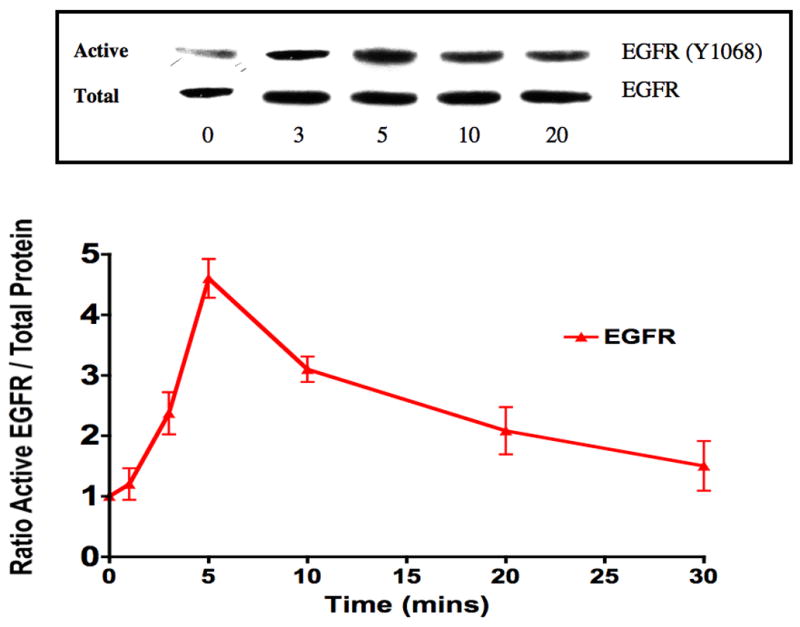
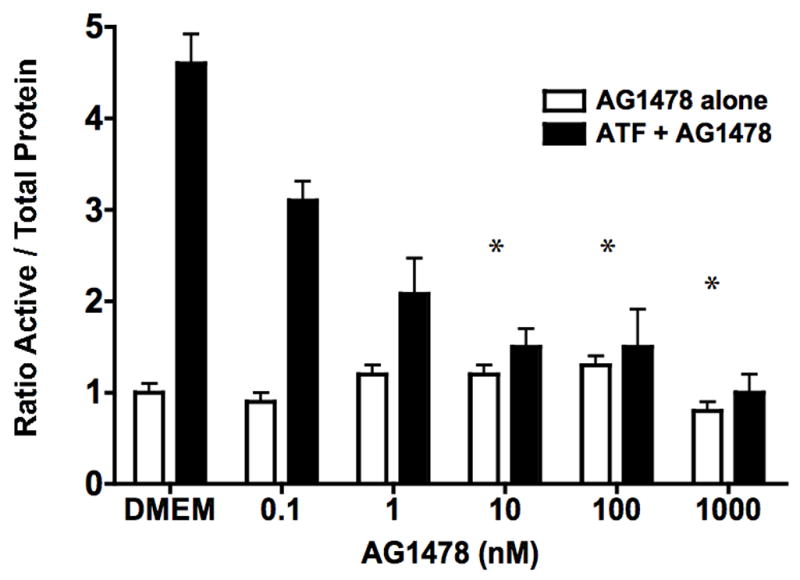
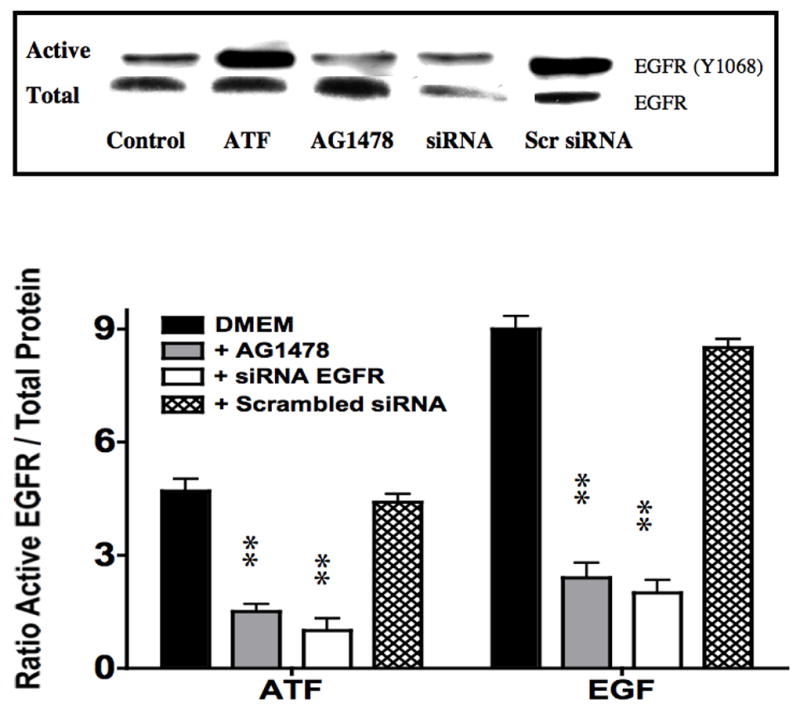
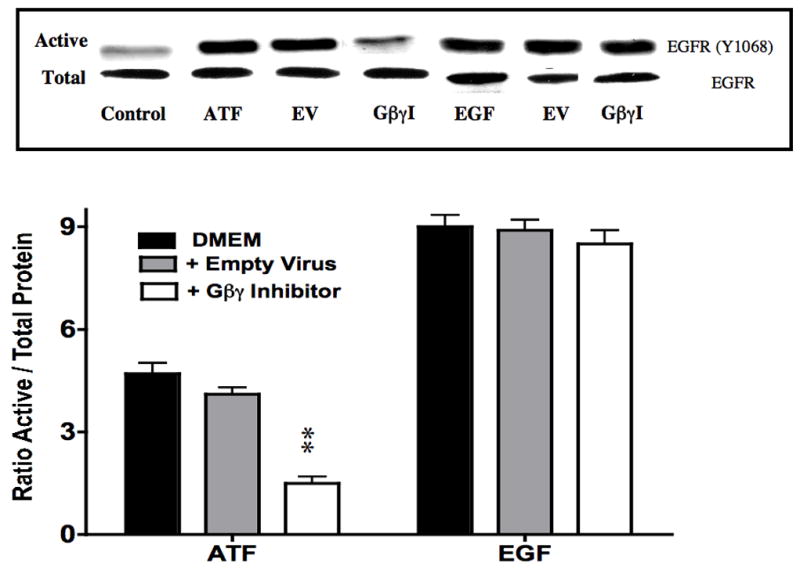
(A) ATF induced a 4-fold increase in EGFR phosphorylation and (B). this response was inhibited in a concentration-dependent manner by increasing concentrations of AG1478 (0.1–1000nM, 1 hr pre-stimulation). (C) Pre-incubation with siRNA to EGFR inhibited EGFR activation in response to ATF and also inhibited EGFR phosphorylation in response to EGF. Response to AG1478 is shown for comparison (D). Transfection with adenovirus containing the Gβγ inhibitor βARKCT (GβγI) inhibited EGFR activation in response to ATF, but had no effect in response to EGF. Values are the mean±SEM of the ratio of the phosphorylation of EGFR relative to the total unphosphosphorylated EGFR (n=6, * p<0.05, **p<0.01 compared to DMEM control). A representative Western blot is shown above the graphs.
