Abstract
Background
The ability to redirect the path of the foot during walking is critical for responding to perturbations and maintaining upright stability. The purpose of the current study was to compare mechanisms of reactive stepping adjustments in young versus older adults when responding to an unexpected perturbation during voluntary step initiation.
Methods
We tested 13 healthy community-dwelling older adults and an equal number of young control participants performing stepping movements onto a visual target on the floor. In some trials, perturbations were introduced by unexpectedly shifting the target, at various time points, from its usual location to a new location 20 cm to the right. We measured ground reaction forces under the supporting leg and three-dimensional kinematics of the stepping leg in baseline and target shift trials.
Results
During target shift trials, that is, when reactive adjustments were required, older adults demonstrated the following: delayed responses in modifying the lateral propulsive forces under the supporting foot, reduced rates of lateral force production, delayed responses in modifying the stepping foot trajectory, and prolonged movement execution times.
Conclusions
The current study quantitatively distinguishes between healthy older and young adults in generating reactive stepping adjustments to an unpredictable shift of a visual target. The decreased capability for rapidly planning and executing an effective voluntary step modification could reveal one potential cause for the increased risk of falls in the older population.
Keywords: Aging, Postural control, Falls, Biomechanics, Gait
FALLS represent a serious threat to elderly individuals. In the United States, 30%–60% of older adults fall each year, and 10%–20% of these falls result in injury or death (1). Accidents, environmental hazards, poor postural control, and gait problems have been shown to account for 50% of falls in elderly individuals (1,2). Therefore, it is important to assess gait function in older adults, as well as the ability to perform quick and accurate corrective movements when gait is perturbed.
To control balance during gait, centrally organized patterns of muscle activity are modulated based on the availability of sensory information, conditions of the support surface, biomechanical constraints, specific behavioral goals, and learning (3–8). Both anticipatory and reactive postural control mechanisms are regularly employed for this purpose. Anticipatory mechanisms are based on a feed-forward movement plan, whereas reactive mechanisms are generated by the use of sensorimotor feedback. Anticipatory, or feed-forward, mechanisms therefore are utilized in situations when current body dynamics and the environment are predictable and well learned. Conversely, reactive, or feedback, mechanisms are necessary in unpredictable situations. In novel or unpredictable circumstances, or in cases when the predictive feed-forward plan is incorrect, reactive postural control can be used to modify movements already in progress. Reactive mechanisms can be either automatic (reflexive), as in the case of automatic postural responses like recovering balance after a trip, or volitional, as in the case of a self-initiated correction of foot placement during walking in a cluttered environment (9).
One important source of sensory feedback for reactive control during walking is vision. Previous studies have shown that vision plays an important role in human locomotion by providing information about upright orientation, walking direction, speed, avoiding obstacles, and other critical environmental factors (10–12). During gait, visual information from a few steps ahead of the current location is used to form a feed-forward plan for limb movements (13–15). In addition, online visual feedback is also required to modulate, or update, the plan for limb movements on a step-by-step basis to avoid an inappropriate foot landing (12). This occurs frequently when an obstacle either initially goes undetected (eg, an icy patch not seen until almost upon it) or inserts itself into the previously clear walking path (eg, a cat darts into the walking path). When this happens, not only a quick but also an accurate adjustment to the feed-forward foot placement plan must be made that, by itself, can cause a perturbation of balance. Hence, the ability to efficiently and accurately adjust volitional stepping movements in response to unexpected visual feedback is an important skill that may help prevent falls during walking.
In the current study, we utilized a novel step initiation paradigm where the location of a visual target is shifted after the step has already begun, to examine the ability of older adults to modify their ongoing foot trajectory while controlling upright stability and forward progression of the body. Previous studies have shown that young adults utilize movements of the stepping foot while in air to maintain balance, which may impose limitations on the ability to adjust the foot trajectory (16,17). Despite the clear relevance for balance recovery during gait, little evidence is available regarding older adult’s performance on this sort of task. The purpose of this study was to determine how healthy older adults make voluntary stepping adjustments in response to an unpredictable visual target shift. We hypothesized that older participants would show impaired reactive control of stepping adjustments and that these deficits would systematically increase as the available time to respond decreased.
