Abstract
The blood-brain barrier (BBB) is a structural and functional barrier that regulates the passage of molecules into and out of the brain to maintain the neural microenvironment. We have previously developed the in vitro BBB model with human brain microvascular endothelial cells (HBMEC). However, in vivo HBMEC are shown to interact with astrocytes and also exposed to shear stress through blood flow. In an attempt to develop the BBB model to mimic the in vivo condition we constructed the flow-based in vitro BBB model using HBMEC and human fetal astrocytes (HFA). We also examined the effect of astrocyte conditioned medium (ACM) in lieu of HFA to study the role of secreted factor(s) on the BBB properties. The tightness of HBMEC monolayer was assessed by the permeability of dextran and propidium iodide as well as by measuring the transendothelial electrical resistance (TEER). We showed that the HBMEC permeability was reduced and TEER was increased by non-contact, co-cultivation with HFA and ACM. The exposure of HBMEC to shear stress also exhibited decreased permeability. Moreover, HFA/ACM and shear flow exhibited additive effect of decreasing the permeability of HBMEC monolayer. In addition, we showed that the HBMEC expression of ZO-1 (tight junction protein) was increased by co-cultivation with ACM and in response to shear stress. These findings suggest that the non-contact co-cultivation with HFA helps maintain the barrier properties of HBMEC by secreting factor(s) into the medium. Our in vitro flow model system with the cells of human origin should be useful for studying the interactions between endothelial cells, glial cells, and secreted factor(s) as well as the role of shear stress in the barrier property of HBMEC.
Keywords: Brain endothelial cells, astrocytes, astrocyte-conditioned medium, In vitro BBB model, permeability, tight junctions
1. Introduction
The blood-brain barrier is a unique capillary barrier that is formed by brain microvascular endothelial cells and separates the blood from the central nervous system. The barrier plays an important role in the homeostatic regulation of the brain microenvironment. In mammals and higher vertebrates, the complex cell-cell contacts between microvascular endothelial cells comprised of tight and adherens junction proteins prevent the paracellular migration of hydrophilic molecules from blood into the brain [10,59].
Tightness of the intercellular junctions can be monitored in vitro using several techniques which include the trans-membrane recording of electrical resistance and the diffusion of dyes or labeled macromolecules across an endothelial cell monolayer. The disruption of the blood-brain barrier integrity has been shown to occur in many neurological disorders such as Alzheimer's disease, stroke and HIV-1 encephalopathy [34,54,63] but the mechanisms involved in BBB disruption remain incompletely understood. A major limiting factor is the lack of reliable models of the human blood-brain barrier.
The structure and physiological properties of the BBB have been extensively studied in isolated microvessels and in primary cultures of brain microvascular endothelial cells derived from non-human origin such as rodents and cows [30,39,45,75]. We have previously developed the in vitro BBB model with HBMEC [25,53,72]. The HBMEC were found to be >99% pure endothelial cells, based on specific marker studies [72]. However, in vivo HBMEC are shown to interact with astrocytes and also exposed to shear stress through blood flow. Several lines of evidence suggest that non-human brain and non-brain endothelial tight junctions are enhanced when co-cultured with astrocytes or in the presence of ACM [1,5,56,61,62,69,81], and blood flow associated shear stress have been shown to modify the endothelial barrier [67,70,74].
The purpose of the paper was to examine the effects of co-cultivation of HBMEC with HFA/ACM and to investigate the effect of shear stress on the barrier properties of HBMEC in the presence of HFA/ACM. We designed the in vitro flow chamber made of thick plastic chamber connected with peristaltic pump and reservoir. The whole setup was kept inside the CO2 chamber. The advantages of our in vitro flow cell culture system include 1) comparison between the dynamic and stationary system is possible by using the snapwell inserts; 2) continuous medium exchange like in vivo; 3) continuous oxygenation of the cell culture medium within the chamber by keeping inside the CO2 chamber; 4) opportunity to perform continuous or repeat exposure to a test compound; 5) continuous sampling of out-flowing medium for analysis of the metabolic products; 6) the whole system is sterilize-able and reusable; 7) the volumes on the two sides of the membrane can be easily controlled which allows for drug transport studies; 8) electrophysiology studies are feasible because a uniform current can be applied and 9) different combination of experiments can be performed using the multiple chambers attached together.