To determine if ATF activation of EGFR is via a transactivation mechanism, we examined the role of HB-EGF on EGFR activation in response to ATF. EGFR phosphorylation by ATF was blocked by pre-incubation with heparin and by blockade of the ligand HB-EGF with the HB-EGF inhibitor CRM197 (Fig 4). This suggests that HB-EGF is involved and that it is being ligated by a protease, can bind the EGFR extracellularly and induce EGFR phosphorylation. Incubation with an antibody against HB-EGF blocked the ATF but not the EGF-mediated activation of EGFR (Fig 4). Direct blockade of the EGFR with an anti-EGFR antibody prevented activation by both ATF and EGF (Fig 4). We tested whether plasmin had a role in ATF signaling to EGFR. Blockade of metalloprotease activity with the general inhibitor GM6001, at a level that inhibited MMP-2/MMP-9 activity on gelatin zymography, did significantly inhibit EGFR phosphorylation (Fig 5). EGFR phosphorylation by EGF was not interrupted by the presence of inhibitors of plasmin or MMPs (Fig 5). Given the fact that we demonstrated that intracellular processes (Gβγ) and MMPs are involved, we tested whether A Disintegrin And Metalloproteinase Domains (ADAM) are involved. ADAMs span the membrane and can be activated intracellularly to mediate an extracellular MMP action. VSMC were incubated with the ADAM inhibitors TAPI-0 (20 μM) and TAPI-1 (10 μM) and both blocked EGFR activation by ATF but had no effect on EGFR activation by EGF (Fig 5). The VSMC cells express ADAM-9, ADAM-10 and ADAM-12. We examined their contribution to EGFR activation by the use of siRNA to ADAM-9, ADAM-10 and ADAM-12. SiRNA reduced ADAM-9, -10, -12 proteins levels by >50% (Fig 6).. Incubation with siRNA ADAM-9 and -10 significantly reduced EGFR activation in response to ATF. Scrambled siRNA and siRNA against ADAM-12 had no effect (Fig 6). When the quantity of HB-EGF released by ATF was measured in the presence of the metalloprotease inhibitor, GM6001 and the ADAM inhibitors, TAPI-0 and TAPI-1, there was a significant reduction in the concentration of HB-EGF in the media (Fig 6).. This response was reproduced in the presence of siRNA to ADAM-9 and -10; siRNA to ADAM-12 and a scrambled control had no effect (Fig 6). When we pre-incubated VSMC with siRNA to ADAM-9 and -10 in the Boyden chamber, we saw a 70% decrease in migration compared to the scrambled siRNA and control in response to ATF (Fig 6).
Figure 4.
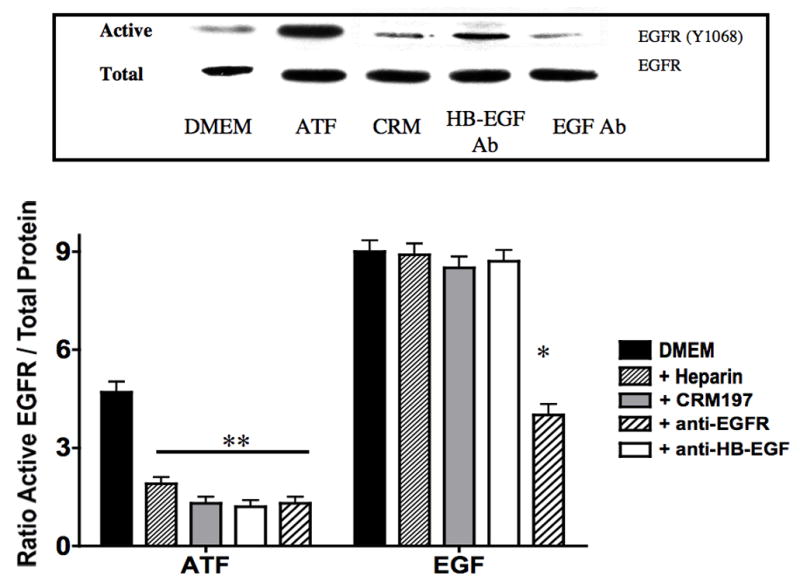
EGFR phosphorylation by ATF (10nM) was inhibited by pre-incubation with heparin (100U/ml), blockade of the ligand HB-EGF with the HB-EGF inhibitor CRM197 and an Anti-HB-EGF antibody. IgG had no effect. EGF-mediated activation of EGFR was not blocked by these inhibitors. Direct blockade of the EGFR prevented activation by both ATF and EGF. Values are the mean±SEM of the ratio of the phosphorylation of EGFR relative to the total unphosphosphorylated EGFR (n=6, **p<0.01 compared to control). Representative Western blots are shown above the graphs.
Figure 5.
