Abstract
Mannan-binding lectin (MBL) is a plasma protein implicated in innate immune defence against a broad range of microorganisms, including viruses. It is also thought that MBL plays a role in the recruitment of the specific clonal immune response. This was studied by injecting soluble hepatitis B surface antigen (HBsAg) intravenously into mice deficient in both MBL-A and MBL-C (MBL DKO mice). The MBL DKO animals on mixed genetic background (SV129EvSv × C57BL/6) produced higher antibody titres than the wild-type littermates. After primary challenge with the antigen the immunoglobulin M anti-HBsAg antibody titres were threefold higher in the MBL DKO mice than in the wild-type mice. Following the boost, the immunoglobulin G anti-HBsAg antibody titres were 10-fold higher in the MBL DKO mice, suggesting that MBL plays a role in a negative feedback regulation of adaptive immunity. However, the modulating effect of MBL was dependent on the genetic environment. The MBL DKO mice backcrossed on a C57BL/6 background showed the opposite response with the MBL DKO mice now producing fewer antibodies than the wild-type animals, whereas MBL deficiency in mice with the SV129EvSv background did not show any effect in antibody production. These findings indicate that the modifying effect of MBL on the humoral immune response is influenced by the genetic environment.
Keywords: complement, hepatitis B surface antigen, immune response, mannan-binding lectin, mannan-binding lectin double knock-out mice
Introduction
Mannan-binding lectin (MBL) binds to patterns of carbohydrates with terminal non-reducing mannose, N-acetylglucosamine or fucose,1 and so recognizes a number of viruses, e.g. herpes simplex virus (HSV), human immunodeficiency virus (HIV), severe acute respiratory syndrome virus and influenza A virus.2,3 The binding of MBL to enveloped virus surface glycoproteins triggers complement activation via the MBL pathway independently of C1q or antibodies.4 In plasma, MBL is associated with the MBL-associated serine proteases (MASPs): MASP-1,5 MASP-26 and MASP-3,7 as well as a 19 000 molecular weight protein (MAp19).7 After binding of MBL to its ligands, autoactivation of MASP-2 occurs, followed by MASP-2-mediated cleavage of complement factors C4 and C2, leading to formation of the C3 convertase, C4bC2b, and triggering of the complement cascade.8,9 Complement activation will provide an initial barrier for viral proliferation and spread via several mechanisms: complement activation contributes to viral neutralization, and activated complement products modulate humoral immunity to infectious hepatitis B virus (HBV) by facilitating immunoglobulin class-switching and promoting the formation of neutralizing antibodies.10,11
In humans the MBL level in blood is strongly influenced by single nucleotide polymorphisms (SNPs) in the MBL gene (MBL2).12 The polymorphisms are located within the promoter region and in exon 1 encoding parts of a collagen-like region of MBL. The mutations lead to disruption of the Gly-Xaa-Yaa pattern of this region and result in decreased circulating levels of MBL. Low MBL levels correlate with low lectin pathway activity. Conversely, high MBL levels ensure high levels of lectin pathway activation as established by an in vitro C4 fixation assay.13 In mice, the MBL is encoded by two different unlinked genes, MBL-A and MBL-C, and polymorphisms that may modulate the MBL levels have not been described. While the individual MBL levels in humans remain virtually constant after a rise until 1 month of age, the levels show a modest and sluggish acute-phase response in mice and man.14
Polymorphisms in MBL reportedly influence the clinical manifestations of HBV infections in humans.15 Mutant MBL allotypes were found at a higher frequency among HBV-infected Vietnamese patients than in controls, and carriage of MBL2 mutant alleles was increased among patients with fulminant liver failure caused by HBV infection.16 Patients infected with HBV who were homozygous for the combination of promoter and exon 1 genotypes that produce low amounts of functional MBL had decreased chances of recovering from the HBV infection.17 Low MBL genotypes are associated with the occurrence of cirrhosis and hepatocellular carcinoma in progressed Hong Kong Chinese hepatitis B surface antigen (HBsAg) carriers.18 In contrast, studies on German and Korean HBV-infected patients revealed no difference in the frequency of the mutant MBL alleles and disease progression.19,20 Therefore, the modulatory role of MBL on the clinical course of HBV infection is still an open question requiring analysis of larger patient groups.
A possible scenario for MBL in facilitating recovery from HBV infection is clearance of virus-infected cells through the activation of the complement system. Alternatively, the lectin pathway may modulate the adaptive immune response to HBV. We focused on HBsAg as a model glycoprotein because control over the HBV infection is currently achieved by vaccination with HBsAg and the HBsAg contains N-linked glycosylation sites, which makes the glycoprotein a potential ligand for MBL.
Materials and methods
Animals
Homozygous MBL-A-and MBL-C-deficient mice were derived as described by Takahashi et al.21 and Shi et al.22 Homozygous double-deficient MBL-A−/− MBL-C−/− mice (DKO) and wild-type (WT) littermates were derived by inter-crossing heterozygous MBL-A and MBL-C mice (of C57BL/6 × SV129EvSv mixed background). The knock-out status was established by genotyping 21 and verified by sandwich immunoassays with rat anti-mouse MBL-A and anti-mouse MBL-C monoclonal antibodies (mAbs).14 To obtain double-deficient MBL-A−/− and MBL-C−/− mice on C57BL/6 and SV129EvSv genetic backgrounds, the MBL-A+/− and MBL-C+/− mice were backcrossed onto C57BL/6 or SV129EvSv mice respectively for 12 generations and then intercrossed to establish an MBL-A−/− MBL-C−/− F12 strain. Male mice (6–8 weeks old) were used throughout the experiments. All animals had free access to food and water. Animal experiments were approved by and were performed as required by Danish national and institutional regulations.
