Abstract
Silencing of Stellate genes in Drosophila melanogaster testes is caused by antisense piRNAs produced as a result of transcription of homologous Suppressor of Stellate (Su(Ste)) repeats. Mechanism of piRNA-dependent Stellate repression remains poorly understood. Here, we show that deletion of Su(Ste) suppressors causes accumulation of spliced, but not nonspliced Stellate transcripts both in the nucleus and cytoplasm, revealing post-transcriptional degradation of Stellate RNA as the predominant mechanism of silencing. We found a significant amount of Su(Ste) piRNAs and piRNA-interacting protein Aubergine (Aub) in the nuclear fraction. Immunostaining of isolated nuclei revealed co-localization of a portion of cellular Aub with the nuclear lamina. We suggest that the piRNA–Aub complex is potentially able to perform Stellate silencing in the cell nucleus. Also, we revealed that the level of the Stellate protein in Su(Ste)-deficient testes is increased much more dramatically than the Stellate mRNA level. Similarly, Su(Ste) repeats deletion exerts an insignificant effect on mRNA abundance of the Ste-lacZ reporter, but causes a drastic increase of β-gal activity. In cell culture, exogenous Su(Ste) dsRNA dramatically decreases β-gal activity of hsp70-Ste-lacZ construct, but not its mRNA level. We suggest that piRNAs, similarly to siRNAs, degrade only unmasked transcripts, which are accessible for translation.
INTRODUCTION
Short RNA molecules are implicated in the regulation of gene expression, defence against viruses and transposable elements and maintenance of genome integrity (1–4). To date, three main classes of repressive short RNA molecules are known in animals: short-interfering RNAs (siRNAs), microRNAs (miRNAs) and PIWI interacting RNAs (piRNAs). siRNAs and miRNAs are bound by proteins related to the Ago clade of the Argonaute family and guide them to induce silencing of complementary targets by mRNA degradation, repression of transcription or translation (5,6). piRNAs are bound by the PIWI clade of Argonaute family proteins and differ from miRNAs and siRNAs in length and in the mechanisms of biogenesis and functioning (7–9). The evolutionarily conserved role of piRNAs consists in the regulation of transposable elements and genomic repeats in the germline (7–9). PIWI clade proteins have endonuclease activity and are able to degrade mRNAs of repetitive elements (10–12). Drosophila piRNAs can also lead to chromatin silencing of retroelements in ovaries (13). However, the current data poorly elucidate the mechanisms of the repression mediated by piRNAs as compared to those for siRNAs and miRNAs.
Before the acceptance of the ‘piRNA’ term, Aravin and colleagues (14,15) described in testes of Drosophila a short RNA species produced by the Y-chromosome linked Su(Ste) (Suppressor of Stellate) repeats. The authors revealed that Su(Ste) short RNAs are longer than siRNAs and perform silencing of Su(Ste) homologous Stellate tandem repeated genes located on the X chromosome, which is assisted by the PIWI related Aubergine (Aub) protein. Deletion of the Su(Ste) repeats or a mutation in the aub gene leads to the elimination of Su(Ste) piRNAs and a significant increase of Stellate mRNA abundance in testes (14,15). Stellate mRNA overexpression causes a drastic accumulation of Stellate protein in crystals, resulting in the disturbance of spermatogenesis and male sterility (16). Recently, it was shown that the Aub protein–Su(Ste)–piRNA complex is able to degrade Stellate mRNA in vitro (11). However, it remains unknown whether Su(Ste) piRNAs suppress expression of Stellate genes by degradation of mRNA or by repression of transcription. Here, we show that the predominant mechanism of Stellate genes silencing is post-transcriptional degradation of mRNA, which occurs both in the nucleus and in the cytoplasm. Our results also suggest that the piRNA dependent machinery is able to degrade only a pool of unmasked transcripts, which are accessible for translation.
MATERIALS AND METHODS
Drosophila strains
To produce males carrying the cry1Y chromosome (deletion of the bulk of Su(Ste) repeats on the Y chromosome), Df(1)w67c23(2)y females were crossed to X/cry1BsYy+males, described in Palumbo et al. (17). As a control we used Df(1)w67c23(2)y, designated as wild type. Strain bearing the aubsting-1 was y1ac1sc1w1Ste+;P{lacW}aubsting-1/Cy (18). P-element transformed flies carrying the Ste703-lacZ construct (contains the complete Stellate promoter and 141 nt of transcribed Stellate sequence) were kindly provided by A. A. Aravin. Ste134-lacZ transgenic construct containing a shortened (101 nt) Stellate promoter and 33 nt of transcribed Stellate sequence was described previously (14). All the compared strains carry the same X chromosome.
