Abstract
The mechanisms involved in simvastatin-mediated attenuation of cerebral vasospasm after subarachnoid hemorrhage (SAH) are unclear. We investigated the role of the phosphatidylinositol 3-kinase/Akt (PI3K/Akt) pathway and endothelial nitric oxide synthase (eNOS) in the cerebral vasculature in statin-mediated attenuation of cerebral vasospasm using wortmannin, an irreversible pharmacological PI3K inhibitor, and a rat SAH endovascular perforation model. Simvastatin was administered intraperitoneally in two dosages (1 mg/kg and 20 mg/kg) at 0.5, 24, and 48 hr after SAH and histological parameters of ipsilateral intracranial carotid artery (ICA) were assessed at 24 and 72 hr. SAH significantly decreased ICA diameter and perimeter while increasing wall thickness at both 24 and 72 hr. High-dosage simvastatin prevented the reduction of ICA diameter and perimeter following SAH, whereas both high and low dosages reduced wall thickness significantly at 24 and 72 hr. The effects of simvastatin were significantly reversed by wortmannin. High-dosage simvastatin increased pAkt and peNOS (phosphorylated forms) levels without increasing Akt and eNOS expression compared with the SAH group and also improved neurological deficits at 24 and 72 hr. Simvastatin did not affect protein levels by itself compared with untreated sham group. The present study elucidates the critical role of the PI3K activation leading to phosphorylation of Akt and eNOS in simvastatin-mediated attenuation of cerebral vasospasm after SAH.
Keywords: cerebral vasospasm, experimental subarachnoid hemorrhage, statin, PI3K/Akt, eNOS
Cerebral vasospasm is a known sequela of subarachnoid hemorrhage (SAH) with the potential for severe consequences, such as delayed ischemic neurological deficits (DINDs), that often lead to an unfavorable prognosis (de Oliveira et al., 2007). However, the mechanisms leading to cerebral vasospasm are still unclear. Previous studies, using the rat perforation model of SAH, suggest that protecting cerebral vascular tissues, particularly endothelial cells, during acute brain injury after SAH can attenuate cerebral vasospasm (Cahill et al., 2006). Recent experimental and clinical studies have shown that statins are efficacious in ameliorating cerebral vasospasm (McGirt et al., 2002, 2006a,b; Lynch et al., 2005; Tseng et al., 2005). This protective effect of statins against cerebral vasospasm is currently thought to be mediated by improving endothelial and vascular smooth muscle functioning via inhibition of the Rho-kinase signaling pathway (Laufs and Liao, 1998) and decreasing oxidative stress and inflammation (Wassmann et al., 2001; Erdos et al., 2006; McGirt et al., 2006a). Statins also improve endothelial function by maintaining the nitric oxide supply (McGirt et al., 2002). Previous studies have highlighted the importance of a statin-mediated phosphatidylinositol 3-kinase (PI3K)/Akt pathway and endothelial nitric oxide synthase (eNOS) phosphorylation in cardiovascular physiology by presenting evidence suggesting that statins increase PI3K activity, which leads to Akt phosphorylation, in turn leading to the phosphorylation of eNOS and a subsequent increase in production of NO by endothelial cells (Kureishi et al., 2000; Urbich et al., 2002; Wolfrum et al., 2004; J. Wang et al., 2005). The physiological relevance of this pathway is that it plays a key role in the maintenance of vascular function through the promotion of endothelial cell survival as well as the NO-mediated regulation of vascular tone (Kureishi et al., 2000; J. Wang et al., 2005). In vivo, the maintenance of this pathway by statins has been shown to be cytoprotective in ischemic cardiac injury (Wolfrum et al., 2004). However, this pathway has not been investigated in statin-mediated attenuation of cerebral vasospasm. We suspect that Akt phosphorylation followed by eNOS phosphorylation plays an important role in statins’ reversal of vasospasm and hypothesized that simvastatin attenuates cerebral vasospasm by up-regulating PI3K leading to Akt (also known as protein kinase B) and eNOS phosphorylation in cerebral arteries after SAH.
MATERIALS AND METHODS
Induction of SAH
All procedures and experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University. The endovascular perforation model of SAH in rats was used for this study as previously described (Bederson et al., 1995; Kusaka et al., 2004; Ostrowski et al., 2006b). Briefly, general anesthesia was induced with ketamine (100 mg/kg i.p.) and xylazine hydrochloride (10 mg/kg i.p.), followed by atropine (0.1 mg/kg s.c.). After intubation, the animals were ventilated with an animal ventilator (Harvard Apparatus). A heating pad and a heating lamp were used to maintain the rectal temperature at 36.0°C ± 0.5°C. SAH was induced by endovascular perforation of the internal carotid artery (ICA) bifurcation with a sharpened 4-0 nylon suture. After exposing the left common carotid artery (CCA), external carotid artery (ECA), and ICA through a midline skin incision, the ECA was ligated, cut, and shaped into a 3-mm stump. The suture was advanced rostrally into ICA from the ECA stump until resistance was felt (~ 15–18 mm from the common carotid bifurcation) and then pushed 3 mm farther to perforate the bifurcation of the anterior cerebral and middle cerebral arteries. Immediately after puncture, the suture was withdrawn into the ECA stump, and the ICA was reperfused. Operative procedures were exactly same for the sham group, except that the suture was removed once resistance was felt, without puncture. The incision was then closed, and rats were housed individually following their recovery from anesthesia. All rats received 3 ml normal saline intraperitoneally to prevent dehydration, and animals had free access to food and water until euthanization.