MBL-A and MBL-C binding to HBsAg
Binding of MBL-A and MBL-C to HBsAg was analysed as described 23 with minor modifications. Briefly, microtitre plates (Maxisorb; Nunc, Kamstrup, Denmark) were coated with 5 µg HBsAg, subtype ad, purified (American Research Products, Inc., Belmont, MA) per millilitre phosphate-buffered serum (PBS), and blocked with 1 mg human serum albumin (Statens Serum Institut, Copenhagen, Denmark) per millilitre Tris-buffered saline (TBS; 10 mm Tris–HCl, 140 mm NaCl, 15 mm NaN3, pH 7·4). The plates were washed with TBS, 0·05% (v/v) Tween-20 (TBS/Tween) and then incubated with sera from WT and DKO animals diluted in TBS containing 5 mm CaCl2 or 10 mm ethylenediaminetetraacetic acid (EDTA) or mannose. The plates were developed with biotinylated anti-MBL-A or MBL-C mAbs, and europium-labelled streptavidin followed by measurement of bound europium using time-resolved fluorometry.
Immunization protocol
The MBL DKO mice and WT littermates were immunized intravenously (i.v.) in the tail with different doses (8 or 20 µg per animal) of HBsAg in 0·1 ml 0·9% NaCl at day 0 and boosted 4 weeks later with the same dose. Serum samples were collected weekly. The MBL DKO F6 and C57BL/6 control animals were similarly immunized and doses of 2 and 0·5 µg were also tested. Animals backcrossed for 12 generation on C57BL/6 or SV129EvSv were immunized with dose of 0·5 µg HBsAg. The same procedure was applied for immunization with human serum albumin (HSA) at various concentrations. Intraperitoneal (i.p.) immunizations were carried out in MBL DKO and WT littermates with 8 µg of HBsAg. Reconstitution experiments were carried out by immunizing MBL DKO mice with 8 µg HBsAg in the presence of 100 µg/ml recombinant MBL (rMBL) (kindly provided by NatImmune, Copenhagen, Denmark).24 Mice were immunized at week 0, rested for 3 weeks, rechallenged at week 4 and rested for an additional 3 weeks. Serum samples were collected weekly.
Measurement of immunoglobulin M (IgM) and IgG anti-HBsAg antibody
Microtitre plates (Maxisorb) were coated with 1 µg HBsAg/ml PBS overnight, blocked with HSA (1 mg/ml) TBS and incubated for 3 hr at room temperature with preimmune or immune animal sera serially diluted in TBS containing 5 mm EDTA and 1 mg HSA/ml. The plates were washed with TBS/Tween and bound antibodies were detected with either affinity isolated and biotinylated goat anti-mouse IgG (whole molecule) (product number: B6649; Sigma, St Louis, MI) or affinity-purified and biotinylated rabbit anti-mouse IgM (Bethyl Laboratories, Montgomery, TX). The assay was modified for analysis by time-resolved immunofluorometry (TRIFMA).25 The plates were developed with europium-labelled streptavidin (Perkin Elmer, Waltham, MA) and, after addition of enhancement solution, they were counted on a Victor 3 (Perkin Elmer) reader.
Measurement of preimmune IgM and IgG titre
Microtitre plates were coated with 1 µg goat anti-mouse immunoglobulin (Southern Biotechnology Associates, Inc., Birmingham, AL) per millilitre PBS overnight, blocked with 1 mg HSA/ml TBS and incubated for 3 hr at room temperature with preimmune sera serially diluted in TBS containing 5 mm EDTA and 1 mg HSA/ml. IgG and IgM were detected as described above.
Immunofluorescent analysis of spleen sections
Cryosections (5 µ;m) from snap-frozen spleens were caught on SuperFrost® Plus slides (Menzel-Glaser, Braunschweig, Germany), dried for 1 hr and stored at −80°. When needed the cryosections were fixed in cold acetone (4°) for 4 min, blocked by incubation for 30 min in PBS containing 1 µg FcR Block (rat anti-mouse FcγIII/II receptor mAb; Pharmingen, San Diego, CA) per millilitre and 5% (volume/volume; v/v) heat-treated normal fetal calf serum (FCS), followed by a wash with PBS. Germinal centres were visualized by staining with fluorescein isothiocyanate-labelled peanut agglutinin (Vector Laboratories, Burlingame, CA) at a 1:100 final dilution.23 Deposited C3 was detected by incubation with 1 µg biotinylated rabbit anti-human C3d antibody (Dako Cytomation, Glostrup, Denmark) per millilitre PBS containing 2·5% (v/v) heat-inactivated FCS followed by a wash in PBS and incubation with streptavidin Alexa Fluor R 546 (Molecular Probes, Leiden, the Netherlands) at 2 µg/ml.
Determination of circulating HBsAg
Mice were injected i.v. with 8 or 20 µg HBsAg and bled at 15, 30, 45 and 60 min after the injection. Both non-immunized mice and mice which had been immunized 3 weeks before were used. The levels of HBsAg in serum were determined using the Murex HBsAg enzyme-linked immunsorbent assay kit (Abbott, Abbott Park, IL) according to the instructions provided with the kit.