METHODS
Twenty-six healthy individuals, 13 young and 13 older adults (Table 1), participated in the study. Participants were excluded if they had any history of serious orthopedic, cardiovascular, neurological, or other medical disease or dysfunction. Participants in the older group were required to meet additional inclusion and exclusion criteria (Table 2), including passing a brief clinical examination (Table 3). All gave informed consent prior to participation, and a human studies committee approved the study.
Table 1.
Demographics and Anthropometry by Group
| Young Adult (N = 13) | Older Adult (N = 13) | ||
| Age | 28.4 ± 4.0 years (range 23–38) | 73.9 ± 4.6 years (range 66–81) | |
| Gender | 8 female participants, 5 male participants | 8 female participants, 5 male participants | |
| Height | 1.69 ± 0.11 m | 1.65 ± 0.08 m | F(1,24) = 1.04, p = .32 |
| Mass | 67.4 ± 19.4 kg | 67.8 ± 16.1 kg | F(1,24) = 0, p = .96 |
Note: Data shown are means ± 1 SD.
Table 2.
Entry Criteria for the Older Adult Group
| Older Adult Group Inclusion Criteria | Older Adult Group Exclusion Criteria |
| • Independent with all activities of daily living | • Use of any walking mobility aid |
| • Independent in community | • Any current or significant history of orthopedic, cardiovascular, neurological or other medical disease or dysfunction |
| • Engaged in some form of regular (≥2 days/week) physical activity | • 1 or more falls within past ≥1 year |
| • Pass a brief clinical exam (Table 3) | • Any chronic pain or any pain at test time |
| • Able to see visual targets and perform the task |
Table 3.
Brief Clinical Examination Components
| Test | Required Score | |
| Strength | Manual muscle testing* | ≥4 (on a 0–5 scale) on 3 of 3 attempts |
| Cutaneous sensation | Semmes–Weinstein monofilaments† | Threshold, handle mark ≤3.84 |
| Proprioception | Side-to-side position matching test‡ | Discrepancy <5° on 5 of 5 attempts |
| Vision | Self-reported acuity | 20/40 or better with or without corrective lenses |
| Balance | Maintain tandem stance with eyes closed ≥30 seconds | Able to perform on ≥1 of 2 attempts |
Notes: *Manual muscle testing (here, bilateral quadriceps and hamstrings) involves a “break” test; an examiner manually resists movement of a limb segment and rates the level of force applied at the point where the participant can no longer hold the position. Values of 4 or 5 indicate good or normal strength for a typical adult (18).
Semmes–Weinstein monofilaments (Wood Dale, IL) are thin fibers made of hair pressed on the skin surface (here, bilateral feet and lower legs). With eyes closed, the participant indicates if and when he/she can feel the monofilament. Monofilament handle mark ratings are determined by the filament thickness and its force capabilities, with larger numbers indicating greater thresholds for detection. A value of 3.84 or better (lower) on the lower leg or dorsum of the foot is considered the borderline between normal and diminished touch sensation (19).
The side-to-side position matching test assesses limb position sense (here, bilateral ankles and knees). An examiner places a joint at a particular angular position and, with eyes closed, the participant is asked to move the homologous extremity on the opposite side to the identical position.
We compared young versus older adults as they made reactive adjustments in response to an unpredictable shift of a target during a volitional, visually guided stepping movement. Participants stood with feet about pelvis-width apart, arms crossed, and body weight evenly distributed between the two feet. A force plate (AMTI, Watertown, MA) was under the left (supporting) foot; the right foot initiated the stepping response. During each stepping trial, a visual target (a red light of approximately 2 cm diameter projected onto the floor by a laser) appeared. Participants were instructed to “step so that you aim the center of your foot on the center of the target. Step as fast and accurately as you can”.
