Abstract
Signal transducer and activator of transcription 6 (STAT6) expression in lung epithelial cells plays a central role in asthma pathogenesis, with its activation driving the development of airway hyper-reactivity and local inflammation. Therefore, inhibition of local STAT6 expression provides a rationale for therapeutic intervention in bronchial asthma. Given the absence of specific inhibitory drugs, we tested the ability of small interfering RNAs (siRNAs) to target STAT6 gene expression through the molecular process of RNA interference (RNAi). At pico-molar concentrations, STAT6-specific siRNAs potently inhibited STAT6 mRNA expression in lung epithelial cells (50% inhibitory concentration range = 134–861 pm) without inducing cellular interferon responses. Detectable STAT6 protein expression was rapidly abolished within 48 hr of treatment (t1/2 range = < 12–37 hr) and this was unaffected by pretreatment with STAT6-activating cytokines. Furthermore, STAT6 suppression by RNAi produced downstream functional inhibitory effects in that interleukin (IL)-13-or IL-4-driven eotaxin chemokine family [chemokine (C-C motif) ligand 11 (CCL11), CCL24 and CCL26] mRNA expression was markedly inhibited. Induction of detectable CCL26 protein synthesis was completely ablated by pretreating cells with STAT6-specific siRNA. The therapeutic potential of this approach is further demonstrated by novel findings that cells pre-exposed to IL-13 or IL-4 and subsequently treated with STAT6-targeting siRNA exhibited a rapid and significant attenuation of ongoing CCL26 protein expression, suggesting that chronic asthma-associated lung inflammation will be responsive to this approach.
Keywords: asthma, epithelium, RNAi, STAT6
Introduction
Bronchial asthma is a complex inflammatory disorder intimately associated with allergy and the local expression of T helper type 2 (Th-2) cytokines.1 In particular, interleukin (IL)-13 has been identified as a major driver of asthma pathology,2 with experimentally induced local IL-13 expression producing eosinophilic inflammation, mucus hypersecretion, subepithelial fibrosis and airway hyper-responsiveness (AHR).3 Findings that IL-13 expression is markedly up-regulated in bronchial tissues from asthma patients 4–6 further indicate the importance of this inflammatory pathway to active disease. IL-13 binds to a heterodimeric cell-surface receptor composed of the IL-4 receptor α (IL-4Rα) (signalling) chain and the IL-13Rα1 (IL-13-binding) chain,7 with receptor engagement leading to Janus kinase-mediated tyrosine phosphorylation of the IL-4Rα chain with subsequent binding and activation of the transcription factor signal transducer and activator of transcription 6 (STAT6). Analogous to IL-13 data, gene-knockout mouse studies have shown that STAT6 expression plays an obligatory role in allergen-induced airway inflammation 8–10 and, similarly to IL-13, STAT6 expression is up-regulated in bronchial tissues from asthma patients.11,12 Furthermore, STAT6-deficient animals are protected from the pro-asthmatic effects of IL-13 and, importantly, reconstitution of STAT6 expression only in lung epithelial cells was sufficient to allow IL-13-induced AHR and mucus production.9 This illustrates not only the importance of STAT6 expression, but also the recently recognized important role of epithelial cells in asthma pathogenesis.13,14
These findings have identified the IL-13 pathway as a key therapeutic target in asthma.15–17 However, in addition to IL-13, IL-4 can also activate STAT6 via the IL-4 type I receptor (IL-4Rα/γc heterodimer); targeting the principal cellular source of IL-4 – local and circulating Th2 cells – not only poses significant technical challenges but also raises concerns with regard to host immunosuppression. Therefore, given its role as the central regulator of both IL-4 and IL-13 signalling, targeting STAT6 expression in local lung epithelial cells is a more appropriate therapeutic strategy. However, to date, there are no inhibitors capable of targeting STAT6 expression in a specific and non-toxic manner. Given the reported ability of RNA interference (RNAi) to potently inhibit expression of cellular genes,18–21 we hypothesized that appropriately designed small interfering RNA (siRNA) molecules would specifically inhibit STAT6 mRNA in appropriate human lung cells. To test this, we characterized the activities of STAT6-targeting siRNAs in cultured lung epithelial cells. We demonstrate potent inhibition of STAT6 mRNA expression with consequent loss of detectable STAT6 protein expression, even in the presence of ongoing stimulation with STAT6-activating cytokines. Potent suppressive effects on downstream pro-inflammatory pathways are illustrated in that cytokine-mediated induction of eotaxin [chemokine (C-C motif) ligand 11 (CCL11), CCL24 and CCL26] gene expression was markedly inhibited in epithelial cells treated with STAT6-specific siRNA. The therapeutic potential of this approach is further highlighted by findings that siRNA treatment significantly attenuated CCL26 protein expression in cells pre-exposed to eotaxin-inducing cytokines.
Materials and methods
Cell cultures and transfection with siRNA
The human A549 lung epithelial cell line was obtained from European Collection of Cell Cultures (ECACC, Salisbury, UK) and routinely cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) containing 10% fetal calf serum (FCS). Subconfluent cultures were split using trypsin/ethylenediaminetetraacetic acid (EDTA). In supplementary experiments, patient-derived normal human small airway epithelial cells (SAECs) and bronchial smooth muscle cells (BSMCs) were cultured as directed using appropriate Clonetics™ growth medium and BulletKits® (Cambrex BioScience, Workingham, UK). All culture media and reagents, unless otherwise stated, were obtained from Invitrogen (Paisley, UK). For transfection with siRNA, cells were seeded in six-well culture plates at 1·0 × 105 cells/well in appropriate antibiotic-free medium and cultured (37°/5% CO2) for 24 hr prior to transfection. In all cases, adherent cells were transfected with siRNA using Lipofectamine™ (Invitrogen) as recommended by the manufacturers. In certain experiments, cells were cultured in the presence of recombinant human IL-4, IL-13 or interferon (IFN)-γ (PeproTech EC, London, UK) at the final concentrations indicated. Poly (I:C) was obtained from Sigma-Aldrich (Poole, UK). siRNA duplexes targeting human STAT6 mRNA (sequence ID: NM 003153) were designed using recommended guidelines 22 and their specificity was confirmed using NCBI BLAST® (National Center for Biotechnology Information, US National Library of Medicine, Bethesda, MD). Negative control siRNA consisting of a scrambled control (SC) nucleotide sequence with no known homology to the human or mouse genome was similarly designed. All siRNAs were chemically synthesized by Ambion Inc. (Austin, TX). Details of siRNA sequences can be found in the published patent (PCT – GB2005/000721; Materials and Methods for the Treatment of Allergic Disease; Walker W. & Hopkin J. M.).