Radioactive labelling of HBsAg and positron emission tomography analysis
Radiolabelling of HBsAg was designed to yield the incorporation of 0·5–1 atom of 125I per HBsAg molecule. Two hundred micrograms of HBsAg was dually labelled with 124I (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and 125I (Hammersmith Hospital, London, UK) for 1 min at room temperature in a reaction mixture containing the following: 10 µl of 1 µCi 124I in 10 mm NaOH, 80 µl 3·4 µCi 125I in 42 mm NaOH, 8 µl 420 mm HCl (to neutralize the NaOH), 20 µl 0·5 m phosphate pH 7·5, 133 µl of HBsAg at 1·5 mg/ml and 20 µl of Chloramine T at 0·5 mg/ml. Twenty microlitres of Na2S2O3 at 0·5 mg/ml and 10 µl 10 mm KI were added upon completion of the reaction, followed by 50 µl HSA at 20 mg/ml. The labelled HBsAg was separated from low molecular weight material by passage through a desalting column (PD10; GE Healthcare, Hillerød, Denmark), preconditioned with HSA at 10 mg/ml and washed with 0·9% NaCl. The radioactively labelled HBsAg was eluted with 0·9% NaCl in 200-µl fractions. Labelling efficiency was about 70%. Each mouse was injected i.v. with 20 µg of the labelled HBsAg.
Positron emission tomography scanning was carried out at three time-points after injection: 5 min, 4 hr and 20 hr. The first scan was for 45 min, the second and third scannings were performed for 30 min. During the scanning procedure, the animals were anaesthetized by i.p. injection with 300 µl Dormicum (Roche, Basel, Switzerland) (6·64 mg/kg) i.p. to reduce the subsequent usage of isofluorane for maintaining the anaesthesia during the scanning.
Statistical analysis
All data are presented as means with standard deviation. Differences between groups were analysed using the non-parametric Mann–Whitney U-test (Stata Software; Stata Corporation, College Station, TX) or Student's t-test. Significance was judged when P < 0·05.
Results
MBL binding to HBsAg
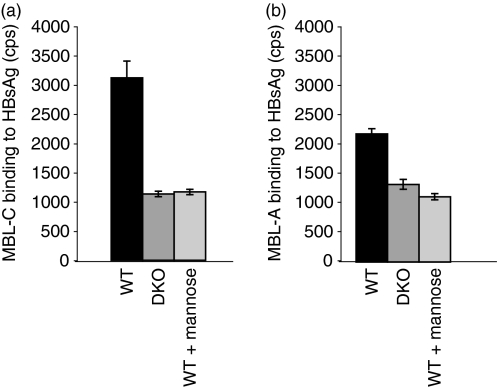
Previous reports showed that both murine and human MBL bind to HSV-2 virions.23 We extended these observations to HBsAg. Both MBL-A and MBL-C recognized HBsAg via the carbohydrate recognition domains as the binding of MBL-A and MBL-C to HBsAg could be inhibited by mannose to background levels comparable to those present in the sera from MBL DKO (Fig. 1a,b). The interaction was dependent on the presence of Ca2+ because the EDTA in the buffer inhibited the binding (data not shown).
Figure 1.
Mannan-binding lectin A (MBL-A) and MBL-C bind specifically to hepatitis B surface antigen (HBsAg). MBL-A and MBL-C binding to HBsAg was analysed in a time-resolved immunofluorometry assay, where HBsAg were coated on an enzyme-linked immunsorbent assay plate and then exposed to sera from WT and MBL DKO animals, followed by development with specific anti-mouse MBL-A and MBL-C antibodies. The sera were diluted in CaCl2-containing buffer to enable the MBL interaction with ligands or inhibited by mannose-containing buffer. The y-axis represents counts per second (cps) measured in the wells.
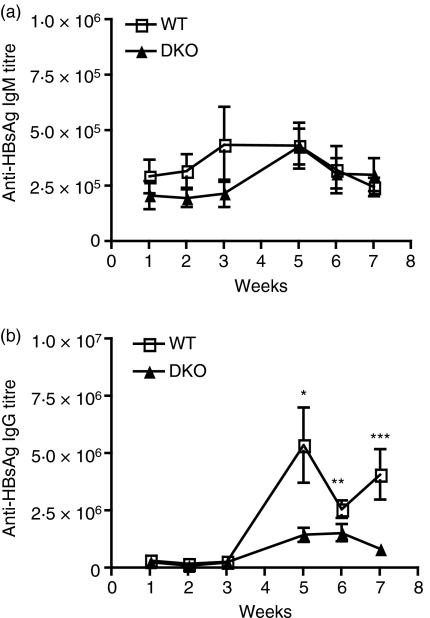
Antibody responses to HBsAg in MBL DKO on mixed background
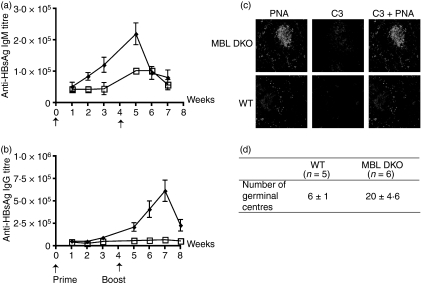
To analyse whether the lectin pathway had an effect on antibody response, groups of MBL DKO (SV129EvSv × C57BL/6) and corresponding WT animals were immunized with 8 µg HBsAg per animal using either the i.v. or the i.p. route. The antibody titres were followed for 3 weeks after the priming and 3 weeks after the boost. One week after priming, the IgM anti-HBsAg antibody titres were significantly elevated (about twofold) in the MBL DKOs, but not in the WT mice (Fig. 2a). A further increase was seen after boost, and now also the WT mice showed a twofold increase in IgM anti-HBsAg. The difference between the two groups of mice was more marked when looking at anti-HBsAg IgG response (Fig. 2b). In the MBL DKOs, there was a gradual increase in the antigen-specific titres from the time of the boost and reached maximum at 3 weeks after the boost. In contrast, there was a very small increase in the WT mice after boost. The higher levels of anti-HBsAg antibody titres in the MBL DKO mice correlated with increased numbers of germinal centres (GC) in the spleens of MBL DKO compared with in WT mice (Fig. 2d). Complement deposition in the spleen, visualized with anti-complement C3d antibody, revealed a larger number of GC and stronger staining in the MBL DKO when compared with the GC of the WT mice (Fig. 2c).
