Abstract
Signal transducer and activator of transcription (STAT) 6 is a molecule involved in interleukin (IL)-4 and-13 signalling. We investigated the role of STAT6 signalling in Toxoplasma gondii-infected mice using STAT6-deficient (STAT6−/−) and wild-type (WT) mice. A significantly larger number of cysts were recovered from the brain in STAT6−/− than in WT mice on days 28 and 56 post-infection. CD8+ T cells in cerebrospinal fluid and spleen stimulated with T. gondii antigen produced higher levels of interferon (IFN)-γ in WT than in STAT6−/− mice. CD8+ T-cell function, estimated by expression of CD25 and cytotoxic activity, was lower in STAT6−/− than in WT mice. Transfer of CD8+ but not CD4+ T cells, purified from infected WT mice, into STAT6−/− mice successfully prevented formation of cysts in the brain. However, transfer of naïve CD8+ T cells from WT into STAT6−/− mice did not show either activation of CD8+ T cells or a decrease in the number of cysts in the brain. Transfer of splenic adherent cells from WT into STAT6−/− mice induced activation of CD8+ T cells and decreased the number of cysts in the brain. Expression of CD86 on splenic dendritic cells and IL-12 p40 production were weaker in STAT6−/− than in WT mice after T. gondii infection. These results indicate that STAT6 signalling is important in CD8+ T-cell activation, possibly through regulation of antigen-presenting cells, which could suppress T. gondii infection in the brain.
Keywords: CD8+ T cell, signal transducer and activator of transcription 6, Toxoplasma gondii
Introduction
Toxoplasma gondii is an intracellular protozoan parasite that infects humans and other mammals. After peroral infection, T. gondii affects multiple organs including the spleen, liver, heart, lung and brain. The ensuing immune responses eliminate the pathogen from most organs, but not from the brain, where the parasite persists with development of chronic toxoplasmic encephalitis (TE).1 This intracerebral parasite is regulated by interferon-γ (IFN-γ)-producing CD4+ and CD8+ T cells, which are recruited into the brain.2 CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to TE.3,4 CD8+ T cell-or IFN-γ-deprived mice are unable to control both acute and chronic toxoplasmosis.5,6
In contrast to the important role of IFN-γ, the role of interleukin-4 (IL-4) in T. gondii infection is still unclear. Significantly greater acute focal inflammation with tachyzoites and a larger number of cysts in the brain were observed in IL-4-deficient (IL-4−/−) than in wild-type (WT) mice on days 28 and 56 post-infection (pi). Mortality was also higher in IL-4−/− mice compared with WT mice during the late stage of infection. These results indicate that IL-4 is protective against TE by preventing formation of cysts and proliferation of tachyzoites in the brain.7 In contrast, another group reported that IL-4−/− mice were resistant to T. gondii infection, showing a higher survival rate than WT mice during the early acute phases of infection. Pathology in the small intestine was less severe in IL-4−/− mice although conversely liver pathology was greater than in WT mice.8
Intracellular signalling mechanisms provide the link between binding of a cytokine with its receptor and the effect of the cytokine on cellular function. The janus kinase (JAK) and signal transducer and activator of transcription (STAT) family plays a critical role in the signalling of many cytokine receptors. The IL-4 receptor (IL-4R) is associated with JAK1-3 and STAT6. STAT6-deficient (STAT6−/−) mice are unable to process IL-4R-induced signals.9,10 Furthermore, IL-13, which is closely related to IL-4 in biological function, shares receptor components and signalling through the STAT6 pathway with IL-4.11
In this study, the role of STAT6 signalling in cyst formation, TE in the brain and the immune response following T. gondii infection was investigated.
Materials and methods
Animals
STAT6−/− mice were donated by Dr S. Akira (Osaka University, Suita City, Japan)10 and backcrossed to C57BL/6 (B6) mice at least 10 times. Six-week-old WT B6 mice were purchased from Clea Japan (Tokyo, Japan). Animals were housed in polycarbonate cages and fed with a commercial diet (Funabashi Farm, Chiba, Japan) in the Shinshu University Animal House. All mice were maintained under a 12:12 hr light/dark cycle (lights on at 9:00 am) at 24 ± 2° and 55 ± 10% relative humidity. The Animal Ethics Committee of Shinshu University approved all protocols used in this study.