Gene expression analysis
Total RNA was isolated using Tri® Reagent (Sigma-Aldrich) and first-strand cDNA synthesized from 1 μg of RNA using oligo-dT primers (Protoscript® First Strand cDNA Synthesis Kit; New England BioLabs Inc., Hitchin, UK). The reverse transcription reaction product was amplified in a polymerase chain reaction (PCR) containing SYBR®-Green (iQ SYBR® Green Supermix; Bio-Rad Laboratories, Hemel Hempstead, UK) with accumulation of PCR product quantified in real time using an iCycler iQ Real-time PCR Detection System (Bio-Rad Laboratories). Forward (for) and reverse (rev) primers used for measurement of individual genes were as follows:
STAT6: for 5′-CTTTCCGGAGCCACTACAAG, rev 5′-AGGAAGTGGTTGGTCCCTTT
GAPDH: for 5′-TGCACCACCAACTGCTTAGC, rev 5′GGCATGGACTGTGGTCATGAG
Eotaxin-1: for 5′-CAGCTTCTGTCCCAACCAC, rev 5′-TATCCTTGGCCAGTTTGGTC
Eotaxin-2: for 5′-GGAGTGGGTCCAGAGGTACA, rev 5′-GTGGTTTGGTTGCCAGGATA
Eotaxin-3: for 5′-CCTCCTGAGTCTCCACCTTG, rev 5′-TGGGAGCAGCTGTTACTGGT
2′5′-Oligoadenylate synthetase (OAS)-1: for 5′-AGGTGGTAAAGGGTGGCTCC, rev 5′-ACAACCAGGTCAGCGTCAGAT
Standard PCR reaction conditions were: 95° for 5 min followed by 40 cycles of 95° for 30 seconds/60° for 30 seconds. The expression of each gene was normalized against the expression of the housekeeping gene GAPDH. Gene expression in the STAT6 or SC siRNA treatment was compared with relative expression in the presence of transfection reagent (negative control) using the method described by Pfaffl et al.,23 i.e. normalized fold-change using GAPDH as a reference housekeeping gene (change in cycle threshold, Ct) relative to treatment with transfection reagent (ΔΔCt). For IC50 (i.e. the concentration at which 50% of maximal was achieved) determinations, STAT6 mRNA expression was quantified using the multiplex real-time RT-PCR TaqMan® Gene Expression Assay (Applied Biosystems, Warrington, UK) using STAT6-specifc (FAM) and GAPDH-specific (VIC-TAMRA) probe-primer sets.
Measurement of protein expression by western blotting/enzyme-linked immunosorbent assay (ELISA)/flow cytometry
Western blotting: Protein extracts were prepared from cells treated with Tri® Reagent (Sigma-Aldrich) as described by the manufacturer. Briefly, after removal of the RNA fraction, the organic phase proteins were precipitated using 1·5 ml of isopropanol per ml of Tri® Reagent. After subsequent washes with 0·3 m guanidine hydrochloride in 95% ethanol and finally 100% ethanol, pelleted proteins were solubilized in 1% sodium dodecyl sulphate (SDS) containing protease inhibitor cocktail (P8340; Sigma-Aldrich). The protein cell extract (10 μg) was resolved on denaturing 7% NuPage® Novex Tris-Acetate gels (Invitrogen) using Cruz molecular weight markers (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and Jurkat cell lysate (BD Biosciences, Oxford, UK) as control reference markers. Electrophoresed proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Hybond™-P; Amersham Biosciences, Little Chalfont, UK) using an XCell II blot module (Invitrogen). Human STAT6, phosphorylated STAT6 and GAPDH proteins were detected using rabbit polyclonal antibodies from Santa Cruz Biotechnology Inc. (sc-981, sc-11762 and sc-25778, respectively). Proteins were visualized using Luminol Reagent (sc-2048; Santa Cruz Biotechnology Inc.). Individual band density was determined by scanning with a Molecular Imager Gel Doc XR System (Bio-Rad Laboratories) and analysed using Quantity One1-D analysis software (Bio-Rad). The per cent STAT6 protein expression in treated samples was calculated with reference to transfection reagent-treated cultures (i.e. no siRNA treatment) and was normalized to the expression (density) of GAPDH for each individual treatment.
ELISA: Human eotaxin-1 (CCL11), eotaxin-2 (CCL24), and eotaxin-3 (CCL26) were measured in undiluted harvested samples using the appropriate DuoSet® ELISA Development System (R&D Systems Europe Ltd, Abingdon, UK). In all cases, bound cytokine was visualized using streptavidin-horseradish peroidase (HRP) in conjunction with BD OptEIA™ TMB substrate reagent (BD Biosciences). The cytokine concentration was determined by measuring the optical density (absorbance) of samples at 450 nm and extrapolating values against a standard curve of known cytokine concentrations.
Flow cytometry: Phospho-specific intracellular staining of siRNA-treated cells was performed using standard protocols and analysed using a BD FACSAria™ flow cytometer (BD Biosciences) in conjunction with BD FACSDiVa™ software. Briefly, cells were stimulated with IL-4 or IL-13 (100 ng/ml) for 1 hr at 37° prior to harvest, fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS), permeabilized with 90% methanol and subsequently stained with appropriate phospho-specific antibody: anti-phospho-STAT6 (Y641)-Alexa Fluor® 488 and anti-phospho-STAT1 (Y701)-Alexa Fluor® 488 (BD Biosciences) in staining buffer (PBS containing 1% FCS and 0·1% sodium azide). Appropriate isotype controls were used in all staining protocols.
