Abstract
Human neutrophils express Toll-like receptor 4 (TLR4) at low levels, and the role of this receptor in neutrophil responses to microbial stimuli has been questioned. Genetic manipulation of these cells to enable the study of the role of proteins such as TLR4 in their function is challenging. Here, we show that primary human neutrophils rapidly express novel proteins such as enhanced green fluorescent protein (eGFP) after transduction with lentivirus. Stimulation of transduced neutrophils with lipopolysaccharide (LPS) resulted in increased cell survival, which was inhibited when neutrophils were transduced with a lentivirus encoding a dominant negative (dn) TLR4 protein. LPS-induced survival was also inhibited by lentiviruses encoding dnMyD88 or a truncated TRIF (Toll/interleukin-1R homologous domain-containing adapter protein inducing interferon-β) molecule, whilst, in contrast, neutrophil survival was enhanced by overexpression of kinase-mutated interleukin-1 receptor-associated kinase 1 (kmIRAK-1), which activated nuclear factor (NF)-κB. These studies provide proof of the role of TLR4 in human neutrophil biology, have begun to elucidate TLR-dependent pathways regulating neutrophil survival, and demonstrate that neutrophils can be genetically manipulated to enhance or inhibit survival.
Keywords: inflammation, neutrophil, Toll-like receptor 4
Introduction
The neutrophil is the most numerous of professional phagocytes, with a major role in host defence against bacterial and fungal pathogens. Detection of pathogens is mediated by a variety of pattern-recognition systems, foremost amongst which are the Toll-like receptors (TLRs), and thus these receptors are likely to have important roles in the regulation of neutrophil function.1 We and others have shown that neutrophils express TLR4,2–4 the signalling receptor for lipopolysaccharide (LPS). Whilst several studies have shown that TLR4 signalling results in a range of neutrophil functional responses such as enhanced survival, respiratory burst, and cytokine generation,2–4 other work has questioned the importance of TLR4 in human neutrophil function.5 Interpretation of these data is complicated by the increasing realization that small numbers of contaminating monocytes often present in neutrophil preparations can have a profound influence on the resulting cell phenotype.3,4,6 Dissecting the roles of individual TLRs is also made more challenging by variation in their function between cell types and species.7 For example, purified LPS, selectively activating TLR4, causes transient delays in neutrophil apoptosis,3,4 but in monocytic cells TLR signalling also couples to pro-apoptotic pathways.8
Neutrophils are terminally differentiated and have a short life span. Dissection of the signalling pathways regulating human neutrophil survival has been severely hindered by the challenges of manipulating the gene expression of these cells, and by problems with contamination of cell preparations with highly LPS-responsive monocytes.3 Protein transduction techniques have some experimental utility,9 but much of our knowledge of neutrophil survival pathways either arises from studies in knockout mice, or is inferred from analysis of protein or RNA expression patterns.10
Accordingly, we set out to provide further evidence for a role for TLR4 in human neutrophil function. As part of these studies, we investigated the pathways used by LPS in the regulation of survival in primary human neutrophils, and determined whether a lentiviral gene delivery system could be exploited to investigate the regulation of neutrophil survival.
Materials and methods
Neutrophils were prepared from peripheral blood of healthy volunteers with written informed consent (approved by the South Sheffield Research Ethics Committee), and residual contaminating leucocytes were depleted by negative selection using a custom antibody cocktail as described previously.4 Constructs encoding a dominant negative (dn) TLR4 or the Toll/interleukin-1R homologous (TIR) domain of the adapter TRIF (TIR domain-containing adapter protein inducing interferon-β) were from Invivogen (San Diego, CA), whilst dnMyD88 was a generous gift from Dr Kim Burns (Gent University, Gent, Belgium).11 Constructs for kinase-mutated K239S interleukin-1 receptor-associated kinase 1 (kmIRAK-1)12 and enhanced green fluorescent protein (eGFP) coupled to the Bcl-2 homology 3 (BH3) interacting death domain agonist (GFP-Bid)13 were made in-house. Constructs were subcloned into the pLenti6 destination vector (Invitrogen, Paisley, UK) and packaged using the Invitrogen ViraPower™ lentiviral system. Lentiviruses were concentrated and purified using tangential flow filtration (Minimate™; PAL Corp., Hampshire, UK), and titred in HT1080 cells or by p24 enzyme-linked immunosorbent assay (ELISA) (Cell Biolabs, San Diego, CA).