Figure 2.
Antibody titres against hepatitis B surface antigen (HBsAg) are elevated in mannose-binding lectin double knockout (MBL DKO; 129 SvEv × C57BL/6) mice compared with wild-type (WT) control littermates. MBL DKO and WT mice were challenged with 8 μg HBsAg intravenously (a and b) The antigen-specific immunoglobulin M (IgM) and IgG titres were determined by time-resolved immunofluorometry and followed for 3 weeks after priming and boost. At least five animals in each group were used. The data shown are representative of two individual experiments. (c) Immunofluorescent detection of C3 deposits within the GC. Spleens were harvested 5 weeks after the boosting. Cryosections were treated with PNA and anti-C3d antibody. Sections are representative of spleens analysed from at least five mice per group. (d) Quantification of the number of germinal centres per splenic section. MBL DKO and WT mice were harvested after the boost at the peak of the IgG response. Splenic sections were stained with PNA and the number of germinal centres was counted per section. At least five animals per group and five individual sections per spleen were analysed.
To determine if MBL deficiency affects the generation of antibody responses to non-MBL ligands, e.g. non-glycosylated protein antigens, groups of mice were challenged with HSA. The HSA induced weak antibody responses, but there was no difference between the MBL DKO and WT littermates (data not shown).
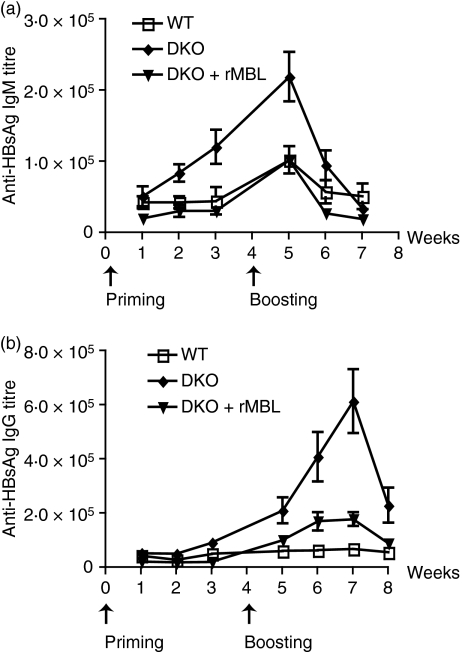
When using knock-out mice to test various biological functions, it is always a concern if the observed phenotype is a direct effect of the lack of the gene of interest, or if it is the result of modifications of loci residing in close proximity to the gene of interest. To examine this issue reconstitution experiments with rMBL were performed. The MBL DKO mice were immunized with either HBsAg alone or rMBL-HBsAg (Fig. 3a,b). Since a dose of 100 µg/ml rMBL efficiently reconstituted MBL activity in the ischaemia–reperfusion model,24 a similar dose of rMBL was premixed with HBsAg before immunization. The reconstitution with rMBL resulted in a decrease in the HBsAg-specific IgM responses and HBsAg-specific IgG responses.
Figure 3.
Recombinant mannose-binding lectin (rMBL) reconstitutes the antibody responses to hepatitis B surface antigen (HBsAg) in MBL double knockout (DKO; 129 SvEv x C57BL/6) mice. MBL DKO, MBL DKO supplemented with rMBL at the time of the antigen challenge and wild-type (WT) mice were immunized with 8 μg HBsAg intravenously (a and b) The antigen-specific IgM and IgG titres were determined by time-resolved immunofluorometry and followed for 3 weeks after priming and boost. At least 10 animals in each group were used.
Preimmune levels of IgM and IgG anti-HBsAg antibody
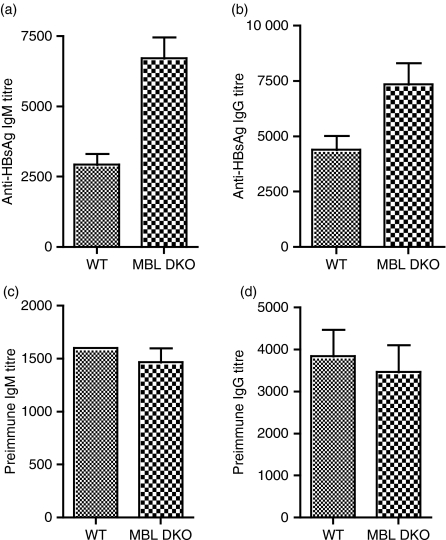
Sera from MBL DKOs (129SvEv × C57BL/6) and WT mice were compared with determine whether differences in antibody responses might be reflected in differences in the levels of pre-existing, natural anti-HBsAg antibody. Significantly higher antigen-specific preimmune antibody titres were found in the MBL DKO when compared with WT mice (Fig. 4a,b). Yet, the titres of total serum IgM and IgG were comparable (Fig. 4c,d).