Experimental Animals and Groups
One hundred eighty-two adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing between 250 and 350 g were divided randomly into six weight-matched groups: sham-operated treated with vehicle (sham n = 24), SAH treated with vehicle (SAH n = 45), SAH treated with low-dosage simvastatin (Calbiochem, La Jolla, CA; 1 mg/kg, S-1, n = 33) or high-dosage simvastatin (20 mg/kg, S-20, n = 35), SAH treated with simvastatin plus PI3K inhibitor (wortmannin 15 μg/kg; W + S-20, n = 41), and sham operated plus PI3K inhibitor with vehicle (sham + W n = 4, only for histology). An additional 18 animals were used separately to ascertain whether simvastatin alone affects protein levels (Akt, eNOS, pAkt, and peNOS).
Animals experiencing mild SAH were excluded from the study as per the SAH grading system criteria, because mild SAH does not affect the arteries sufficiently in experimental studies (Sugawara et al., 2007). In brief, the SAH grading system is as follows: the basal cistern is divided into six segments, and each segment is allotted a grade from 0 to 3 depending on the amount of subarachnoid blood clot in the segment; grade 0: no subarachnoid blood; grade 1: minimal subarachnoid blood; grade 2: moderate blood clot with recognizable arteries; grade 3: blood clot obliterating all arteries within the segment. The animals received a total score ranging from 0 to 18 after adding the scores from all six segments. Mild SAH is categorized as all animals that received a total score of 5 or less. The SAH grading was done in a blinded fashion.
Drug Administration
Thirty minutes after the procedure, treatment groups received either a high (20 mg/kg) or low (1 mg/kg) dosage of simvastatin via an intraperitoneal injection. The high dosage of simvastatin (20 mg/kg) was selected on guidance from previous literature (McGirt et al., 2002), whereas the low dosage (1 mg/kg) is comparable to that used in current clinical settings. All simvastatin dosages were dissolved in ethanol and adjusted to a final concentration 10%, with a total volume of 1.5 ml, when they were administered. Sham, SAH, and sham + W groups received vehicle (1.5 ml of 10% ethanol in normal saline). This concentration of ethanol is not likely to affect the production of vasospasm by after SAH (Barry and Scott, 1979). The animals sacrificed at 72 hr also received treatment at 24 and 48 hr after surgery. Wortmannin was administered intravenously 15 min before IC perforation at a dosage of 15 μg/kg in W + S-20 and sham + W groups as previously described (Gao et al., 2002).
Neurological Scoring
Neurological scores were evaluated 24 and 72 hr after SAH with a modification of the scoring system reported by Garcia et al. (1995) in a blinded fashion. An 18-point scoring system was used to evaluate the sensorimotor deficits.
Morphological Assessment of Cerebral Vasospasm
Cerebral vasospasm was evaluated at 24 and 72 hr after SAH as established in previous studies (Cahill et al., 2006). The ipsilateral intracranial ICA is close to the puncture point in this model, which ensures its physical contact with extravasated blood. Additionally, the ICA is straight, consistent in diameter, and perpendicular to coronal sections in the rat brain, allowing accurate measurements of diameter, perimeter, and wall thickness, which are critical in assessing cerebral vasospasm. Therefore, the ipsilateral intracranial ICA was chosen for the histological assessment of diameter, perimeter, and arterial wall thickness 24 and 72 hr after SAH. Six animals from each group (except for sham + wortmannin group, n = 4) were used for histological assessment at 24 hr, and six animals from SAH and S-20 groups were used for histological assessment at 72 hr. Under deep anesthesia, the rats were transcardially perfused with 0.1 mol/liter PBS (pH 7.4) for 15 min, followed by 15 min of 10% paraformaldehyde for fixation. Perfusion pressures were between 60 and 80 mmHg as described previously (Parra et al., 2002). Whole brains were quickly removed and postfixed in 10% paraformaldehyde for 7 days, followed by 30% sucrose (wt/vol) for an additional 3 days. Ten-micrometer-thick coronal sections cut by a cryostat (Leica Microsystems LM3050S) were mounted on poly-L-lysine-coated slides (Richard Allen, Kalamazoo, MI). The intracranial ICA was sectioned every 200 μm, and the narrowest segment most affected by SAH was selected to assess the vasospasm. These sections were stained with hematoxylin and eosin (H&E). Measurement of the major and minor axis, perimeter, and wall thickness of the ipsilateral intracranial ICA was done in Image J freeware from the National Institutes of Health. The diameter of the intracranial ICA is defined as the average of the major and minor axes.