GFP expression in transduced cells was determined by flow cytometry using a FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ), and data were analysed using flowjo software (Tree Star Inc., Ashland, OR). In experiments investigating cell survival, neutrophils were incubated with lentiviruses for 4 hr to allow novel protein expression, and then stimulated for a further 4 hr with 0·1 ng/ml purified Escherichia coli R515 LPS (Alexis, San Diego, CA) or 50 U/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ). Survival was determined by examination of nuclear morphology on duplicate cytospins at each point as described previously.14 Additionally, modulation of the anti-apoptotic myeloid cell leukaemia-1 (Mcl-1) protein was determined by western blot (anti-Mcl-1; Santa Cruz Biotechnology Inc., Santa Cruz, CA). Protein expression was also determined by western blotting of lysates from neutrophils transduced with empty vector, eGFP, GFP-Bid, kmIRAK-1 or dnTRIF encoding lentiviruses for 4 hr, using antibodies to GFP (Roche Diagnostics, Burgess Hill, UK), Bid, IRAK-1 or TRIF (Cell Signalling, Boston, MA). Blots were probed with anti-actin (Sigma-Aldrich, Dorset, UK) as a loading control. Activation of nuclear factor (NF)-κB was determined by detection of phosphorylated IκBα. Cells were incubated with buffer, 0·1 ng/ml purified LPS, empty vector (eV) or kmIRAK-1 for 1 hr, and then lysates were prepared for detection of phosphorylated IκBα by western blot (anti-phosphorylated IκBα; Cell Signalling). Additionally, cells were incubated with buffer, 10 ng/ml LPS, eV or kmIRAK-1 for 8 hr and then the supernatant was assayed for interleukin (IL)-8 content by ELISA (R&D Systems, Minneapolis, MN). Statistical analysis was performed using prism v5 (GraphPad Software, San Diego, CA) and the tests indicated in the figure legends.
Results
Neutrophils can be transduced with lentivirus to express novel proteins
We sought to determine if neutrophils could be manipulated by lentiviral transduction, with a view to using these techniques to investigate the role of TLR4 in these cells. Initial experiments exposed neutrophils to lentiviral constructs encoding eGFP. The results demonstrated that peripheral blood primary human neutrophils could be successfully induced to express GFP following lentiviral gene delivery. GFP expression was detectable very rapidly, within 1 hr after viral transduction (Fig. 1), and was proportional to the amount of virus applied. In further experiments, the ability of lentiviruses to infect cells from a large panel of donors was examined. The levels of GFP expression achieved varied between donors (Fig. 2), although expression occurred in all donors tested.
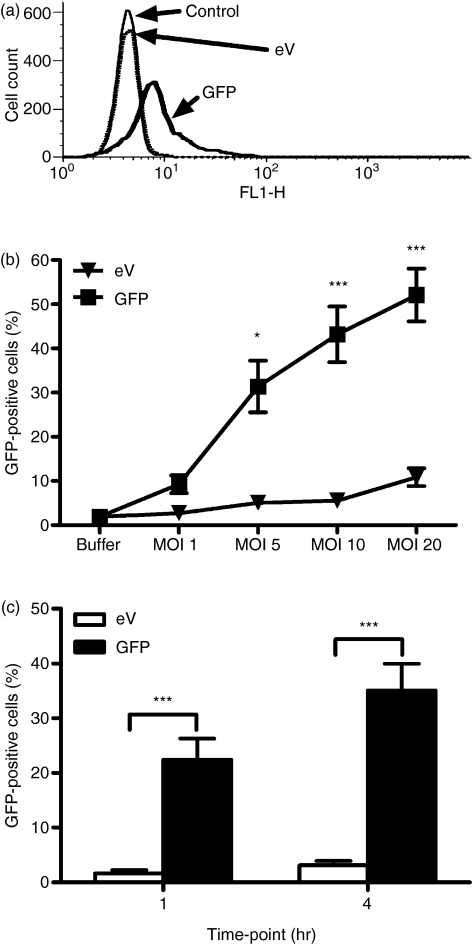
Figure 1.