Monoclonal and polyclonal antibodies
Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) against murine CD4 (RM4-5), CD8 (53-6·7), B220 (RA3-6B2), CD11b (M1/70), CD11c (HL3), CD25 (7D4), CD44 (IM7), CD62L (MEL-14), H-2Db (KH95) and IFN-γ (XMG1·2) were purchased from BD Biosciences (San Jose, CA). Phycoerythrin (PE)-conjugated mAbs against CD4 (RM4-5), CD8 (53-6·7), CD11c (HL3), I-Ab (25-9-7), CD40 (3/23), CD80 (16-10A1) and CD86 (GL1) and PE-Cy5-conjugated anti-CD4 (RM4-5) mAb were also purchased from BD Biosciences. Rat immunoglobulin G (IgG) was purchased from Sigma-Aldrich (St Louis, MO).
Monoclonal Abs against murine CD4 (GK1·5) and CD8 (53-6·7) were purified as described previously.12 Mice were injected intraperitoneally with anti-CD4 and anti-CD8 mAbs (0·5 mg/week) to deplete CD4+ and CD8+ T cells, respectively. Control mice received rat IgG. Depletion was confirmed by flow cytometric analysis.
Infection, cyst count and antigen preparation of T. gondii
An avirulent Fukaya strain and a virulent RH strain of T. gondii were donated by Dr N. Watanabe (Jikei Medical School, Tokyo, Japan). The Fukaya strain was maintained in B6 mice. Brains of infected mice were gently homogenized with a tissue homogenizer (UltraTurax; IKA-WERK, Staufen, Germany). Cysts were enriched by centrifugation of the homogenate. They were counted under a microscope in portions of precipitates that were smeared on cover-slipped glass slides. Mice were orally inoculated with brain homogenate containing 10 cysts using a stomach tube with a 1-ml syringe.13
Tachyzoites of the RH strain were collected from the peritoneal cavity of B6 mice injected 4 days previously. Toxoplasma gondii crude antigen was prepared by sonication of the tachyzoites followed by centrifugation.14 The supernatant was stored as T. gondii antigen at −30° until use.
Histopathological evaluation
Mice were killed on days 14, 28 and 56 pi and their heads were fixed in 10% neutral buffered formalin solution. The cranium was then decalcified by immersion in K-CX (Fujisawa Co., Osaka, Japan) for 16 hr, washed with tap water for 12 hr and embedded in paraffin. Sections of 5 μm thickness were cut. Serial sections at 500-μm intervals were stained with haematoxylin and eosin. The histopathological changes in the brain were evaluated under a microscope.13
Collection of cerebrospinal fluid (CSF)
Mice were anaesthetized with an intraperitoneal injection of pentobarbital sodium (30 mg/kg; Dainippon Sumitomo Pharma, Osaka, Japan) and perfused through the heart with phosphate-buffered saline (PBS) to remove contaminating intravascular leucocytes. CSF was harvested by suboccipital puncture as follows.13 The dura mater above the cisterna magna was exposed and cut. Then, 10–15 μl of CSF per mouse was aspirated with a micropipette. Total CSF cells were stained with Turk solution and counted under a microscope. The percentages of CD4+, CD8+, B220+ and CD11b+ cells were determined by flow cytometric analysis on cells stained with FITC-conjugated respective mAbs.
Culture of CSF cells and splenocytes
CSF cells (2 × 104 cells/well) in 200 μl or a single suspension of splenocytes (4 × 106 cells/well) in 1 ml of RPMI-1640 medium containing 10% fetal calf serum (FCS), penicillin (100 IU/ml), streptomycin (100 μg/ml), and amphotericin B (0·25 mg/ml) were incubated with T. gondii antigen (12 μg/ml) at 37° in a humidified atmosphere of 5% CO2 and 95% air for 48 hr. In order to block activation of CD4+ and/or CD8+ T cells in culture conditions, mAbs against murine CD4 (GK1·5) and/or CD8 (53–6·7) were added to the culture medium at 5 μg/ml before T. gondii antigen stimulation.12 Supernatants were then collected and stored at −30° until use.
Flow cytometric analysis
Single nucleated cell suspensions of spleen, CSF and peripheral blood were stained with fluorescence-labelled mAbs for 30 min on ice in the dark after lysing erythrocytes with ammonium chloride solution. Cell populations were analysed by FACSCalibur (BD Biosciences).
Determination of cytokines using cytometric bead array (CBA)
The cytokine concentrations in CSF and culture supernatants of splenocytes and CSF cells were determined using a CBA mouse inflammation kit (BD Biosciences) according to the manufacturer's instructions.15 The concentration of IFN-γ was also determined using the CBA Flex set (BD Biosciences). IL-12 p40 was determined using an OptEIA enzyme-linked immunosorbent assay (ELISA) set (BD Biosciences).