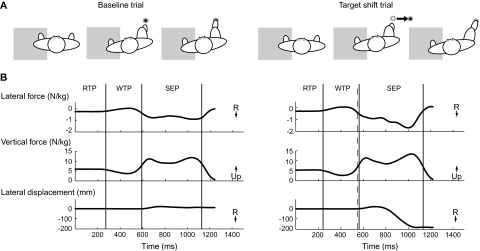
In baseline trials, the target was located directly in front of and at a distance of 40% of body height away from the stepping leg. In target shift trials, the target first appeared in this position but was replaced, instantaneously and without warning, by a second target in a new location 20 cm to the right (Figure 1A). These target shifts occurred 450, 550, or 650 ms (early, middle, or late) after the initial target presentation. A block of 20 baseline stepping trials was completed first to familiarize participants with the task; these data were not analyzed. Participants then performed 90 trials, within which a total of nine target shift trials (three trials for each target shift time) were pseudo-randomly inserted between nine blocks of 6–13 baseline trials, such that participants could not predict when a target shift might occur. Three infrared-emitting markers (Optotrak, NDI, Waterloo, ON), placed on the fifth metatarsal head, the lateral base of the calcaneus and the lateral malleolus of the right foot, measured foot movement. Footswitches (Motion Lab Systems, Inc., Baton Rouge, LA) recorded the timing of footfalls. Kinematic data were collected at 100 Hz; footswitch and ground reaction forces were collected at 1,000 Hz and time synchronized with the kinematics. Offline, data were low-pass filtered at 10 Hz. Subsequent analyses were conducted using custom MATLAB (Mathworks, Natick, MA) software.
Figure 1.
(A) Overhead view of the experimental setup for baseline and target shift trials. The supporting leg was on a force plate (gray rectangle). After an auditory “ready” cue, the appearance of the visual target acted as the “go” cue. In baseline trials, the target (filled circle) was positioned directly in front of the stepping foot. In target shift trials, the target unpredictably and instantaneously shifted from its initial location to a new location 20 cm to the right. (B) Lateral and vertical ground reaction forces under the supporting foot and movement trajectories of the stepping foot in the medial/lateral direction from a typical young control participant during a baseline (left) and target shift (right) trial. Vertical lines delineate the boundaries of the response time (RTP), weight transfer (WTP), and step execution (SEP) phases. Response time phase = time from initial target illumination to onset of the first decrease in the vertical force under the supporting leg to below 5% of the mean vertical force during quiet standing. Weight transfer phase = time from the end of response time phase to onset of heel-off on the stepping leg. The weight transfer time encompasses the very well characterized and stereotyped lateral weight shift, first toward the stepping side, then the supporting side, that rapidly unloads the stepping leg before step initiation (20–22). Stepping execution phase = time from the end of weight transfer phase to subsequent initial contact of the stepping leg onto the target. Dashed vertical line indicates the time of the target shift (in this case, 550 ms after initial target illumination).
A step trial was defined as the time from initial target illumination to lift-off on the supporting leg. We identified three movement phases within each trial (Figure 1B): response time, weight transfer, and stepping execution phases. For analysis, we quantified the (a) movement phase durations, (b) stepping endpoint accuracy, (c) time required to modify the stepping foot trajectory following a target shift, (d) time required to modify the lateral force under the supporting foot following a target shift, (e) peak lateral force achieved, and (f) rate of lateral force production. Stepping accuracy was quantified by the medial-lateral endpoint errors in the stepping foot position. The time required to modify the stepping foot trajectory (or the lateral force) was measured as the time from the onset of the target shift to the initial deviation of the foot trajectory (or lateral force) from baseline. The time of the initial deviation was identified as the point when the foot trajectory (or lateral force) during the target shift trial first deviated from the mean of that during baseline trials by more than 2 SD. The peak lateral force was measured during the stepping execution phase. The rate of lateral force production was measured as the slope of a line connecting minimal and maximal lateral forces during stepping execution. All variables were averaged over the three target shift trials for each target shift condition and over three selected baseline trials (those that immediately preceded the second target shift trial for each of the three target shift conditions) for each participant.
Statistical comparisons were made using SAS/STAT software (SAS Inc., Cary, NC). A two-way (Group × Condition) mixed model analysis of variance (ANOVA) with repeated measures on one factor (condition) was used. The older group demonstrated substantially greater variance than the young on stepping execution phase duration, time to modify foot trajectory, and time to modify lateral force, so these data were log transformed prior to statistical analyses. When the ANOVA was significant, post hoc analyses were performed using Tukey's honestly significant difference test (level for statistical significance, p ≤ .05).
RESULTS
There were no differences in height or body mass between young and older adults (Table 1). As expected, most demonstrated a consistent pattern of increased lateral force under the supporting foot and a shift of the stepping foot position toward the right after target shifts. Figure 1B shows traces of ground reaction forces and the stepping trajectory from a typical young participant in a baseline and a target shift trial.