Neutrophils rapidly express enhanced green fluorescent protein (eGFP) following lentiviral transduction. Highly purified neutrophils were incubated with buffer alone, or lentiviruses encoding either empty vector (eV) or GFP. Expression of GFP was detected by flow cytometry. In (a), fluorescence of neutrophils incubated with the indicated lentiviruses at a multiplicity of infection (MOI) of 10 for 4 hr is shown, measured by flow cytometry, and plotted as a histogram. Data are representative of 19 individual donors shown in Fig. 2. The control histogram (fine line) and eV (dotted line) show similar MFI values, whereas GFP lentivirus (bold line) causes a shift in FL1 fluorescence. For (b) and (c), the percentage of cells taken as being GFP positive was quantified using a marker drawn on the histogram set so that 1% of control (untransduced) cells lay within it. Cells found within the marked region were labelled as GFP positive. (b) Neutrophils were incubated with lentiviruses at an MOI of 1, 5, 10 or 20 for 4 hr and GFP expression analysed by flow cytometry. Data are the mean ± standard error of the mean (SEM) for three (eV) and 10 (GFP) experiments, each from an independent donor. (c) Neutrophils were incubated for 1 or 4 hr with lentiviruses at an MOI of 5. GFP was expressed after 1 hr of viral transduction and expression was increased at 4 hr. Data are the mean ± SEM for seven experiments, each for an independent donor. Significant effects of treatment are indicated by *P < 0·05 and ***P < 0·001, determined by two-way analysis of variance (anova) and Bonferroni post-tests.
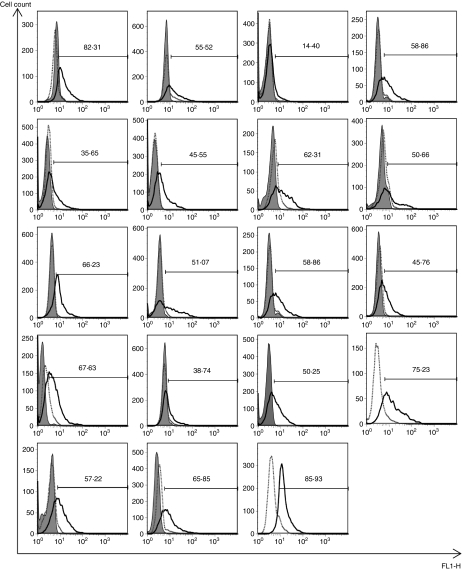
Figure 2.
Expression of enhanced green fluorescent protein (eGFP) in neutrophils from a panel of donors. Highly purified neutrophils were incubated with buffer alone, or with lentiviruses [multiplicity of infection (MOI) of 10] encoding either empty vector (eV) or eGFP. Expression of GFP was detected by flow cytometry after 4 hr. Results are shown for 19 donors (and include donors shown in Fig. 1) where the fluorescence of the cells incubated with media is shown by filled histograms, that of the eV-treated cells by dotted lines, and that of GFP virus-treated cells by a solid-line histogram. The percentage of cells taken as being GFP positive was quantified using a marker drawn on the histogram set so that 1% of control (untransduced) cells lay within it.