RT-PCR analysis
Total RNA was isolated from dissected testes or cell culture using Trizol reagent (Invitrogen). The first strand of cDNA was synthesized using the SuperScript II reverse transcriptase (Gibco) and oligo(dT) primer according to a standard procedure. cDNAs were analyzed by real-time quantitative PCR (Chromo4, Bio-Rad) using SYBR Green for detection of Stellate cDNA or by semiquantative PCR with αP32 dATP for detection of Ste-lacZ cDNA. For PCR the following primers were used: 5′-AAGTCTGATACACAGCTGGACGGAGCG-3′ (spl), 5′-GTAATTCTCCGAATATAGTC-3′ (non-spl), 5′-CGCTTGCACTTGCAGTACCTAG-3′ (non-spl2), 5′-CCTGACCAATATTCCGATATTCTTTGGC-3′ (euSte), 5′-GGGTCGTCCAGGGGCGATC-3′ (hetSte2) and 5′-CGATTTGAGTTGCATCAAGGCTTTCA-3′ (hetSte); primers spl/euSte (eu sum), non-spl2/euSte (eu non) were used for amplification of a total of and of nonspliced euchromatic Stellate cDNAs, respectively (although the eu sum pair of primers amplifies both spliced and non-spliced PCR products it can be used for detection of spliced transcripts in real-time analysis because its quantity is about 5-fold bigger than quantity of nonspliced transcripts); primers spl/hetSte2 (het sum), non-spl/hetSte (het non) used for amplification of a total of and of nonspliced heterochromatic Stellate cDNAs, respectively (for Stellate sequences see Supplementary Data); primer specificity was checked by PCR analysis using plasmids carrying eu [plasmid pSX1.3 kindly provided by K.J. Livak (19)] or heterochromatic Stellate genes (20) or Su(Ste) repeat (Gen Bank accession number Z11735). Other primers were used: 5′-CGGCATCTAAGAAGTGATACTCCCAAAA-3′ (Adh-d3) and 5′-TGAGTGTGCATCGAATCAGCCTTATT-3′ (Adh-r3) for the Adh gene (used as a loading control for quantitative real-time RT-PCR of Stellate cDNA); 5′-CAGGCCCAAGATCGTGAAG-3′ (rp49d) and 5′-TGAGAACGCAGGCGACC-3′ (rp49r) for the rp49 gene (used as a loading control for semiquantative RT-PCR of Ste-lacZ cDNA); 5′-GTGGTTATGCCGATCGCGT-3′ (lacZ1) and 5′-ATATCGGTGGCCGTGGTGT-3′ (lacZ2) for lacZ (used for amplification of Ste-lacZ cDNA obtained from testes RNA); 5′-GGCGAGGAGCTGTTCACC-3′ (GFP1) and 5′-TGCTCAGGTAGTGGTTGTCG-3′ (GFP2) for GFP; 5′-GGCATGATTCACGCCCGATACAT-3′ (Ste) and 5′-CGATTAAGTTGGGTAACGCCAG-3′ (lacZ3) for Ste-lacZ, lacZ1 and 5′-ACCGCCAAGACTGTTACCCAT-3′ (lacZ4) for lacZ (used for amplification of Ste-lacZ or lacZ cDNA obtained from cell culture RNA).
X-gal staining and β-gal activity assay
X-gal staining and β-gal activity assays were performed according to protocols described previously (14,15). Five to 10 testes dissected from 1–3-day- old males or cell culture were used for β-gal activity assay. To normalize measurements of β-gal activity we equalized the total testes protein evaluated by the Bio-Rad protein assay kit or analyzed an equal quantity of the cell culture cells.
Separation of nuclear and cytoplasmic fractions
Testes were manually dissected with needles in 1× PBS solution (2 mM KH2PO4, 10 mM Na2HPO4, 2.7 mM KCl, 137 mM NaCl, pH 7.3), precipitated, transferred to lysis buffer [350 mM sucrose, 15 mM HEPES (pH 7.6), 10 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 0.5 mM EGTA, 1 mM DTT, Protease inhibitor cocktail (Roche), 0.5 U RNasin Plus (Promega)] and homogenized by Dounce homogenizer on ice. Lysate was filtered through Mirocloth membrane (Calbiochem) and spun at 1800 g for 10 min at 4°C. Pellet 1 and supernatant 1 fractions were separated from each other. Pellet 1 (nuclei) was washed by lysis buffer producing pellet 2 and supernatant 2. Pellet 2 was pipetted in resuspension buffer [290 mM sucrose, 5 mM Tris–HCl (pH 7.4), 1.5 mM KCl, 5 mM MgCl2, 1 mM EGTA, Triton X-100 0.04%, Protease inhibitor cocktail (Roche), 0.5 U RNasin Plus (Promega)], incubated at rocking for 12 min at 4°C for removing nuclear membranes according to (21) and spun at 200 g for 6 min at 4°C. Pellet 3 was considered as nuclear fraction and resuspended in lysis buffer. Supernatants 1, 2 and 3 were pooled and considered as cytoplasmic fraction. One-fifth of the nuclear and cytoplasmic fractions was used for western analysis and the remaining material was used for northern analysis.
RNA isolation and detection by northern blot
RNA was isolated from nuclear and cytoplasmic fractions using Trizol reagent (Invitrogen). Total RNA was quantified by absorbance at 260 nm, and 0.4–8 µg of total RNA was resolved by 20% denaturing polyacrylamide/urea gel electrophoresis (Mini-PROTEAN Tetra Electrophoresis System, Bio-Rad) in 1× TBE. 5′-33P-radiolabeled RNA oligonucleotides were used as size markers. After electrophoresis, the polyacrylamide gel was transferred to Hybond N+membrane (Amercham) in 0.5×TBE by electrophoretic transfer (Mini Trans-Blot Cell, Bio-Rad) at 250 mA for 1 h. The RNA was cross-linked to the membrane by UV irradiation (1200 µjoules/cm; Biolink DNA Crosslinker, Biometra). Membrane was prehybridized in Church buffer [0.5 M phosphate buffer (pH 7.5), 1 mM EDTA, 7% SDS] for 2 h at 37°C for oligonucleotide probes and in prehybridization buffer [50 mM phosphate buffer (pH 7.5), 0.5 M NaCl, 0.1% Ficol400, 0.1% polyvinilpyrolidone, 0.1% pyrophosphate, 50 µg/ml heparin, 25 mM EDTA, 1% sarcosyl, 150 µg/ml denaturated DNA] for 2 h at 50°C for riboprobes. Fifteen picomoles of DNA probe (Syntol, Moscow, Russia) was 5′-32P-radiolabeled (81 µCi γ-32P-dATP) with polynucleotide kinase (NEB) and purified using Sephadex G-25 spin column (Bio-Rad). 32P-labeled riboprobe was transcribed by T7 RNA polymerase (Ambion) with 27 µCi α-32P-dATP using the Su(Ste) fragment as a template, producing an antisense RNA probe for hybridization. After synthesis, the labeled RNA was partially hydrolyzed during 1-h incubation at 60°C in the presence of 80 mM NaHCO3, 160 mM Na2CO3. The 32P-radiolabeled probes were hybridized for 16–20 h in Church buffer or in prehybridization buffer with 25% formamide for oligonucleotide probe or riboprobe, respectively. After hybridization, membranes were sequentially washed with 2× SSC/0.1% (w/v) SDS, 1× SSC/0.1% (w/v) SDS and 0.5× SSC/0.1% (w/v) SDS for 10 min and analyzed by phosphorimager (Storm, Amersham) using Image Quant computational tool. To strip probes, membranes were incubated in Church buffer for 2 h at 70°C and then re-exposed to confirm probe removal. The following DNA probes were used: 5′-GGGTATGAACCCAGTAGCTTAA-3′ (mt tRNA M), 5′-AGATTAAGAGTCTCATGCTCTA-3′ (tRNA K), 5′-TCGGGCTTGTTCTACGACGATG-3′ (Su(Ste)-4 piRNA).