Fluorescence Immunohistochemical Staining
Double-fluorescence labeling was performed in four groups (sham, SAH, SAH + S-20, and SAH + W + S-20) at 24 hr after SAH and in two groups (SAH and SAH + S-20) at 72 hr after SAH, as described previously (Tsubokawa et al., 2006). The following primary antibodies were used: 1) mouse polyclonal phospho-Akt (Ser473) antibody (Cell Signaling Technology, Beverly, MA), 2) rabbit polyclonal phospho-eNOS (Ser1177) antibody (Cell Signaling Technology). Appropriate fluorescein isothiocyanate- or Texas red-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were also used. The sections were visualized with a fluorescence microscope (Olympus), and pictographs were recorded and analyzed with MagnaFire SP 2.1B software.
Western Blotting Analysis
Arteries from the basal cisterns were harvested with the aid of a microscope and were snap frozen in liquid nitrogen. These arteries were preserved at −80°C. Eighteen arteries [three arteries harvested per blot to obtain sufficient protein, as previously described (Scott et al., 2007)] were collected and assessed by Western blot in each group. In a separate experiment to examine effects of simvastatin by itself, nine (three per blot) arteries were used per group (sham and sham + simvastatin 20 mg/kg). Western blot analysis was performed as described previously (Park et al., 2004; Cahill et al., 2006). Briefly, artery samples were homogenized, and aliquots of each fraction were used to determine the protein concentration of each sample using a detergent compatible assay (Bio-Rad, Hercules, CA). Equal amounts of protein samples (50 μg) for Akt, eNOS, phosphorylated Akt (pAkt), and phosphorylated eNOS (peNOS) were loaded on a Tris glycine gel, electrophoresed, and transferred to a nitrocellulose membrane. Membranes were then blocked with a blocking solution, followed by incubation overnight at 4°C with the primary antibodies. The following primary antibodies were purchased from Cell Signaling Technology: 1) rabbit polyclonal Akt antibody, 2) rabbit polyclonal phospho-Akt (Ser473) antibody, 3) rabbit polyclonal eNOS antibody, 4) rabbit polyclonal phospho-eNOS (Ser1177) antibody. Immunoblots were processed with secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hr at room temperature and probed with a chemiluminescence reagent kit (ECL Plus kit; Amersham Bioscience, Arlington Heights, IL). The chemiluminescent signal was captured on X-ray films that were scanned and the optical density was determined in Quantity One software (Bio-Rad). β-Actin was used as an internal control for every experiment before normalizing with the shams.
Statistical Analysis
The data are expressed as mean ± SEM. Statistical differences among the various groups were assessed with a one-way analysis of variance (ANOVA) with Holm-Sidak post hoc analysis. Statistical differences between two groups were assessed with Student’s t-test. Mortality was analyzed by χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Physiological Data
Physiological parameters were monitored before, during, and after surgery. No statistical differences were observed between the SAH group (n = 6) and the S-20 group (n = 6) with regard to mean arterial blood pressure, arterial blood gases; paO2, paCO2, pH levels, and glucose levels before, immediately after puncture and 30 min after SAH (Table I).
TABLE I.
Physiological Parameters*
| Mean BP (mmHg) | paO2 (mmHg) | paCO2 (mmHg) | pH | Glucose (mg/dl) | ||
|---|---|---|---|---|---|---|
| SAH (n = 6) | Pre-SAH | 86.83 ± 2.63 | 71.83 ± 3.83 | 37.33 ± 2.24 | 7.35 ± 0.02 | 176.1 ± 10.5 |
| Post-SAH | 102.00 ± 5.77 | 73.00 ± 5.63 | 35.83 ± 2.19 | 7.37 ± 0.02 | 189.6 ± 8.8 | |
| 30 Min after SAH | 85.66 ± 2.60 | 73.83 ± 2.75 | 36.16 ± 2.44 | 7.34 ± 0.03 | 205.6 ± 14.6 | |
| SAH + simvastatin (n = 6) | Pre-SAH | 83.50 ± 2.59 | 73.83 ± 4.65 | 35.83 ± 1.35 | 7.38 ± 0.03 | 179.1 ± 8.8 |
| Post-SAH | 100.50 ± 2.51 | 72.83 ± 4.72 | 35.66 ± 1.47 | 7.34 ± 0.02 | 214.6 ± 8.3 | |
| 30 Min after SAH | 81.50 ± 1.56 | 74.66 ± 4.69 | 36.16 ± 2.00 | 7.34 ± 0.01 | 234.6 ± 4.2 |
There is no significant difference between SAH and SAH + simvastatin groups in the physiological parameters.
Mortality and Exclusion
Twenty-nine rats died before the intended sacrifice time and were excluded from assessment of vasospasm and neurological deficits at 24 hr and 72 hr. However, these rats were included in mortality statistics. The 24-hr mortality rates in each group were as follows: sham 0.0%, SAH 17.2%, SAH + S-1 11.1%, SAH + S-20 4.0%, SAH + W + S-20 35.1%, and sham + W 0.0%. The SAH groups (both treated and untreated) did not differ significantly in their mortality rates: SAH + S-1; P = 0.51; SAH + S-20; P = 0.12; SAH + W + S-20; P = 0.10 vs. SAH. The 72-hr mortality rates were 45.5% in SAH group and 25% in S-20 group. There is no significant difference between these two groups (P = 0.36).