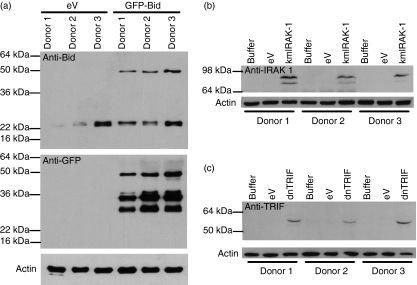
To provide further evidence that novel protein expression in neutrophils could be achieved, neutrophils were transduced with a lentivirus encoding a GFP-Bid fusion protein,13 kmIRAK-112 and dnTRIF, the expression of which was detectable by western blotting in transduced cells (Fig. 3). In the anti-Bid blot, the band at 50 kDa was consistent with expression of the GFP-Bid protein. A band consistent with endogenous Bid (∼22 kDa) was also detected in all lysates. The GFP-Bid fusion protein was also recognized by anti-GFP, with the addition of further bands likely to have arisen from cleavage of the protein. In the anti-IRAK-1 blot, protein was detected only in the transduced cells, consistent with expression of the kmIRAK-1 construct. Full-length TRIF is 98 kDa in size, but here, we used a truncated TRIF, which generated a band appearing at approximately 55 kDa in lysates from dnTRIF-transduced cells probed with anti-TRIF antibody.
Figure 3.
Novel protein expression by neutrophils delivered by lentiviral constructs. Highly purified neutrophils were incubated with (a) buffer, empty vector (eV), or green fluorescent protein (GFP) coupled to the Bcl-2 homology 3 (BH3) interacting death domain agonist (GFP-Bid), (b) kinase-mutated interleukin-1 receptor-associated kinase 1 (kmIRAK-1) or (c) dominant negative Toll/interleukin-1R homologous domain-containing adapter protein inducing interferon-β (dnTRIF) for 4 hr at a multiplicity of infection (MOI) of 5. Following incubation, protein lysates were made and these lysates were analysed by western blot with antibodies to (a) Bid and GFP, (b) IRAK-1 and (c) TRIF. In all cases, actin was run as a loading control. These blots show protein expression for three independent donors, with actin as a loading control.
Role of TLR4 signalling components in induction of neutrophil survival by LPS
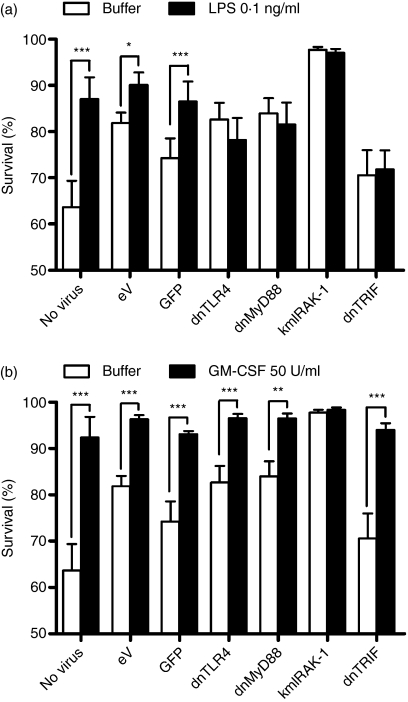
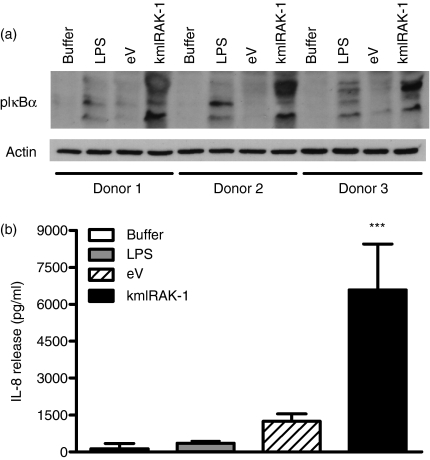
TLR signalling pathways show important variations between primary human cell types. Targeting the TLR signalling adapters impacts substantially on the response to TLR agonists in many tissue cell types.7 In human macrophages, however, manipulation of TLR adapter function is without effect on responses to TLR agonists, for reasons that are as yet poorly understood.7 We therefore examined the role of TLR4 and its signalling pathways in human neutrophil survival, a key mechanism regulating neutrophil function.9,10,14,15 Here (Fig. 4), consistent with our previous data, we show that LPS can induce neutrophil survival. Transduction with lentiviruses induced variable neutrophil survival, evident in the cells that did not receive LPS or GM-CSF (survival was significantly different in untransduced cells versus cells transduced with eV; P < 0·05, n = 5). These observations are consistent with