Intracellular IFN-γ staining
Splenocytes and CSF cells were incubated with T. gondii antigen for 24 hr as described above. Brefeldin A (10 μg/ml) was added during the last 4 hr of incubation. Cells were harvested, and stained with PE-Cy5-labelled anti-CD4 mAb and PE-labelled anti-CD8 mAb, followed by fixation with paraformaldehyde. After washing, cells were permeabilized with saponin and stained with FITC-labelled anti-IFN-γ mAb. Double-stained cells were analysed by flow cytometric analysis.
Separation and transfer of CD8+ T cells, splenic adherent cells (SACs) and dendritic cells (DCs)
CD8+ T cells were purified from splenocytes using a nylon wool column and magnetic beads. Briefly, 1 × 108 splenocytes were added to an autoclaved nylon wool column and incubated at 37° for 1 hr. The non-adherent T cells were collected by washing the column. Then, CD8+ T cells were separated by indirect negative selection using Dynabeads M-450 (Dynal, Oslo, Norway), which bind to sheep anti-rat IgG after treatment of the cells with anti-CD4 mAb (GK1·5). The purity of CD8+ T cells was > 95% as determined by flow cytometric analysis. Then, 5 × 106 CD8+ T cells were injected into the tail vein of STAT6−/− mice at the indicated time.
SACs were separated from splenocytes by attachment to a polystyrene dish. Bone marrow (BM)-derived DCs were generated as described previously.16 Briefly, BM cells were harvested from the femur of mice, and cultured at 1 × 105 cells/ml in the presence of 10 ng/ml recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF; Endogen, Woburn, MA). Every 2 days, non-adherent cells were discarded and the remaining cells were fed with fresh medium containing 10 ng/ml GM-CSF. On day 6, loosely adherent cells were harvested by gentle pipetting. The purity of the cell population was determined to be more than 80% CD11c+ by flow cytometry. SACs (1 × 106 cells/mouse) and DCs (1 × 106 cells/mouse) were injected into the tail vein of STAT6−/− mice before T. gondii infection.
Cytotoxic analysis
Cytotoxic activity of CD8+ T cells was analysed as described previously with some modifications.17 Briefly, peritoneal macrophages were harvested by lavage from untreated B6 mice. They were washed three times with PBS and labelled with PKH67 (Sigma-Aldrich). The cells were incubated with an optimal concentration of T. gondii antigen in 5% CO2 and 95% air at 37° for 1 hr. They were then washed three times and incubated with purified CD8+ T cells at various effector to target cell (E:T) ratios at 37° for 3·5 hr. Dead cells were determined by flow cytometry following labelling with 7-amino-actinomycin D (7-AAD) (BD Biosciences).
Statistical analysis
Statistical analysis of the data was performed using Student's t-test. A value of P <0·05 was accepted as indicating significance.
Results
Histopathological evaluation and cyst burden in the brain
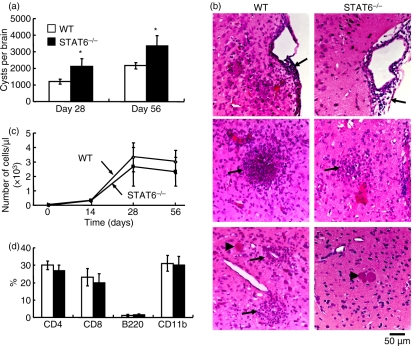
STAT6−/− and WT mice were orally infected with 10 cysts of an avirulent Fukaya strain of T. gondii. Less than 10% of infected mice died between days 10 and 14 pi. The number of cysts in the brain was greater in STAT6−/− than in WT mice on days 28 and 56 pi (Fig. 1a). Before day 14 pi, little evident inflammatory change was observed in the brains of STAT6−/− and WT mice (data not shown). However, on day 28 pi, cellular infiltrate was observed in the subarachnoid space and cerebral cortex in both STAT6−/− and WT mice (Fig. 1b). It was relatively mild in STAT6−/− compared with WT mice, in contrast to cyst burden. Cysts were not surrounded by inflammatory cells. The number of CSF cells increased after infection and peaked on day 28 pi, and then decreased in both STAT6−/− and WT mice (Fig. 1c). The total number of CSF cells in STAT6−/− mice was comparable to that in WT mice. Monocytes and CD4+ and CD8+ T cells were present in CSF, and there was no difference in the the percentage of each population between STAT6−/− and WT mice (Fig. 1d).
Figure 1.