Western blot analysis
Quantity of protein lysates used for western analysis was equalized by the Bio-Rad protein assay kit. To estimate the efficiency of nuclear and cytoplasmic fractions separation mouse monoclonal antibodies to lamin DmO ADL67.10 (Developmental Studies Hybridoma Bank) and rabbit monoclonal antibodies to γ-tubulin (Sigma) and rabbit polyclonal antibodies to calnexin were used at 1:1000, 1:7000 and 1:2000 dilution, respectively. To detect the Aub protein rabbit polyclonal antibodies to N-terminal 16 AA of Aub kindly provided by G. Hannon were used at 1:2000 dilution. To detect Stellate protein in testis extracts anti-Stellate mouse polyclonal antibodies (Egorova K., manuscript in preparation) were used at 1:3000 dilution. To estimate loading mouse monoclonal antibodies to beta-actin ab8224 (Abcam, Cambridge, UK) were used at 1:800 dilution. Alkaline-phosphatase-conjugated anti-mouse or anti-rabbit IgG (whole molecules) (Sigma–Aldrich Chemie GmbH, Steinheim, Germany) were used as a secondary reagent. Samples were resolved by electrophoresis in 12% or 8% PAGE/SDS and blotted onto the PVDF membrane Immobilon-P (Sigma–Aldrich Chemie GmbH, Steinheim, Germany). Blots were developed using the Immun-Star AP detection system (Bio-Rad Laboratories, Hercules, CA, USA) according to the recommendations of the manufacturer.
RNAi in cell culture
dsRNA was transcribed by T7 RNA polymerase (Ambion) using the Su(Ste), Ste, lacZ or GFP PCR products with two opposite T7 promoters as templates. To obtain dsRNA duplexes, mixtures were heated to 60°C and slowly cooled in water bath. Drosophila S2 cells (5×106) were transfected by the Ca transformation method (22) with hsp70-GFP (pHSBJ) (15 µg), hsp70-Ste-lacZ (pCaSpeR, construct contain 585 nt of transcribed Stellate sequence) (15 µg) or hsp70-lacZ (pCaSpeR) (15 μg) and one of the dsRNAs (GFP, Su(Ste), Ste or lacZ) 300–600 nt in length (1.5 µg). Three days after transfection, heat-shock procedure of cells was performed for 20 min at 37°C. Four hours later, β-gal activity assays and RNA isolation for RT-PCR were done.
Immunocytochemistry of testes and nuclei
Testes were dissected in phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde in PBS at room temperature for 1 h. Testes were incubated two times for 30 min in PBTD solution (PBS+0.1% Tween-20+0.3% Triton X-100+0.3% sodium deoxycholate), rinsed three times in PBT (PBS+0.1% Tween-20) for 5 min and blocked in 3% goat antiserum in PBT plus 0.3% Triton X-100 for 1–2 h at room temperature. Testes were incubated with primary antibodies diluted 1:500 in PBT plus 0.3% Triton X-100 for 1 h at room temperature. We used rabbit anti-Aub polyclonal antibodies to N-terminal 16 AA of Aub kindly provided by G. Hannon and mouse monoclonal antibodies to lamin DmO ADL67.10 (Developmental Studies Hybridoma Bank). Then testes were rinsed three times for 15 min in PBT, incubated with secondary antibodies for 3 h at room temperature or at 4°C overnight, stained with DAPI for 15 min in PBT and washed for 15 min in PBT again. We used Alexa Fluor 647 conjugated anti-rabbit antibody and Alexa Fluor 488 conjugated anti-mouse antibody (Invitrogen). All secondary antibodies were diluted 1:1000. Then PBT was replaced by SlowFade Gold antifade reagent (Invitrogen) and testes were transferred to glass slides and analyzed by a confocal microscope (Carl Zeiss).
The nuclear fraction was spread on a work surface of microscope slides (SuperFrost Plus Gold, Menzel-Glaser) and incubated for 15 min to allow nuclei to settle down. Nuclear fraction was fixed in 1.8% paraformaldehyde in PBS at room temperature for 15 min, rinsed three times in PBT for 5 min and blocked in 3% goat antiserum in PBT plus 0.3% Triton X-100 for 1–2 h at room temperature. The incubations with antibodies were the same as for testes analysis.