During target shift trials, shifts were timed to occur early, middle, or late in the trial, but were not synchronized to participants’ movements. For most trials and most individuals, each target shift condition occurred generally at the same time within the movement. Early target shifts almost always occurred during the weight transfer phase (young, 84% of trials; older adults, 97%). Middle target shifts most often occurred during the weight transfer phase (young, 56%; older adults, 74%), but sometimes in the stepping execution phase. Late target shifts nearly always occurred during the stepping execution phase (young, 100%; older adults, 84%). Overall, there was a clear trend for target shifts to occur somewhat earlier in older compared with young participants. This is consistent with older participants reacting to the movement cue and/or executing movements slower than young participants.
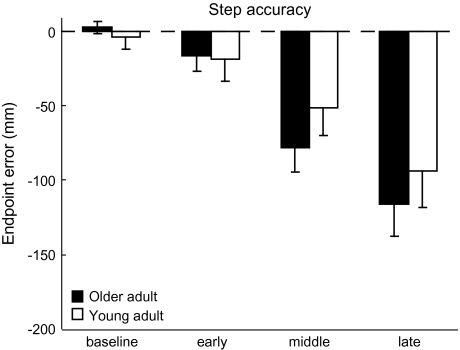
Figure 2 shows the averaged lateral stepping endpoint accuracy. As the available response time decreased, stepping errors incrementally increased (condition effect, F(3,22) = 13.86, p < .001). Stepping errors in the middle and late conditions were both significantly larger than those in the others (post hoc, both p ≤ .001). Although there was a visible trend for the older group to show increased stepping errors, particularly in the later target shift conditions, there were no significant differences between groups (F(1,24) = 0.39, p = .54) and no interaction effect (F(3,22) = 0.65, p = .59).
Figure 2.
Stepping foot endpoint accuracy. Average step accuracy across all conditions and groups. Accuracy is depicted as the medial/lateral error in the foot endpoint position. Negative numbers indicate errors in the leftward (undershoot) direction. Error bars, ±1 SEM.
Average movement phase durations are depicted in Figure 3. Older adults had consistently longer response time phase durations, approximately 30 ms longer, across all conditions (group effect, F(1,24) = 9.88, p = .004; Figure 3A). There were no differences across target shift conditions (F(3,72) = 0.74, p = .53) nor any interaction effect (F(3,72) = 1.04, p = .38). For the weight transfer durations (Figure 3B), there were no group (F(1,24) = 0.37, p = .55), condition (F(3,72) = 1.9, p = .14), or interaction effects (F(3,72) = 0.32, p = .81). Interestingly, comparisons of the stepping execution phase (Figure 3C) showed significant group (F(1,24.2) = 9.65, p = .005), condition (F(3,45.8) = 10.32, p < .01), and interaction effects (F(3,45.8) = 3.15, p = .03). The young adults were able to maintain a relatively constant stepping execution phase duration across all conditions, whereas older adults showed substantial prolongation whenever a target shift occurred (post hoc comparing baseline with early, middle, or late target shifts, respectively, in the older adult group, p < .001, p = .005, p = .03). Older adults were about 50 ms slower than the young during the baseline condition, but became approximately 200 ms slower during any of the target shift conditions.
Figure 3.
Movement phase durations. Average duration of the (A) response time phase, (B) weight transfer phase, and (C) stepping execution phase across all conditions and groups. Asterisks show specific significant post hoc differences. Error bars, ±1 SEM.
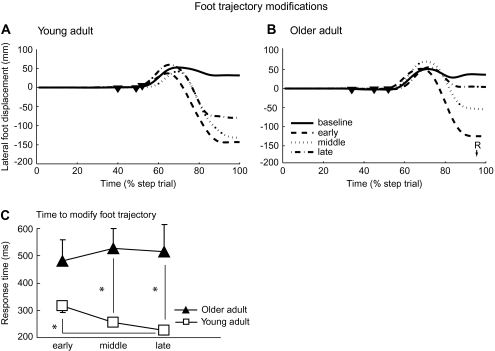
Figure 4A and B show individual stepping foot trajectories from two typical participants, one from each group. Both had a similar pattern of later foot trajectory modifications when the target shift occurred later. Figure 4C illustrates average foot trajectory modification times, or the times between target shift onset and the earliest modification of the foot trajectory in the medial/lateral direction. There were significant group and interaction effects (F(1,23.3) = 17.92, p = .0003, F(2,35.8) = 4.72, p = .015, respectively), but no condition effect (F(2,35.8) = 1.38, p =.26). When target shifts occurred later, young adults progressively shortened the modification time (post hoc comparing early with late target shift condition, p = .03). However, this was not the case for older adults; the post hoc showed no differences across three target shift conditions in older adults. Compared with the young, modification times were significantly longer for the older adults during the middle and late target shift conditions (post hoc, p = .001 and p = .006, respectively). Therefore, the young group was better able to execute foot trajectory modifications in response to target shifts, particularly when the available response time was reduced.