Statistical analysis
Data from multiple experiments are expressed as mean ± standard deviation. For mRNA fold-change data, differences between groups were examined for statistical significance using the Mann–Whitney U-test. Quantitative measurements were compared using Student's t-test. In all cases, P values ≤0·05 were considered significant.
Results
Characterization of STAT6 siRNA activity
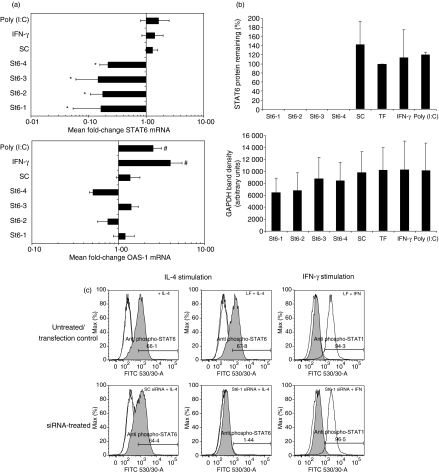
Four siRNAs with sequence complementarity to STAT6 mRNA were initially screened for inhibitory activity. The ability to inhibit STAT6 expression at the mRNA and protein levels was measured using real-time reverse transcription–polymerase chain reaction (RT-PCR), western blotting, and flow cytometric analysis, respectively. When compared with non-specific sequence scrambled control (SC) siRNA, all STAT6-specific siRNA molecules (St6-1–St6-4) were shown to significantly down-regulate STAT6 mRNA expression (Fig. 1a). Confirmation of STAT6 mRNA knockdown was demonstrated in that STAT6 protein levels were undetectable by western blotting in siRNA-treated cultures, 72 hr post-siRNA transfection (Fig. 1b). Furthermore, consistent with these observations, IL-4-driven phosphorylation of STAT6 was abolished in siRNA-treated cells (Fig. 1c). The inhibitory effects were siRNA-specific as neither transfection reagent nor SC siRNA had any significant effect on STAT6 gene expression or cytokine-driven STAT6 activation. Although the siRNA duplexes consist of short double-stranded RNA (21 nucleotides), IFN responses associated with long double-stranded RNA have been reported in some siRNA studies.24,25 We therefore routinely measured expression of the IFN-response gene OAS-1 by RT-PCR and monitored IFN-driven STAT1 activation in cultured cells by flow cytometry. Under standard transfection conditions, siRNA treatment did not up-regulate OAS-1 mRNA expression (Fig. 1a) or induce STAT1 phosphorylation (Fig. 1c), indicating a lack of IFN response induction in siRNA-treated cells. Control cultures indicated that cells were capable of normal IFN responses in that poly (I:C)/IFN-γ treatment induced significant up-regulation of OAS-1 mRNA expression and STAT1 phosphorylation. It should be noted that these observations were consistently reproducible and identical in cultures of normal SAECs and bronchial smooth muscle cells (Supporting information Figs S1 and S2). However, given the expense and relatively short life span associated with these primary cultures, further detailed characterization of siRNA activity was performed in the A549 cell line, which exhibits identical STAT6 expression characteristics to those observed in primary SAECs and BSMCs.
Figure 1.
Signal transducer and activator of transcription 6 (STAT6)-targeting small interfering RNAs (siRNAs) specifically down-regulate STAT6 gene expression without inducing cellular interferon (IFN) responses. A549 cells were transfected with four STAT6-targeting (St6-1, St6-2, St6-3 and St6-4) and scrambled control (SC) siRNAs (20 nm) as described in the text. As controls for IFN response induction, parallel cultures were stimulated in the presence of 50 ng/ml IFN-γ or 10 μg/ml poly (I:C) for 72 hr. (a) Real-time reverse transcription–polymerase chain reaction (RT-PCR) analysis demonstrated significant down-regulation of STAT6 mRNA expression in cells treated with STAT6-targeting siRNA compared with cells treated with SC siRNA (*P ≤ 0·05). Analysis of 2′5′-oligoadenylate synthetase (OAS)-1 expression by RT-PCR showed that only cells treated with IFN-γ or poly (I:C) exhibited significant up-regulation of OAS-1 mRNA expression compared with siRNA-treated cells (#P ≤ 0·05). (b) Densitometry data from western blot experiments (n = 3) illustrating loss of detectable STAT6 protein expression in cells treated with STAT6-targeting siRNA (top panel), 72 hr post-treatment. TF, transfection reagent control. The lower panel shows that mean GAPDH levels (band densities) were not significantly different in siRNA-treated versus non-siRNA-treated cultures (P ≥ 0·05). (c) Flow cytometric analysis of staining with phospho-STAT6 (shaded histograms) or phospho-STAT1 antibodies (unshaded histograms). Cells treated with transfection reagent (LF) or SC siRNA exhibited interleukin (IL)-4 responsiveness (phospho-STAT6 staining) comparable with that of untreated cells (top left panel). In contrast, cells pretreated with STAT6-specific siRNA (representative data showing St6-1 treatment) did not exhibit detectable STAT6 phosphorylation upon stimulation with IL-4. STAT1 activation was only detectable in cultures treated with IFN-γ.