Cyst burden, histopathological findings and immune responses in the brain. (a) Brains were removed on days 28 and 56 pi, and the number of cysts was counted under a microscope. Data are expressed as mean ± standard deviation (SD) (n = 5). Similar results were obtained from five replicate experiments. (b) Representative photographs of the brain on day 28 post-infection (pi) are shown. Sections were fixed and stained with haematoxylin and eosin. Arrows indicate cellular infiltration in the subarachnoid space and cerebral cortex. Arrow heads indicate Toxoplasma gondii cysts. Note that the area of inflammation is wider in wild-type (WT) than in STAT6−/− mice. (c) Cerebrospinal fluid (CSF) was obtained from uninfected and infected mice on days 14, 28 and 56 pi. Nucleated cells were stained with Turk solution, and the total number of cells was counted. (d) Classification of cells in CSF was determined by flow cytometric analysis. Data are expressed as mean ± SD (n = 5). Similar results were obtained from three replicate experiments. *Significantly different from WT mice (P <0·05).
IFN-γ concentration in CSF and in vitro production by CD8+ T cells were reduced in T. gondii-infected STAT6−/− mice
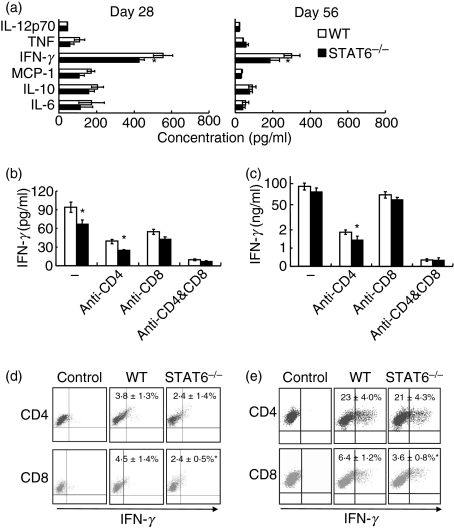
Inflammatory cytokine levels in CSF were determined on days 14, 28 and 56 pi. On day 14 pi, no significant difference was observed in levels of IL-6, IL-10, monocyte chemotactic protein (MCP)-1, IFN-γ, and tumour necrosis factor (TNF), which were very low in STAT6−/− and WT mice. However, on days 28 and 56 pi, their concentrations in CSF were markedly increased, and the IFN-γ level was significantly lower in STAT6−/− than in WT mice (Fig. 2a). After in vitro cultivation of CSF cells with T. gondii antigen, IFN-γ production by CD8+ but not CD4+ T cells and the percentage of IFN-γ-producing cells in CD8+ T cells were greater in WT than in STAT6−/− mice (Fig. 2b and 2d). Splenocytes recovered on day 28 pi were cultured with T. gondii antigen in vitro. IFN-γ production by CD4+ T cells in STAT6−/− mice was comparable to that in WT mice. The percentage of IFN-γ-producing CD4+ T cells in STAT6−/− mice was not different from that in WT mice, whereas IFN-γ production by CD8+ T cells was significantly lower in STAT6−/− mice than in WT mice. The percentage of IFN-γ-producing CD8+ T cells was also significantly lower in STAT6−/− mice than in WT mice (Fig. 2c and 2e). These results indicate that IFN-γ production by CD8+ T cells, but not by CD4+ T cells, was systemically reduced in T. gondii-infected STAT6−/− mice.
Figure 2.
Production of interferon (IFN)-γ by T cells in the cerebrospinal fluid (CSF) and spleen. (a) CSF was taken from signal transducer and activator of transcription (STAT) 6−/− and wild-type (WT) mice on days 28 and 56 post-infection (pi). Concentrations of cytokines in CSF were determined using a cytometric bead array (CBA) kit. IFN-γ level was significantly lower in STAT6−/− as compared with WT mice. Data are mean ± standard deviation (SD) (n = 5). CSF cells (b) and splenocytes (c) were taken from STAT6−/− and WT mice on day 28 pi, and were incubated with Toxoplasma gondii antigen. Anti-CD4 and/or anti-CD8 monoclonal antibodies (mAbs) (5 μg/ml) were added to the culture to block activation of CD4+ and/or CD8+ T cells. The IFN-γ concentration in culture supernatants was determined using a CBA kit. Data are mean ± SD (n = 5). CSF cells (d) and splenocytes (e) were incubated with T. gondii antigen, and stained for T-cell markers and IFN-γ. Cells without antigen stimulation were used as a control. Numbers indicate the percentage of IFN-γ-producing cells in CD4+ and CD8+ T cells. Data are mean ± SD (n = 5). Experiments were carried out three times with similar results. *Significantly different from WT mice (P <0·05).