Figure 4.
Stepping foot trajectory data. Individual foot trajectories in the medial/lateral direction for each target shift condition from a typical (A) young and (B) older participant. Time is shown as a percentage of the total step trial. Onset times of the target shifts in each of the three target shift conditions are indicated by filled inverted triangles. (C) Average time to initiate a stepping foot trajectory modification following a target shift, shown for all target shift conditions and groups. Asterisks show specific significant post hoc differences. Error bars, ±1 SEM.
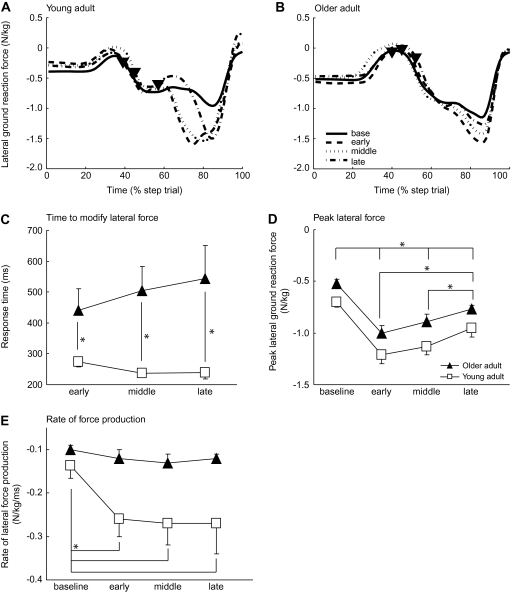
Figure 5A and B show individual traces of lateral ground reaction forces for two typical participants. The increased lateral force late in the stepping movement propels the center of mass to the right and over the stepping foot during target shift trials. Generally, the peak lateral force occurred later when the target shifts occurred later. For the time required to modify lateral forces (Figure 5C), there were group (F(1,23.7) = 21.87, p < .0001) and interaction effects (F(2,35.7) = 3.66, p = .036), but no condition effect (F(2,35.7) = 0.16, p = .85). Specifically, force modification times were significantly longer for older adults compared with the young across all target shift conditions (post hoc, early, p = .03; middle, p = .0007; late, p = .003). Thus, older adults also showed deficits in the ability to modify lateral forces under the supporting foot in response to unpredictable target shifts, and this deficit was greatest when the available response time was shortest (ie, during late target shifts).
Figure 5.
Support foot force data. Individual lateral ground reaction forces under the support foot for each target shift condition from a typical (A) young and (B) older participant. Time is shown as a percentage of the total step trial. Onset times of the target shifts in each of the three target shift conditions are indicated by filled inverted triangles. Average (C) time to initiate a lateral force modification, (D) peak lateral force amplitude, and (E) rate of lateral force production under the supporting foot, shown for all target shift conditions and groups. Asterisks show specific significant post hoc differences. Error bars, ±1 SEM.
The peak lateral force (Figure 5D) was significantly reduced in older adults compared with the young (group effect, F(1,24) = 0.02, p = .02) and reduced across target shift conditions (condition effect, F(3,22) = 55.8, p < .001), but there was no interaction (F(3,22) = 0.25, p = .86). We also found similar significant results for the anterior/posterior force (not shown). The rate of force (Figure 5E) was also reduced in older adults (group effect, F(1,24)=5.99, p = .022) and across target shifts (condition effect, F(3,40.4)=13.46, p < .001). Here, there was also a significant interaction (F(3,40.4)=5.26, p = .004), such that the young significantly increased their rate of lateral force development when there was a target shift (early, middle, and late target shifts compared with baseline within young group post hoc, all p ≤ .001), whereas older adults showed no change in their rate of force during any target shift compared with baseline (post hoc, all p ≥ .80).