RESULTS
Expression of Stellate repeats is regulated at the post-transcriptional level
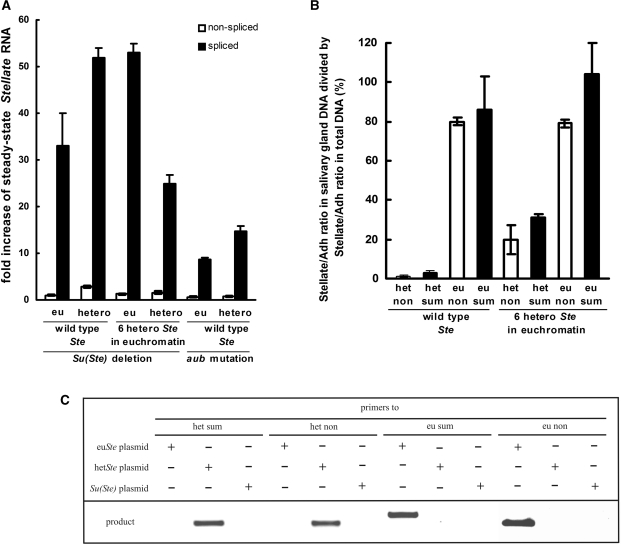
To elucidate whether the repression of Stellate genes by Su(Ste) piRNAs occurs at the transcriptional or post-transcriptional level, we estimated the effect of Su(Ste) deletion or the aubsting-1 mutation on the abundance of spliced and nonspliced Stellate transcripts in testes by quantitative RT-PCR. Deletion of the Su(Ste) locus or aubsting-1 lead to the disappearance of Su(Ste) piRNAs (14,15). If Su(Ste) piRNAs suppressed transcription of Stellate genes, the loss of silencing would cause a comparable increase of spliced and newly transcribed nonspliced transcripts quantity. We found that both Su(Ste) deletion and aubsting-1 lead to an insignificant increase of nonspliced transcript amount, while the level of spliced transcripts is dramatically increased (10–50 times) (Figure 1A). Thus, we consider posttranscriptional mRNA degradation as the predominant mechanism of Stellate silencing.
Figure 1.
The effect of Su(Ste) repeats deletion or aub mutation on spliced and non-spliced Stellate mRNA abundance in the testes of Drosophila melanogaster. (A) Deletion of Su(Ste) repeats (compared with wild type) and the aubsting-1 (homozygous flies compared with heterozygous ones) lead to an increase of spliced euchromatic (eu) and heterochromatic (hetero) Stellate transcripts abundance (dark bars), but not nonspliced transcripts abundance (light bars). Quantitative RT-PCR was done using primers detecting either nonspliced (pairs of primers designated as eu non or het non in the text) or a sum of spliced and nonspliced Stellate transcripts (designated as eu sum or het sum). The quantity of spliced transcripts was calculated by subtracting the nonspliced transcript quantity from the sum. We were able to detect euchromatic and heterochromatic Stellate transcripts separately, since the 3′ end nucleotides of the primers were complementary to variants of single nucleotide polymorphisms, which distinguish types of Stellate genes (see Supplementary Data). The middle four bars show the effect of Su(Ste) deletion causing derepression of Stellate genes in the fly strain carrying a transgenic construct with six heterochromatic Stellate genes in the euchromatin of chromosome 3. (B) PCR analysis with plasmids carrying eu- or heterochromatic Stellate genes or a Su(Ste) repeat confirms primers specificity. (C) Verification of localization of detected Stellate copies. The diagram shows values, which correspond to the results of dividing the Stellate/Adh ratio in salivary gland DNA by the Stellate/Adh ratio in total DNA. This test confirms that het non and het sum or eu non and eu sum primers detect Stellate copies located in the hetero- or euchromatin, correspondingly. Using of fly strain with six heterocromatic Stellate genes in the euchromatin leads to increasing of the values for primers specific to heterochromatic Stellate genes confirming validity of the test.
The genome of Drosophila melanogaster contains two clusters of Stellate genes located in the 12D euchromatic region and in the distal X-heterochromatin (17,19,20,23). The designed primers allowed us to separately detect Stellate transcripts derived from both clusters, owing to the single nucleotide polymorphisms (19,20) (see Supplementary Data). PCR analysis of plasmids, containing euchromatic or heterochromatic Stellate copies or a Su(Ste) repeat, confirmed the specificity of primers (Figure 1B). To verify that detected Stellate copies have eu- or heterochromatic localization in the genome, we used a well-known approach based on the phenomenon of heterochromatic DNA under-replication in the nuclei of salivary glands (24), where euchromatic DNA undergoes multiple polytenization, while heterochromatic DNA is not replicated. We related quantities of euchromatic or heterochromatic Stellate genes to the euchromatic Adh gene using quantitative Real-Time PCR of DNA isolated from whole flies and from larvae salivary glands. We evaluated euSte/Adh and hetSte/Adh ratios for the salivary glands DNA and calculated the percentage to the corresponding values for DNA from whole flies (Figure 1C). It was shown, that amount of heterochromatic Stellate PCR products, amplified by two different pairs of primers, is dramatically decreased in salivary gland DNA compared to total DNA. Thus, the used primers are able to discriminate between euchromatic or heterochromatic Stellate copies. For both types of Stellate genes, Su(Ste) deletion and aubsting-1 mutation cause an increase of spliced but not nonspliced transcripts level (Figure 1A). Thus, our data suggests that the chromatin state does not affect the mode of Stellate silencing.