Seventeen (14 for 24 hr and 3 for 72 hr) rats were excluded from the study because of mild SAH. Our previous study on the SAH grading system shows evidence that mild SAH is unsuitable to assess therapeutic applications and pharmacological agents in this animal model (Sugawara et al., 2007). The grading was performed in a blinded manner in this study. The SAH-induced plus treatment and/or wortmannin groups had no significant differences in their average SAH grades in comparison with the untreated SAH group, indicating an equal degree of injury among all groups (SAH + S-1: P = 0.25, SAH + S-20; P = 0.79; and SAH + W + S-20; P = 0.40 vs. SAH). After all exclusions, 146 rats and 19 rats were used for assessment of mortality at 24 hr and 72 hr, respectively, and 124 rats and 12 rats were used for assessment of vasospasm and neurological deficits at 24 hr and 72 hr, respectively.
Morphological Evaluation of Cerebral Vasospasm
24-Hour assessment
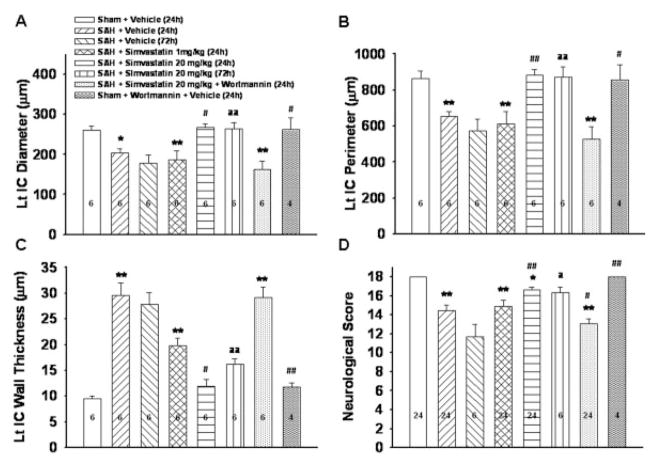
The significant decreases in ICA diameter and perimeter observed in the SAH group (203.2 ± 10.3 μm: P < 0.05, 652.7 ± 29.0 μm; P < 0.01) compared with sham (259.7 ± 10.6 μm, 865.4 ± 39.5 μm) were significantly attenuated by high-dosage simvastatin (267.4 ± 8.0 μm: P < 0.01 vs. SAH, 882.4 ± 30.0 μm; P < 0.01 vs. SAH). The significant increase in arterial wall thickness following SAH (SAH 29.50 ± 2.42 μm; P < 0.01 vs. sham 9.52 ± 0.56 μm) was significantly attenuated by both high- and low-dosage simvastatin (11.87 ± 1.56 μm: P < 0.01 vs. SAH, 19.75 ± 1.40 μm; P < 0.01 vs. SAH). These effects were significantly reversed by wortmannin (162.7 ± 20.6 μm, 528.9 ± 65.9 μm, 29.19 ± 1.97 μm; P < 0.01, 0.01, 0.01 vs. S-20). The sham + W group (treated with wortmannin alone) did not significantly differ in any of these parameters compared with the sham group (261.9 ± 29.1 μm, 855.1 ± 86.3 μm, 11.76 ± 0.86 μm; P = 0.936, 0.902, 0.392 vs. sham; Figs. 1A,B, 2A–C).
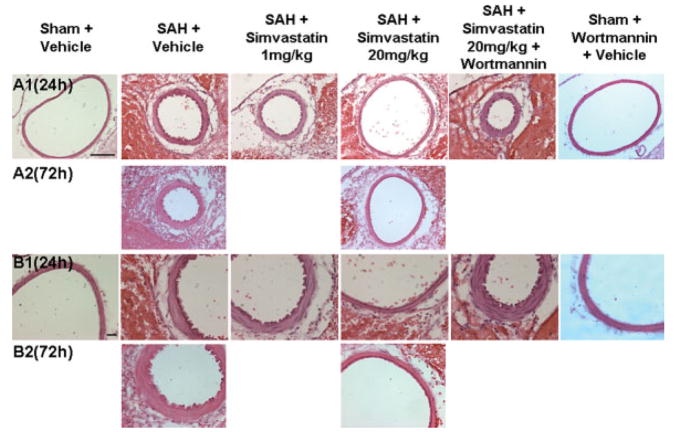
Fig. 1.
Morphological evaluation of cerebral vasospasm. A,B show representative (n = 6, except for sham + W group, in which n = 4) hematoxylin-eosin-stained cross-sections of the ipsilateral ICA at 24 and 72 hr after SAH. These panels depict high-dose simvastatin amelioration of vasospasm at 24 and 72 hr after SAH and how wortmannin, a PI3K inhibitor, reversed the effect of simvastatin. Wortmannin alone did not differ significantly from sham group. ICA, internal carotid artery; SAH, subarachnoid hemorrhage. Scale bars = 100 μm in A; 20 μm in B. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 2.