These advantages allowed us (a) to measure the permeability across the HBMEC monolayer exposed to shear stress by influx or traversal of fluorescent molecules, (b) to assess the role of ACM and shear stress on the barrier property of HBMEC (c) to fix the snap well membrane with paraformaldehyde for immunofluorescent studies of the spatial distribution and expression of cellular proteins and tight junction proteins and (d) to set up the whole system with tissues derived from humans. In our model system, we measured the transendothelial permeability, TEER and expression of tight junction protein ZO-1, as the markers for the BBB characteristics. We showed that HBMEC co-cultivation with HFA/ACM and exposure to shear stress exhibited decreased permeability and increased resistance and also exhibited increased expression of ZO-1 compared with HBMEC without ACM and flow. Our findings suggest non-contact co-cultivation of HBMEC with HFA/ACM and shear stress enhances the barrier functions, by up-regulating the tight junctional protein ZO-1, and reducing the transendothelial permeability across the HBMEC.
2. Results
2.1 Co-cultivation with HFA decreased the permeability of HBMEC
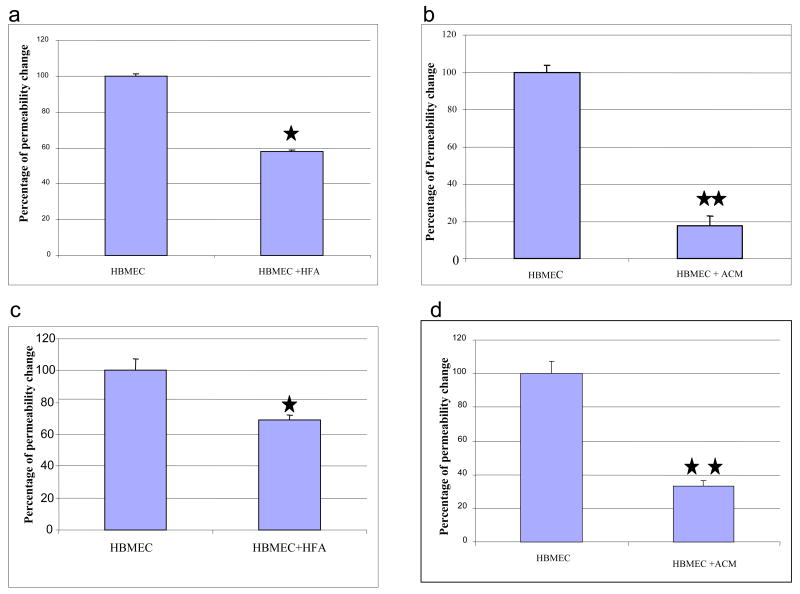
It has been previously shown that co-cultivation of bovine, rat and porcine brain and non-brain endothelial cells with rat brain astrocytes decrease the permeability of endothelial cells [13,37,52,70]. However, there are no reports for examining the effects of human astrocytes on the HBMEC barrier properties such as transendothelial permeability and TEER. In the present study, we examined the effect of HFA co-cultivation on the permeability of HBMEC monolayers. To investigate the effect of HFA, HBMEC were cultivated on transwell inserts and HFA on the lower chamber of the 24-well plates as described in the Materials and Methods section, without direct contact and the results were compared with HBMEC without HFA. As shown in Fig 2a penetration of low molecular weight propidium iodide across HBMEC monolayer was significantly reduced by HFA co-cultivation by 30% when compared to the HBMEC alone, suggesting HFA co-cultivation decreased the permeability of HBMEC. This suggests that the co-cultivation of HBMEC with human astrocytes can enhance the barrier properties such as decreased permeability.
Fig.2.
Static co-cultivation of HBMEC with HFA or ACM significantly reduces the permeability of fluorescein-conjugated dextran (a, b) and propidium iodide (c, d) across the endothelial cell monolayer. HBMEC grown on the transwell inserts were co-cultivated with HFA (a, c) and ACM (b, d). The fluorescein dextran and propidium iodide permeability was determined as described in Materials and Methods section. An average of 3 experiments are shown in each panel and the results are presented as mean + SEM. *P<0.05, **P<0.01.