In vitro pharmacokinetics of STAT6-specific siRNA
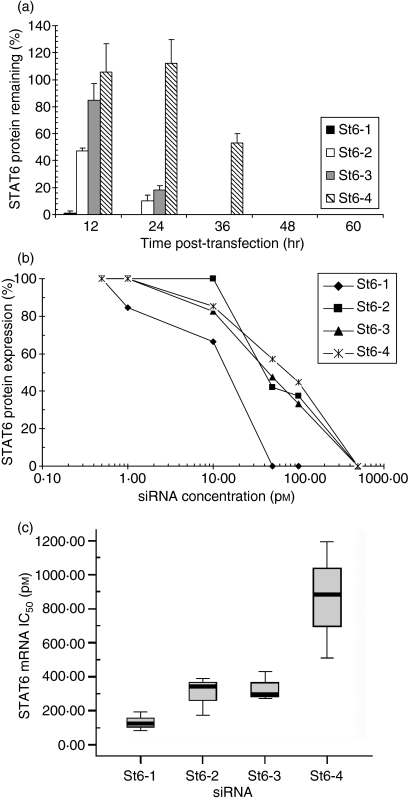
Having demonstrated inhibitory activity, we performed further in vitro analyses to assess the individual efficacy of STAT6-specific siRNA. To determine the kinetics of STAT6 inhibition by individual siRNAs, time–course experiments were performed in which STAT6 protein expression was measured in lung epithelial cells at various time-points after siRNA treatment. This analysis showed that all four siRNAs induced maximal down-regulation of STAT6 protein expression within 48 hr (Fig. 2a). St6-1 siRNA abolished STAT6 expression more rapidly than other tested siRNAs, with loss of detectable STAT6 at 24 hr post-transfection (t1/2 < 12 hr). In contrast, St6-4 siRNA treatment did not induce loss of detectable STAT6 expression until 48 hr post-transfection (t1/2 = 37 ± 0·6 hr). In further experiments, comparative dose–response testing (down to femtomolar quantities) was performed to determine 50% inhibitory concentrations (IC50) for each siRNA at the protein (Fig. 2b) and mRNA levels (Fig. 2c). In agreement with time–course data, this analysis showed that St6-1 siRNA was the most potent inhibitor of STAT6 protein (IC50 = 10 pm) and mRNA expression (IC50 = 134 ± 53 pm). By comparison, St6-4 siRNA was the least potent in terms of inhibitory activity (protein IC50 = 74 pm; mRNA IC50 = 861 ± 342 pm). Differences in IC50 at the protein and mRNA levels presumably reflect differences in the sensitivity of the individual assays. These analyses allow ranking of the siRNAs in terms of inhibitory potency: St6-1 > St6-3 > St6-2 > St6-4. It should be noted that these siRNA molecules are, in general terms, significantly more efficacious than antisense DNA oligonucleotides which typically exhibit IC50 values in the μm range. As St6-1 exhibited superior pharmacokinetics, this candidate siRNA was used in further detailed biological testing of effects on functional IL-13 activity.
Figure 2.
Signal transducer and activator of transcription 6 (STAT6)-specific small interfering RNAs (siRNAs) exhibit potent suppressive effects on STAT6 gene expression. Comparative testing was performed on individual STAT6-targeting siRNAs to determine relative efficacy. (a) Time–course analysis demonstrated rapid elimination of detectable STAT6 protein (western blotting) after treatment with STAT6-targeting siRNA (St6-1, St6-2, St6-3 and St6-4; 20 nm). Per cent protein remaining data (band densities) are normalized to GAPDH and calculated relative to the transfection reagent (TF) control. St6-1 eliminated STAT6 expression within 24 hr (t1/2 < 12 hr) whereas St6-4 exhibited significantly slower STAT6 elimination kinetics (t1/2 = 37 ± 0·6 hr). (b) Dose–response analysis of STAT6 protein expression by western blotting at 72 hr post-transfection showed that all STAT6-targeting siRNAs have activity in the picomolar range: St6-1, 50% inhibitory concentration (IC50) = 10 pm; St6-4, IC50 = 74 pm. The protein data (band density) are normalized to GAPDH expression, with per cent STAT6 expression calculated relative to the TF control. (c) Quantitative analysis of relative STAT6 mRNA expression by TaqMan® RT-PCR. IC50 values were determined from normalized per cent STAT6 mRNA remaining at each siRNA concentration, relative to the TF control. St6-1, IC50 = 134 ± 53 pm; St6-4, IC50 = 861 ± 342 pm.
RNAi of STAT6 is unaffected by the presence of STAT6-activating cytokines
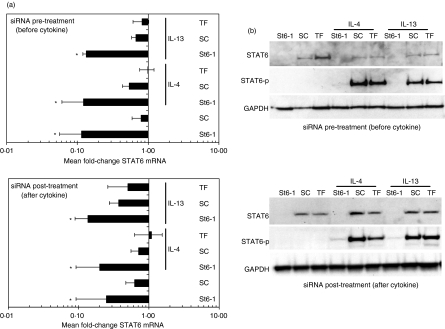
In active disease, mucosal lung cells would be pre-exposed to the predominant pro-asthmatic cytokine IL-13.6 However, as the principal Th2-inducing cytokine IL-4 can also activate (phosphorylate) STAT6, we therefore tested the ability of St6-1 siRNA to suppress STAT6 expression in the presence of either of these cytokines. To allow temporal analysis, A549 cells were either treated with siRNA for 48 hr prior to cytokine stimulation (IL-4 or IL-13: 50 ng/ml) for 24 hr, i.e. siRNA pretreatment, or treated with siRNA after 24 hr of stimulation with cytokine, i.e. siRNA post-treatment. Note that the cytokine was not removed from cultures after addition and in both cases cells were harvested after 72 hr in culture. The kinetics of STAT6 mRNA and protein knockdown in control cultures not stimulated with cytokine were identical to those found in previous experiments, confirming the reproducibility of our observations (Figs 1 and 3). Irrespective of the timing of cytokine treatment, St6-1 siRNA significantly down-regulated STAT6 mRNA expression when compared with SC siRNA (Fig. 3a). These findings were mirrored at the protein level in that St6-1 treatment abolished STAT6 protein expression, irrespective of cytokine stimulation (Fig. 3b). Furthermore, ablation of functional STAT6 activity was confirmed by the absence of phosphorylated STAT6 in St6-1 siRNA-treated cultures by western blotting. In contrast, cells treated with either SC siRNA or transfection reagent exhibited normal cytokine-induced STAT6 activation and expression. Therefore, the capacity of STAT6-specific siRNA to inhibit STAT6 gene expression in lung epithelial cells is unaffected by the presence of the STAT6-activating cytokines IL-13 and IL-4. Importantly from a therapeutic perspective, pre-activation of these cytokine signalling pathways did not have a detrimental effect on this capacity.
