Abstract
Women giving birth at advanced reproductive ages in natural fertility conditions have been shown to have superior postmenopausal longevity. It is unknown whether improved survival is more likely among relatives of late-fertile women. This study compares survival past age 50 of men with and without a late-fertile sister in two populations: Utahns born in 1800–1869 identified from the Utah Population Database and Québec residents born in 1670–1750 identified from the Programme de recherche en démographie historique. Male survival was greater for those with, rather than without, a sister reproducing after age 45, particularly among men with at least three sisters (Utah rate ratio [RR] = .801, 95% CI = 0.687–0.940; Quebec RR = .786, 95% CI = 0.664–0.931). Survival of wives was unaffected by whether their husbands had a late-fertile sister, suggesting a weak influence of unmeasured socioenvironmental factors. These results support the hypothesis that late female fertility and slow somatic aging may be promoted by the same genetic variants.
Keywords: Survival, Longevity, Reproduction, Aging, Natural fertility
ADVANCING adult age is associated with increasing mortality rates and decreasing fertility rates (1). This age-related deterioration in functioning is partly subject to genetic regulation (2,3). Several observational studies of human populations have demonstrated how these two life history traits are linked. Women bearing children at advanced ages have been shown to have better postmenopausal survival in natural fertility conditions (4–9). For women born in 1896, childbearing after age 40 was found to be four times more frequent in centenarian women than in control women who died in their early seventies (10). Contemporary women who reach advanced ages at natural menopause were also shown to experience significantly improved survival chances (7,11–13), Experimental designs are also useful in demonstrating the close connection between fertility and mortality patterns. Experiments that select for late reproduction in female mice (14) and Drosophila melanogaster (15,16) have generated longer lived strains, which suggest that the timing of reproductive cessation in females is determined in part by the rate of senescence.
An important question in these studies is that the association measured between late female fertility and longevity within the same individual may arise for factors that are complex and often unobservable. Late female fertility and excess survival in the same woman may be observed because of a beneficial environment or selection of healthier women to continue with additional births. Some investigations have made serious efforts to consider the effects of these confounders, but this issue remains a concern in observational studies of human populations. For example, one study using genealogies of the British peerage from 1603 to 1959 found that the relationship between fertility and late-life mortality was obtained only when the influences of health differentials and mortality selection during reproductive ages were taken into account (17). Another analysis also considered the effects of mortality selection in three natural fertility populations and found that it did not appreciably affect the association between fertility and subsequent survival (18).
This body of work has not determined whether improved survival longevity is more likely in the relatives of late-fertile (or late-menopausal) women, as would be predicted if genes that slow aging also enhance both late fertility in women and longer life spans in both sexes. It is plausible that late female fertility might enhance survival prospects in female relatives, especially in those with no or few children. For most women in natural fertility settings, however, their own reproductive schedules are likely to mask the potentially beneficial influences of having a late-fertile female kin. We are aware of only one study that has considered the association between late female reproduction and longevity among female relatives, but this was found to be insignificant, perhaps a result of the small sample size used (19). We hypothesize that improved survival is partly attributable to genetic variants that slow the rate of aging in both sexes via a mechanism that also facilitates late female fertility. To test this hypothesis, we compare the survival of men who have a late-fertile sister to men with sisters who were not fertile late. To evaluate the possible role of socioenvironmental factors that may affect this hypothesized association, we examine survival among wives married to brothers of late and non–late-fertile women. This is the first study we are aware of that has considered fraternal longevity as a function of late fertility among female relatives.
This analysis uses brothers to assess familial aggregation of longevity associated with late female fertility for two primary reasons. First, men do not experience the physical challenges associated with pregnancy, childbirth, and their aftermath. Although men are not immune to the influences of their own fertility on their life span (4,9), men represent an excellent population to study because the effects of late female (sister) fertility can be examined as a marker for slower aging but without the complications of their own fertility behaviors that are inherent in studies of women. Second, an examination of brothers’ survival as a function of their sister's late fertility focuses the attention on the possibility of shared genetic predisposition for slower aging. This particular sibling-based design (looking at brother's longevity as a function of sister's fertility) is preferred because the putative longevity benefits to women of having a late-fertile sister is complicated by a woman's own fertility history.