2.2 ACM decreased the permeability of HBMEC
Our co-cultivation system did not allow direct contact between HFA and HBMEC, suggesting that the above-noted effect of astrocyte co-cultivation may be mediated by secreted factor(s). Our next experiment was to use ACM derived from HFA instead of glial cells and to examine its effect on HBMEC permeability. HBMEC in the presence of ACM also showed decreased propidium iodide permeability across the HBMEC monolayer by 70% (Fig.2d), when compared to HBMEC alone. These findings support the concept that the secreted factor(s) into the medium by astrocytes helps maintain the barrier properties of HBMEC by decreasing the transendothelial permeability of small and large molecules.
2.3 Co-cultivation with HFA/ACM increased the resistance of HBMEC
We next examined the role of HFA or ACM in another barrier parameter, TEER. To examine the role of HFA and ACM on TEER, SMC and SMC-conditioned medium were used as a control in the TEER measurement to replace the HFA and ACM, respectively. Fig 3a using Endohm showed that both HFA and ACM exhibited the increased resistance, but the resistance decreased with SMC. Transendothelial resistance was also measured by ECIS. Figure 3b revealed that after an overnight incubation in low serum containing medium the resistance of HBMEC was gradually decreased but restored with the addition of ACM, not with SMC CM. We, however, cannot exclude the possibility that the failure to increase the resistance by rat SMC or SMC-conditioned medium might be related to xenogeneic issues. Taken together, these findings indicate that HFA and ACM were able to maintain the barrier properties of HBMEC via secreted factor(s) into the medium.
Fig.3.
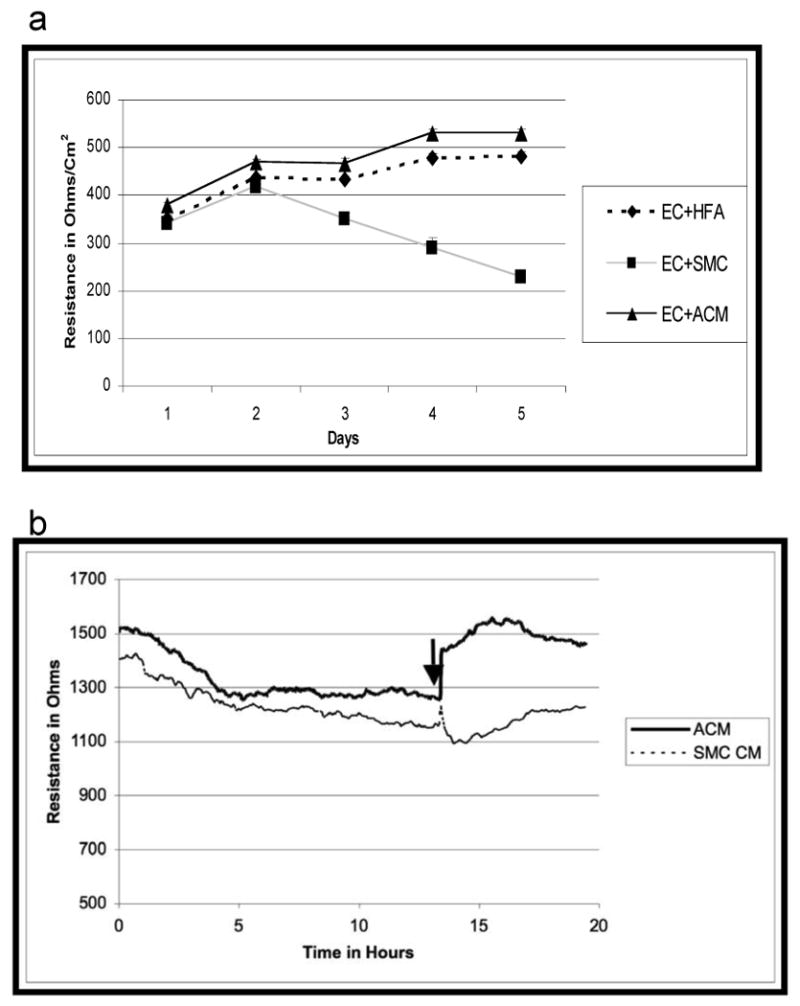
Static co-cultivation of HBMEC with HFA and ACM increases the TEER. (a) Endohm TEER measurements across HBMEC monolayer grown on the transwell insert membrane in the presence of HFA, ACM or SMC. (b) HBMEC grown on collagen-coated gold electrodes of the ECIS culture-ware were maintained on low serum containing media for overnight prior to the addition of ACM or SMC-CM. Arrow represents the addition of conditioned medium. Representative traces of 3 independent experiments are shown.
