Abstract
We have recently shown that α-C-galactosylceramide (α-C-GalCer) stimulates invariant natural killer T (iNKT) cells and preferentially induces a T helper 1 (Th1)-type response in mice. However, α-C-GalCer was found to be a rather weak ligand against human iNKT cells in vitro. Therefore, in this study, we sought to identify a compound that displays a strong stimulatory activity against human iNKT cells, by determining the biological activities of several C-glycoside analogues. From the in vitro screening assays, we found that almost all C-glycoside analogues, which have an E-alkene linker between sugar and lipid moieties, are able to activate human iNKT cells and to induce the maturation and activation of human dendritic cells through iNKT-cell activation. In summary, although α-galactosylceramide (α-GalCer) remains the strongest iNKT-cell ligand, our study identified E-alkene-linked C-glycoside analogues as potent human iNKT-cell stimulants, and indicated that these analogues could be used as a therapeutic agent in the future for diseases resolved by Th1-type responses.
Keywords: CD1d, C-glycoside, mouse and human iNKT cells, T helper 1-type response
Introduction
The invariant natural killer T (iNKT) cell is a unique CD1d-restricted T cell with an invariant T-cell receptor (TCR). In mice, the TCRs of most NKT cells comprise an invariant α chain encoded by Vα14 and Jα18 gene segments and a β chain encoded by Vβ8.2, Vβ7 or Vβ2 gene segments. In humans, the semi-invariant TCRs of NKT cells are encoded by Vα24 and Jα18 gene segments, paired with a β chain encoded by the Vβ11 gene.1 Unlike conventional T cells, iNKT cells do not recognize peptide antigens presented by polymorphic major histocompatibility complex (MHC) class I or class II molecules, but rather recognize glycolipid antigens presented by the non-polymorphic MHC class I-like molecule, CD1d.
α-Galactosylceramide (α-GalCer), a glycolipid originally derived from a marine sponge extract, is a well-characterized compound that activates iNKT cells. When stimulated with α-GalCer, iNKT cells rapidly secrete both T helper 1 (Th1)-type cytokines [interferon-γ (IFN-γ)] and T helper 2 (Th2)-type cytokines [interleukin (IL)-4)]. Subsequently, iNKT-cell activation results in the trans-activation of bystander cells, including NK cells that produce IFN-γ 2–24 hr postactivation.2,3 Dendritic cells (DCs) are also trans-activated by α-GalCer.4 Several maturation markers, such as MHC class II, CD80 and CD86, are up-regulated as early as 24 hr postadministration of α-GalCer. Of importance is that α-GalCer exhibited these properties in both murine and human NKT cells in in vitro experiments.
Although α-GalCer appears to possess the potential for a broad application in clinical settings, the concomitant stimulation of IL-4 may counteract the effects of IFN-γ and limit its therapeutic effects.5,6 It has been shown that some α-GalCer analogues with modifications in the lipid chain stimulate a Th1-biased response.7–9 Recently, we reported that a synthetic C-glycoside analogue of α-GalCer, α-C-galactosylceramide (α-C-GalCer), acts as an iNKT cell ligand in vivo, resulting in the induction of a higher level of Th1-type cytokines, IFN-γ and IL-12, and a lower level of IL-4 in mice compared with α-GalCer.10–12 In addition, α-C-GalCer displayed a more potent antimalarial activity and antitumour activity in mice. However, in contrast to α-GalCer, α-C-GalCer did not demonstrate parallel activity in the human iNKT cells in vitro. Interestingly, one study has shown that a 1-methylene-linked C-glycoside analogue, albeit being a less potent agonist for human iNKT cells than α-GalCer and α-C-GalCer, induced cytokine production with the highest IFN-γ:IL-4 and IFN-γ:IL-13 ratios in vitro.13 In the present study, we determined the biological activities of several C-glycoside analogues by a series of immunoassays in order to identify a compound that displays a potent stimulatory activity against human iNKT cells.
Materials and methods
Glycolipid analogues and animals
An α-C-GalCer (CRONY) analogues library was synthesized, as described previously.14 Six-to 8-week-old BALB/c mice and C57BL6 mice were purchased from Taconic (Germantown, NY) and maintained under standard conditions in The Laboratory Animal Research Center of The Rockefeller University.