Figure 4.
Preimmune hepatitis B surface antigen (HBsAg)-specific immunoglobulin M (IgM) levels are elevated in the mannose-binding lectin double knockout (MBL DKO) mice. The preimmune HBsAg-specific IgM (a) and IgG (b) titres were determined by a time-resolved immunofluorometry (TRIFMA) assay where microtitre wells were coated with HBsAg and exposed to different dilutions of non-immune sera. At least 10 animals per group were tested. Groups were compared using the Mann–Whitney test for significance. Significant differences were observed for anti-HBsAg IgM titres (P = 0·007) and anti-HBsAg IgG titres (P = 0·03). Preimmune IgM (c) and IgG (d) levels. The preimmune serum levels of IgM and IgG were determined by a TRIFMA assay where microtitre wells were coated with anti-mouse immunoglobulin and exposed to different dilutions of non-immune sera. Groups were compared using the Mann–Whitney test for significance. In both cases, no significant differences were found between the groups (P > 0·05).
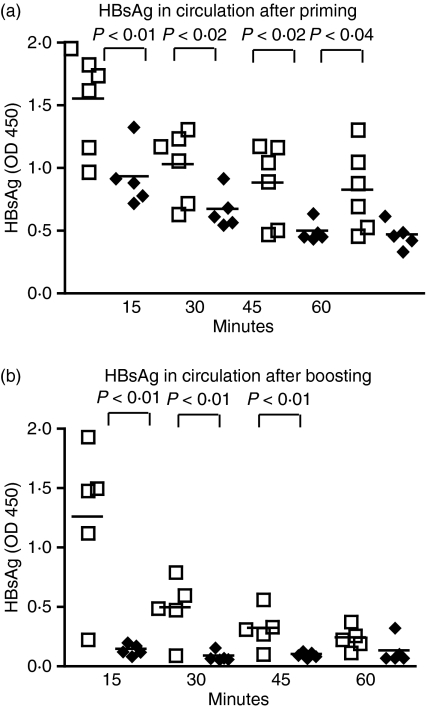
Clearance of HBsAg antigen from the circulation
To determine possible differences in soluble antigen sequestering between MBL DKO (SV129EvSv × C57BL/6) and WT mice, groups of at least six animals were injected i.v. with HBsAg and the concentration of circulating antigen was estimated at 15, 30, 45 and 60 min after challenge. The results in Fig. 5(a) show a significantly faster clearance of HBsAg in the MBL DKO than in the WT mice. When immunized mice were infused with HBsAg a more rapid clearance was seen in the MBL DKO mice (Fig. 5b), as might be expected because of the presence of increased antibody levels (Fig. 2). On the other hand, there was hardly any increased clearance in the WT mice, confirming the difference determined by antibody quantification.
Figure 5.
Hepatitis B surface antigen (HBsAg) is cleared from circulation more efficiently in mannose-binding lectin double knockout (MBL DKO) mice than in the wild-type (WT) animals. Non-primed (a) and primed (b) MBL DKO and WT animals were challenged with HBsAg intravenously and serum samples were collected at different time-points after the challenge. The levels of HBsAg in circulation were determined by enzyme-linked immunosorbent assay. Individual animals are shown as individual symbols. The different groups were compared by Mann–Whitney test analysis for significance and P-values are noted in the figure.
It was of interest to determine if the differences in HBsAg concentration in circulation between the MBL DKO and WT mice could be the result of differences in antigen trafficking or antigen capture. To examine the pattern of antigen trafficking non-immunized and primed MBL DKO and WT animals were infused with 124I-labelled HBsAg and the antigen localization was visualized by positron emission tomography analysis at different time-points after antigen infusion (5 min, 4 hr and 20 hr). No gross differences were observed between the different genotypes. Five minutes after challenge antigen could be detected in the livers and bladders of the mice. At the later time-points (4 and 20 hr) antigen presence could still be found in the liver. The analysis of HBsAg uptake demonstrated the liver as the major site of antigen retention (data not shown). Comparable levels of radioactively labelled antigen were present in the livers or kidneys of MBL DKO and WT mice, which is suggestive of comparable antigen trafficking in both WT and MBL DKO (129SvEv × C57BL/6) mice. To establish if differences in the antigen retention exist in other organs like spleens, livers or kidneys, total radioactivity of the organs was compared with the weight of the organ. Although a small number of labelled HBsAg-infused animals (two per genotype) were analysed a tendency for increased antigen presence in the spleens of the MBL DKO mice was observed (data not shown). However, examination of larger numbers of animals is required for conclusions to be made.
Antibody responses to HBsAg in back-crossed MBL DKO mice
To examine the influence of the genetic background on a phenotype, MBL DKO mice on the mixed background were backcrossed for six or 12 generations onto a C57BL/6 background or for 12 generations onto an SV129EvSv background. The mice were immunized by i.v. injection of different doses of HBsAg. When challenged with 20 µg HBsAg, the WT C57BL/6 and MBL DKO F6 mice mounted comparable IgM and IgG anti-HBsAg antibody responses. Since the effect of complement on antibody responses is usually seen when animals are challenged with low antigen doses, WT and MBL DKO F6 and F12 mice were challenged with lower amounts of HBsAg (8, 2 or 0·5 µg per animal). The responses in both groups (F6 or F12) were an order of magnitude higher than those detected in the mixed background mice, indicating an enhancing effect of background genes in C57BL/6. Remarkably, after backcrossing, the MBL DKO F12 mice showed a tendency to produce less antigen-specific IgG antibody titres than the WT animals (Fig. 6a,b). This difference was significant at 5–7 weeks after the boost (Mann–Whitney test P = 0·1).