Nuclear compartment contributes to piRNA-dependent Stellate silencing
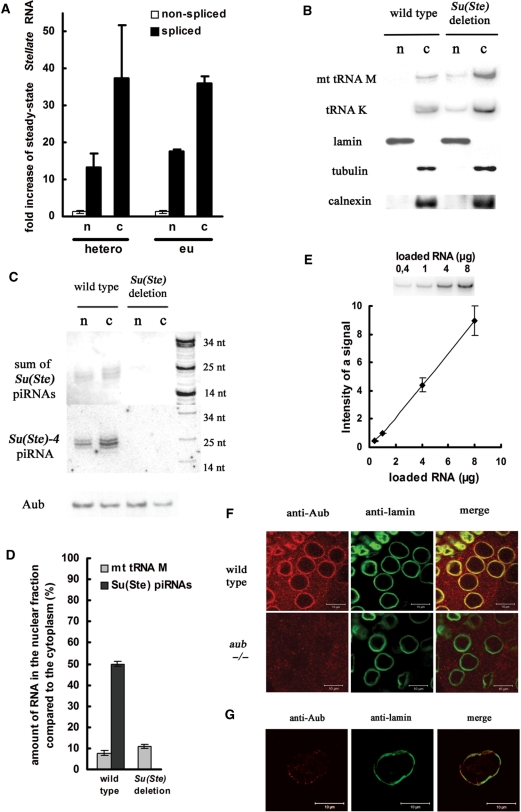
It was shown that siRNA-mediated degradation of transcripts may occur in the cytoplasm (25,26) and in the nucleus (27,28), but it is poorly understood where piRNA-mediated degradation takes place. We examined the effect of Su(Ste) deletion on Stellate transcript abundance in the nuclei and the cytoplasm separately. Nuclear and cytoplasmic fractions were obtained from lysates of Su(Ste)-deficient and wild-type testes. Quantitative RT-PCR revealed that Su(Ste) deletion leads to 38- and 13-fold increase of spliced heterochromatic Stellate mRNA amount in the cytoplasmic and nuclear fraction, respectively (Figure 2A). A similar result was obtained for euchromatic Stellate mRNA. The purity of the fractions was evaluated by western analysis using antibodies against lamin and γ-tubulin, which are nuclear and cytoplasmic proteins, respectively (Figure 2B). According to the procedure of fractionation nuclear fraction should be free of nuclear membranes (21). In order to control this, western analysis with an antibody against the endoplasmic reticulum membrane marker calnexin protein was done (Figure 2B). Contamination of the nuclear fraction by the cytoplasm was also estimated by northern analysis with a probe complementary to cytoplasmic lysine tRNA and mitochondrial methionine tRNA (Figure 2B). According to both western and northern analyses, the extent of nuclear fraction contamination by the cytoplasm does not exceed 10% (Figure 2D). Thus, the observed significant accumulation of Stellate transcripts in the nuclear fraction can not be explained by cytoplasmic contamination. We conclude that Stellate mRNA degradation occurs both in the nucleus and the cytoplasm. This result suggests that the complex of the Aub protein and Su(Ste) piRNAs performing silencing of Stellate genes (11,14) may be found both in the nuclear and cytoplasmic fractions. Using northern hybridization, we detected Su(Ste) piRNAs in both fractions (Figure 2C). We used a riboprobe detecting the sum of Su(Ste) piRNAs, or an oligonucleotide probe complementary to the individual Su(Ste)-4 piRNA, which was shown to be the most abundant among testes piRNAs immunoprecipitated with the Aub protein (11). In the nuclear fraction, Su(Ste) piRNAs are only 2-fold less abundant than in the cytoplasm (Figure 2D). Western analysis revealed the presence of the Aub protein also both in the nuclear and cytoplasmic fractions (Figure 2C). We also performed immunostaining to examine the localization of Aub in whole testes (Figure 2F) and isolated nuclei (Figure 2G). In the spermatocytes Aub is detected as a bright perinuclear ring (Figure 2F) representing the so-called nuage structure (29). In the isolated nuclei it was weaker, but a significant signal remained to be co-localized with the lamina (Figure 2G). Taking into account Aub co-localization with the lamina, we suggest that Aub is localized not only in the perinuclear organelle nuage, but also on the inner side of the nuclear membrane. Thus, fractionation experiments and immunostaining indicate that the piRNA–Aub complex is located both in the nucleus and cytoplasm.
Figure 2.
Degradation of Stellate mRNA by the piRNA machinery occurs both in the nucleus and the cytoplasm. (A) Deletion of Su(Ste) repeats leads to an increase of spliced euchromatic (eu) and heterochromatic (hetero) Stellate transcripts quantity both in the nuclear (n) and cytoplasmic (c) fractions. (B) Estimation of the purity of nuclear and cytoplasmic fractions. Upper rows: northern analysis with probes complementary to mitochondrial methionine tRNA (mt tRNA M) and cytoplasmic lysine tRNA (tRNA K) in nuclear (n) and cytoplasmic (c) fractions. Lower rows: western analysis with antibodies against nuclear lamin, cytoplasmic γ-tubulin and membrane marker calnexin proteins. (C) Su(Ste) piRNAs and the Aub protein are found both in nuclear and cytoplasmic fractions. Upper row: northern analysis with a riboprobe complementary to a pool of Su(Ste) piRNAs. Middle row: northern analysis with a DNA oligonucleotide complementary to a unique Su(Ste)-4 piRNA. Lower row: Western analysis with antibodies against Aub. (D) Amount (%) of mitochondrial methionine tRNA (from B) and Su(Ste) piRNAs (from C) in the nuclear fraction as compared to the cytoplasm. (E) Northern analysis confirms the proportionality of the hybridization signal to the amount of loaded RNA (methionine tRNA probe). (F) Localization of Aub in Drosophila testis. Testes were immunostained with anti-Aub (shown in red) and anti-lamin (shown in green). Specificity of anti-Aub was verified by immunostaining of testes of aubHN/aubQC42 (–/–) trans-heterozygous mutant flies. Scale bars: 10 µm. (G) Aub co-localizes with lamina in the nuclei. Nuclear fraction was immunostained with anti-Aub and anti-lamin. A dot-like signal of Aub co-localizes with lamina.