Isolation and generation of immature human DCs and human iNKT-cell lines
Peripheral blood mononuclear cells (PBMCs) were obtained from Leukopaks (New York Blood Center, New York, NY) and separated by Ficoll–Paque Plus (GE Healthcare Life Sciences, Piscataway, NJ) density centrifugation. For the generation of immature DCs, CD14+ monocytes were first isolated from PBMCs by using CD14 magnetic microbeads (Miltenyi Biotec, Auburn, CA), and then cultured for 5 days in the presence of 20 ng/ml of recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN) and 25 ng/ml of recombinant human IL-4 (R&D Systems). Human iNKT cell lines expressing Vα24 T-cell receptor were generated, as previously reported, with a slight modification.15 Briefly, Vα24+ cells were isolated from PBMCs by using anti-mouse immunoglobulin G (IgG) magnetic beads (Miltenyi Biotec) coupled to a mouse anti-human Vα24 TCR antibody, 6B11 (Beckman Coulter, Fullerton, CA). Vα24+ cells were then cultured with mitomycin C (Sigma-Aldrich, St Louis, MO)-treated homologous immature DCs for 24 hr in the presence of 100 ng/ml of α-GalCer and 10 IU/ml of recombinant human IL-2 (R&D Systems), and further cultured for 7–10 days in the presence of 10 IU/ml of human IL-2.
Quantification of the amount of cytokines produced by human iNKT cell lines by enzyme-linked immunosorbent assay
The levels of IFN-γ, IL-4 and IL-12 produced by human iNKT cell lines were determined by enzyme-linked immunosorbent assay (ELISA) (eBiosciences, San Diego, CA). Twenty-thousand human iNKT cells were co-cultured either with 2 × 104 HeLa cells transfected with the human CD1d gene, or with immature DCs, in the presence of an indicated concentration of each glycolipid. After 24 hr of incubation, the culture supernatants were collected and the concentrations of IFN-γ, IL-12 and IL-4 in the supernatants were determined by ELISA.
Quantification of the relative number of human PBMCs secreting IFN-γ in an enzyme-linked immunosorbent spot-forming cell assay
The relative number of human PBMCs that secrete IFN-γ were determined by an enzyme-linked immunosorbent spot-forming cell assay (ELISPOT), as described previously.16 Briefly, after coating a 96-well Multiscreen-HA plate (Millipore, Billerica, MA) with anti-IFN-γ capture immunoglobulin IgG (Mabtech, Mariemont, OH), 500 000 PBMCs were co-cultured with 5 × 104 autologous immature DCs in the presence of 0·1 μg/ml of each glycolipid. After 24 hr of incubation, the plate was washed and incubated with biotinylated anti-IFN-γ immunoglobulin IgG, followed by the addition of 3-amino-9-ethyl carbazole (ACE) substrate to develop the plate (Mabtech). The numbers of spot-forming cells were counted under a stereomicroscope.
Staining of human iNKT cells with human CD1d:Ig dimeric protein loaded with glycolipids
One microgram of a recombinant human CD1d dimer protein fused to mouse IgG Fc fragment (human CD1d:Ig dimer) was incubated overnight with 0·5 μg of each glycolipid in 20 μl of phosphate-buffered saline (PBS) at 37°. Then, glycolipid-loaded human CD1d:Ig dimer was further incubated with a human iNKT cell line on ice for 30 min. As a positive control, 1 μg of a recombinant mouse CD1d dimer protein fused to mouse IgG Fc fragment (mouse CD1d:Ig dimer) was loaded overnight with 0·5 μg of CRONY, and the complex was further incubated with a mouse iNKT cell hybridoma 1.2.15 After washing with fluorescence-activated cell sorter (FACS) buffer, the cells were stained with phycoerythrin (PE)-conjugated anti-mouse IgG (BD Biosciences, San Jose, CA), as well as with allophycocyanin-conjugated anti-mouse CD3 immunoglobulin IgG (BD Biosciences) in the case of the mouse iNKT cell hybridoma. Acquisition of cells was determined using a fluorescence-activated cell sorter (FACS Calibur; Becton Dickinson, Franklin Lakes, NJ).
Determination of maturation of human DCs: a flow cytometric analysis
Dendritic cell maturation was determined as previously described.10 Briefly, immature DCs were generated as described previously and plated in 96-well flat-bottomed culture plates at a concentration of 3 × 105 cells/well. After incubation with 1 μg/ml of each glycolipid for 2 hr, DCs were co-cultured with human iNKT cells (3 × 104 cells/well) for 16 hr in the presence of 10 IU/ml of recombinant human IL-2. One microgram per millilitre of lipopolysaccharide (LPS) (Sigma) was used as a positive control. Dendritic cell maturation was then monitored by flow cytometry based on the different profile of expression of surface molecules induced. Briefly, we first eliminated dead cells by staining with LIVE/DEAD® Fixable Dead Cell Stain (Invitrogen, Carlsbad, CA), gated out iNKT cells using anti-Vα24–PE (Beckman Coulter) and defined DCs using anti-CD11c–APC (BD Biosciences). Anti-CD86–fluorescein isothiocyanate (FITC) or anti-human leucocyte antigen (HLA)-DR–peridinin chlorophyll protein (PerCP) (BD Biosciences) was used to identify the maturation marker of DCs. Acquisition was performed on a FACS Calibur (Becton Dickinson), and data were analyzed using summit software (DAKO, Glostrup, Denmark).