an ability of viral signals to delay neutrophil apoptosis.16 Nonetheless, LPS was still able to enhance survival of neutrophils transduced with the eV, but neutrophils transduced with dnTLR4 showed no additional survival in response to LPS. Expression of the anti-apoptotic protein Mcl-1 was also investigated by western blot but was not found to be increased by LPS, or modulated by eV or dnTLR4 lentiviruses at 8 hr (data not shown). TLR4 uses two principal adapter-coupled signalling pathways, depending in the first on MyD88 and MyD88 adapter-like (Mal), and in the second on TRIF and TRIF-related adapter molecule (TRAM).17 Here, transduction with dnMyD88 or truncated TRIF lentiviruses also inhibited LPS-induced cell survival. None of these viruses inhibited survival induced via the cytokine GM-CSF (Fig. 4). In contrast, neutrophils transduced with a kmIRAK-1 construct that activates IL-1-like signalling 12,18,19 caused enhancement of neutrophil survival, consistent with a role for NF-κB in neutrophil survival.15 Further investigation, using the kmIRAK-1-encoding lentivirus, showed that this construct significantly increased phosphorylation of IκBα, to a greater extent than LPS, and induced IL-8 release from purified neutrophils (Fig. 5).
Figure 4.
Lipopolysaccharide (LPS)-mediated neutrophil survival involves Toll-like receptor 4 (TLR4) and its signalling pathway. Highly purified neutrophils were treated with buffer or the indicated lentiviral particles at a multiplicity of infection (MOI) of 10 for 4 hr, and then stimulated with (a) LPS at 0·1 ng/ml or (b) granulocyte–macrophage colony-stimulating factor (GM-CSF) at 50 U/ml for a further 4 hr. Survival was determined by nuclear morphology on duplicate cytospins for each point. Data are the mean ± standard error of the mean (SEM) for five experime nts [except for green fluorescent protein (GFP) data, which are n = 4], each for an independent donor. (a) LPS caused increased survival in cells treated with buffer, empty vector (eV), or GFP lentiviruses. Cells transduced with dominant negative (dn) TLR4, dnMyD88, or truncated Toll/interleukin-1R homologous domain-containing adapter protein inducing interferon-β (dnTRIF) showed no increased survival in response to LPS. Neutrophils transduced with kinase-mutated interleukin-1 receptor-associated kinase 1 (kmIRAK-1) showed increased survival. (b) GM-CSF induced significant enhancement of neutrophil survival in all cases, except for kmIRAK-1, where there was minimal death at this time-point. Data showing effects of constructs on responses to buffer are repeated in panels (a) and (b), with panels being separated to aid visualization of responses to LPS and GM-CSF. Significant effects of treatment are indicated by *P < 0·05, **P < 0·01 ***P < 0·001, determined by two-way analysis of variance (anova) and Bonferroni post-tests.
Figure 5.