Figure 3.
RNA interference (RNAi) of signal transducer and activator of transcription 6 (STAT6) is undiminished in the presence of STAT6-activating cytokines. A549 cells were either pretreated or post-treated with St6-1 small interfering RNA (siRNA) relative to stimulation with cytokine [interleukin (IL)-4 or IL-13; 50 ng/ml] and STAT6 expression was analysed at the mRNA and protein levels by real-time reverse transcription–polymerase chain reaction (RT-PCR) (a) and western blotting (b), respectively. (a) Irrespective of the timing of cytokine addition, St6-1 siRNA treatment induced a significant down-regulation of STAT6 mRNA compared with scrambled control (SC) siRNA-treated cultures (*P ≤ 0·05). The fold-change in STAT6 mRNA expression was similar in siRNA pretreatment versus siRNA post-treatment cultures. (b) Representative western blots showing absence of detectable STAT6 protein expression in cultures either pretreated with St6-1 siRNA (48 hr prior to cytokine stimulation) or post-treated with St6-1 siRNA (24 hr after cytokine stimulation). Phosphorylated STAT6 (STAT6-p) was undetectable in St6-1 siRNA-treated cultures.
RNAi of STAT6 potently suppresses ongoing pro-inflammatory events
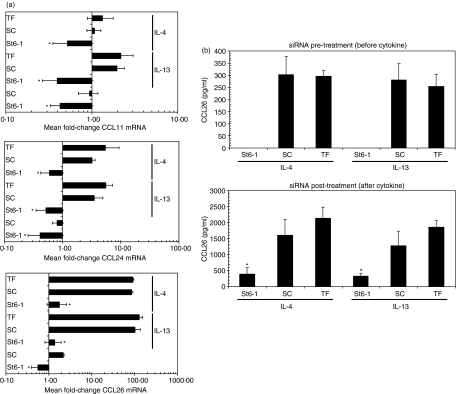
The local production of eotaxin chemokines is a major determinant of eosinophilic inflammation in asthma, and a previous study with STAT6 expression vectors demonstrated that eotaxin-1 (CCL11) expression in lung epithelial cells is up-regulated in a STAT6-dependent manner.26 We therefore utilized such observations as a functional downstream test of the robustness of STAT6 deletion in our model system. Furthermore, we expanded this to include measurement of the other known human eotaxin family members, eotaxin-2 (CCL24) and eotaxin-3 (CCL26). In A549 cells, CCL26 mRNA expression was predominantly up-regulated by cytokine stimulation whereas CCL24 and CCL11 mRNA exhibited significantly weaker induction; for example, mean fold-changes in SC + IL-13 cultures were as follows: CCL11 = 2·0 ± 0·4, CCL24 = 3·5 ± 1·4 and CCL26 = 103·3 ± 30·1. Notably, there was no significant difference between IL-13 and IL-4 in terms of ability to up-regulate eotaxin mRNA expression. Irrespective of the extent of individual eotaxin mRNA induction, pretreatment with St6-1 siRNA for 48 hr effectively blocked subsequent IL-4-or IL-13-mediated up-regulation of all three eotaxin family members (Fig. 4a). To test whether these effects were reflected at the protein level, we analysed culture supernatants by ELISA. Consistent with their weaker induction at the mRNA level, secreted CCLL1 and CCL24 proteins were undetectable under the culture conditions employed (not shown). Consistent with its marked induction at the mRNA level, CCL26 protein was readily detectable after cytokine stimulation and this expression was abolished by pretreating cells with St6-1 siRNA (Fig. 4b). It should be noted that these observations of CCL26 inhibition were consistently reproducible and identical in cultures of normal SAECs and bronchial smooth muscle cells. Indeed, despite SAEC cultures exhibiting a 10-fold higher induction of CCL26 mRNA expression in response to IL-13 stimulation, this was significantly inhibited by appropriate siRNA treatment (Supporting information Fig. S3).
Figure 4.
RNA interference (RNAi) of signal transducer and activator of transcription 6 (STAT6) potently inhibits ongoing cytokine-driven eotaxin family expression. (a) A549 cells pretreated with St6-1 small interfering RNA (siRNA) for 48 hr prior to cytokine stimulation [interleukin (IL)-4 or IL-13; 50 ng/ml for 24 hr] were subjected to reverse transcription–polymerase chain reaction (RT-PCR) analysis for expression of eotaxin family [chemokine (C-C motif) ligand 11 (CCL11), CCL24 and CCL26] mRNA expression. Although all eotaxin genes were up-regulated by both cytokines, CCL26 mRNA was predominant; for example, scrambled control (SC) siRNA + IL-13, mean fold-change = 103·3 ± 30·1 (note smaller x-axis scale on CCL11 and CCL23 graphs). St6-1 siRNA treatment abolished the ability of IL-4 or IL-13 to up-regulate all three eotaxin mRNA family members; for example, CCL26: St6-1 + IL-13, mean fold-change = 1·4 ± 0·6; St6-1 + IL-4, 1·8 ± 0·8 (*P ≤ 0·05). (b) Enzyme-linked immunosorbent assay (ELISA) of culture supernatants demonstrated the absence of detectable CCL26 protein expression in cytokine-stimulated cultures pretreated with St6-1 siRNA (top panel). Analysis of supernatants from cells post-treated with siRNA (lower panel) showed that St6-1-treated cultures had significantly reduced levels of CCL26 (St6-1 + IL-13: 326·9 ± 85·1 pg/ml), compared with either SC or transfection reagent (TF) control cultures (*P ≤ 0·05). These reduced levels were comparable to levels found in control cultures (SC and TF) after 24 hr of stimulation, pre-siRNA treatment (e.g. SC + IL-13: 280·4 ± 67·3 pg/ml, top panel; P > 0·05).