Determination of serum cytokine concentration
C57BL6 mice were administrated, intravenously, 1 μg of each glycolipid. The sera were collected from mice 2, 6, 12, 24, 48 and 72 hr after the glycolipid treatment, and the serum concentrations of IFN-γ, IL-4 and IL-12 were measured using a sandwich ELISA (eBioscience).
Quantification, by ELISA, of the level of IFN-γ produced by murine splenocytes
Splenocytes were obtained from spleens of BALB/c or C57BL/6 mice, as described previously.10 For the generation of immature DCs, mouse bone marrow cells were cultured for 6 days in the presence of 20 ng/ml of mouse GM-CSF (R&D Systems) and 10 ng/ml of mouse IL-4 (R&D Systems). Culture medium was replaced every other day, and after 6 days of culture, 90% of cultured cells became CD11c+ cells, as determined by FACS analysis. Approximately 5 × 105 splenocytes were co-cultured with 5 × 104 syngeneic immature DCs in the presence of 0·1 μg/ml of glycolipids in a 96-well culture plate. In some experiments, 50 μg/ml of a blocking anti-CD1d immunoglobulin IgG, 1B1 (BD Biosciences), was added to the culture. After 24 hr of incubation, supernatants were collected, and the concentrations of IFN-γ were determined using ELISA.
Data analysis
All data were expressed as the mean ± standard deviation (SD) of triplicate wells from each sample. Statistical analysis of experimental and control data were evaluated using the Student'#x2019;s t-test. A P-value of<0·05 was considered statistically significant.
Human subjects
Human PBMCs from anonymous blood donors were obtained from leukopacks provided by the New York Blood Center (NYBC). The NYBC does not select donors on the basis of gender or race but ensures that all donors are above 18 years of age. Therefore, the work we performed did not require approval from the Institutional Review Board.
Results
Th1/Th2 cytokine production by human iNKT cell lines reacting against glycolipids presented by HeLa-CD1d cell lines
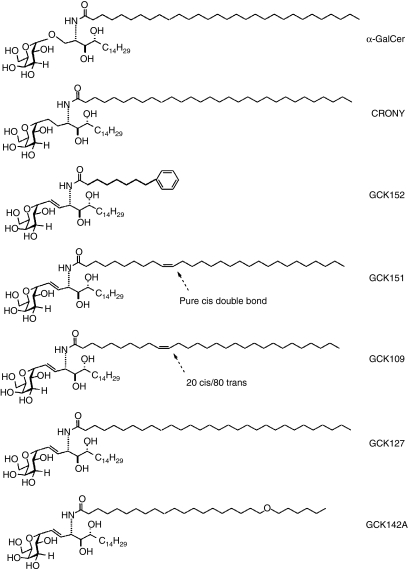
A series of α-C-GalCer analogues was synthesized, all of which consisted of galactose with an α-linked E-alkene connecting to the ceramide portion, which displays a variety of fatty acids in its amide sector (Fig. 1). For the purpose of identifying analogues that can activate human iNKT cells, we first used a human HeLa cell line that expresses human CD1d molecules as an antigen-presenting cell, and co-cultured it with a human iNKT cell line in the presence of several glycolipid analogues. Twenty-four hours later, the concentrations of IFN-γ and IL-4 produced in the supernatants were determined by ELISA. From the first set of screening assays, we found that certain C-glycoside analogues, namely GCK109, GCK151 and GCK152, had strong dose-dependent abilities to induce Th1 and Th2 cytokines, whereas other analogues, such as GCK142A and GCK127, had weaker ability to stimulate iNKT cells to produce Th1 and Th2 cytokines (Fig. 2a). Surprisingly, in contrast to α-GalCer, α-C-GalCer (the parent C-glycoside named CRONY) was unable to induce Th1/Th2 cytokine production by human iNKT cells. Based on these results we selected GCK142A, GCK127, GCK109, GCK151 and GCK152 as candidate compounds and investigated their biological activities in greater detail by additional immunoassays.
Figure 1.
Structures of α-C-galactosylceramide (α-C-GalCer; CRONY) and its analogues, as well as α-galactosylceramide (α-GalCer), tested in this study.
Figure 2.