Activated CD8+ T cells were decreased in STAT6−/− mice
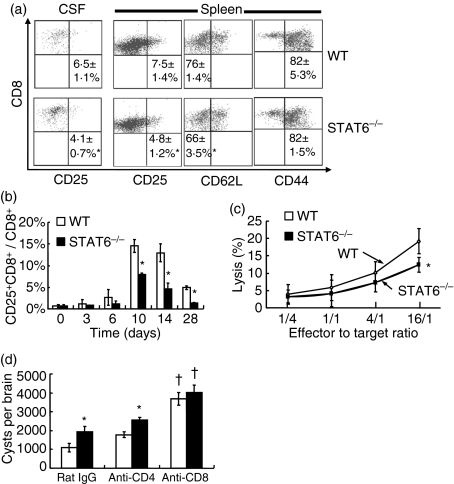
We further investigated CD8+ T cell activation and function during T. gondii infection in STAT6−/− and WT mice. CD25+, CD62Llow and CD44+ CD8+ T cells increased in CSF, spleen and peripheral blood after infection. The percentage of CD25+ CD8+ and CD62Llow CD8+ T cells in CD8+ T cells was significantly lower in STAT6−/− than in WT mice in the CSF and spleen (Fig. 3a). The percentage of CD25+ CD8+ T cells in CD8+ T cells peaked on day 10 pi, and was significantly lower in STAT6−/− mice than in WT mice in peripheral blood (Fig. 3b). In addition, cytotoxic activity of CD8+ T cells in infected mice was significantly lower in STAT6−/− than in WT mice (Fig. 3c). The role of CD8+ T cells in protection against T. gondii cyst formation in the brain was then studied. As shown in Fig. 1c, infiltration of T cells into the brain started on day 14 pi. Therefore, we injected anti-CD8 mAb on days 14 and 21 pi to deplete CD8+ T cells. Injection of the mAb before day 14 pi induced the death of mice. A significantly larger number of cysts formed in the brains of the anti-CD8 mAb-treated group than in the rat IgG-treated control group in STAT6−/− and WT mice, respectively (Fig. 3d). Depletion of CD4+ T cells had little influence on the number of cysts. No significant difference in number of cysts was observed between CD8+ T cell-depleted STAT6−/− and WT mice.
Figure 3.
Activation of CD8+ T cells after Toxoplasma gondii infection. (a) Cerebrospinal fluid (CSF) cells and splenocytes were taken from mice on day 28 post-infection (pi), and stained with fluorescein isothiocyanate (FITC)-anti-CD25, FITC-anti-CD62L, FITC-anti-CD44 and phycoerythrin (PE)-anti-CD8 monoclonal antibodies (mAbs) and then analysed by flow cytometry. Numbers indicate the percentage of CD25+ CD8+, CD62Llow CD8+ and CD44+ CD8+ T cells in CD8+ T cells in the CSF and spleen. Data are mean ± standard deviation (SD) (n = 4). (b) The percentage of CD25+ CD8+ T cells in CD8+ T cells in peripheral blood is shown. Data are mean ± SD (n = 5). (c) Purified CD8+ T cells and peritoneal macrophages labelled with PKH67 were co-cultured at various effector to target cell (E:T) ratios. Dead cells were determined by flow cytometry after staining with 7-amino-actinomycin D (7-AAD). Data are mean ± SD (n = 4). (d) Toxoplasma gondii-infected mice were intraperitoneally injected with 0·5 mg of anti-CD4 mAb, anti-CD8 mAb or rat immunoglobulin G (IgG) on days 14 and 21 pi. Cysts were counted on day 28 pi. Data are mean ± SD. (n = 5). Experiments were carried out three times with similar results. *Significantly different from WT mice (P <0·05). †Significantly different from rat IgG-treated mice (P <0·05).
Transfer of immune CD8+ T cells was effective in reducing cyst number in the brain of STAT6−/− mice
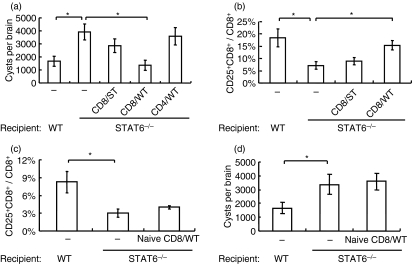
Activation of CD8+ T cells was considered to be important for preventing cyst formation in the brain. Therefore, we transferred CD8+ T cells from either infected or uninfected WT mice into STAT6−/− mice to determine the effects of cyst burden in the brain. Transfer of CD8+ T cells but not of CD4+ T cells, recovered from infected WT mice, into STAT6−/− mice on day 14 pi successfully decreased the formation of cysts in the brain. The number of cysts in the brain of WT CD8+ T cell-transferred mice was comparable to that of WT mice without cell transfer. Transfer of CD8+ T cells from infected STAT6−/− mice showed only a slight reduction in cyst number (Fig. 4a). The percentage of CD25+ CD8+ T cells in CSF CD8+ T cells was higher in WT CD8+ T cell-transferred mice than in STAT6−/− CD8+ T cell-transferred mice (Fig. 4b). Interestingly, transfer of native WT CD8+ T cells (5 × 106 cells/mouse) into STAT6−/− mice that were infected with T. gondii simultaneously resulted in neither an increase in the percentage of CD25+ CD8+ T cells in peripheral blood nor a decrease in the number of cysts in the brain (Fig. 4c and 4d). These results indicate that STAT6 signalling in CD8+ T cells is not important in their activation and that activated CD8+ T cells predominantly suppress cyst formation in the brain.