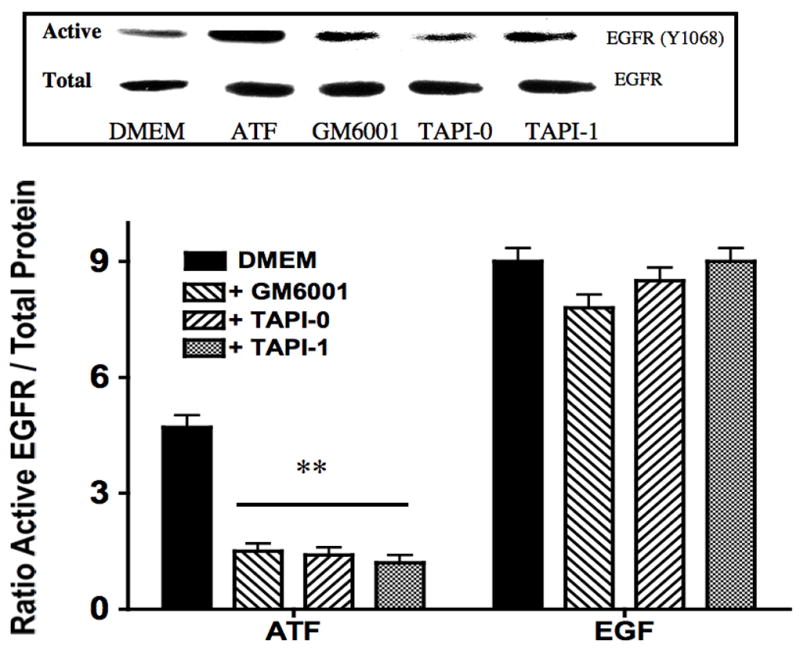
ATF (10nM) induced EGFR phosphorylation which was inhibited by the matrix metalloprotease inhibitor (GM6001) and the A Disintegrin And Metalloproteinase Domains (ADAM) inhibitors (TAPI-0 and TAPI-1). Values are the mean±SEM of the ratio of the phosphorylation of EGFR relative to the total unphosphosphorylated EGFR (n=6, *p<0.05 **p<0.01 compared to control). Representative Western blots are shown above the graphs.
Figure 6.
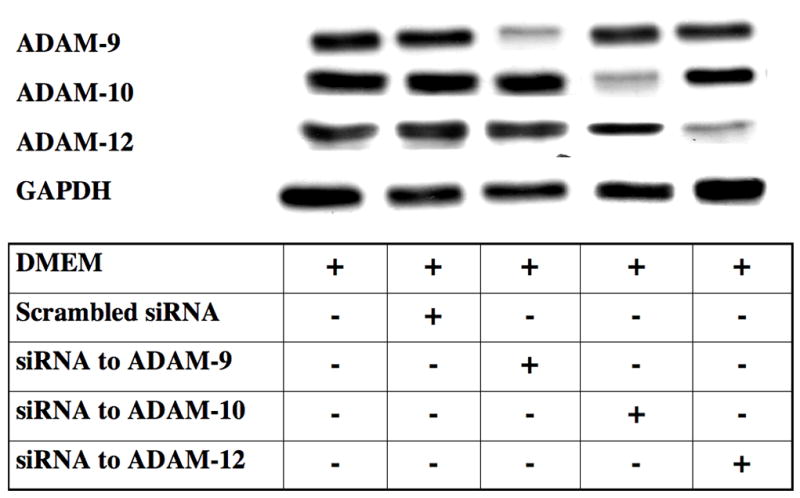
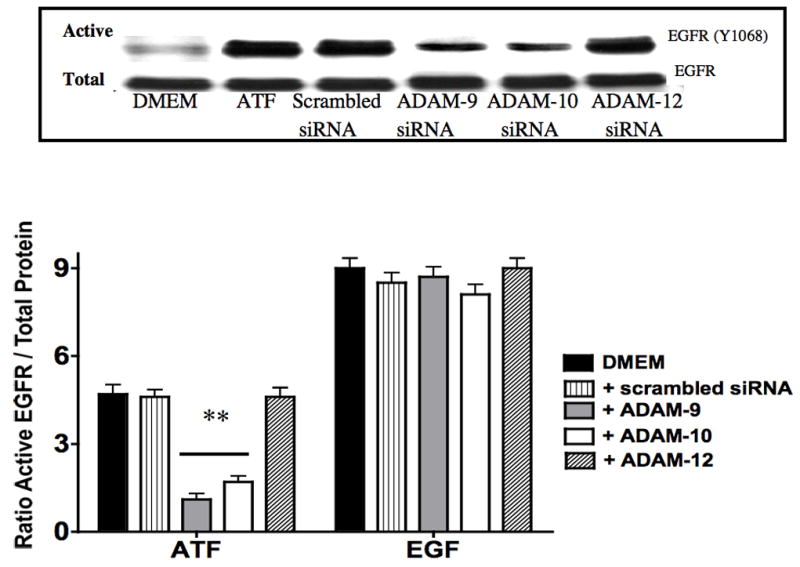
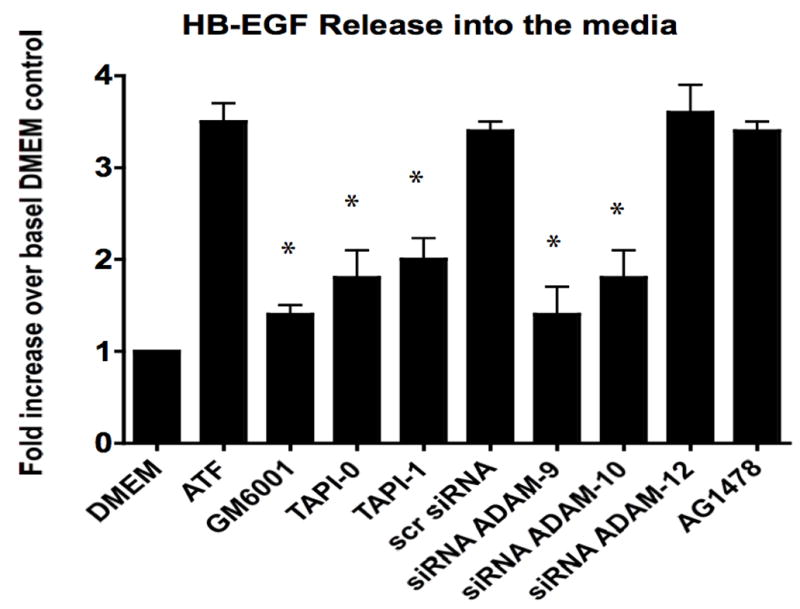
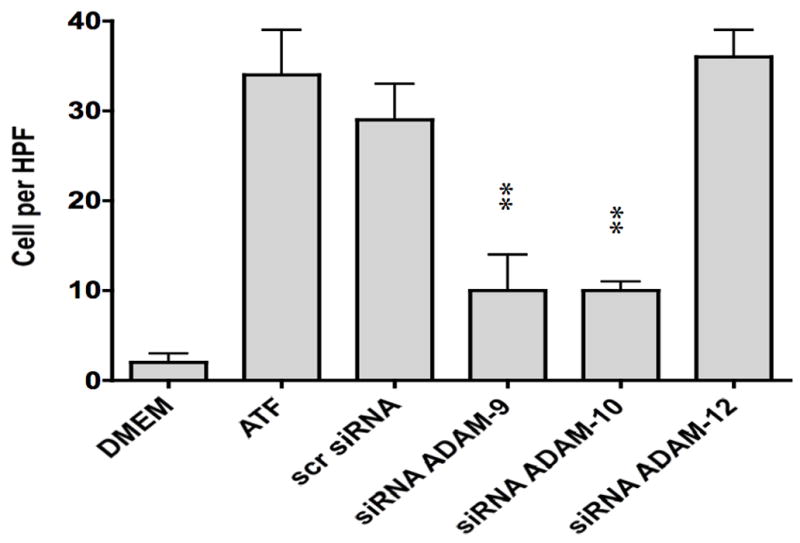
(A) siRNA to ADAM-9, -10 and -12 resulted in a ~50% decrease in their respective A Disintegrin And Metalloproteinase Domains (ADAM) protein expression compared to DMEM control or scrambled siRNA. This was specific. (B) Incubation with siRNA ADAM-9 and -10 significantly reduced EGFR activation in response to ATF. Scrambled siRNA and siRNA against ADAM-12 had no effect. (C) Incubation with GM6001, TAPI-0 and TAPI-1 and siRNA to ADAM-9 and -10 markedly decreased HB-EGF release from smooth muscle cells into the media. (D) Incubation with siRNA to ADAM-9 and -10 markedly decreased smooth muscle cells migration compared to scrambled siRNA in the Boyden chamber in response to ATF. Values are the mean±SEM (n=6, * p<0.05, **p<0.01 compared to control).