Activation of nuclear factor (NF)-κB by kinase-mutated interleukin-1 receptor-associated kinase 1 (kmIRAK-1)-encoding lentivirus. Highly purified neutrophils were incubated with buffer, lipopolysaccharide (LPS), empty vector (eV) or kmIRAK-1 lentiviruses at a multiplicity of infection (MOI) of 5. In (a), protein lysates were made from cells incubated with viruses for 1 hr, and then analysed by western blot, probing the blots with anti-phospho-IκBα. Phosphorylation of IκBα was induced by both LPS stimulation and transduction with kmIRAK-1. In (b), cells were incubated with the indicated viruses or stimuli for 8 hr, and cell-free supernatants were analysed for interleukin (IL)-8 content. The kmIRAK-1 lentivirus induced a significant amount of IL-8 release compared with cells treated with buffer alone. Data are for three independent donors with a significant effect of treatment indicated by ***P < 0·001, determined by one-way analysis of variance (anova) and Dunnett's post-test.
Discussion
We show here that we could achieve efficient gene delivery to primary human neutrophils with a range of constructs, encoding GFP, GFP-fusion proteins, or dominant negative constructs targeting TLR signalling. Whilst the levels of new gene expression showed variation among donors, neutrophils from all donors studied could be transduced with the lentiviral constructs. Previously, the ability to genetically manipulate primary human neutrophils has been very limited. A semliki forest virus vector has been used to deliver constructs to neutrophils 20 and nucleofection can result in low-level modification of neutrophils.21 Modification of these cells is also possible by delivering modified proteins to neutrophils using transactivator (TAT) protein of HIV.9,22 Our data are the first to show reliable transduction with a readily available vector system, potentially allowing dissection of primary neutrophil function by genetic manipulation.
Given uncertainty about the roles of TLR4 in the neutrophil, and a lack of understanding of the coupling of LPS signalling to transient delays in neutrophil apoptosis, we sought to use dominant negative signalling constructs 23 to investigate the mechanisms of LPS-induced neutrophil survival. We found that exposure of neutrophils to lentiviruses caused some non-specific induction of neutrophil survival, consistent with known antiviral responses of neutrophils.16 Nonetheless, exposure of neutrophils to LPS caused an increased survival in cells treated with control constructs, but not with dnTLR4. Non-TLR responses induced by GM-CSF were not impaired by these viruses, showing specificity of this inhibition. These data provide the first molecular proof of a role for TLR4 in human neutrophil responses to purified LPS and show that the low levels of neutrophil TLR42–5 are indeed important and functional in these cells. Dominant negative constructs targeting MyD88 and TRIF, representing the two major arms of signalling activated by TLR4,17 impaired responses to LPS. We also showed that a construct activating NF-κB, a key neutrophil survival pathway,15 resulted in enhanced neutrophil survival. These data show that LPS-mediated survival involves the TLR4/MyD88/IRAK-1 pathway, which can be manipulated to reduce or enhance survival in response to microbial stimuli.
In conclusion, our work shows for the first time that a readily available gene delivery system can be used to manipulate human neutrophil function. We show that TLR4 is important and functional in these cells, mediating responses to LPS, and that neutrophil survival in response to LPS is mediated by signalling through both the TRIF and MyD88 signalling arms.