(a) The levels of cytokines produced by a human invariant natural killer T (iNKT)-cell line co-cultured with a CD1d-expressing HeLa cell line in the presence of glycolipids. After generating human iNKT cell lines from healthy human peripheral blood mononuclear cells (PBMCs), 2 × 104 iNKT cells were co-cultured with 2 × 104 CD1d-expressing HeLa cells in the presence of 0·01, 0·1 or 1 μg/ml of glycolipids in a 96-well culture plate. After 24 hr of incubation, the supernatants were collected and the concentrations of interferon-γ (IFN-γ) and interleukin-4 (IL-4) were determined by enzyme-linked immunosorbent assay (ELISA). (b) The levels of cytokine produced by the human iNKT cell line co-cultured with autologous immature dendritic cells (DCs) in the presence of glycolipids. Immature DCs were generated from CD14+ PBMCs after 5 days of incubation with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4. Approximately 2 × 104 cells of a human iNKT cell line were co-cultured with 2 × 104 immature DCs in the presence of 0·1 μg/ml of glycolipids in a 96-well culture plate. After 24 hr of incubation, supernatants were collected, and the concentrations of IFN-γ, IL-4 and IL-12 were determined by ELISA. In both (a) and (b), error bars represent the standard deviation between triplicate wells. P = NS (indicated by an asterisk) indicates non-significant compared with a negative control as determined using the Student's t-test. The results represent one of three similar experiments using iNKT cell lines derived from different donors.
Cytokine production by human iNKT cell lines reacting against glycolipids presented by immature DCs
It is known that immature mouse DCs can present α-C-GalCer (CRONY) in vivo.10 Therefore, we used autologous immature DCs as an APC to stimulate human iNKT cells. By measuring the levels of the cytokines secreted from activated human iNKT cells, we found that all of the candidate analogues could stimulate human iNKT cells to secrete IFN-γ, IL-4 and IL-12 (Fig. 2b). These results indicate not only that DCs can present the selected compounds to iNKT cells, but also that activated human iNKT cells can, in turn, induce activation of DCs to secrete IL-12. Among the candidates, the highest levels of IFN-γ production by human iNKT cells were induced by GCK109, GCK127, GCK151 and GCK152, and GCK142A induced the production of a lower level of cytokines. CRONY again failed to induce significant levels of the cytokines. These results were corroborated by experiments in which HeLa-CD1d cells were used.
IFN-γ production by human PBMCs reacting against glycolipids presented by immature DCs
In an attempt to determine the relative number of PBMCs that react with glycolipids, we performed an ELISPOT assay ex vivo. In this assay, we co-cultured human PBMCs and autologous immature DCs as APCs in the presence or absence of the glycolipids for 24 hr. Because human PBMCs were used in this assay rather than iNKT cell lines, the assay reflected a more physiological way of assessing the biological activity of the glycolipids in a human system. As a source of PBMCs, we used blood from two different donors. In both donors, GCK109, GCK151 and GCK152 stimulated a high number of PBMCs to secrete IFN-γ, which is comparable to that induced by stimulation with α-GalCer. GCK127 and GCK142A induced a smaller number of PBMCs secreting IFN-γ production (Fig. 3). It is noteworthy that although we were unable to detect any biological activity of CRONY in the preceding bioassays, we found, in an ELISPOT assay, that CRONY stimulated a significant number of PBMCs to secrete IFN-γ. To determine the proportion of iNKT cells that are capable of secreting IFN-γ among PBMCs, we measured the percentage of human iNKT cells by fluorescence-activated cell sorting, using anti-Vα24 immunoglobulin IgG. Peripheral blood mononuclear cells from donor 1 contained 0·45% Vα24+ cells, whereas 0·17% of PBMCs in donor 2 were Vα24+ cells (data not shown). These results indicate that 14 and 10% of Vα24+ cells in donor 1 and donor 2, respectively, secreted IFN-γ in response to α-GalCer, as determined by an ELISPOT assay.
Figure 3.
Relative number of human peripheral blood mononuclear cells (PBMCs) secreting interferon-γ (IFN-γ) in response to glycolipids presented by autologous immature dendritic cells (DCs), as determined by an enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). Approximately 5 × 105 CD14− PBMCs were co-cultured with 5 × 104 immature DCs in the presence of 0·1 μg/ml of glycolipids in a 96-well Multiscreen-HA plate coated with a captured anti-IFN-γ immunoglobulin IgG. After 24 hr of incubation, biotinylated anti-IFN-γ detection immunoglobulin IgG was added to the plate, and the relative number of IFN-γ-secreting cells was determined by developing the color with 3-amino-9-ethyl carbazole (ACE) substrate and counting the number of spot-forming cells under the stereomicroscope. Error bars represent the standard deviation between triplicate wells, and a P-value of<0·05 (shown by an asterisk) indicates a significant difference compared with a negative control, as determined by the Student's t-test. The results show two independent experiments using PBMCs from two different donors.