Figure 4.
Effects of transfer of CD8+ T cells on cyst burden in the brain. (a) Wild-type (WT) CD8+ T cells (CD8/WT), WT CD4+ T cells (CD4/WT) and signal transducer and activator of transcription (STAT) 6−/− CD8+ T cells (CD8/ST) were purified from infected mice on day 14 post-infection (pi). Then 5 × 106 of these cells were injected intravenously into STAT6−/− mice that had been infected 14 days previously. The cyst burden in the brain was then determined on day 28 pi. (b) The percentage of CD25+ CD8+ T cells in CD8+ T cells in cerebrospinal fluid (CSF) was determined on day 28 pi. Data are mean ± standard deviation (SD) (n = 5). (c) Five million CD8+ T cells from uninfected WT mice (naïve CD8/WT) were transferred into STAT6−/− mice which were simultaneously infected with Toxoplasma gondii. The percentage of CD25+ CD8+ T cells in CD8+ T cells in peripheral blood was determined on day 14 pi. (d) Cysts in the brains of these mice were determined on day 28 pi. Data are mean ± SD (n = 5). Experiments were carried out three times, with similar results. *Significantly different (P <0·05).
Activation of CD8+ T cells by antigen-presenting cells (APCs) may be impaired in STAT6−/− mice
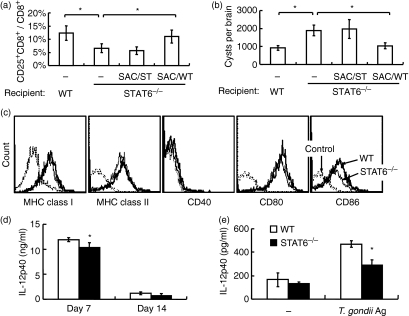
Transfer of SACs from uninfected WT mice into STAT6−/− mice 10 days before infection successfully increased the percentage of CD25+ CD8+ T cells in peripheral blood, and decreased the number of cysts in the brain (Fig. 5a and 5b). Transfer of SACs from uninfected STAT6−/− mice into STAT6−/− mice did not change the percentage of CD25+ CD8+ T cells in peripheral blood or the number of cysts in the brain. Transfer of BM-derived DCs from uninfected WT mice also resulted in a decreased cyst burden in STAT6−/− mice (data not shown). The expression level of CD86 on splenic DCs after T. gondii infection was lower in STAT6−/− than in WT mice (17·5 ± 1·3 versus 21·0 ± 1·1, n = 4, P <0·05) (Fig. 5c). The concentration of IL-12 p40 in serum was significantly lower in STAT6−/− mice on day 7 pi (Fig. 5d). In addition, SACs from infected STAT6−/− mice produced significantly lower IL-12 p40 than those from WT mice after incubation with T. gondii antigen (Fig. 5e).
Figure 5.
Effects of transfer of splenic adherent cells (SACs) on CD8+ T-cell activation and cyst burden. (a) One million SACs derived from uninfected wild-type (WT) (SAC/WT) and signal transducer and activator of transcription (STAT) 6−/− (SAC/ST) mice were injected into STAT6−/− mice. Ten days after injection, mice were infected with Toxoplasma gondii. The percentage of CD25+ CD8+ T cells in CD8+ T cells in peripheral blood was determined on day 14 post-infection (pi). *Significantly different (P <0·05). (b) Cysts in the brains of these mice were determined on day 28 pi. Data are mean ± standard deviation (SD) (n = 5). (c) On day 28 pi, splenocytes were prepared and stained with anti-CD11c and the indicated monoclonal antibodies (mAbs). CD11c+ cells were gated and analysed by flow cytometry. Expression of CD86 was reduced in STAT6−/− as compared with WT mice. Unstained cells were used as controls. The mean fluorescence intensity (MFI) for expression of the molecules in STAT6−/− and WT mice were 154 ± 23 and 190 ± 36 [major histocompatibility complex (MHC) class I], 72 ± 10 and 83 ± 7 (MHC class II), 2·1 ± 0·3 and 1·9 ± 0·1 (CD40), 56 ± 3 and 58 ± 4 (CD80), and 17 ± 1 and 21 ± 1 (CD86), respectively (n = 4). (d) The interleukin (IL)-12 p40 concentration in serum was determined by enzyme-linked immunosorbent assay (ELISA) on days 7 and 14 pi. Data are mean ± SD (n = 5). (e) The IL-12 p40 concentration in culture supernatants of splenocytes was determined by ELISA on day 7 pi. Data are mean ± SD (n = 5). Experiments were carried out three times, with similar results. *Significantly different from WT mice (P <0·05).