DISCUSSION
This study demonstrates that ATF requires EGFR for cell migration and that this response is mediated by Gβγ G-proteins and by ADAM-9 and -10. ADAMs in turn release the tethered ligand HB-EGF by a metalloprotease mediated process to activate EGF. This is distinct from our published findings with the findings with the intact molecule (6). Kalmes et al have shown that thrombin, another coagulation factor, will also mediate EGFR dependent VSMC migration and induce EGFR activation through an extracellular pathway, which involves MMP-mediated, HB-EGF release, and subsequent activation of the EGFR (16). The EGFR/ErbB family is composed of four receptors tyrosine kinases: ERB1/EGFR, ErbB2/neu, ErbB3 and ErbB4, each of which consists of a glycosylated ligand binding domain, a single transmembrane domain and a cytoplasmic tyrosine kinase domain (17, 18). Eleven different ligands have been identified for this receptor family; EGF, TGF-α, amphoregulin, HB-EGF, betacellulin, epiregulin, epigen bind to activate EGFR/ErbB1; betacellulin and in some cases HB-EGF activate ErbB4 in addition to ErbB1. Neuregulins are ligands for ErbB3 and ErbB4. Although no ligand for ErbB2 has been identified it appears to act as a cofactor for ErbB1, ErbB3 and ErbB4 forming heterodimers with the respective receptors. These heterodimers enhance and diversify downstream signal transduction pathways. ErbB1 has been studied in VSMC and is expressed in the injured vessel wall (19, 20). In the rat carotid injury model, blockade of EGF with an antibody will reduce intimal hyperplasia development (21). In a murine injury model, deletion of uPA reduced intimal hyperplasia while deletion of PAI-1 enhanced intimal hyperplasia (22–25). Thus EGFR and uPA both appear to be necessary for vessel remodeling. All ErbB ligands are synthesized as transmembrane precursors and reside in a tethered fashion on the exxtracellular membrane. They can then in turn be proteolytically cleaved to release biologically active soluble growth factors, which act in an autocrine and/or paracrine manner. HB-EGF is expressed in atherosclerotic lesions (26, 27) and in restenotic lesions following angioplasty (28–30). The present study examined HB-EGF as HB-EGF has been shown to stimulate the growth and migration of VSMC under appropriate conditions. (29, 31). Activation of HB-EGF requires that it be cleaved from the membrane and also requires cell surface heparan sulphate proteoglycans to act as a co-receptors; HB-EGF binding to EGFR can be antagonized in a dose dependent manner by heparin (32). We saw a similar inhibitory effect with heparin in this study. Our data using CRM197 and antibodies against HB-EGF and EGFR support the presence of the extracellular pathway using HB-EGF.
Receptor transactivation is the process, whereby activation of a given receptor activates a heterologous receptor (12). Both G-protein coupled receptors and receptor linked tyrosine kinases can induce rapid phosphorylation of the EGFR, and suppression of this EGFR activation leads to reduced MAPK activation (12–14). At present, the triple membrane passing signaling (TMPS) mechanism of GPCR induced EGFR activation is a widely accepted model of receptor linked tyrosine kinases transactivation (12). In this model, there is a sequence of three transmembrane signaling events: G-protein coupled receptor activation followed by MMP activation, and subsequent activation of the EGFR by HB-EGF, or other latent ligands of the EGFR. Several different MMPs have been identified with HB-EGF release: ADAM-10 (A Disintegrin And Metalloproteinase Domain), ADAM-12, ADAM-17 (TNF-α converting enzyme) and MMP3 (33). Activation of these proteases pathway has been shown to involve one or more of the following signaling molecules: src, intracellular calcium, and protein kinase C (12). An alternative mechanism may be inactivation of protein tyrosine phosphatases due to the generation of oxygen free radicals by the NAD(P)H oxidase complex. In the current study, we have identified both intracellular and extracellular components of transactivation. Transmembrane molecules such as ADAM are attractive molecules for the transmembrane portion of the signaling pathway. ADAM are approximately 70 to 90 kDa (mature proteins; the unprocessed precursors are about 20 kDa larger due to their prodomain). They feature a common modular ectodomain structure, encompassing a variable stalk region; a cysteine-rich domain, a disintegrin domain binding to integrin-class cell adhesion molecules and a Zn-binding metalloprotease domain. ADAM-12, ADAM-9, ADAM-10, ADAM-17 have been reported to be involved in the ectodomain shedding of proHB-EGF (34, 35). We have shown that the VSMC express ADAM-9, ADAM-10 and ADAM-12. Furthermore, we have identified that the predominant pathways that lead to HB-EGF release are ADAM-9 and ADAM-10 in these particular VSMC. Little is known of the role of ADAM in atherogenesis and vessel remodeling.