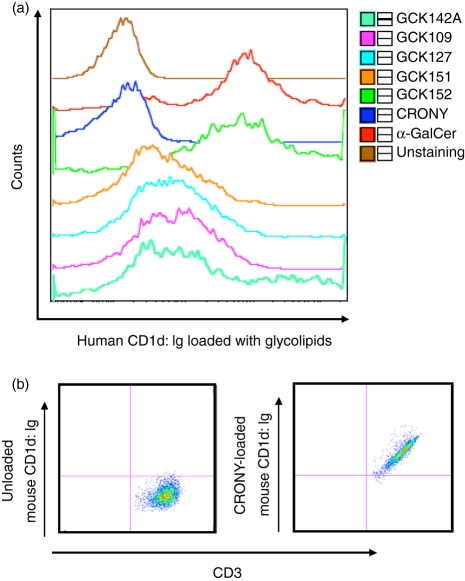
Staining of a human iNKT cell line with human CD1d:Ig dimer loaded with a glycolipid
In order to confirm that human iNKT cells, in fact, recognize the glycolipids in the context of human CD1d molecules through the TCR, we incubated human iNKT cells with a human CD1d-mouse IgG dimeric protein loaded with the glycolipid. Then, we stained the cells with PE-labelled anti-mouse IgG and analyzed them by FACS. With the exception of CRONY, all analogues loaded onto the human CD1d:Ig dimer were able to bind to a human iNKT cell line (Fig. 4a). Notably, a human CD1d:Ig dimer loaded with GCK152 was able to stain human iNKT cells with a much higher intensity than the dimer loaded with other glycolipids. In fact, the staining pattern of human iNKT cells by human CD1d:Ig dimer loaded with GCK152 was superimposable to that loaded with α-GalCer. Consistent with other bioassays, CRONY was found to be a very weak ligand to human iNKT cells, as the human CD1d:Ig dimer loaded with CRONY did not appear to stain human iNKT cells. This lack of apparent staining was not caused by the insoluble nature of CRONY because the mouse CD1d:Ig dimer loaded with CRONY was able to stain mouse NKT cells (Fig. 4b).
Figure 4.
Staining of invariant natural killer T (iNKT) cells with CD1d:Ig dimeric protein loaded with glycolipids was determined using FACS. (a) Approximately 2 × 105 human iNKT cells were incubated for 1 hr at room temperature with 1 μg of a recombinant human CD1d:mouse Ig dimeric protein preloaded overnight with 0·5 μg of each glycolipid. The iNKT cells were then stained with phycoerythrin (PE)-conjugated anti-mouse IgG and analyzed by FACS. The results represent one of three similar experiments in which different human iNKT cell lines were generated from three different donors. (b) Approximately 2 × 105 cells of murine iNKT hybridoma 1.2 cells were incubated for 1 hr at room temperature with 1 μg of a recombinant mouse CD1d:mouse Ig dimeric protein preloaded overnight with 0·5 μg of α-C-galactosylceramide (α-C-GalCer; CRONY). The hybridoma cells were then stained with allophycocyanin-conjugated anti-CD3 immunoglobulin IgG and with PE-conjugated anti-mouse IgG and analyzed by FACS. The results represent one of two similar experiments.
Maturation of human monocyte-derived DCs are promoted by glycolipids
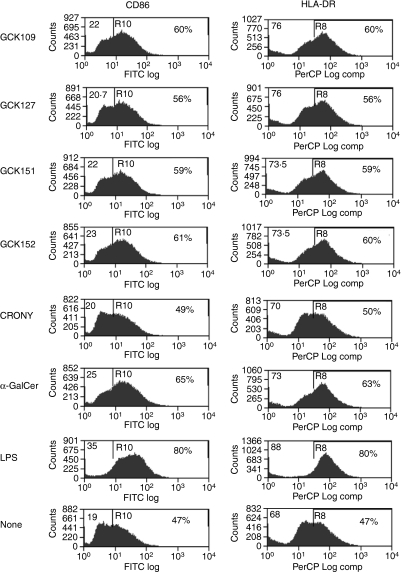
A previous study has shown that CRONY induced the maturation of murine DCs via activation of iNKT cells in vivo.10 From the series of our in vitro assays described earlier, we selected certain glycolipids (i.e. GCK109, GCK127, GCK151 and GCK152), based on their ability to induce a higher level of IFN-γ secretion. These compounds were subjected to an assay that determines their abilities to induce the maturation of human DCs in vitro. For these purposes, we isolated immature human DCs and co-cultured them with human iNKT cells in the presence or absence of glycolipids. By FACS analysis, we found that the expression of DC maturation markers, in particular CD86 and HLA-DR, was strongly up-regulated upon incubation with GCK109, GCK151 and GCK152, and the level of expression was similar to that observed upon incubation with α-GalCer (Fig. 5). These results were in agreement with those obtained when the number of IFN-γ-secreting PBMCs was counted using an ELISPOT assay Fig. 3. GCK127 induced a modest increase in the levels of DC markers, whereas CRONY did not change the levels of any DC markers (Fig. 5). These results indicate that GCK109, GCK151 and GCK152 are potent inducers of human DC maturation.
Figure 5.