Discussion
Our current study has demonstrated that STAT6 signalling is important in activation of CD8+ T cells, resulting in a decrease in cyst number in the brain of T. gondii-infected mice. CD8+ T-cell activation by APCs is possibly impaired in a STAT6-deficient environment.
Recent in vivo studies have shown that IL-4 is critical for the development of protective CD8+ T-cell memory responses against tumours and infections with protozoan parasites such as Leishmania and Plasmodium.18 Cytotoxic T lymphocyte-mediated immune responses against mammary and colon carcinoma were abrogated or did not develop in the absence of IL-4.19 More recently, vaccination studies demonstrated that the development of CD8+ T cell-mediated protective immune responses against Leishmania donovani in mice was fully dependent on IL-4.20 Studies using parasite-specific T cell receptor-transgenic CD8+ T cells revealed a critical role of IL-4 in the generation of memory CD8+ T-cell responses against the liver stages of the rodent malaria parasite Plasmodium yoelii.21 In the present study, we also observed that STAT6 signalling was important in CD8+ T-cell activation in T. gondii-infected mice. However, the underlying mechanism of STAT6 signalling in CD8+ T-cell activation is still unclear.
Two possible mechanisms have been considered for the role of STAT6 signalling in CD8+ T-cell responses. One is that STAT6 signalling might be involved in maturation of APCs such as DCs, and contribute to CD8+ T-cell activation. DCs are the most potent APCs that can activate T cells to induce a primary immune response.22 Recently, IL-4R/IL-13R-associated STAT6 signalling in DC maturation has been well studied and has been shown to have important roles in IL-12 production and activation marker expression by DCs.23–25 STAT6 signalling is constitutively activated in primary immature DCs and progressively declines as the cells differentiate into mature DCs.26 These results suggest that STAT6 signalling may be important in DC maturation. Our current results also suggested an important role of STAT6 signalling in DC maturation and in CD8+ T-cell activation. Expression of an activation marker, CD86, on spleen DCs was lower in STAT6−/− than in WT mice. IL-12 p40 production was lower in STAT6−/− mice. In addition, activation of CD8+ T cells in STAT6−/− mice was impaired, and was restored after transfer of SACs or BM-derived DCs from WT mice.
Another possible mechanism is that STAT6 may directly influence CD8+ T cells. As reported by Marsland et al., CD8+ T cells possess the IL-4 receptor, and STAT6 signalling in these cells is stimulated by IL-4.27 In our experiments, STAT6−/− CD8+ T cells were activated by transfer of WT SACs into STAT6−/− mice. However, naïve WT CD8+ T cells transferred into STAT6−/− mice were not activated. These results indicate that STAT6 signalling not in CD8+ T cells but in APCs is important in the activation of CD8+ T cells in T. gondii-infected mice.
Our results agree with those previous reported by Suzuki et al.7 They demonstrated that cyst number in the brain was greater in IL-4−/− mice than in WT mice in the chronic stage of infection. Their results also showed that IFN-γ production by splenocytes from IL-4−/− mice was comparable to that in WT mice in spite of the expected up-regulation of T helper type 1 (Th1) responses in IL-4−/− mice. However, they did not report IFN-γ production by CD8+ T cells. Mortality was significantly higher, with more severe TE and heavier cyst burden in IL-4−/− than in WT mice. In contrast, Roberts et al. reported that IL-4−/− mice showed significantly higher mortality, with less severe TE and decreased cyst burden compared with WT (129/Sv × B6)F2 mice.28 The same group reported that IL-4−/− mice on a B6 background showed a higher survival rate in spite of a heavier cyst burden and more severe histopathological changes in the liver than WT mice.8 Brain inflammatory responses were less severe in STAT6−/− mice than in WT mice, with a similar mortality rate to that in our study. Taken together, these findings suggest that the virulence of the T. gondii strain and the susceptibility of the mouse strain might influence mortality and pathology. Reciprocal effects of IL-4 on the production of IFN-γ might also be involved in this discrepancy.