Acknowledgments
This work was supported by an MRC Senior Clinical Fellowship (IS), an MRC Clinician Scientist Fellowship (SAR), and a University of Sheffield Studentship (EPD). We thank Dr Lisa Parker and Professor Steven Dower for helpful discussions, and Dr Kathryn Vaughan for assistance with counting cytospins.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Parker LC, Whyte MK, Dower SK, Sabroe I. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J Leukoc Biol. 2005;77:886–92. doi: 10.1189/jlb.1104636. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 3.Sabroe I, Jones EC, Usher LR, Whyte MKB, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 4.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MKB. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 5.Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, Finberg RW. Role of Toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–8. [PubMed] [Google Scholar]
- 6.Sabroe I, Prince LR, Dower SK, Walmsley SR, Chilvers ER, Whyte MKB. What can we learn from highly purified neutrophils? Biochem Soc Trans. 2004;32:468–9. doi: 10.1042/BST0320468. [DOI] [PubMed] [Google Scholar]
- 7.Sacre SM, Lundberg AM, Andreakos E, Taylor C, Feldmann M, Foxwell BM. Selective use of TRAM in lipopolysaccharide (LPS) and lipoteichoic acid (LTA) induced NF-kappaB activation and cytokine production in primary human cells: TRAM is an adaptor for LPS and LTA signaling. J Immunol. 2007;178:2148–54. doi: 10.4049/jimmunol.178.4.2148. [DOI] [PubMed] [Google Scholar]
- 8.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–36. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi AG, Ward C, Dransfield I. Getting to grips with the granulocyte: manipulation of granulocyte behaviour and apoptosis by protein transduction methods. Biochem Soc Trans. 2004;32:452–5. doi: 10.1042/BST0320452. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi SM, Dockrell DH, Renshaw SA, Sabroe I, Whyte MK. Granulocyte apoptosis in the pathogenesis and resolution of lung disease. Clin Sci (Lond) 2006;110:293–304. doi: 10.1042/CS20050178. [DOI] [PubMed] [Google Scholar]
- 11.Burns K, Martinon F, Esslinger C, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–9. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 12.Ross K, Yang L, Dower S, Volpe F, Guesdon F. Identification of threonine 66 as a functionally critical residue of the interleukin-1 receptor-associated kinase. J Biol Chem. 2002;277:37414–21. doi: 10.1074/jbc.M205160200. [DOI] [PubMed] [Google Scholar]
- 13.Renshaw SA, Dempsey CE, Barnes FA, Bagstaff SM, Dower SK, Bingle CD, Whyte MK. Three novel Bid proteins generated by alternative splicing of the human Bid gene. J Biol Chem. 2004;279:2846–55. doi: 10.1074/jbc.M309769200. [DOI] [PubMed] [Google Scholar]
- 14.Renshaw SA, Timmons SJ, Eaton V, Usher LR, Akil M, Bingle CD, Whyte MK. Inflammatory neutrophils retain susceptibility to apoptosis mediated via the Fas death receptor. J Leukoc Biol. 2000;67:662–8. doi: 10.1002/jlb.67.5.662. [DOI] [PubMed] [Google Scholar]
- 15.Ward C, Chilvers ER, Lawson MF, Pryde JG, Fujihara S, Farrow SN, Haslett C, Rossi AG. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem. 1999;274:4309–18. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- 16.Lindemans CA, Coffer PJ, Schellens IM, de Graaff PM, Kimpen JL, Koenderman L. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-kappaB-dependent mechanism. J Immunol. 2006;176:5529–37. doi: 10.4049/jimmunol.176.9.5529. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 18.Knop J, Martin MU. Effects of IL-1 receptor-associated kinase (IRAK) expression on IL-1 signaling are independent of its kinase activity. FEBS Lett. 1999;448:81–5. doi: 10.1016/s0014-5793(99)00322-1. [DOI] [PubMed] [Google Scholar]
- 19.Maschera B, Ray K, Burns K, Volpe F. Overexpression of an enzymically inactive interleukin-1-receptor-associated kinase activates nuclear factor-kappaB. Biochem J. 1999;339:227–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Gardiner EM, Pestonjamasp KN, Bohl BP, Chamberlain C, Hahn KM, Bokoch GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr Biol. 2002;12:2029–34. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JL, Ellis BA, Munafo DB, Brzezinska AA, Catz SD. Gene transfer and expression in human neutrophils. The phox homology domain of p47phox translocates to the plasma membrane but not to the membrane of mature phagosomes. BMC Immunol. 2006;7:28. doi: 10.1186/1471-2172-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi M, Rolle S, Wellner M, Cardoso MC, Scheidereit C, Luft FC, Kettritz R. Inhibition of NF-kB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosis. Blood. 2003;102:2259–67. doi: 10.1182/blood-2002-09-2960. [DOI] [PubMed] [Google Scholar]
- 23.Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv. 2003;3:466–77. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]