If evidence exists for genetic codetermination of late fertility in women and longevity in both sexes, then researchers can select for study the long-lived individuals most likely to carry genes for slower aging, that is, those from families containing late-fertile women. Furthermore, late fertility, along with other biomarkers of slower aging that can be measured in middle-aged women and their family members, may constitute a trait specific for slow aging to allow the identification of the relevant genes even when long-lived research participants are not available.
METHODS
We use two historical sources of high-quality and comprehensive data on sibships with complete survival and fertility information when little or no effective birth control existed. The first population is drawn from the Utah Population Database (UPDB) at the University of Utah. The full UPDB includes genealogical data on 1.6 million individuals who comprise the founding Utah population and their descendants (see http://www.huntsmancancer.org/groups/ppr/). The second data set is compiled by the Programme de recherche en démographie historique (PRDH) at the Université de Montréal and includes data on more than 400,000 individuals who lived in Québec between 1608 and 1850 (see http://www.genealogie.umontreal.ca/en/). For the UPDB, sibships are included in which all siblings were born before 1870, whereas PRDH siblings were born between 1670 and 1750. Both samples comprise extinct cohorts. We further restricted the sample to once-married individuals and those who survived to age 50 to minimize variability in survival unrelated to aging (e.g., accidental deaths). Requiring that individuals survive to 50 ensured that female fertility was largely complete.
We conduct two distinct analyses. We first assess how late female reproduction affects women's own survival in these two populations. These results are based on Cox proportional hazard regressions that include women who were married once, lived to age 50, had husbands who were alive when these women were age 50, and had at least one child. To ensure consistency with the brother survival analysis shown later, these women also had to have all brothers born during 1800–1870 for the Utah sample or born during 1650–1750 for the Quebec sample. For both samples, included covariates are year of birth, age at death of spouse, age at first birth, and parity; the Utah sample also adjusts for religious affiliation (active member of the Church of Jesus Christ of Latter-day Saints, inactive member, or a nonmember). The sample size of women for Utah is N = 14,123 and that for Québec is N = 4,666.
The second analysis compares male survival classified according to the age at last birth of their latest fertile sister using the same percentile categorization as mentioned earlier: more than or equal to 85th percentile, 50th–84th percentile, and the reference category comprising the bottom 50th percentile. Choosing the maximum value of age at last birth in a group of sisters maximizes the effects of detecting slower reproductive aging. This strategy also reduces the unknown effects of nonrandom selection arising because some sisters will not appear in the sample due to maternal mortality; this mortality selection occurs but is far less likely to occur in multiple sisters.
RESULTS
Late Female Fertility and Risk of Female Mortality
We compare women with late ages at last birth with a reference group of women whose ages at last birth did not occur at an advanced age, which we define here as below the median (i.e., the 50th percentile). For Utah, the reference group comprises those with an age at last birth before age 41, whereas it is before age 42 for the Quebec sample. In relation to the reference group, Utah women with an age at last birth between ages 41 and 44 (50th–84th percentile) had a mortality hazard rate ratio (HRR) of 0.94 (95% CI = 0.90–0.98), whereas those with an age at last birth of 45 years or later (≥85th percentile) had an HRR of 0.86 (95% CI = 0.81–0.91). For Quebec, the HRR = 0.93 (95% CI = 0.86–0.99) for women between the 50th and 84th percentile (ages 42–44.5), whereas the HRR = 0.83 (95% CI = 0.76–0.91) for those at or above the 85th percentile (age ≥ 44.5). Across both populations, therefore, late fertility reduced women's own mortality risk after age 50 by about 15% in relation to their respective reference group.