CD8+ T cells, once activated, functioned also in STAT6−/− mice, because transfer of CD8+ T cells recovered from infected WT mice into STAT6−/− mice on day 14 pi resulted in a decrease in the number of cysts in the brain, whereas transfer of CD8+ T cells from uninfected WT mice did not show any effect on the formation of cysts in the brain. These results imply that CD8+ T cells, once activated in infected WT mice, regulate the formation of cysts in the brain. Activation of CD8+ T cells is important in the acute phase of T. gondii infection.3,4 Therefore, STAT6−/− mice were considered to be less resistant than WT mice in the acute phase. Surprisingly, anti-CD8 mAb treatment on days 14 and 21 pi resulted in a similar cyst burden in the brain in STAT6−/− and WT mice on day 28 pi, indicating the possibility of no difference in parasite burden in the brain on day 14 pi. This needs to be further clarified.
Our results clearly demonstrate that STAT6 signalling is important in CD8+ T-cell activation, possibly through regulation of APCs, which could suppress T. gondii infection in the brain.
References
- 1.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–22. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–92. [PubMed] [Google Scholar]
- 3.Brown CR, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–41. [PubMed] [Google Scholar]
- 4.Shirahata T, Yamashita T, Ohta C, Goto H, Nakane A. CD8+ T lymphocytes are the major cell population involved in the early gamma interferon response and resistance to acute primary Toxoplasma gondii infection in mice. Microbiol Immunol. 1994;38:789–96. doi: 10.1111/j.1348-0421.1994.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 5.Parker SJ, Roberts CW, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991;84:207–12. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Yang Q, Yang S, Nguyen N, Lim S, Liesenfeld O, Kojima T, Remington JS. IL-4 is protective against development of toxoplasmic encephalitis. J Immunol. 1996;157:2564–9. [PubMed] [Google Scholar]
- 8.Nickdel MB, Lyons RE, Roberts F, Brombacher F, Hunter CA, Alexander J, Roberts CW. Intestinal pathology during acute toxoplasmosis is IL-4 dependent and unrelated to parasite burden. Parasite Immunol. 2004;26:75–82. doi: 10.1111/j.0141-9838.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Tanaka T, Shi W, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Kishimoto T, Akira S. STAT6: its role in interleukin 4-mediated biological functions. J Mol Med. 1997;75:317–26. doi: 10.1007/s001090050117. [DOI] [PubMed] [Google Scholar]
- 12.Takamoto M, Kusama Y, Takatsu K, Nariuchi H, Sugane K. Occurrence of interleukin-5 production by CD4−CD8− (double negative) T cells in lungs of both normal and congenitally athymic nude mice infected with Toxocara canis. Immunology. 1995;85:285–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Harada T, Takamoto M, Jin DH, Tada T, Sugane K. Young C3H mice infected with Toxoplasma gondii are a novel experimental model of communicating hydrocephalus. Neurol Res. 2007;29:615–21. doi: 10.1179/016164107X164201. [DOI] [PubMed] [Google Scholar]
- 14.Lin TM, Halbert SP, O’Connor GR. Standardized quantitative enzyme-linked immunoassay for antibodies to Toxoplasma gondii. J Clin Microbiol. 1980;11:675–81. doi: 10.1128/jcm.11.6.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP., Jr Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–9. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 16.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lecoeur H, Fevrier M, Garcia S, Riviere Y, Gougeon ML. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 2001;253:177–87. doi: 10.1016/s0022-1759(01)00359-3. [DOI] [PubMed] [Google Scholar]
- 18.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med. 2005;202:551–60. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J Exp Med. 1999;189:803–10. doi: 10.1084/jem.189.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stager S, Alexander J, Kirby AC, Botto M, Rooijen NV, Smith DF, Brombacher F, Kaye PM. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med. 2003;9:1287–92. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–70. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Deszo EL, Brake DK, Kelley KW, Freund GG. IL-4-dependent CD86 expression requires JAK/STAT6 activation and is negatively regulated by PKCdelta. Cell Signal. 2004;16:271–80. doi: 10.1016/s0898-6568(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 24.Lutz MB, Schnare M, Menges M, Rossner S, Rollinghoff M, Schuler G, Gessner A. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574–80. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson SH, Yu CR, Mahdi RM, Ebong S, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol. 2004;172:2307–15. doi: 10.4049/jimmunol.172.4.2307. [DOI] [PubMed] [Google Scholar]
- 27.Marsland BJ, Schmitz N, Kopf M. IL-4Ralpha signaling is important for CD8+ T cell cytotoxicity in the absence of CD4+ T cell help. Eur J Immunol. 2005;35:1391–8. doi: 10.1002/eji.200425768. [DOI] [PubMed] [Google Scholar]
- 28.Roberts CW, Ferguson DJ, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]