DISCUSSION
In the current study, older adults with no known pathology showed significant impairments of reactive control during voluntary, visually guided step initiation. Following an unpredictable shift of a visual target location, older participants demonstrated delayed responses in modifying lateral propulsive forces, reduced rates of lateral force production, delayed responses in modifying the stepping foot trajectory, and prolonged movement execution times. To our knowledge, this is the first study to quantitatively distinguish between healthy young and older adults in generating reactive stepping adjustments to an unpredictable shift of a visual target during midstep. Our results reveal clear deficits in the ability of healthy older adults to rapidly and accurately execute an effective voluntary stepping modification and could reveal one potential cause for the increased risk of falls in this vulnerable population.
It is important to note that these deficits are beyond any general impairments of step initiation. That is, older adults showed slowness and reduced forces that were much greater whenever reactive control was required (ie, target shift condition) to alter a previously preplanned stepping movement compared with unperturbed (ie, baseline condition) preplanned movements. This suggests that these deficits of reactive control are not solely the result of age-related peripheral sensory losses (eg, reduced neural conduction velocities, slowed reaction times), though these problems can and do exist in older adults. Rather, poor reactive control in this case may be the result of a convergence of multiple factors, including sensory losses, strength or power deficits, cognitive changes, psychological and emotional factors (eg, fear of falling), neural processing deficits, and difficulty managing dual-task situations.
Force and Force Rate Modification Deficits
The force data suggest that postural stability is degraded in the medial/lateral dimension during voluntary stepping modifications in older adults. Substantially increased lateral forces are required to propel the body to the right to achieve the new target location. Our older participants were unable or unwilling to increase the rate of lateral force production in these reactive control conditions. They also produced force modifications later when the time available to make such adjustments shortened. It has been proposed that ineffective muscle activations (23,24) or reduced rates of force production (24–26) degrade locomotion and increase the incidence of falls in elderly individuals. Studies utilizing electromyography have shown that older adults may have degraded neural control that diminishes their capability to make appropriate postural and force adjustments during gait initiation (23,24). Unfortunately, we cannot determine from the current study whether impaired neural control or reduced power-generating capability is responsible for the decreased force rate seen here. A strategy we observed in our older participants was to exert a smaller peak force but prolong its duration. This is ineffective for situations when speed is essential, which is typical of cases when reactive postural control mechanisms are utilized.
Foot Trajectory Modification Deficits
For both young and older groups, stepping accuracy declined systematically when the target shift occurred later in the preplanned movement. However, only young participants increased the speed at which they began to adjust the ongoing foot trajectory in response to a decreased available response time. It is well known that during visually guided stepping, an estimate of intended foot placement and associated body motion are coupled and preplanned before lifting the foot off the ground (16,27). Thus, when the intended target location shifts (or the nervous system reestimates its position) after the stepping foot moves forward, both the planned foot trajectory and the accompanying postural adjustments must be quickly altered. We hypothesize that the difficulty in increasing lateral forces and lateral force rates seen in our older adults could explain the deficit in foot trajectory modifications observed: Reduced and slowed lateral forces under the support foot could prevent lateral progression of the stepping foot toward its new target. The inability to shift foot locations rapidly following a target shift probably represents a serious impairment that could lead to falls in circumstances when reactive control is required to maintain posture.
Interestingly, young adults appeared to show slight decreases in both the force and the foot trajectory modification response times when less time was available (Figures 4C and 5C). Thus, younger participants seem to be able to speed up their reactive responses when required, whereas older adults do not. It is not clear whether older adults are already working at their maximal capacity and therefore cannot reduce response times any further or whether they elect not to speed up in an attempt to avoid “self-perturbing” movements that could lead to greater postural instability.
Movement Execution Durations
We observed significant increases in response time phase durations in older adults, which is consistent with other studies showing slower step initiation in this population (26,28,29). A more striking difference was the very large increase in stepping execution phase durations during target shift conditions. Because reduced lateral ground reaction forces likely reduce the rate of center of mass shift to the stepping side, we speculate that stepping execution phase prolongation in the older group may represent a compensation to regulate the rate of step relative to the rate of center of mass shift.