The degree of dendritic cell (DC) maturation/activation induced by glycolipids, as determined by fluorescence-activated cell sorter (FACS) analysis. Human immature DCs were first incubated with 1 μg/ml of each glycolipid for 2 hr, as previously described.11 Then, the DCs were co-cultured with human invariant natural killer T (iNKT) cells for 16 hr and the degree of DC maturation/activation was determined by FACS analysis. The CD11c marker was used to gate on DCs, and the expression of DC maturation markers, including CD86 and HLA-DR, was determined. DAKO summit software was used to analyze the data. The results represent one of two similar experiments.
Cytokine production profile of glycolipids in vivo
We determined the levels of cytokines produced in mice as described previously.12 Upon in vivo administration, GCK109 and GCK127 induced a significantly higher level of IFN-γ, IL-12 and IL-4 secretion in the sera than CRONY (Fig. 6a). It is noteworthy that GCK109 and GCK127 induced a twofold higher level of IL-12 than CRONY. GCK151 also induced a significantly higher level of Th1 cytokines than CRONY, but less than that induced by GCK109 and GCK127. Finally, GCK152 failed to induce significant secretion of cytokines.
Figure 6.
(a) The levels of cytokines in the sera of mice administered glycolipids. One microgram of each glycolipid was administered to wild-type C57BL/6 mice intravenously, and the sera were collected at the indicated time-points after administration. The concentrations of cytokines, including interferon-γ (IFN-γ), interleukin (IL)-4 and IL-12, in the sera were determined by enzyme-linked immunosorbent assay (ELISA). The data were expressed as the mean ± standard deviation (SD) of sera from five mice, and the results represent one of two similar experiments. (b) The level of IFN-γ produced by murine splenocytes co-cultured with immature dendritic cells (DCs) in the presence of glycolipids. Immature DCs were generated from bone marrow cells after 6 days of incubation with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4. Approximately 5 × 105 splenocytes from BALB/c or C57BL/6 mice were co-cultured with 5 × 104 syngeneic immature DCs in the presence of 0·1 μg/ml of glycolipids in a 96-well culture plate (black bar). To confirm the restriction specificity to CD1d molecules, 50 μg/ml of a blocking anti-CD1d immunoglobulin IgG, 1B1, was added to the culture (white bar). After 24 hr of incubation, supernatants were collected, and the concentrations of IFN-γ were determined by ELISA. Error bars represent the standard deviation between triplicate wells, and P = NS (indicated by an asterisk) indicates non-significant compared with a negative control, as determined by the Student's t-test.
IFN-γ production by murine splenocytes reacting against glycolipids presented by immature DCs
In order to determine that murine iNKT cells produce IFN-γ in response to glycolipids presented by immature DCs, we co-cultured splenocytes from BALB/c or C57BL/6 mice and respective syngeneic DCs in the presence or absence of each glycolipid for 24 hr, and determined the level of IFN-γ secreted in the culture supernatant by ELISA. Interestingly, GCK109 and GCK127, both of which induced a high level of IFN-γ when administered to mice, were also able to stimulate mouse splenocytes to secrete a high level of the cytokine, whereas GCK152, a none-inducerin vivo, was unable to stimulate the splenocytes to secrete IFN-γin vitro (Fig. 6b). These results indicate that the ability of the glycolipids to stimulate murine iNKT cells to secrete IFN-γin vitro closely correlates with their ability to induce IFN-γin vivo. The addition of a blocking anti-CD1d immunoglobulin IgG to the culture completely abolished the IFN-γ response, confirming the restriction specificity of a glycolipid-induced response by mouse splenocytes (Fig. 6b)
Discussion
We have previously identified α-C-GalCer as a superior iNKT cell agonist because it induced a more Th1-biased response and displayed more potent antimalarial and antitumour activities in mice than α-GalCer.12 The improved antitumour efficacy of α-C-GalCer over α-GalCer appeared to be a result of the ability of α-C-GalCer to bind more stably to DCs, resulting in more prolonged production of Th1 cytokines, such as IL-12 and IFN-γ, but not of IL-4 or tumour necrosis factor-α (TNF-α).10 Our current study has confirmed the previously published data and has shown that α-C-GalCer induced a potent and a prolonged Th1 cytokine profile in mice. Surprisingly, however, when we assessed the activity of α-C-GalCer against human iNKT cells in vitro, this glycolipid was unable to induce a significant level of cytokine production by human iNKT cells. This finding was corroborated by the experiments in which human CD1d dimer loaded with α-C-GalCer was unable to stain human iNKT cells strongly. It is unlikely that the inability of α-C-GalCer to bind to and stimulate human iNKT cells was caused by the solubility of the glycolipid because we demonstrated that α-C-GalCer can stimulate mouse NKT cells considerably to secrete IFN-γin vitro in a CD1d-dependent manner, and that mouse CD1d dimer loaded with α-C-GalCer could stain mouse iNKT hybridoma.