Cerebral vasospasm parameters and neurological score. A–C show the quantitative assessment of the diameter, perimeter, and wall thickness of the ipsilateral ICA at 24 and 72 hr after SAH (n = 6 except for sham + W group, in which n = 4). These panels reveal that high-dosage simvastatin ameliorated vasospasm at 24 and 72 hr SAH and that wortmannin reversed the effect of simvastatin at 24 hr. Wortmannin, a PI3K inhibitor, alone did not differ significantly from sham group. D shows that the SAH group has significant neurological deficits compared with sham group. Neurological deficits significantly improved in the high-dosage group at 24 and 72 hr after SAH but not in the low-dosage group. These effects of high-dosage simvastatin were reversed by adding wortmannin. (*P < 0.05, **P < 0.01 vs. sham, #P < 0.05, ##P < 0.01 vs. SAH, aP < 0.05 vs. SAH (72 hr), aaP < 0.01 vs. SAH (72 hr).
72-Hour assessment
High-dosage simvastatin significantly attenuated the SAH induced decrease in IC diameter (SAH + S-20: 263.5 ± 16.4 μm vs. SAH: 178.3 ± 19.4 μm; P < 0.01) and perimeter (SAH + S-20: 872.1 ± 56.4 μm vs. SAH: 571.7 ± 66.5 μm; P < 0.01) at 72 hr. High-dosage simvastatin also significantly attenuated the SAH-induced increase in IC wall thickness at 72 hr (SAH + S-20: 16.20 ± 0.99 μm vs. SAH: 27.81 ± 2.28 μm; P < 0.01; Figs. 1A,B, 2A–C).
Neurological Assessment
The SAH group has significantly reduced neurological function as compared with the sham group (P < 0.01 vs. sham). Neurological deficits significantly improved in the SAH + S-20 group both at 24 and 72 hr (P < 0.01, P < 0.05 vs. SAH respectively), but not in SAH + S-1 group at 24 hr (P = 0.49 vs. SAH). The improvement of neurological deficit by high dose simvastatin was reversed by wortmannin (P < 0.01 SAH + W + S-20 vs. SAH + S-20; Fig. 2D).
Expression of Akt, eNOS, Phosphorylated Akt, and Phosphorylated eNOS
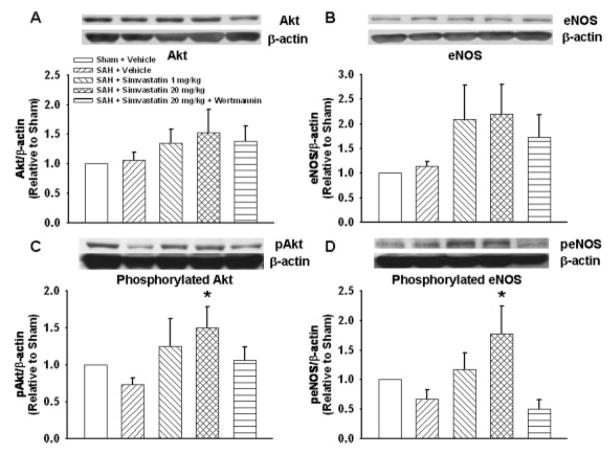
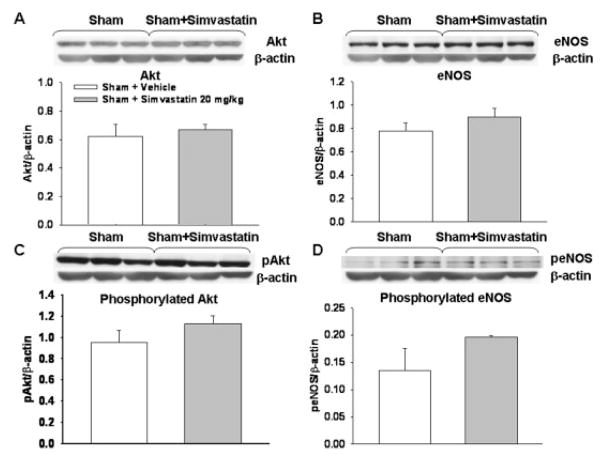
Western blotting analyses at 24 hr showed that Akt and eNOS expression was unchanged between sham and SAH groups; a trend toward increased expression was seen in low- and high-dosage simvastatin treated groups, but this trend did not reach significance (for Akt, sham: P = 0.87, SAH + S-1: P = 0.41, SAH + S-20: P = 0.18, SAH + W + S-20: P = 0.35 vs. SAH; for eNOS, sham: P = 0.83, SAH + S-1: P = 0.16, SAH + S-20: P = 0.12, SAH + W + S-20: P = 0.37 vs. SAH; Fig. 3A,B). However, both pAkt and peNOS (phosphorylated forms) were significantly increased in the high-dose simvastatin group, SAH + S-20, compared with the SAH group (pAkt P < 0.05, peNOS P < 0.01 vs. SAH). There were no significant differences in pAkt and peNOS levels in all other groups compared with the SAH group (for pAkt, sham: P = 0.41, SAH + S-1: P = 0.12, SAH + W + S-20: P = 0.31 vs. SAH; for peNOS, sham: P = 0.38, SAH + S-1: P = 0.20, SAH + W + S-20: P = 0.66 vs. SAH; Fig. 3C,D). Simvastatin alone did not significantly change any protein levels compared with the sham group (Fig. 4).