Study Limitations
The current study has several limitations worthy of mention. First, the timing of the target shifts was not synchronized to any kinematic events such as weight shifting or step initiation. It is possible that some of the target shifts occurred earlier in the step cycle for the older participants. If true, they would have more time to make modifications than young participants, and this could explain why they took longer times for many of our measured variables. We argue against this possibility because (a) if true, we would still expect older participants to show a gradual decrease in foot trajectory and force modification times as the available response time decreases, just as controls do, yet, they do not (Figures 4C and 5C) and (b) when comparing the data from the older group responding to the middle target shift with the young group responding to the early shift (in this comparison, the older group received the target shift later), we still see clear differences in response times (Figures 4C and 5C). Therefore, we do not think this difference substantially alters our results or our interpretation. Another limitation is that we did not measure forces under the stepping leg; this could have revealed interesting details of how the center of mass is stabilized during the landing phase of the step. An assessment of the anticipatory postural adjustments (APAs) that precede step initiation may also have revealed interesting trends. Note that APAs are predictive in nature and occur before the target shifts; therefore, they would not be expected to be affected by the unpredictable target shifts. However, some individuals may have executed larger APAs naturally. They would then have experienced slower rates of center of mass movement and perhaps not have had to execute stepping adjustments as quickly. Thus, it is possible that unique individual or age-related differences in APAs could have altered participants’ responses to the unpredictable perturbations. This presents an interesting question that should be addressed in future research. Another limitation is that, although the forward distance to the target was normalized to participant height, the lateral distance was not. Because the step trajectory was not likely to be purely along the diagonal, this could have provided some advantage to taller participants. We do not think this presents a serious problem with our results, however, given there were no height differences between groups. Finally, inclusion criteria for our older adults were relatively generous. It is possible that some participants may have had mild disease burden that we did not detect with our questionnaire and clinical exam. Nevertheless, the sample probably reasonably represents many typical independent, community-dwelling older adults who consider themselves healthy and not at risk for falling.
Conclusions
Older adults show systematic degradation of stepping adjustments during unpredictable target shifts coinciding with a reduction in available response times. The ability to initiate quick, effective stepping modifications such as these may be critical for altering the base of support, preserving upright stability, and preventing falls. Future studies should specifically address relationships between these deficits and falls.
FUNDING
This study was supported by National Institutes of Health, National Institute of Child Health and Human Development grant HD050369 and a predoctoral grant from the International Society of Biomechanics.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
Acknowledgments
We would like to thank D. Savin for helpful discussion about this project and S. Hartman for assistance with data collection.
References
- 1.Rubenstein L. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Aging. 2006;35:ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 2.Ashley MJ, Gryfe CI, Amies A. A longitudinal study of falls in an elderly population II. Some circumstances of falling. Age Ageing. 1977;6:211–220. doi: 10.1093/ageing/6.4.211. [DOI] [PubMed] [Google Scholar]
- 3.Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Shepard J, Rowell L, editors. Handbook of Physiology. New York, NY: Oxford University Press; 1996. pp. 255–292. [Google Scholar]
- 4.Horak FB, Henry SM, Shumway-Cook A. Postural perturbations: new insights for treatment of balance disorders. Phys Ther. 1997;77:517–533. doi: 10.1093/ptj/77.5.517. [DOI] [PubMed] [Google Scholar]
- 5.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 6.Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev. 1998;22:465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 7.Massion J, Alexandrov A, Frolov A. Why and how are posture and movement coordinated? Prog Brain Res. 2004;143:13–27. doi: 10.1016/S0079-6123(03)43002-1. [DOI] [PubMed] [Google Scholar]