Recently, a crystal structure study revealed some key characteristics of the conformation of the human TCR–α-GalCer–CD1d complex.17–19α-GalCer actually binds to the CD1d antigen-binding groove through its hydrophobic backbone and through hydrogen bonds that involve hydroxy groups in the sugar moiety and in the phytosphingosine backbone, and these interactions stabilize the complex with CD1d. The galactose ring of the lipid extends above the surface of CD1d and is precisely configured for recognition of the α-GalCer–CD1d complex by the TCR of iNKT cells. The X-ray also revealed significant H-bonding interactions between CD1d and the TCR of iNKT cells that were brought into alignment by the galactose recognition event. It is known that α-GalCer and α-C-GalCer have the same lipid moiety, and the only difference is the replacement of the glycosidic O with CH2 in the linker region, thus substituting a weak H-bond acceptor with a bulkier hydrophobic residue. Therefore, we presumed that the alkyl chain and sphingosine chain of α-C-GalCer may fit well in the antigen-binding cleft of both human CD1d and mouse CD1d molecules. However, the activity of α-C-GalCer observed against mouse iNKT cells, but not against human iNKT cells suggests that the O/CH2 group switch in the linker region causes one of two possible alterations: (i) distortion of the galactose so that the galactose head group of α-C-GalCer is accessible to the semi-invariant TCR of murine iNKT cells, but does not fit as well with that of human iNKT cells; or (ii) the CH2 induces changes in conformation of the CD1d-binding surface so it is less well recognized by the TCR of iNKT cells. Interestingly, our study has shown that GCK127, an α-C-GalCer analogue that uses an E-olefin linker instead of a glycosidic CH2 linker, not only exhibited its activity in mice, but also induced a potent stimulatory activity against human iNKT cells. Pertinent to the research on the activities of α-GalCer analogues outlined in this report, the bound conformation of the linker region between the galactose and the ceramide revealed ‘anti’ dihedral angles of ∼170° in the X-ray structure. Our E-analog has this angle fixed at 180°. This indicates that the olefin linker may fit precisely into the CD1d groove and restore the best conformation of sugar moiety, thus allowing the analogue to be recognized well by the TCR of both human and mouse iNKT cells. A complementary explanation might be found in the relative steric effect of the three linkers being compared. The steric repulsion energy of OCH2, or standard glycolipid linker, can be estimated as close to 31 kcal/mole (a recent value for OCH3), whereas the estimate for CH2CH2, our parent C-glycolipid linker, would be close to 37 kcal/mole (the value for CH2CH3), and hence larger and more repulsive than the O material. In contrast, the estimate for CH = CH is 18. This is smaller and less likely to cause repulsions in the hydrophobic CD1d groove where it must fit.20
Some structure–activity related studies suggested that modification on the alkyl and sphingosine chains of α-GalCer may result in different cytokine profiles in human iNKT cells and mouse models.7,9,21–23 OCH, a sphingosine-truncated analogue of α-GalCer, preferentially induced Th2 cytokines from iNKT cells, and administration of OCH suppressed experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) by inducing a Th2 bias in autoantigen-reactive T cells,23,24 because OCH was less stable in binding the CD1d molecule than α-GalCer and exerted short-lived stimulation on iNKT cells.22 Another study suggested that there is a correlation between lipid chain length and cytokine-release profiles, and the chain-shortened glycolipids bias cytokine release toward an immunomodulatory response. This study also suggested that modification of the lipid chain in the α-GalCer structure might favor a Th1 response.9 In addition to shortening the lipid length, the introduction of double bonds into the fatty acyl chain of α-GalCer, C20:1 cis/trans and C20:2 analogues seemed to promote a bias towards Th2 responses.25 Our previous study showed that introduction of an aromatic group to the fatty acyl chain greatly enhanced IFN-γ secretion and displayed significantly greater anticancer potency than α-GalCer, possibly through the alteration of glycolipid–CD1d complex stability.7,21
In the present study, some modifications were introduced to the alkyl chain of GCK127, an E-alkene-linked analogue of α-C-GalCer. For example, GCK152 (an analogue with an aromatic ring in the tail of a shorter alkyl chain), as well as GCK109 and GCK151 (analogues with the addition of an olefin linkage in the alkyl chain), induced a higher level of Th1 cytokine production by human iNKT cells compared with GCK127. GCK152 induced the highest level of Th1 cytokine production by human iNKT cells in vitro. Furthermore, GCK152 and GCK109 were able to induce a higher level of human DC activation than other analogues. On the contrary, GCK142A, an analogue with ether linkage failed to enhance any significant production of Th1 cytokines, which was consistent with our previous findings.14 Most interestingly, although both GCK109 and GCK152 possessed strong biological activity against human iNKT cells, they displayed a sharply contrasting degree of activity against mouse iNKT cells. GCK109, among all analogues tested, not only induced the highest level of Th1 cytokine production in mice, but also exhibited a most potent stimulatory activity against murine NKT cells in vitro. On the contrary, GCK152 failed to induce robust Th1 cytokine production by murine NKT cells either in vivo or in vitro. It is our assumption that the differential activities observed between GCK109 and GCK152 against mouse and human iNKT cells were caused by the structural differences, not only between the TCRs of mouse versus human iNKT cells, but also between human CD1d and mouse CD1d molecules. In this regard, it is noteworthy that by modifying the sugar head group of the ligand, Silk et al. demonstrated a different recognition of ligands by mouse versus human NKT cells.26 We are currently carefully investigating which structural elements dictate the strength of the biological activity of the CD1d-binding glycolipids.