Su(Ste) piRNAs affect Stellate protein level much more dramatically than mRNA level
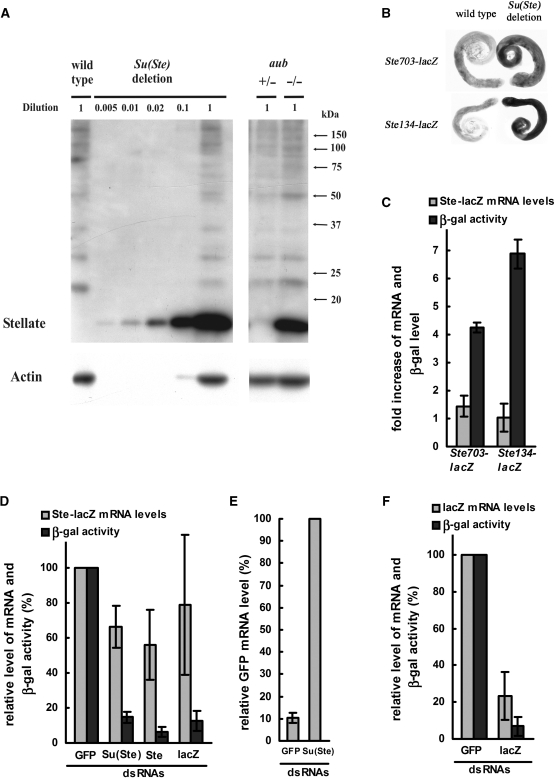
Western assay with anti-Stellate antibodies reveals that Stellate protein level in Su(Ste)-deficient testes increases much more dramatically (>200-fold) than the Stellate mRNA level (20- to 50-fold) (Figures 1 and 3A). Similar results we obtained using transgenic flies carrying Ste-lacZ reporter constructs driven by the Stellate promoter (Ste703-lacZ or Ste134-lacZ constructs contain the whole or 101 nt of Stellate promoter and first 141 or 33 nt of transcribed Stellate sequences, respectively). Deletion of Su(Ste) repeats exerts an insignificant effect on Ste-lacZ mRNA abundance (no more than 1.5-fold), but causes 4–7-fold increase of β-gal activity (Figure 3B and C). Slight increase of Ste-lacZ expression as compared to the endogenous Stellate genes level may be attributed to the structure of Ste-lacZ constructs, which contain incomplete Stellate sequences.
Figure 3.
(A) Deletion of Su(Ste) repeats or aubsting-1 lead to a more than 200-fold increase of the Stellate protein level. Different quantities of lysates from testes of Su(Ste)-deficient flies were used for western analysis with antibodies against the Stellate protein and actin. (B) X-gal staining of wild-type and Su(Ste)-deficient testes, carrying transgenic reporters Ste703-lacZ or Ste134-lacZ. (C) Fold increase of Ste-lacZ mRNA level (light bars) and β-gal activity (dark bars) in testes of Su(Ste)-deficient males relatively to the wild-type ones. (D–F) S2 cell culture was transfected by plasmids encoding a Ste-lacZ reporter construct (D), or the GFP gene (E) or a lacZ reporter construct (F) driven by a heat-shock promoter and one of dsRNAs [GFP, Su(Ste), Ste or lacZ in (D) and GFP or Su(Ste) in (E) and (F)]. Decrease of abundance of mRNA (D–F) and β-gal activity (D and F) owing to transfection by homologous dsRNAs is shown.
A fraction of Ste-lacZ mRNA is protected from siRNAs in S2 cells
The usage of reporter constructs allowed us to compare the peculiarities of piRNA- and siRNA-mediated repression. We performed dsRNA-mediated knockdown of the hsp70-Ste-lacZ construct in S2 cell culture. The hsp70-Ste-lacZ plasmid contains the first 585 nt of transcribed Stellate sequence fused to lacZ driven by the hsp70 promoter. Cotransfection of the hsp70-Ste-lacZ plasmid and Su(Ste), Ste or lacZ dsRNAs leads to a 7-10-fold decrease of β-galactosidase activity, but no more than 1.5–2-fold decrease of the mRNA level (Figure 3D). This result revealed similarities of piRNA and siRNA effects on the expression of Ste-lacZ constructs, resulting in a drastic decrease of protein level without a significant decrease of the abundance of Ste-lacZ mRNA. Note that in cell culture dsRNA digestion produces siRNAs, which are incorporated into Ago2 complexes and catalyze degradation of complementary mRNAs, but cannot perform repression of translation (30,31). To estimate the efficiency of mRNA degradation by dsRNA in our system, knockdown of hsp70-GFP plasmid by GFP dsRNA was done and 10-fold decrease of GFP mRNA quantity was observed (Figure 3E). Thus, we conclude that a significant fraction of the Ste-lacZ mRNA is not degraded by siRNA-containing effector complexes. To estimate whether the Stellate sequence per se is responsible for Ste-lacZ mRNA protection from degradation, a knockdown of hsp70-lacZ plasmid by lacZ dsRNA was also done, leading to a 5-fold decrease of lacZ mRNA quantity (Figure 3F), that is significantly stronger than the observed effect on the Ste-lacZ mRNA (about 1.3-fold decrease). Presumably, Ste-lacZ transcripts are protected by putative masking proteins, interacting with specific sequence motifs of Stellate RNA. Similarity of the results, which we obtained studying piRNA- and siRNA-dependent Ste-lacZ repression, allow us to suggest that only an unprotected fraction of total cytoplasmic Stellate RNA in testes is accessible both for piRNA-mediated degradation and translation.