Late Female Fertility and Risk of Mortality Among Their Brothers
The Cox regression models also control for other important confounders. The longevity of the longest lived sisters is controlled using again the same percentile trichotomy that was used for the age-at-last-birth distribution. The remaining variables included in the Cox regressions are similar but not identical between the two samples (owing to differences in variables available for each population and missing data). For the Utah sample, statistical controls include birth year, age at death of longest lived sister, paternal and maternal age at death, and religious affiliation. In the Quebec sample, the covariates comprise birth year, age at death of longest lived sister, age at death of spouse, urban/rural status, and region of residence in the colony (East or West).
Given that selection for extreme values of traits is associated with larger sibships, we examine how the number of sisters affects the association between late female fertility and survival of brothers. Stratifying the analysis by the number of sisters also addresses the possibility that there are social support benefits for brothers who have more adult sisters.
The precise ages that define the categories of age-at-last-birth vary as the analysis restricts the sample by the number of sisters. For Utah, the 50th percentiles are at ages 41.0, 42.3, and 43.1 for samples with 1 or more, 2 or more, and 3 or more sisters, respectively. For Quebec, the 50th percentiles are at ages 42.5, 43.2, and 43.8 for the three comparable samples. For these three subsamples, the 85th percentiles are at ages 44.4, 45.1, and 46.3 for Utah and at ages 44.9, 45.4, and 45.6 for Quebec.
Statistical adjustments to account for correlated survival among brothers are introduced to address the possibility that the brothers later life survival is due to some common early-life and mid-life environmental circumstances that enhanced their health and robustness (i.e., the estimated association between late female fertility in sisters and superior survival in brothers is confounded by some shared environment that promoted both late female fertility [the independent variable] and enhanced male longevity [the dependent variable]). Because of patrilocality in Quebec, shared circumstances among brothers not only exist when they are young but can also extend into their adult years. In Quebec, brothers often congregated in the same region, living their lives not far apart from one another (20).
Although the analyses shown here are for brothers only, statistical control for shared frailty among these brothers is an effort to adjust for variations in favorable or deleterious environments. It also controls for shared but unobserved genetic factors that promote better adult survival. With Cox proportional hazards models, allowance for the effects of shared frailty means that members of a group (brothers) are possibly correlated because they have some common propensity to experience death (21). This family-specific propensity is not directly observable, but its distribution can be estimated and tested as to its significance. As the estimated variance of this distribution increases, there is evidence that shared but unmeasured factors among brothers are present. If the variance is zero, then no relevant shared factors are detected given the model and the data. Estimating Cox regressions as though shared frailty effects are not present (when they are) can lead to biased estimates of the covariates (22,23). These adjustments for shared fraternal frailty yield results (not shown) that have effect sizes that are slightly larger than the more conservative results reported later.
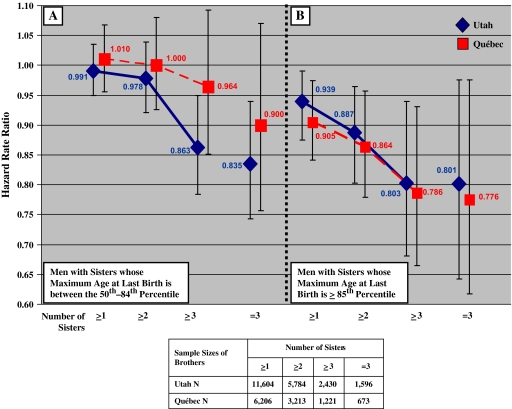
Figure 1 shows that the relative survival after age 50 was greater for men with a late-fertile sister. The male survival advantage increases with increasing numbers of sisters because additional sisters provide more opportunities to detect sisters with slower reproductive senescence. Therefore, the probability that control sibships (sibships lacking sisters with older ages at the time of their last birth) will lack genes for slower reproductive senescence should rise with the number of sisters in those sibships. The largest effects occur for men with a late-fertile sister (out of at least three sisters). These men have a mortality rate that is approximately 20% lower than that for men without such sisters (for Utah HRR = 0.801, 95% CI = 0.687, 0.940; for Quebec HRR = 0.786, 95% CI = 0.664, 0.931).