Fig. 3.
Western blotting analysis for Akt, eNOS, pAkt, and peNOS. A,B: Akt and eNOS expression were marginally increased in the low-and high-dosage simvastatin group, but there was no significant difference between groups. C,D: Both pAkt and peNOS (phosphorylated forms) were significantly increased in the high-dosage simvastatin-treated group compared with the SAH group. However, there was no significant difference in pAkt and peNOS levels in other compared groups. Vessels from three animals were pooled for each Western blotting experiment. Thus in total six experiments from a total of 18 animals were performed in each group. β-Actin was used as an internal control for every experiment before normalizing with their shams (*P < 0.05 vs. sham). pAkt, phosphorylated Akt; peNOS, phosphorylated eNOS.
Fig. 4.
Simvastatin effects on protein expression in sham arteries. There was no significant difference in the expression of Akt, eNOS, pAkt, and peNOS protein levels (A–D) between the sham and sham-treated with simvastatin (20 mg/kg) groups. Vessels from three animals were pooled for each Western blotting experiment. The data presented are analyzed from three experiments from a total of nine animals in each group. β-Actin was used as an internal control for every experiment.
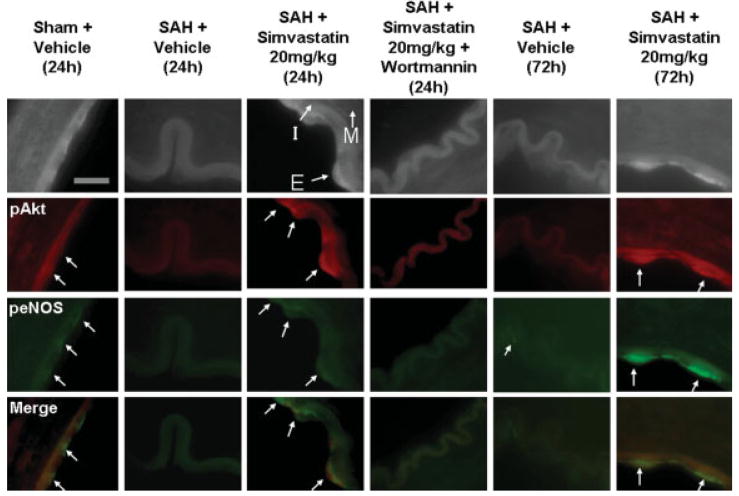
These results were supported by double-labeling fluorescent immunohistochemical data showing that expression of pAkt and peNOS in endothelial cells was decreased in the SAH and SAH + W + S-20 groups while being maintained in the SAH + S-20 group compared with sham at 24 hr after SAH. pAkt and peNOS expression in endothelial cells was also maintained at a higher level in the SAH + S-20 group compared with the SAH group at 72 hr. pAkt expression was also observed in vascular smooth muscle at 72 hr but not at 24 hr (Fig. 5).
Fig. 5.
Double-labeling fluorescent immunohistochemistry for pAkt and peNOS. Red and green stain shows pAkt and peNOS expression, respectively. These pictures show qualitatively that the expression of pAkt and peNOS in the endothelial cells was decreased in vehicle-treated and high-dosage simvastatin- + wortmannin-treated groups; however, it was maintained in the high-dosage simvastatin-treated group compared with sham. These decreased expression of pAkt and peNOS in vehicle-treated group, and the maintained expression of pAkt and peNOS in high-dosate simvastatin-treated group continued to 72 hr after SAH. I, intima; M, media; E, endothelial cell. Scale bar = 10 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
3-Hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly known as statins, are widely used clinically for their lipid-lowering properties (Prager, 1989; Czuriga and Edes, 2006). Recent evidence shows that statins are also effective in ameliorating cerebral vasospasm, which occurs as a sequela of SAH (McGirt et al., 2002, 2006a). Two randomized clinical trials investigating the use of statins for vasospasm after aneurysmal SAH showed that acute treatment with statins after SAH is safe and ameliorates vasospasm (Lynch et al., 2005; Tseng et al., 2005). Statins have been established as pleiotropic in nature, mediating their effects through several second messenger pathways (Liao and Laufs, 2005). In studies of cardiovascular disease, the therapeutic effects of statins have been found to be mediated through PI3K/Akt (Kureishi et al., 2000; Wolfrum et al., 2004; H. Wang et al., 2005). Wolfrum et al. (2004) reported that simvastatin acutely reduced the extent of myocardial necrosis in an NO-dependent manner by activating the PI3K/Akt pathway. The activation of this pathway by statins in the amelioration of cerebral vasospam has not been previously established. In the present study, we demonstrate for the first time that simvastatin increases Akt phosphorylation, followed by eNOS phosphorylation and the amelioration of cerebral vasospasm.