DISCUSSION
Silencing of the testes expressed Stellate genes has been shown to be associated with short piRNAs recognized by the PIWI clade protein Aub (11,32). However, it remains unclear whether this mechanism is operated at the transcriptional or post-transcriptional level. Here, we show that deletion of Su(Ste) repeats, producing piRNAs, or aub mutation causes accumulation of spliced, but not nascent Stellate transcripts, revealing post-transcriptional degradation of Stellate RNA as the predominant mechanism of silencing. At the same time, the role of piRNAs in the transcriptional silencing of mobile elements in mammals and Drosophila has been suggested (9,13,33–35). In Drosophila ovaries a mutation decreasing piRNA production results in the opening of chromatin of transposable elements (13). Suggested piRNA-depending chromatin silencing is presumably caused by another PIWI clade protein Piwi (9), that is localized in the nuclei of ovarian cells, but is not expressed in the testes spermatocytes (10,11,36), where Aub-dependent Stellate repression occurs (Refs 11 and 15 and in this work). Thus, the piRNA machinery may be involved both in chromatin closing and post-transcriptional silencing.
We found that degradation of Stellate mRNA may occur not only in the cytoplasm, but also in the nuclei. The Aub protein, which performs silencing of the Stellate genes by Su(Ste) piRNAs (11,32), was detected in the cytoplasm and perinuclear structure called nuage in ovarian germinal cells (29,37,38). We found a significant amount of Aub in testis nuclear fraction, which was free of nuclear membranes according to the procedure of isolation (21) and western analysis with an antibody against endoplasmic reticulum membrane marker calnexin. Immunostaining of the testes or nuclei showed co-localization of Aub and lamin, which is an inner nuclear-membrane-associated protein. Patterns of Aub localization are very similar to those detected by previous authors (29,38). Using of antibodies to lamin allowed us to visualize a detectable amount of Aub on the inner side of nuclear membrane in isolated nuclei. We suggest that the piRNA–Aub complex is potentially able to perform Stellate silencing in the nucleus and cytoplasm.
Our previous data have shown the accumulation of antisense Su(Ste) transcripts in the nuclei of early stages of spermatocyte maturation (15). This observation emphasizes the possibility of antisense piRNA production in the nuclei and its immediate involvement in Stellate silencing.
We found that the loss of piRNAs in testes caused by the removal of Su(Ste) repeats leads to a significantly more strong increase of the Stellate protein level (more than 200-fold) than Stellate mRNA abundance (30–50-fold). The same effect we observed using transgenic Ste-lacZ constructs carrying fragments of the Stellate gene fused to lacZ. In this case, Su(Ste) deficiency also causes a drastic upregulation of β-gal activity (4–7-fold) whereas the Ste-lacZ transcript abundance increases insignificantly (no more than 1.5-fold). The usage of Ste-lacZ constructs allowed us to compare the response of their expression to Su(Ste) piRNA in vivo and to siRNA in cell culture. Knockdown of Ste-lacZ construct by siRNAs in S2 cells causes a drastic decrease of β-gal activity (7–10-fold), but does not significantly affect the amount of Ste-lacZ mRNA (about 1.5-fold). Taking into account the much more strong decrease of GFP or lacZ mRNA levels caused by GFP or lacZ siRNAs (10- and 5-fold, respectively), we propose that the bulk of Ste-lacZ mRNA is associated with putative ubiquitous masking proteins, interacting with specific sequence motifs of Stellate RNA and preventing siRNA-mediated degradation. These data suggest that piRNAs in testes, similarly to siRNAs in S2 cells, cause degradation of translation accessible unmasked transcripts, rather than repression of translation.
The proposed mode of piRNA-mediated posttranscriptional silencing could occur in the course of silencing of transposable elements. It was shown that the disruption of the piRNA machinery leads to a drastic increase of transposition rate of the mdg1 retrotransposon, but a slight increase of mdg1 transcript level (39). We suppose that the piRNA system may be aimed predominantly at elimination of translation accessible mRNAs, preventing the synthesis of reverse transcriptases, transposases and other transposon-encoded proteins.
Our results show that piRNAs-mediated post-transcriptional silencing of Stellate genes takes place both in the nucleus and in the cytoplasm and Su(Ste) piRNAs slicing activity may be preferentially directed to the cytoplasmic transcripts, which are accessible for translation. These peculiarities of piRNA action seem to be similar to those described for siRNAs.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
The RAS program for Molecular and Cell Biology, Russian Foundation for Basic Research (08-04-00087-a); and the program of Scientific School Support (3464.2008.4). Funding for open access charge: Russian Foundation for Basic Research (08-04-00087-a).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank G. Hannon and A. Aravin for the Aub antibodies, A. Aravin and A. Tulin for stocks carrying transgenic Ste-lacZ constructs, V. Vagin for his generous help at the beginning of this study, Yury Y. Shevelyov for advice and improving the text of the manuscript, Anastasia D. Stolyarenko for the useful discussion.