Figure 1.
Mortality hazard rate ratios (HRRs) for brothers living to age 50 by age-at-last-birth of sisters who also survived to age 50 based on Cox proportional hazards models. HRR estimates are diamonds for Utah and squares for Quebec. Vertical bars represent HRR (95% confidence intervals) that adjust for familial clustering. See text for other variables controlled in the models. The comparison group comprises brothers whose sisters are all in the bottom 50th percentile. (A) Survival of men with sisters whose maximum age-at-last-birth was in the 50th–84th percentile of the female age-at-last-birth distribution. (B) Survival of men with a sister whose age at last birth is more than or equal to 85th percentile of the female age-at-last-birth distribution.
Survival benefits for brothers associated with late female fertility may arise for unmeasured socioenvironmental reasons. If true, and because spouses share environmental circumstances, wives of brothers of late-fertile women might also experience a survival advantage. However, using the same set of controls as mentioned earlier, we find that the survival of wives was unaffected by whether their husbands had a late-fertile sister in both populations. For Utah, women married to men with at least three sisters living to age 50 where one sister gave birth at age 46.3 or later (the 85th percentile) had an HRR = 0.94 (95% CI = 0.78–1.15; N = 1,637) in relation to women married to men with no late-fertile sisters. The comparable figure for Quebec women (85th percentile for age at last birth is 45.6 years) is HRR = 1.12 (95% CI = 0.92–1.38; N = 861).
DISCUSSION
The association reported here between increased male survival and late fertility in their sisters is consistent with the hypothesis that genetic variants exist that might simultaneously promote late female fertility and slow somatic aging. Genetic analyses of longevity may be more powerful in families with late-fertile or late-menopausal women. It may also be useful to substitute or supplement phenotyping for late fertility with phenotyping for late menopause, if it can be established that late menopause is associated with increased sibling survival to extreme ages. Mapping such genes without long-lived research participants is feasible, if heritable quantitative traits that are as good or better indicators of slow rates of aging can be measured in middle-aged individuals.
Social factors as well as the hypothesized genetic factors may play a role in explaining the association between longevity and late fertility. It is possible, for example, that an individual whose sister has children late in life will have a lower risk of death at extreme ages because of social benefits conferred by the presence of younger adult kin. An individual who has long-lived siblings may also expect to have above-average longevity because of the support such siblings might provide late in life, brothers and sisters alike. Our analysis of spouse longevity suggests that socioenvironmental variables contribute less than genetic factors to the familial patterns of longevity we have observed. However, it is clear that biological relatives often share socioenvironmental factors more than nonbiological relatives, and it is not possible to definitively distinguish social and genetic transmission of longevity in these data. Nevertheless, the results presented here should encourage researchers to continue to pursue genetic analyses of slower aging and longevity in humans.
FUNDING
This work was supported by National Institutes of Health grant AG022095 (The Utah Study of Fertility, Longevity, and Aging to K.R.S., R.M.C., G.P.M.), a University of Western Ontario grant (to A.G. and R.M.), and by the Social Sciences and Humanities Research Council of Canada (to A.G. and B.D.).
Acknowledgments
We wish to thank the Pedigree and Population Resource (funded by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance, and support of the UPDB.