As siRNA-mediated inhibition of STAT6 was demonstrable in cells pretreated with cytokine (Fig. 3), we also tested the ability of STAT6-specific siRNA to modulate CCL26 protein expression under the same conditions – i.e. siRNA treatment after cytokine stimulation for 24 hr. In these experiments, cells were maintained in IL-4 or IL-13 for the duration of culture and this is reflected in the significantly increased levels of detectable CCL26 (Fig. 4b). Although CCL26 expression was detectable in St6-1 siRNA-treated cultures after cytokine stimulation (i.e. siRNA post-treatment), this was significantly attenuated when compared with control, cytokine-stimulated cultures. Indeed, CCL26 levels in St6-1 siRNA post-treated cultures were comparable to levels obtained in control cultures stimulated for 24 hr; for example, St6-1 (post-treatment) + IL-13: = 326·9 ± 85·1 pg/ml versus SC (pretreatment) + IL-13: 280·4 ± 67·3 pg/ml. This suggests rapid down-regulation of ongoing CCL26 synthesis upon St6-1 siRNA treatment. RNAi of STAT6 therefore effectively inhibits the ability of Th2 cytokines to drive downstream expression of eotaxin family gene expression in lung epithelial cells. Furthermore, this effect was demonstrable in epithelial cells pre-exposed to pro-asthmatic cytokines, suggesting potential therapeutic efficacy in pre-existing disease where local cytokine levels are known to be markedly up-regulated in vivo.
Discussion
Mainstream asthma therapy consists of control with glucocorticosteroid-based drugs that, although anti-inflammatory, can induce important side effects including weight gain, osteoporosis and oro-pharyngeal candidiasis. These problems, combined with limited knowledge of how steroid asthma therapy operates in vivo, point to a real demand for more specific and efficacious treatments.27 Recent developments in the understanding of asthma pathogenesis at the molecular level have led to the identification of potential novel drug targets, with IL-13 and its signalling partners being recognized as particularly important.7,15,16 STAT6 is central to the IL-13 signalling pathway, and studies with gene-knockout mice 8,9 and analysis of human disease 11,12 have illustrated the importance of STAT6 expression to asthma pathology. However, targeting intracellular transcription factors with specificity is technically problematical. For example, previous antisense-based techniques were not capable of completely inhibiting STAT6 expression 28 and anti-STAT6 DNA oligonucleotides elicited significant immuno-toxicity in vivo.29 With the discovery of the endogenous RNAi pathway as a potent gene-silencing mechanism in mammalian cells,18–21 we postulated that an RNAi-based strategy would offer superior targeting of STAT6 gene expression. Given findings that STAT6 expression in lung epithelial cells is essential for the development of IL-13-driven asthma symptoms,9 it was important to determine whether this cell type was amenable to this approach. We therefore characterized the ability of STAT6-specifc siRNAs to mediate RNAi of STAT6 in the A549 lung epithelial cell line – a well-characterized and accepted surrogate cellular model of human lung epithelium. In support of the relevance of the A549 model, we found STAT6 expression, IL-13 responsiveness and knockdown of STAT6 mRNA by RNAi to be similar among A549 cells, cultured primary epithelial cells (SAECs) and primary bronchial smooth muscle cells (Supporting Information Figs S1–S3). Given the expense and short life span (2–3 weeks) associated with primary cultures, we therefore performed extensive characterization of individual siRNA (St6-1 to St6-4) pharmacokinetics in the more amenable A549 model. In contrast to previous antisense studies which employed micromolar concentrations of DNA oligonucleotide,28 we show that picomolar siRNA concentrations can routinely mediate significant down-regulation of STAT6 mRNA with resultant loss of STAT6 protein expression. Furthermore, this strategy did not modulate cellular OAS-1 mRNA levels, STAT1 phosphorylation, or GAPDH expression, confirming the absence IFN responses or off-target effects, previously reported in some RNAi studies.24,25
Allergic asthma is characterized by accumulation of eosinophils at peribronchial sites where subsequent degranulation and mediator release promote tissue damage and airway remodelling.30 Allergen-specific Th2 cells are central to this process in which lymphocyte-derived IL-13 induces local production of eotaxin chemokines that subsequently attract eosinophils into the airways. In support of this, cultured bronchial epithelial cells have been shown to synthesize eotaxin family molecules (CCL11, CCL24 and CCL26) in response to IL-1331 and studies of lung tissue from asthmatics have revealed significant up-regulation of these chemokines and their specific cognate receptor (CCR3) in lung epithelium.32–34 Interestingly, these latter studies 33,34 also demonstrated that, by virtue of their CCR3 expression, these airway cell types are themselves functionally regulated by eotaxins, producing mediators that promote remodelling and fibrosis, leading to the suggestion that blockade of these eotaxin pathways will need to be an essential component of any future asthma therapeutic. Therefore, our finding that RNAi of STAT6 prevented IL-13-mediated eotaxin family mRNA induction and ablated functional CCL26 protein expression is highly relevant. Although previous studies focused on eotaxin-1 (CCL11) expression by epithelial cells, our analysis showed eotaxin-3 (CCL26) to be the most predominant IL-13-regulated eotaxin gene, and we confirmed this finding in normal SAECs, which exhibited a 10-fold greater IL-13-mediated induction of CCL26 expression when compared with normal BSMCs (Supporting Information Fig. S3). This is in agreement with recent findings that CCL26 is the predominant eotaxin chemokine expressed by normal lung epithelial cells 31,35 and that it is associated with persistence of allergen-induced bronchial eosinophilia;32 both observations point to this chemokine having an important role in the maintenance of human asthma symptoms. To date, there has been one other study published addressing inhibition of epithelial STAT6 by RNAi.36 However, this previous study used a single high dose of siRNA (100 nm) which did not completely eliminate detectable STAT6 mRNA or protein expression. In contrast, our extensive dose–response and time–course analyses show unequivocally that STAT6 expression is rapidly lost in siRNA-treated cells, without evidence of IFN response induction. Furthermore, consistent with functional elimination of STAT6, we have shown that siRNA pretreatment abolishes the ability of either IL-13 or IL-4 to induce CCL26 protein synthesis, despite the potent up-regulation of this gene by these cytokines. This contrasts with the previous study in which IL-4-induced CCL26 expression was still detectable after siRNA treatment, presumably reflecting the incomplete inhibition of STAT6.36 More importantly, the present study has shown that cells prestimulated with IL-13 or IL-4 do not become refractory to siRNA-mediated knockdown of STAT6, and moreover such treatment produces a significant and rapid attenuation of ongoing CCL26 protein expression. From a therapeutic perspective, these novel findings predict that pre-existing allergic lung inflammation will be responsive to STAT6 targeting by RNAi.