Overall, in this study, we were able to identify α-C-GalCer analogues that possess a superior biological activity, not only in mice but also against human iNKT cells. We hope that this finding will contribute not only to our knowledge on the structure–activity relationship of CD1d-binding glycolipids that act as an iNKT cell ligand, but also to the development of a new therapeutic tool in humans based on these iNKT-stimulating glycolipids.
Acknowledgments
We thank Drs Takayuki Shiratsuchi and Mitchell Kronenberg for providing us with a human CD1d:Ig dimeric protein and a mouse iNKT hybridoma 1.2, respectively, used in this study. We also thank Kirin Brewery Co. for providing α-GalCer. This work was supported by Cytheris and the NIAID Tetramer Facility located at Emory University via a subcontract from NO1-AI-25456-MOD#5.
Glossary
Abbreviations:
- ACE
3-amino-9-ethyl carbazole
- APC
antigen-presenting cell
- CIA
collagen-induced arthritis
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- ELISA
enzyme-linked immunosorbent assay
- ELISPOT
enzyme-linked immunosorbent spot-forming assay
- FACS
fluorescence-activated cell sorter
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HLA-DR
human leucocyte antigen-DR
- IFN-γ
interferon-γ
- IgG
immunoglobulin G
- IL
interleukin
- iNKT
invariant natural killer T cell
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- NYBC
New York Blood Centre
- PBMC
peripheral blood mononuclear cell
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- TCR
T-cell receptor
- Th1
T helper 1
- Th2
T helper 2
- TNF-α
tumour necrosis factor-α
- α-C-GalCer
alpha-C-galactosylceramide
- α-GalCer
alpha galactosylceramide
References
- 1.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–88. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu KO, Porcelli SA. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 4.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa A, Motohashi S, Ishikawa E, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–7. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 6.Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–9. [PubMed] [Google Scholar]
- 7.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci USA. 2007;104:10299–304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savage PB, Teyton L, Bendelac A. Glycolipids for natural killer T cells. Chem Soc Rev. 2006;35:771–9. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]
- 9.Goff RD, Gao Y, Mattner J, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–3. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 10.Fujii S, Shimizu K, Hemmi H, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci USA. 2006;103:11252–7. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franck RW, Tsuji M. Alpha-c-galactosylceramides: synthesis and immunology. Acc Chem Res. 2006;39:692–701. doi: 10.1021/ar050006z. [DOI] [PubMed] [Google Scholar]
- 12.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–41. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Song L, Metelitsa LS, Bittman R. Synthesis and evaluation of an alpha-C-galactosylceramide analogue that induces Th1-biased responses in human natural killer T cells. Chembiochem. 2006;7:1750–6. doi: 10.1002/cbic.200600197. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Chien M, Tsuji M, Franck RW. E and Z alpha-C-galactosylceramides by Julia-Lythgoe-Kocienski chemistry: a test of the receptor-binding model for glycolipid immunostimulants. Chembiochem. 2006;7:1017–22. doi: 10.1002/cbic.200500386. [DOI] [PubMed] [Google Scholar]
- 15.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 16.Fujii S, Shimizu K, Steinman RM, Dhodapkar MV. Detection and activation of human Valpha24+ natural killer T cells using alpha-galactosyl ceramide-pulsed dendritic cells. J Immunol Methods. 2003;272:147–59. doi: 10.1016/s0022-1759(02)00497-0. [DOI] [PubMed] [Google Scholar]
- 17.Borg NA, Wun KS, Kjer-Nielsen L, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–9. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 18.Koch M, Stronge VS, Shepherd D, et al. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 2005;6:819–26. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 19.Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–8. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White D, Anthony J, Oyefeso A. Computational measurement of steric effects: the size of organic substituents computed by ligand repulsive energies. J ORG CHEM. 1999;64:7707–16. [Google Scholar]
- 21.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–3. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 22.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–40. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 24.Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum. 2004;50:305–13. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 25.Yu KO, Im JS, Molano A, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–8. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silk JD, Salio M, Reddy BG, et al. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180:6452–6. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]