References
- 1.Finch CE. Longevity, Senescence, and the Genome. Chicago, University of Chicago Press; 1990. [Google Scholar]
- 2.Jazwinski SM. Genetics of longevity. Exp Gerontol. 1998;33:773–783. doi: 10.1016/s0531-5565(98)00027-8. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel JW, Carey JR, Christensen K, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- 4.Gagnon A, Mazan R, Desjardins B, Smith KR. Post-reproductive longevity in a natural fertility population. In: Bengtsson T, Mineau GP, editors. Kinship and Demographic Behavior in the Past. New York, NY: Springer; 2008. pp. 225–241. [Google Scholar]
- 5.Grundy E, Kravdal O. Reproductive history and mortality in late middle age among Norwegian men and women. Am J Epidemiol. 2008;167:271–279. doi: 10.1093/aje/kwm295. [DOI] [PubMed] [Google Scholar]
- 6.Helle S, Lummaa V, Jokela J. Are reproductive and somatic senescence coupled in humans? Late, but not early, reproduction correlated with longevity in historical Sami women. Proc Biol Sci. 2005;272:29–37. doi: 10.1098/rspb.2004.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mcardle PF, Pollin TI, O'Connell JR, et al. Does having children extend life span? A genealogical study of parity and longevity in the Amish. J Gerontol A Biol Sci Med Sci. 2006;61:190–195. doi: 10.1093/gerona/61.2.190. [DOI] [PubMed] [Google Scholar]
- 8.Muller HG, Chiou JM, Carey JR, Wang JL. Fertility and life span: late children enhance female longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B202–B206. doi: 10.1093/gerona/57.5.b202. [DOI] [PubMed] [Google Scholar]
- 9.Smith KR, Mineau GP, Bean LL. Fertility and post-reproductive longevity. Soc Biol. 2002;49:185–205. [PubMed] [Google Scholar]
- 10.Perls TT, Alpert L, Fretts RC. Middle-aged mothers live longer. Nature. 1997;389:133. doi: 10.1038/38148. [DOI] [PubMed] [Google Scholar]
- 11.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003;157:923–929. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 13.Snowdon DA, Kane RL, Beeson WL, et al. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79:709–714. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagai J, Lin CY, Sabour MP. Lines of mice selected for reproductive longevity. Growth Dev Aging. 1995;59:79–91. [PubMed] [Google Scholar]
- 15.Rose MR, Nusbaum TJ, Fleming JE. Drosophila with postponed aging as a model for aging research. Lab Anim Sci. 1992;42:114–118. [PubMed] [Google Scholar]
- 16.Partridge L, Prowse N, Pignatelli P. Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc Biol Sci. 1999;266:255–261. doi: 10.1098/rspb.1999.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doblhammer G, Oeppen J. Reproduction and longevity among the British peerage: the effect of frailty and health selection. Proc Biol Sci. 2003;270:1541–1547. doi: 10.1098/rspb.2003.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagnon A, Smith KR, Tremblay M, Vézina H, Paré P-P, Desjardins B. Is there a trade-off between fertility and longevity? A comparative study of women from three large historical demographic databases accounting for mortality selection. Am J Hum Biol. doi: 10.1002/ajhb.20893. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettay JE, Kruuk LE, Jokela J, Lummaa V. Heritability and genetic constraints of life-history trait evolution in preindustrial humans. Proc Natl Acad Sci U S A. 2005;102:2838–2843. doi: 10.1073/pnas.0406709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazan R, Gagnon A. Familial and environmental influences on longevity in historical Quebec. Population. 2007;62:271–291. [Google Scholar]
- 21.Horowitz J. Semiparametric estimation of a proportional hazard model with unobserved heterogeneity. Econometrica. 1999;67:1001–1028. [Google Scholar]
- 22.Garibotti G, Smith KR, Kerber RA, Boucher KM. Longevity and correlated frailty in multigenerational families. J Gerontol A Biol Sci Med Sci. 2006;61:1253–1261. doi: 10.1093/gerona/61.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heckman JJ, Singer B. A method for reducing the impact of distributional assumptions in econometric models for duration data. Econometrica. 1984;52:271–320. [Google Scholar]