Figure 6.
The hepatitis B surface antigen (HBsAg)-specific immunoglobulin M (IgM) and IgG titres in mannose-binding lectin double knockout (MBL DKO) F6 and C57BL/6 mice. MBL DKO F6 and C57BL/6 mice were challenged with 2 μg HBsAg intravenously. The antigen-specific IgM (a) and IgG (b) titres were determine by time-resolved immunofluorometry and followed for 3 weeks after priming and after boost. At least five animals in each group were used. The data shown are representative of two individual experiments. Groups were compared using the Mann–Whitney test for significance. The stars annotate the time-points at which significant differences in the titres were seen (P > 0·05).
Preimmune levels of anti-HBsAg antibodies (IgM and IgG) and total serum IgM and IgG were analysed and no difference in antibody titres was observed in MBL DKO F6 mice when compared with WT mice (the data not shown).
To further investigate the difference between the immune response in the mixed background animals (SV129EvSv × C57BL/6), MBL DKO animals backcrossed for 12 generations onto SV129EvSv background were challenged with soluble antigen. No difference in antigen-specific IgM or IgG antibody production was detected in MBL DKO F12 and SV129EvSv mice. However, when compared with C57BL/6 background, SV129EvSv mice developed strikingly lower specific antibody responses (data not shown).
Clearance of HBsAg in the back-crossed mice
Groups of five animals were infused with HBsAg. In contrast to the observations on the mice of mixed background, there was no difference in the clearance of HBsAg in the serum of either primed or non-primed MBL DKO F6 or C57BL6 (data not shown). Within 1 hr after challenge, the levels of HBsAg dropped to non-detectable in circulation.
Discussion
Studies in mice bearing targeted deficiencies in C1q, C4, C3 or CD21/CD35 receptors (receptors for complement fragments) demonstrated the involvement of complement in multiple stages of the regulation of B-cell development.26–28 The complement receptors on the surface of the B cells are of crucial importance in modulating the signal received through the B-cell antigen receptor.29 The blockade of the CR1 or CR2 by mAbs or by gene targeting leads to reduced antibody responses to T-cell-dependent antigens.30–32 C3dg and C3d function as adjuvants to enhance humoral immune responses to antigens, including pneumococcal capsular polysaccharide, hen egg lysozome, streptavidin, HIVgp120, and influenza or measles virus haemagglutinin.33,34 However, complement components may also exhibit immunosuppressive activities.35 C3 depletion by cobra venom factor can inhibit the primary, yet enhance the secondary, humoral immune response to both unmodified and protein-conjugated pneumococcal polysaccharide.33 Furthermore, C3d may enhance or inhibit antigen-specific antibody responses through both CD21-dependent and independent mechanisms depending on the concentration and nature of the antigen–C3d complexes. The mechanisms involve both modulation of B-cell responsiveness through CR2 and capture of C4-or C3-opsonized antigen by the follicular dendritic cell (FDC) network. In addition, recent data demonstrated that in mice CR1/CR2 are responsible for the trapping of T-independent antigens (TI-2 antigens), e.g. group B streptococcus polysaccharide type III (III-PS). C3-and CR1/CR2-deficient animals had impaired IgM and IgG responses to III-PS because of impaired antigen trapping and processing by marginal zone B cells.36 These observations prompted us to investigate the possible involvement of MBL in the humoral responses towards glycoproteins.
In this report, we made a quite unexpected observation. We found that MBL DKO mice on mixed background produced elevated antigen-specific IgM and IgG immune responses to soluble glycoprotein antigen. The observed phenotype could be reverted by reconstitution with rMBL. Consistently, the clearance of the HBsAg antigen from the circulation was more efficient in the MBL DKO (SV129EvSv × C57BL/6) mice when compared with WT littermates. Immunohistochemical examination of splenic sections demonstrated increased numbers of germinal centres and elevated complement deposition in the MBL DKO, which might offer some explanation for the increased antibody response. Interestingly, the preimmune antigen-specific immunoglobulins were elevated in these mice. A plausible hypothesis is that pre-existing antigen specific antibodies may facilitate antigen capture and promote immune response.
The idea that the classical pathway of complement activation may compensate for the absence of MBL under certain conditions is consistent with findings by Roos et al., who demonstrated that mannan can induce complement activation by both the lectin and the classical pathway.37,38 The activation of the classical pathway by mannan was triggered by anti-carbohydrate antibodies and followed by C1 activation. While it is currently not clear if this is the mechanisms through which MBL DKO mice on mixed background generate elevated antibody responses to HBsAg, it is consistent with the presence of elevated levels of preimmune anti-HBsAg titres in MBL DKO mice.
The major source of natural antibody in mice is thought to be the peritoneal B1 cells. Natural, or pre-existing, IgM and, more rarely, IgG are thought to be expressed independently of infection. They represent germ-line-encoded antibodies with low affinities. Interestingly, it has recently been seen that the numbers of B1 cells in MBL DKO mice on mixed background are increased,39 it is therefore likely that the natural antibody repertoire or the levels of natural antibodies are different in the MBL DKO mice on mixed background.