The above results with HFA/ACM were obtained from HBMEC derived from adults. We also examined whether HFA/ACM exhibit similar properties on the HBMEC derived from children. HFA/ACM also caused a decreased permeability in these HBMEC (data not shown). In another experiment we replaced the ACM with human astrocytoma derived conditioned medium. The astrocytoma-conditioned medium had similar effects in increasing and maintaining HBMEC monolayer resistance similar to ACM (data not shown). These findings illustrate that co-cultivation of adult/children HBMEC with HFA/ACM or astrocytoma-derived conditioned medium helps maintain the barrier properties of HBMEC.
2.4 Shear stress decreased permeability of HBMEC monolayer
The static co-cultivation using transwell inserts or flat bottom wells or Petri dishes may not mimic the in vivo situation for endothelial cells, because endothelial cells are likely to be exposed to shear stress through blood flow in vivo. We developed the in vitro flow system to mimic the in vivo situation of BBB (Fig.1) where endothelial cells are exposed to shear stress on apical side and basolateral side is exposed to glial cells or astrocytes.
Fig.1.
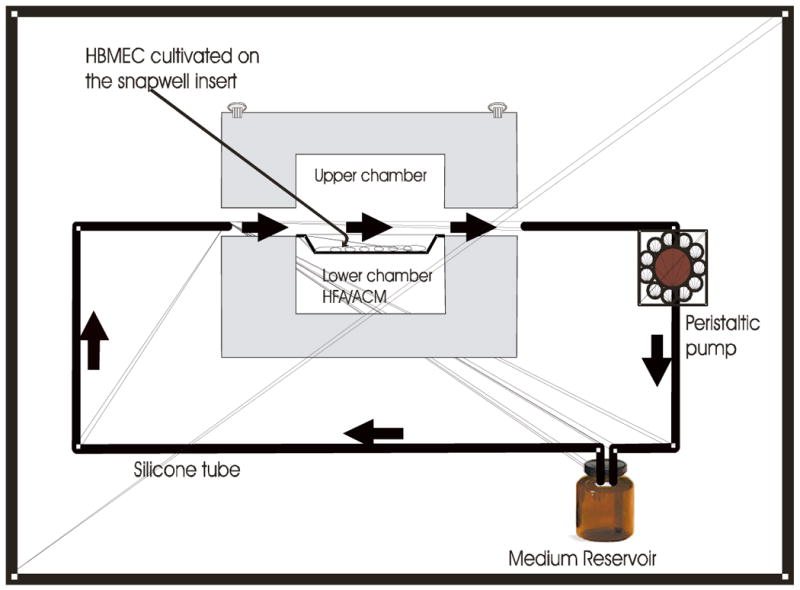
Diagrammatic representation of the in vitro blood brain barrier model. The dynamic in vitro flow system consists of a transparent plastic chamber, with upper and lower chamber, having hollow space in between where a snapwell insert with 12 mm diameter is housed. The upper and lower chambers are tightly sealed with Teflon O-ring and stainless steel screws. Flow chamber is connected to the speed-controllable peristaltic pump with the gas-permeable, platinum-cured silicon tubes. The whole set up is kept inside the temperature controlled CO2 incubator. The arrow indicates the direction of the flow.
To examine the effect of flow on HBMEC permeability, our initial experiments were performed on the 12-mm snap well insert. The results obtained from the cells grown under the static condition were set at 100%. The permeability was significantly reduced by confluent HBMEC monolayer exposed to fluid shear stress of 1-2 dyne/cm2 when compared to the static condition. (Fig.4a). These results suggest that shear stress contributes to decreasing permeability of HBMEC monolayers.
Fig.4.