REFERENCES
- 1.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 2.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Ambros V, Chen X. The regulation of genes and genomes by small RNAs. Development. 2007;134:1635–1641. doi: 10.1242/dev.002006. [DOI] [PubMed] [Google Scholar]
- 5.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 6.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol. Cell. 2007;26:611–623. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Hartig JV, Tomari Y, Forstemann K. piRNAs–the ancient hunters of genome invaders. Genes Dev. 2007;21:1707–1713. doi: 10.1101/gad.1567007. [DOI] [PubMed] [Google Scholar]
- 8.O'D;onnell KA, Boeke JD. Mighty Piwis defend the germline against genome intruders. Cell. 2007;129:37–44. doi: 10.1016/j.cell.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 10.Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, Nagami T, Siomi H, Siomi MC. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006;20:2214–2222. doi: 10.1101/gad.1454806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishida KM, Saito K, Mori T, Kawamura Y, Nagami-Okada T, Inagaki S, Siomi H, Siomi MC. Gene silencing mechanisms mediated by Aubergine piRNA complexes in Drosophila male gonad. RNA. 2007;13:1911–1922. doi: 10.1261/rna.744307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. A slicer-mediated mechanism for repeat-associated siRNA 5' end formation in Drosophila. Science. 2007;315:1587–1590. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 13.Klenov MS, Lavrov SA, Stolyarenko AD, Ryazansky SS, Aravin AA, Tuschl T, Gvozdev VA. Repeat-associated siRNAs cause chromatin silencing of retrotransposons in the Drosophila melanogaster germline. Nucleic Acids Res. 2007;35:5430–5438. doi: 10.1093/nar/gkm576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aravin AA, Naumova NM, Tulin AV, Vagin VV, Rozovsky YM, Gvozdev VA. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D melanogaster germline. Curr. Biol. 2001;11:1017–1027. doi: 10.1016/s0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 15.Aravin AA, Klenov MS, Vagin VV, Bantignies F, Cavalli G, Gvozdev VA. Dissection of a natural RNA silencing process in the Drosophila melanogaster germ line. Mol. Cell Biol. 2004;24:6742–6750. doi: 10.1128/MCB.24.15.6742-6750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozzetti MP, Massari S, Finelli P, Meggio F, Pinna LA, Boldyreff B, Issinger OG, Palumbo G, Ciriaco C, Bonaccorsi S, et al. The Ste locus, a component of the parasitic cry-Ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase 2. Proc. Natl Acad. Sci. USA. 1995;92:6067–6071. doi: 10.1073/pnas.92.13.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palumbo G, Bonaccorsi S, Robbins LG, Pimpinelli S. Genetic analysis of Stellate elements of Drosophila melanogaster. Genetics. 1994;138:1181–1197. doi: 10.1093/genetics/138.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt A, Hollmann M, Schafer U. A newly identified Minute locus, M(2)32D, encodes the ribosomal protein L9 in Drosophila melanogaster. Mol. Gen. Genet. 1996;251:381–387. doi: 10.1007/BF02172530. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ. Detailed structure of the Drosophila melanogaster stellate genes and their transcripts. Genetics. 1990;124:303–316. doi: 10.1093/genetics/124.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tulin AV, Kogan GL, Filipp D, Balakireva MD, Gvozdev VA. Heterochromatic Stellate gene cluster in Drosophila melanogaster: structure and molecular evolution. Genetics. 1997;146:253–262. doi: 10.1093/genetics/146.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vann LR, Wooding FB, Irvine RF, Divecha N. Metabolism and possible compartmentalization of inositol lipids in isolated rat-liver nuclei. Biochem. J. 1997;327 (Pt 2):569–576. doi: 10.1042/bj3270569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nocera PP, Dawid IB. Transient expression of genes introduced into cultured cells of Drosophila. Proc. Natl Acad. Sci. USA. 1983;80:7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shevelyov YY. Copies of a Stellate gene variant are located in the X heterochromatin of Drosophila melanogaster and are probably expressed. Genetics. 1992;132:1033–1037. doi: 10.1093/genetics/132.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abramov YA, Kogan GL, Tolchkov EV, Rasheva VI, Lavrov SA, Bonaccorsi S, Kramerova IA, Gvozdev A. Eu-heterochromatic rearrangements induce replication of heterochromatic sequences normally underreplicated in polytene chromosomes of Drosophila melanogaster. Genetics. 2005;171:1673–1681. doi: 10.1534/genetics.105.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y, Cullen BR. RNA interference in human cells is restricted to the cytoplasm. RNA. 2002;8:855–860. doi: 10.1017/s1355838202020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki H, Taira K. Short hairpin type of dsRNAs that are controlled by tRNA(Val) promoter significantly induce RNAi-mediated gene silencing in the cytoplasm of human cells. Nucleic Acids Res. 2003;31:700–707. doi: 10.1093/nar/gkg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat. Struct. Mol. Biol. 2005;12:133–137. doi: 10.1038/nsmb886. [DOI] [PubMed] [Google Scholar]
- 28.Langlois MA, Boniface C, Wang G, Alluin J, Salvaterra PM, Puymirat J, Rossi JJ, Lee NS. Cytoplasmic and nuclear retained DMPK mRNAs are targets for RNA interference in myotonic dystrophy cells. J. Biol. Chem. 2005;280:16949–16954. doi: 10.1074/jbc.M501591200. [DOI] [PubMed] [Google Scholar]
- 29.Snee MJ, Macdonald PM. Live imaging of nuage and polar granules: evidence against a precursor-product relationship and a novel role for Oskar in stabilization of polar granule components. J. Cell Sci. 2004;117:2109–2120. doi: 10.1242/jcs.01059. [DOI] [PubMed] [Google Scholar]
- 30.Tomari Y, Du T, Zamore PD. Sorting of Drosophila small silencing RNAs. Cell. 2007;130:299–308. doi: 10.1016/j.cell.2007.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell. 2007;130:287–297. doi: 10.1016/j.cell.2007.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–324. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 33.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 34.Carmell MA, Girard A, van de Kant HJ, Bourc'h;is D, Bestor TH, de Rooij DG, Hannon GJ. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell. 2007;12:503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–917. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox DN, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 37.Findley SD, Tamanaha M, Clegg NJ, Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development. 2003;130:859–871. doi: 10.1242/dev.00310. [DOI] [PubMed] [Google Scholar]
- 38.Harris AN, Macdonald PM. Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C. Development. 2001;128:2823–2832. doi: 10.1242/dev.128.14.2823. [DOI] [PubMed] [Google Scholar]
- 39.Kalmykova AI, Klenov MS, Gvozdev VA. Argonaute protein PIWI controls mobilization of retrotransposons in the Drosophila male germline. Nucleic Acids Res. 2005;33:2052–2059. doi: 10.1093/nar/gki323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.