The immunogobulins IgM or IgG1, IgG2a, IgG2b or IgG3 when complexed with an antigen can enhance antibody responses to soluble antigens.40,41 The IgG1 or IgG2a or IgG2b enhancement is dependent on the presence of activating FcγR, expressed on the antigen-presenting cell, and stimulates better antigen presentation to CD4+ T cells. The IgM-mediated and IgG3-mediated enhancement of antibody responses depends on complement activation.42,43 Hence, C3-deficient animals or cobra venom-treated mice or Cr1/2-deficient mice fail to produce elevated antibody response when antigen is administered either alone or along with antigen-specific IgM. One possibility is that in MBL DKO mice with a mixed background the capture of antigen by IgM or IgG3 suffices to start complement activation and to facilitate the primary antibody formation. Once activation of specific B cells has taken place, these will secrete antigen-specific IgM, further enhancing the positive feedback loop.
Differences in antibody responses are often accompanied by differences in antigen trapping. We therefore determined the antigen clearance from circulation and also addressed the antigen distribution. The kinetics of clearance of HBsAg from the blood was faster in MBL DKO on mixed background than in WT controls. Consistent with previously published data the majority of HBsAg was retained in the liver, followed by the spleen. As expected, primed animals demonstrated exacerbated HBsAg capture in the spleens when compared with non-primed. While similar antigen retention in the liver was observed in the primed MBL DKO and WT control littermates, there was a tendency for a twofold increase of HBsAg in the spleens of the MBL DKO mice. Although the number of animals analysed was small, the data correlates with the observed increased antibody levels. In mice, challenged i.v., the HBsAg probably forms immune complexes with circulating antibody, which would facilitate their targeting to the liver or spleen.
When MBL-deficient animals were backcrossed onto C57BL/6 for six or 12 generations and challenged with similar or decreasing levels of HBsAg antigen, the antibody responses were reversed, indicating that on this background MBL may have an enhancing effect on the response against this glycosylated antigen. The antibody response developed in MBL DKO mice backcrossed on an SV129EvSv background was significantly lower than that in mice on a C57BL/6 background, possibly explaining the intermediate level of specific antibodies in mice on mixed background. The antibody titres in the SV129EvSv backcrossed mice were similar in both MBL DKO and WT groups.
Overall these observations suggest that MBL deficiency is a weak modifier of adaptive immunity in the context of certain genetic environment. The identities of the gene products that, in combination with MBL deficiency, lead to increased production of antibodies are unknown. The present study further emphasizes the importance of using backcrossed animals when studying the influence of various genes on the immune response.
Acknowledgments
This work was supported by the Danish Ministry of Finance grant to MG, the Danish Medical Research Council and the Novo Nordic Foundation.
Glossary
Abbreviations:
- DKO
double knockout
- EDTA
ethylenediaminetetraacetic acid
- FCS
fetal calf serum
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- HSA
human serum albumin
- HSV
herpes simplex virus
- IgM
immunoglobulin M
- i.p.
intraperitoneal
- i.v.
intravenous
- mAb
monoclonal antibody
- MASP
mannan-binding lectin associated serine protease
- MBL
mannan-binding lectin
- MBL DKO
MBL double knockout
- PBS
phosphate-buffered saline
- rMBL
recombinant MBL
- SNP
single nucleotide polymorphism
- TBS
Tris-buffered saline
- WT
wild-type
Conflict of interest
None.
References
- 1.Hart ML, Saifuddin M, Spear GT. Glycosylation inhibitors and neuraminidase enhance human immunodeficiency virus type 1 binding and neutralization by mannose-binding lectin. J Gen Virol. 2003;2:353–60. doi: 10.1099/vir.0.18734-0. [DOI] [PubMed] [Google Scholar]
- 2.Thielens NM, Tacnet-Delorme P, Arlaud GJ. Interaction of C1q and mannan-binding lectin with viruses. Immunobiology. 2002;205:563–74. doi: 10.1078/0171-2985-00155. [DOI] [PubMed] [Google Scholar]
- 3.Lubinski J, Nagashunmugam T, Friedman HM. Viral interference with antibody and complement. Semin Cell Dev Biol. 1998;9:329–37. doi: 10.1006/scdb.1998.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman HM, Wang L, Pangburn MK, Lambris JD, Lubinski J. Novel mechanism of antibody-independent complement neutralization of herpes simplex virus type 1. J Immunol. 2000;165:4528–36. doi: 10.4049/jimmunol.165.8.4528. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 7.Dahl MR, Thiel S, Matsushita M, Fujita T, Willis AC, Christensen T, Vorup-Jensen T, Jensenius JC. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15:127–35. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 8.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 9.Vorup-Jensen T, Petersen SV, Hansen AG, et al. Distinct pathways of mannan-binding lectin (MBL)-and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000;165:2093–100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 10.Verschoor A, Brockman MA, Gadjeva M, Knipe DM, Carroll MC. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J Immunol. 2003;171:5363–71. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 11.Zheng ZM, Hsiung GD. Complement-requiring neutralizing antibody in guinea pigs with primary and recurrent genital herpes. Proc Soc Exp Biol Med. 1984;177:332–6. doi: 10.3181/00379727-177-41952. [DOI] [PubMed] [Google Scholar]