We have shown that high-dosage simvastatin significantly reversed the decrease in arterial diameter and perimeter as well as the vessel wall thickening observed in the ICA affected by cerebral vasospasm at 24 and 72 hr after SAH. High-dosage simvastatin also improved neurological status 24 and 72 hr after SAH. These beneficial effects of simvastatin were countered by wortmannin, a PI3K inhibitor. Wortmannin by itself did not influence cerebral vasospasm as indicated by morphological data and neurological scores (compared with sham animals). In addition, pAkt and peNOS were up-regulated in endothelial cells by high-dose simvastatin, and this phosphorylation was inhibited with the addition of wortmannin. The ability of wortmannin to reverse simvastatin’s attenuation of vasospasm (morphological data) and up-regulation of pAkt and peNOS in endothelial cells (molecular data) indicates an important role for increased phosphorylation of Akt and eNOS in statin-mediated prevention of vasospasm. Another study also supports the role of increased phosphorylation of Akt and eNOS in the amelioration of cerebral vasospasm following SAH (Santhanam et al., 2005). Santhanam et al. showed that erythropoietin (EPO) prevented SAH-induced vasospasm and amplified pAkt and peNOS expression. It appears that both EPO- and statin (present study)-treated animals show significant increases in phosphorylated Akt and eNOS levels compared with vehicle-treated animals. However, the findings of Santhanam et al. (2005) differ from ours in that they depicted a slight but significant increase in phosphorylated Akt and eNOS levels in the SAH group by itself, which was not observed in our results. This may be related to the severity of injury due to the use of different species as well as SAH modeling.
In this study, we also observed that simvastatin did not change the expression of Akt and eNOS at 24 hr after SAH. The expression of eNOS (unphosphorylated) following SAH is controversial; Laufs and colleagues showed that statins stabilized eNOS mRNA and up-regulated eNOS expression (Laufs et al., 1997; Laufs and Liao, 1998), but recently some studies reported that the expression of eNOS remained unchanged after statin treatment (Kureishi et al., 2000; McGirt et al., 2002; J. Wang et al., 2005). McGirt et al. (2002) showed that short-term posttreatment of simvastatin did not increase eNOS protein but ameliorated cerebral vasospasm and neurological deficit at 72 hr after SAH. These results and our study support the conclusion that statins do not affect the overall eNOS and Akt levels in the early stage after SAH; rather, they act by phosphorylating the Akt and eNOS in the endothelial cells. The present study also confirms, for the first time in vivo, the role of the activation of PI3K leading to phosphorylation of Akt and eNOS in statin-mediated protection against cerebral vasospasm.
In the present study, simvastatin up-regulates protein levels of phosphorylated forms of Akt and eNOS after SAH. However, the lack of effect of simvastatin alone in altering levels of Akt and eNOS and their phosphorylated forms in sham animals suggests that simvastatin targets the PI3K/Akt signaling pathway that is affected primarily by SAH.
Other putative mechanisms of statin-mediated protection against cerebral vasospasm are mediated via inflammation, oxidative stress, and proteins such as caveolin-1. McGirt et al. previously demonstrated that simvastatin is likely to ameliorate cerebral vasospasm after SAH by mitigating inflammatory events such as perivascular granulocyte migration. Erdos et al. (2006) showed that rosuvastatin improved cerebrovascular function in rats by inhibiting NADPH oxidase-dependent superoxide production, and Pelat et al. (2003) revealed that rosuvastatin decreased caveolin-1 expression and promoted eNOS function in cardiac and aortic cells. These statin-mediated effects could also be involved in prevention of cerebral vasospasm and must be further investigated.
The activation of this pathway may also have therapeutic implications outside the treatment of cerebral vasospasm. Acute brain injury after SAH also plays a significant role in SAH-induced brain injury and is a major contributor to the negative sequelae of SAH (Ostrowski et al., 2006a). The PI3K/Akt/eNOS pathway’s above-mentioned roles in endothelial cell survival and regulation of vascular tone likely play a role in SAH-induced brain injury (Ostrowski et al., 2006a; Sehba and Bederson, 2006), but the pathway may also have more direct implications. Investigators have linked Akt phosphorylation to the inhibition of caspase activation and prevention of neuronal cell death both in vitro and in rodent models of ischemic brain injury (Jin et al., 2000; Shioda et al., 2007; Vauzour et al., 2007). However, the role of eNOS phosphorylation and NO production following brain injury is more controversial, insofar as NO has been related to both neuroprotection and injury (Endres et al., 1998; J. Wang et al., 2005; Ho et al., 2006). Sehba and Bederson (2006) have formulated a hypothesis that may help resolve these conflicting results in the setting of acute injury. Beyond the scope of treating acute sequelae, the prevention of caspase activation and apoptosis following acute insults may prevent the development of long-term CNS disease. Cerebral ischemia (van Groen et al., 2005; Tesco et al., 2007) and CNS exposure to isoflurance anesthetics (Xie et al., 2006, 2007) have been linked to the development of Alzheimer’s disease through caspase activation and apoptosis. Recently, Baki et al. (2008) demonstrated that PI3K/Akt signaling may be beneficial to familial Alzheimer’s disease patients through the suppression of caspase 3 activation.
In conclusion, the present study provides evidence for the role of PI3K activation leading to phosphorylation of Akt and eNOS in simvastatin-mediated attenuation of cerebral vasospasm after SAH and demonstrates the therapeutic implications of the up-regulation of this pathway in cerebral vasospasm following SAH. The up-regulation of this pathway has the potential to provide a therapeutic benefit in other CNS diseases as well.