The data presented here are consistent with STAT6 being a major transcriptional regulator of eotaxin family expression in human lung epithelial cells and as such confirm the importance of STAT6 as a relevant in vivo therapeutic target for asthma. Since the initial description of RNAi in mammalian cells,19 siRNA-based drug development has developed at a remarkable pace, with these molecules exhibiting therapeutic potential.37 The experimental data presented here suggest that STAT6 expression in asthmatic airways is a viable target for this strategy and it is important to note that successful RNAi-mediated gene targeting in mouse lung tissue has recently been described.38,39 Efficient delivery of siRNA in vivo remains an important challenge necessitating various optimization strategies, including chemical modification of siRNA, and use of delivery vehicles and short-hairpin RNA-encoding DNA vectors. However, it is interesting that lung delivery of unmodified siRNA has been reported to produce therapeutic benefit.40 We therefore postulate that local delivery of STAT6-specific siRNA has real potential to suppress asthma pathology with consequent amelioration of symptoms. Of course, asthma is a complex inflammatory disorder and it remains to be determined whether blockade of STAT6 as a single gene target will produce sufficient therapeutic benefit. The observation that many inflammation-related genes up-regulated in asthma exhibit STAT6 dependence, coupled with the proof of principle findings reported here, indicate that further testing of the therapeutic potential of STAT6-targeting siRNA is warranted.
Acknowledgments
This study was in part funded by a generous donation from the Stone-Mallabar Charitable Foundation (Registered Charity No. 1013678).
Supporting information
Additional Supporting Information may be found in the online version of this article.
Figure S1 RNA interference (RNAi) of signal transducer and activator of transcription 6 (STAT6) gene expression in primary human airway cells. Normal small airway epithelial cells (SAECs) or bronchial smooth muscle cells (BSMCs) were transfected with STAT6-targeting (St6-1) and scrambled control (SC) small interfering RNA (siRNA; 20 nM) as described for A549 cells. Real-time reverse transcription–polymerase chain reaction (RTPCR) analysis demonstrated significant and similar downregulation of STAT6 mRNA expression in cells treated with STAT6-targeting siRNA compared with cells treated with SC siRNA (*P ≤ 0·05). Similar to findings with A549 cells, the ability of St6-1 siRNA to down-regulate STAT6 mRNA expression was unaffected by the presence of interleukin (IL)-13 (50 ng/ml) throughout the duration of culture (72 hr).
Figure S2 RNA interference (RNAi) of signal transducer and activator of transcription 6 (STAT6) gene expression in primary human airway cells eliminates functional STAT6 protein expression. Representative western blots from experiments with normal small airway epithelial cells (SAECs) or bronchial smooth muscle cells (BSMCs) show loss of detectable STAT6 protein expression in St6-1 small interfering RNA (siRNA)-treated cultures (20 nM; 72-hr treatment). Loss of functional expression is demonstrated by the inability of interleukin (IL)-13 to induce detectable STAT6 phosphorylation (STAT6-p) in St6-1 siRNA-treated cultures. As with A549 cells, this inhibitory effect was undiminished in the presence of IL-13 (50 ng/ml).
Figure S3 RNA interference (RNAi) of signal transducer and activator of transcription 6 (STAT6) potently inhibits interleukin (IL)-13-driven chemokine (C-C motif) ligand 26 (CCL26) gene expression in primary human airway cells. Normal small airway epithelial cells (SAECs) or bronchial smooth muscle cells (BSMCs) pretreated with St6-1 small interfering RNA (siRNA) for 48 hr prior to IL-13 stimulation (50 ng/ml for 24 hr) were subjected to reverse transcription–polymerase chain reaction (RT-PCR) analysis for expression of CCL26 mRNA. CCL26 mRNA was more predominantly up-regulated in SAECs (approximately 1000-fold) than in BSMCs (100-fold). Compared with scrambled control (SC) siRNA, St6-1 siRNA treatment significantly attenuated the ability of IL-13 to up-regulate CCL26 mRNA expression (*P ≤ 0·05), confirming the relevance of observations in the A549 cell line.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than about missing material) should be directed to the corresponding author for the article.