Conclusion
ATF requires EGFR activation during cell migration. ATF induces transactivation of EGFR by an ADAM-mediated, HB-EGF dependent process. This is the first description of crosstalk via ADAM between ATF and EGFR in VSMC.
Acknowledgments
Funded by the U.S. Public Health Service HL086968 and HL67746 awarded to MG Davies
Supported by: U.S. Public Health Service HL086968 and HL67746
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies MG, Hagen P-O. Pathobiology of Intimal Hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 2.Strauss BH, Lau HK, Bowman KA, Sparkes J, Chisholm RJ, Garvey MB, et al. Plasma urokinase antigen and PAI-1 antigen levels predict angiographic coronary restenosis. Circulation. 1999;100(15):1616–22. doi: 10.1161/01.cir.100.15.1616. [DOI] [PubMed] [Google Scholar]
- 3.Nicholl SM, Roztocil E, Davies MG. Plasminogen Activator System and Vascular Disease. Curr Vasc Pharmacol. 2006;4(2):101–16. doi: 10.2174/157016106776359880. [DOI] [PubMed] [Google Scholar]
- 4.Blasi F, Carmeliet P. uPAR: a versatile signaling orchestrator. Nature Cell Biology. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 5.Preissner KT, Kanse SM, May AE. Urokinase receptor: a molecular organizer in cellular communication. Curr Opin Cell Biol. 2000;12:621–628. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 6.Nicholl SM, Roztocil E, Davies MG. Urokinase (uPA) induced smooth muscle cell responses require distinct signaling pathways: a role for the epidermal growth factor receptor. J Vasc Surg. 2005;41:672–81. doi: 10.1016/j.jvs.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Stepanova V, Mukhina S, Kohler E, Resink TJ, Erne P, Tkachuk VA. Urokinase plasminogen activator induces human smooth muscle cell migration and proliferation via distinct receptor dependent and proteolysis-dependent mechanisms. Mol Cell Biochem. 1999;195:199–206. doi: 10.1023/a:1006936623106. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen DH, Hussaini IM, Gonias SL. binding of uPA to its receptor in MCF-7 cells activates ERK1 and 2 which is required for increased motility. J Biol Chem. 1998;273:8502–8607. doi: 10.1074/jbc.273.14.8502. [DOI] [PubMed] [Google Scholar]
- 9.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, et al. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci U S A. 2002;99(3):1359–64. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanski WJ, Fegley AJ, Roztocil E, Davies MG. Domain dependent actions of urokinase on smooth muscle cell responses. J Vasc Surg. 2004;39:214–22. doi: 10.1016/s0741-5214(03)01031-0. [DOI] [PubMed] [Google Scholar]
- 11.Galaria II, Nicholl SM, Roztocil E, Davies MG. Urokinase-induced smooth muscle cell migration requires both PI3-K/akt and PI3-K/ERK1/2 activation. J Surg Res. 2005;127:46–52. doi: 10.1016/j.jss.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4(8):651–7. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- 13.Pierce KL, Tohgo A, Ahn S, Field ME, Luttrell LM, Lefkowitz RJ. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J Biol Chem. 2001;276(25):23155–60. doi: 10.1074/jbc.M101303200. [DOI] [PubMed] [Google Scholar]
- 14.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 15.Tanski WJ, Nicholl SM, Kim D, Fegley AJ, Roztocil E, Davies MG. Sphingosine-1-Phosphate-induced smooth muscle cell migration involves the Mammalian Target of Rapamycin. J Vasc Surg. 2005;39:91–98. doi: 10.1016/j.jvs.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 16.Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ Res. 2000;92–8(2):92–8. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- 17.Barnes CJ, Kumar R. Biology of the epidermal growth factor receptor family. Cancer Treat Res. 2004;119:1–13. doi: 10.1007/1-4020-7847-1_1. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr Opin Cell Biol. 2003;15(2):143–8. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 19.Pastore CJ, Isner JM, Bacha PA, Kearney M, Pickering JG. Epidermal growth factor receptor-targeted cytotoxin inhibits neointimal hyperplasia in vivo: results of local versus systemic administration. Circ Res. 1995;77:519–529. doi: 10.1161/01.res.77.3.519. [DOI] [PubMed] [Google Scholar]