- 12.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–9. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 13.Thiel S, Moller-Kristensen M, Jensen L, Jensenius JC. Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology. 2002;205:446–54. doi: 10.1078/0171-2985-00145. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Jensen L, Hansen S, et al. Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand J Immunol. 2001;53:489–97. doi: 10.1046/j.1365-3083.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown KS, Ryder SD, Irving WL, Sim RB, Hickling TP. Mannan binding lectin and viral hepatitis. Immunol Lett. 2007;108:34–44. doi: 10.1016/j.imlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Hakozaki Y, Yoshiba M, Sekiyama K, et al. Mannose-binding lectin and the prognosis of fulminant hepatic failure caused by HBV infection. Liver. 2002;22:29–34. doi: 10.1046/j.0106-9543.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- 17.Thio CL, Mosbruger T, Astemborski J, Greer S, Kirk GD, O’Brien SJ, Thomas DL. Mannose binding lectin genotypes influence recovery from hepatitis B virus infection. J Virol. 2005;79:9192–6. doi: 10.1128/JVI.79.14.9192-9196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong WP, To YF, Ip WK, Yuen MF, Poon TP, Wong WH, Lai CL, Lau YL. Mannose-binding lectin in chronic hepatitis B virus infection. Hepatology. 2005;42:1037–45. doi: 10.1002/hep.20891. [DOI] [PubMed] [Google Scholar]
- 19.Hohler T, Wunschel M, Gerken G, Schneider PM, Meyer zum Buschenfelde KH, Rittner C. No association between mannose-binding lectin alleles and susceptibility to chronic hepatitis B virus infection in German patients. Exp Clin Immunogenet. 1998;15:130–3. doi: 10.1159/000019064. [DOI] [PubMed] [Google Scholar]
- 20.Cheong JY, Cho SW, Lim SK, Shin do H, Yoon SK, Lee JE, Hahm KB, Kim JH. Lack of association between hepatitis B virus infection and polymorphism of mannose-binding lectin gene in Korean population. J Korean Med Sci. 2005;20:65–9. doi: 10.3346/jkms.2005.20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Gordon J, Liu H, et al. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 2002;4:773–84. doi: 10.1016/s1286-4579(02)01597-6. [DOI] [PubMed] [Google Scholar]
- 22.Shi L, Takahashi K, Dundee J, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–90. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer PB, Ellermann-Eriksen S, Thiel S, Jensenius JC, Mogensen SC. Mannan-binding protein and bovine conglutinin mediate enhancement of herpes simplex virus type 2 infection in mice. Scand J Immunol. 1994;39:439–45. doi: 10.1111/j.1365-3083.1994.tb03398.x. [DOI] [PubMed] [Google Scholar]
- 24.Moller-Kristensen M, Wang W, Ruseva M, et al. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61:426–34. doi: 10.1111/j.1365-3083.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 25.Hemmila I, Dakubu S, Mukkala VM, Siitari H, Lovgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984;137:335–43. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- 26.Carroll MC. CD21/CD35 in B cell activation. Semin Immunol. 1998;10:279–86. doi: 10.1006/smim.1998.0120. [DOI] [PubMed] [Google Scholar]
- 27.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 28.Carroll MC. The complement system in B cell regulation. Mol Immunol. 2004;41:141–6. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Roozendaal R, Carroll MC. Complement receptors CD21 and CD35 in humoral immunity. Immunol Rev. 2007;219:157–66. doi: 10.1111/j.1600-065X.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 30.Green TD, Montefiori DC, Ross TM. Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d. J Virol. 2003;77:2046–55. doi: 10.1128/JVI.77.3.2046-2055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green TD, Newton BR, Rota PA, Xu Y, Robinson HL, Ross TM. C3d enhancement of neutralizing antibodies to measles hemagglutinin. Vaccine. 2001;20:242–8. doi: 10.1016/s0264-410x(01)00266-3. [DOI] [PubMed] [Google Scholar]
- 32.Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1:127–31. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Test ST, Mitsuyoshi J, Connolly CC, Lucas AH. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect Immun. 2001;69:3031–40. doi: 10.1128/IAI.69.5.3031-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas KM, Toapanta FR, Oliver JA, et al. Cutting edge: C3d functions as a molecular adjuvant in the absence of CD21/35 expression. J Immunol. 2004;172:5833–7. doi: 10.4049/jimmunol.172.10.5833. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, Poe JC, Tedder TF. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol. 2005;175:8011–23. doi: 10.4049/jimmunol.175.12.8011. [DOI] [PubMed] [Google Scholar]
- 36.Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3-and complement receptor 2-deficient mice. J Immunol. 2003;170:84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- 37.Roos A, Bouwman LH, Munoz J, et al. Functional characterization of the lectin pathway of complement in human serum. Mol Immunol. 2003;39:655–68. doi: 10.1016/s0161-5890(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 38.Roos A, Garred P, Wildenberg ME, et al. Antibody-mediated activation of the classical pathway of complement may compensate for mannose-binding lectin deficiency. Eur J Immunol. 2004;34:2589–98. doi: 10.1002/eji.200324401. [DOI] [PubMed] [Google Scholar]
- 39.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 40.Wiersma EJ, Coulie PG, Heyman B. Dual immunoregulatory effects of monoclonal IgG-antibodies: suppression and enhancement of the antibody response. Scand J Immunol. 1989;29:439–48. doi: 10.1111/j.1365-3083.1989.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 41.Heyman B. Feedback regulation by IgG antibodies. Immunol Lett. 2003;88:157–61. doi: 10.1016/s0165-2478(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 42.Diaz de Stahl T, Dahlstrom J, Carroll MC, Heyman B. A role for complement in feedback enhancement of antibody responses by IgG3. J Exp Med. 2003;197:1183–90. doi: 10.1084/jem.20022232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heyman B, Wigzell H. Specific IgM enhances and IgG inhibits the induction of immunological memory in mice. Scand J Immunol. 1985;21:255–66. doi: 10.1111/j.1365-3083.1985.tb01428.x. [DOI] [PubMed] [Google Scholar]