References
- 1.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–63. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brightling CE, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID, Bradding P. Interleukin-4 and-13 expression is co-localised to mast cells within the airway smooth muscle in asthma. Clin Exp Allergy. 2003;33:1711–6. doi: 10.1111/j.1365-2222.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang S-K, Xiao H-Q, Klleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–94. [PubMed] [Google Scholar]
- 6.Wills-Karp M. IL-12/IL-13 axis in allergic asthma. J Allergy Clin Immunol. 2001;107(1):9–18. doi: 10.1067/mai.2001.112265. [DOI] [PubMed] [Google Scholar]
- 7.Izuhara K, Arima K. Signal transduction of IL-13 and its role in the pathogenesis of bronchial asthma. Drug News Perspect. 2004;17:91–8. doi: 10.1358/dnp.2004.17.2.829041. [DOI] [PubMed] [Google Scholar]
- 8.Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, Takeda K, Akira S. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998;187:1537–42. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nature Med. 2002;8:885–9. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie ANJ, Young IG, Rothenberg ME, Foster PS. Interleukin-13 mediates airways hyperreactvity through the IL-4 receptor-alpha chain and STAT6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol. 2001;25:522–30. doi: 10.1165/ajrcmb.25.4.4620. [DOI] [PubMed] [Google Scholar]
- 11.Christodoulopoulos P, Cameron L, Nakamura Y, et al. Th2 cytokine-associated transcription factors in atopic and nonatopic asthma: evidence for differential signal transducer and activator of transcription 6 expression. J Allergy Clin Immunol. 2001;107:586–91. doi: 10.1067/mai.2001.114883. [DOI] [PubMed] [Google Scholar]
- 12.Mullings RE, Wilson SJ, Puddicombe SM, et al. Signal transducer and activator of transcription 6 (STAT-6) expression and function in asthmatic bronchial epithelium. J Allergy Clin Immunol. 2001;108:832–8. doi: 10.1067/mai.2001.119554. [DOI] [PubMed] [Google Scholar]
- 13.Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007;28:248–51. doi: 10.1016/j.it.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Kato A, Schleimer RP. Beyond inflammation: epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:1–10. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izuhara K, Arima K, Kanaji S, Ohta S, Kanaji T. IL-13: a promising therapeutic target for bronchial asthma. Curr Med Chem. 2006;13:2291–8. doi: 10.2174/092986706777935140. [DOI] [PubMed] [Google Scholar]
- 16.Izuhara K, Shirakawa T, Adra CN, Hamasaki N, Hopkin JM. Emerging therapeutic targets in allergy: IL-4Ra and Stat6. Emerg Ther Targets. 1999;3:381–9. [Google Scholar]
- 17.Schaefer G, Venkataraman C, Schindler U. STAT6. Role in IL-4-mediated signaling. In: Hansel TT, Barnes PJ, editors. New Drugs for Asthma, Allergy and COPD. Basel: Karger; 2001. pp. 346–9. [Google Scholar]
- 18.Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA. 2001;98:9742–7. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 20.Mello CC, Conte D. Revealing the world of RNA interference. Nature. 2004;431:338–42. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- 21.Novina CD, Sharp PA. The RNAi revolution. Nature. 2004;430:161–4. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- 22.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–7. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridge AJ, Pebernard S, Ducraux A, Nicoulaz A-L, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34:263–4. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- 25.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BRG. Activation of the interferon system by short-interfering RNA's. Nature Cell Biol. 2003;5:834–9. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 26.Matsukura S, Stellato C, Georas SN, et al. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am J Respir Cell Mol Biol. 2001;24:755–61. doi: 10.1165/ajrcmb.24.6.4351. [DOI] [PubMed] [Google Scholar]
- 27.Barnes PJ, Hansel TT. The need for new therapy. In: Hansel TT, Barnes PJ, editors. New Drugs for Asthma, Allergy and COPD Progress in Respiration Research. Vol. 31. Basel: Karger; 2001. pp. 2–5. [Google Scholar]
- 28.Hill S, Herlaar A, Le Cardinal A, van Heeke G, Nicklin P. Homologous human and murine antisense oligonucleotides targeting STAT6: functional effects on germline Ce transcript. Am J Respir Cell Mol Biol. 1999;21:728–37. doi: 10.1165/ajrcmb.21.6.3709. [DOI] [PubMed] [Google Scholar]
- 29.Danahay H, Hill S, Natt F, Owen CE. The in vitro and in vivo pharmacology of antisense oligonucleotides targeted to murine STAT6. Inflamm Res. 2000;49:692–9. doi: 10.1007/s000110050648. [DOI] [PubMed] [Google Scholar]
- 30.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–25. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 31.Komiya A, Nagase H, Yamada H, et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cellular Immunol. 2003;225:91. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Ravensberg AJ, Ricciardolo FLM, van Schadewijk A, Rabe KF, Sterk PJ, Hiemstra PS, Mauad T. Eotaxin-2 and eotaxin-3 expression is associated with persistent eosinophilic bronchial inflammation in patients with asthma after allergen challenge. J Allergy Clin Immunol. 2005;115:779–85. doi: 10.1016/j.jaci.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 33.Beck LA, Tancowny B, Brummet ME, et al. Functional analysis of the chemokine receptor CCR3 on airway epithelial cells. J Immunol. 2006;177:3344–54. doi: 10.4049/jimmunol.177.5.3344. [DOI] [PubMed] [Google Scholar]
- 34.Joubert P, Lajoie-Kadoch S, Labonte I, et al. CCR3 expression and function in asthmatic airway smooth muscle cells. J Immunol. 2005;175:2702–8. doi: 10.4049/jimmunol.175.4.2702. [DOI] [PubMed] [Google Scholar]
- 35.Yuan Q, Campanella GS, Colvin RA, Hamilos DL, Jones KJ, Mathew A, Means TK, Luster AD. Membrane-bound eotaxin-3 mediates eosinophil transepithelial migration in IL-4-stimulated epithelial cells. Eur J Immunol. 2006;36:2700–14. doi: 10.1002/eji.200636112. [DOI] [PubMed] [Google Scholar]
- 36.Rippman JF, Schnapp A, Weith A, Hobbie S. Gene silencing with STAT6 specific siRNAs blocks eotaxin release in IL-4. TNFa stimulated human epithelial cells. FEBS Lett. 2005;579:173–8. doi: 10.1016/j.febslet.2004.11.071. [DOI] [PubMed] [Google Scholar]
- 37.Dykxhoorn DM, Palliser D, Liberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–52. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 38.Bitko V, Musiyenko O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nature. 2005;11:50–5. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 39.Massaro D, Massaro GD, Clerch LB. Noninvasive delivery of small inhibitory RNA and other reagents to pulmonary alveoli in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1066–70. doi: 10.1152/ajplung.00067.2004. [DOI] [PubMed] [Google Scholar]
- 40.Thomas M, Lu JJ, Chen J, Klibanov AM. Non-viral siRNA delivery to the lung. Adv Drug Deliv Rev. 2007;59:124–33. doi: 10.1016/j.addr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.