- 20.Trieu VN, Narla RK, Myers DE, Uckun FM. EGF-genistein inhibits neointimal hyperplasia after vascular injury in an experimental restenosis model. J Cardiovasc Pharmacol. 2000;35:595–605. doi: 10.1097/00005344-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Chan AK, Kalmes A, Hawkins S, Daum G, Clowes AW. Blockade of the epidermal growth factor receptor decreases intimal hyperplasia in balloon-injured rat carotid artery. J Vasc Surg. 2003;37(3):644–9. doi: 10.1067/mva.2003.92. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Moons L, Herbert J-M, Crawley J, Lupu F, Lijnen R, et al. Urokinase but not tissue plasminogen activator mediates arterial neointima formation in mice. Circ Res. 1997;81:829–839. doi: 10.1161/01.res.81.5.829. [DOI] [PubMed] [Google Scholar]
- 23.Carmeliet P, Moons L, Lijnen HR, Baes M, Lemaitre V, Tipping P, et al. Urokinase-generated plasmin is a candidate activator of matrix metalloproteinases during atherosclerotic aneurysm formation. Nat Genet. 1997;17:439–44. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Moons L, Lijnen R, Janssens S, Lupu F, Collen D, et al. Inhibitory role of PAI-1 in arterial wound healing and neointima formation. Circulation. 1997;96:3180–3191. doi: 10.1161/01.cir.96.9.3180. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P, Moons L, Ploplis V, Plow E, Collen D. Impaired arterial neointima formation in mice with disruption of the plasminogen gene. J Clin Invest. 1997;99(2):200–8. doi: 10.1172/JCI119148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakata A, Miyagawa J, Yamashita SNM, Tamura R, Yamamori K, Nakamura T, et al. Localization of heparin binding growth factor-like growth factor in human coronary arteries: possible roles of HB-EGF in the formation of coronary atherosclerosis. Circulation. 1996;994:2778–2786. doi: 10.1161/01.cir.94.11.2778. [DOI] [PubMed] [Google Scholar]
- 27.Reape TJ, Wilson VJ, Kanczler JM, Ward JP, Burnard KG, Thomas CR. Detection and cellular localization of heparin-binding epidermal growth factor-like growth factor mRNA and protein in human atherosclerotic tissue. J Mol Cell Cardiol. 1997;29:1639–1648. doi: 10.1006/jmcc.1997.0399. [DOI] [PubMed] [Google Scholar]
- 28.Igura T, Kawata S, Miyagawa J, Inui Y, Tamura S, fukuda K, et al. Expression of HB-EGF-like growth factor in neointimal cells induced by balloon injury in rat carotid arteries. Arterioscler Thromb Vasc Biol. 1996;16:1524–1531. doi: 10.1161/01.atv.16.12.1524. [DOI] [PubMed] [Google Scholar]
- 29.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–F199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 30.Miyagawa J, Higashiyama S, Kawata S, Inui Y, Tamura S, Yamamoto K, et al. Localization of heparin EGF-like growth factor in the smooth muscle cells and macrophages of human atherosclerotic plaque. J Clin Invest. 1995;95:404–411. doi: 10.1172/JCI117669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev. 2001;81(3):999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 32.Bresner GE, Whelton D, Crissman-Combs MA, Steffen CL, Kim GY, Brigstock DR. Interaction of heparin-binding EGF-like growth factor (HB-EGF) with the epidermal growth factor receptor: modulation by heparin, heparinase or synthetic heparin-binding HB-EGF fragments. Growth Factors. 1992;7:289–296. doi: 10.3109/08977199209046411. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;1997(272):31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- 34.Nanba D, Higashiyama S. Dual intracellular signaling by proteolytic cleavage of membrane-anchored heparin-binding EGF-like growth factor. Cytokine Growth Factor Rev. 2004;15:13–19. doi: 10.1016/j.cytogfr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]