Acknowledgments
Contract grant sponsor: NIH; Contract grant number: NS45694 (to J.H.Z.); Contract grant number: NS43338 (to J.H.Z.); Contract grant number: NS53407 (to J.H.Z.).
References
- Baki L, Neve RL, Shao Z, Shioi J, Georgakopoulos A, Robakis NK. Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling. J Neurosci. 2008;28:483–490. doi: 10.1523/JNEUROSCI.4067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KJ, Scott RM. Effect of intravenous ethanol on cerebral vasospasm produced by subarachnoid blood. Stroke. 1979;10:535–537. doi: 10.1161/01.str.10.5.535. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- Cahill J, Calvert JW, Solaroglu I, Zhang JH. Vasospasm and p53-induced apoptosis in an experimental model of subarachnoid hemorrhage. Stroke. 2006;37:1868–1874. doi: 10.1161/01.STR.0000226995.27230.96. [DOI] [PubMed] [Google Scholar]
- Czuriga I, Edes I. [Lowering cholesterol: how low is low enough?] Orv Hetil. 2006;147:1349–1356. [PubMed] [Google Scholar]
- de Oliveira JG, Beck J, Ulrich C, Rathert J, Raabe A, Seifert V. Comparison between clipping and coiling on the incidence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurosurg Rev. 2007;30:22–30. doi: 10.1007/s10143-006-0045-5. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdos B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol. 2006;290:H1264–H1270. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, Ma XL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, Lai CC, Liu SH, Liau CS. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal. 2006;18:391–399. doi: 10.1016/j.cellsig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA. Induction of vascular endothelial growth factor receptors and phosphatidylinositol 3′-kinase/Akt signaling by global cerebral ischemia in the rat. Neuroscience. 2000;100:713–717. doi: 10.1016/s0306-4522(00)00331-6. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, Blessing R, Alexander MJ, Graffagnino C, Warner DS, Laskowitz DT. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, Pelligrino DA, Warner DS. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Blessing R, Alexander MJ, Nimjee SM, Woodworth GF, Friedman AH, Graffagnino C, Laskowitz DT, Lynch JR. Risk of cerebral vasopasm after subarachnoid hemorrhage reduced by statin therapy: a multivariate analysis of an institutional experience. J Neurosurg. 2006a;105:671–674. doi: 10.3171/jns.2006.105.5.671. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Pradilla G, Legnani FG, Thai QA, Recinos PF, Tamargo RJ, Clatterbuck RE. Systemic administration of simvastatin after the onset of experimental subarachnoid hemorrhage attenuates cerebral vasospasm. Neurosurgery. 2006b;58:945–951. doi: 10.1227/01.NEU.0000210262.67628.7E. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006a;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006b;37:1314–1318. doi: 10.1161/01.STR.0000217310.88450.c3. [DOI] [PubMed] [Google Scholar]
- Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- Parra A, McGirt MJ, Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res. 2002;24:510–516. doi: 10.1179/016164102101200276. [DOI] [PubMed] [Google Scholar]
- Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480–2486. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- Prager R. [Treatment of hyperlipidemia with HMG-CoA reductase inhibitors] Wien Med Wochenschr Suppl. 1989;105:17–20. [PubMed] [Google Scholar]
- Santhanam AV, Smith LA, Akiyama M, Rosales AG, Bailey KR, Katusic ZS. Role of endothelial NO synthase phosphorylation in cerebrovascular protective effect of recombinant erythropoietin during subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2005;36:2731–2737. doi: 10.1161/01.STR.0000190021.85035.5b. [DOI] [PubMed] [Google Scholar]
- Scott PA, Tremblay A, Brochu M, St Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293:H3713–H3719. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Bederson JB. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- Shioda N, Ishigami T, Han F, Moriguchi S, Shibuya M, Iwabuchi Y, Fukunaga K. Activation of phosphatidylinositol 3-kinase/protein kinase B pathway by a vanadyl compound mediates its neuroprotective effect in mouse brain ischemia. Neuroscience. 2007;148:221–229. doi: 10.1016/j.neuroscience.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2007;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH. Cathepsin and calpain inhibitor E64d attenuates matrix metallo-proteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Zeiher AM, Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- van Groen T, Puurunen K, Maki HM, Sivenius J, Jolkkonen J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36:1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem. 2007;103:1355–1367. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu YB, Du XH. Effect of dexamethasone on nitric oxide synthase and caspase-3 gene expressions in endotoxemia in neonate rat brain. Biomed Environ Sci. 2005;18:181–186. [PubMed] [Google Scholar]
- Wang J, Tokoro T, Matsui K, Higa S, Kitajima I. Pitavastatin at low dose activates endothelial nitric oxide synthase through PI3K-AKT pathway in endothelial cells. Life Sci. 2005;76:2257–2268. doi: 10.1016/j.lfs.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, Itter G, Rosen R, Bohm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Schutt M, Weidtmann B, Heep A, Tempel K, Klein HH, Dominiak P, Richardt G. Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase/Akt pathway. J Cardiovasc Pharmacol. 2004;44:348–355. doi: 10.1097/01.fjc.0000137162.14735.30. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, Crosby G, Tanzi RE. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]