- 8.Shumway-Cook A, Woollacott MH. Motor Control: Theory and Practical Application. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2001. pp. 163–396. [Google Scholar]
- 9.Patla AE. Strategies for dynamic stability during adaptive human locomotion. IEEE Eng Med Biol Mag. 2003;22:48–52. doi: 10.1109/memb.2003.1195695. [DOI] [PubMed] [Google Scholar]
- 10.Hollands MA, Patla AE, Vickers JN. “Look where you're going!”: gaze behaviour associated with maintaining and changing the direction of locomotion. Exp Brain Res. 2002;143:221–230. doi: 10.1007/s00221-001-0983-7. [DOI] [PubMed] [Google Scholar]
- 11.Patla AE, Rietdyk S, Martin C, Prentice S. Locomotor patterns of the leading and the trailing limbs as solid and fragile obstacles are stepped over: some insights into the role of vision during locomotion. J Mot Behav. 1996;28:35–47. doi: 10.1080/00222895.1996.9941731. [DOI] [PubMed] [Google Scholar]
- 12.Patla AE, Prentice SD, Rietdyk S, Allard F, Martin C. What guides the selection of alternate foot placement during locomotion in humans. Exp Brain Res. 1999;128:441–450. doi: 10.1007/s002210050867. [DOI] [PubMed] [Google Scholar]
- 13.Hollands MA, Marple-Horvat DE, Henkes S, Rowan AK. Human eye movements during visually guided stepping. J Mot Behav. 1995;27:155–163. doi: 10.1080/00222895.1995.9941707. [DOI] [PubMed] [Google Scholar]
- 14.Patla AE, Rietdyk S. Visual control of limb trajectory over obstacles during locomotion: effect of obstacle height and width. Gait Posture. 1993;1:45–60. [Google Scholar]
- 15.Patla AE, Vickers JN. How far ahead do we look when required to step on specific locations in the travel path during locomotion. Exp Brain Res. 2003;148:133–138. doi: 10.1007/s00221-002-1246-y. [DOI] [PubMed] [Google Scholar]
- 16.Lyon IN, Day BL. Predictive control of body mass trajectory in a two-step sequence. Exp Brain Res. 2005;161:193–200. doi: 10.1007/s00221-004-2058-z. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds RF, Day BL. Rapid visuo-motor processes drive the leg regardless of balance constraints. Curr Biol. 2005;26:R48–R49. doi: 10.1016/j.cub.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA. Muscles: Testing and Function with Posture and Pain. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005. pp. 359–421. [Google Scholar]
- 19.Weinstein S. Fifty years of somatosensory research: from the Semmes-Weinstein monofilaments to Weinstein Enhanced Sensory Test. J Hand Ther. 1993;6:11–22. [PubMed] [Google Scholar]
- 20.Jensen JL, Brown LA, Woollacott MH. Compensatory stepping: the biomechanics of a preferred response among older adults. Exp Aging Res. 2001;27:361–376. doi: 10.1080/03610730109342354. [DOI] [PubMed] [Google Scholar]
- 21.Maki BE, Edmondstone MA, McIlroy WE. Age-related differences in laterally directed compensatory stepping behavior. J Gerontol. 2000;55:M270–M277. doi: 10.1093/gerona/55.5.m270. [DOI] [PubMed] [Google Scholar]
- 22.McIlroy WE, Maki BE. Age-related changes in compensatory stepping in response to unpredictable perturbations. J Gerontol. 1996;51:M289–M296. doi: 10.1093/gerona/51a.6.m289. [DOI] [PubMed] [Google Scholar]
- 23.Polcyn AF, Lipsitz LA, Kerrigan DC, Collins JJ. Age-related changes in the initiation of gait: degradation of central mechanisms of moment generation. Arch Phys Med Rehabil. 1998;79:1582–1589. doi: 10.1016/s0003-9993(98)90425-7. [DOI] [PubMed] [Google Scholar]
- 24.Brunt D, Santos V, Kim HD, Light K, Levy C. Initiation of movement from quiet stance: comparison of gait and stepping in elderly subjects of different levels of functional ability. Gait Posture. 2005;21:297–302. doi: 10.1016/j.gaitpost.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Patchay S, Gahery Y, Serratrice G. Early postural adjustments associated with gait initiation and age-related walking difficulties. Mov Disord. 2002;17:317–326. doi: 10.1002/mds.10074. [DOI] [PubMed] [Google Scholar]
- 26.Halliday SE, Winter DA, Frank JS, Patla AE, Prince F. The initiation of gait in young, elderly, and Parkinson's disease subjects. Gait Posture. 1998;8:8–14. doi: 10.1016/s0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 27.Lyon IN, Day BL. Control of frontal plane body motion in human stepping. Exp Brain Res. 1997;115:345–356. doi: 10.1007/pl00005703. [DOI] [PubMed] [Google Scholar]
- 28.Lord SR, Fitzpatrick RC. Choice stepping reaction time: a composite measure of falls risks in older people. J Gerontol. 2001;56:M627–M632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- 29.Patla AE, Frank JS, Winter DA, Rietdyk S, Prentice S, Prasad S. Age-related changes in balance control system: initiation of stepping. Clin Biomech. 1993;8:179–184. doi: 10.1016/0268-0033(93)90012-7. [DOI] [PubMed] [Google Scholar]