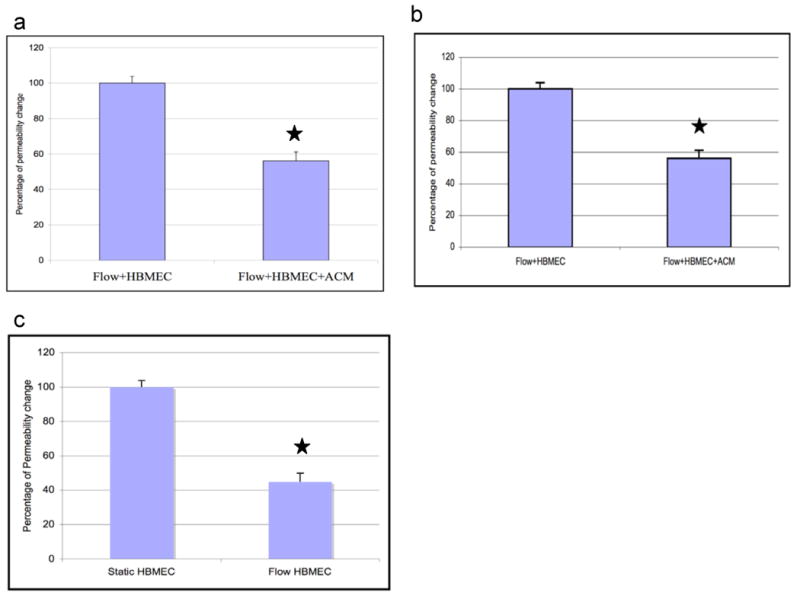
Application of shear stress enhances the barrier properties of HBMEC monolayer. Fluorescein dextran permeability was measured across the HBMEC monolayer grown on snapwell inserts for 5 days under shear stress with HFA or ACM. Flow-exposed HBMEC monolayer (a) significantly decreased the dextran permeability when compared to the static cultured HBMEC. Static values were arbitrarily set at 100%. Flow-exposed HBMEC monolayer co-cultivated with HFA (b) and exposed to ACM on the lower chamber (c) significantly decreased the dextran permeability compared to HBMEC exposed to shear stress. The results are normalized against the permeability of the HBMEC exposed to shear. An average of 3 experiments are shown in each panel and the results are presented as mean + SEM. *P<0.05.
2.5 Shear stress and HFA decreased permeability on HBMEC monolayer
Using the hollow-fiber in vitro BBB model, it has been shown that co-cultivation of rat, bovine and human brain endothelial cells with astrocytes from rat brain and C6 glioma increased the resistance after the introduction of flow with the shear stress of 1 dyne/cm2 [70]. Our earlier experiments using static co-cultivation showed that permeability of HBMEC was decreased with HFA (Fig.2a). Our next experiments were designed to culture HFA on the lower chamber of the flow system in the presence of shear stress. HBMEC exposed to shear stress was considered as a control and the obtained value was arbitrarily set at 100%. Co-cultivation with HFA significantly decreased the permeability by 40% when compared to HBMEC exposed to flow alone (Fig.4a). These findings indicate that HFA decreased the permeability of HBMEC monolayer with and without shear stress.
2.6 Shear stress and ACM decreased permeability of HBMEC monolayer
As shown earlier with our static co-cultivation experiments, ACM decreased the permeability of HBMEC by 70% (Fig.2b). We next examined the effect of ACM along with shear stress on the permeability of HBMEC. HBMEC that are exposed to flow in the presence of ACM decreased the permeability by 50% when compared to HBMEC exposed to flow alone (Fig.4b). Taken together, these findings indicate that HFA/ACM and shear stress are additive in exhibiting decreased permeability of HBMEC monolayer.
2.7 Up-regulation of tight junction protein, ZO-1 in the HBMEC co-cultured with HFA/ACM and exposed to shear stress
Previous studies suggest that permeability of endothelial and epithelial cell is regulated by components of tight and adherens junction proteins, which include occludin [32] VE-cadherin [2,17,38], claudin [26], ZO-1 [29,62,71] ZO-2 [31], cingulin [16], 7H6 antigen [65,82] and symplekin [40]. We examined whether the permeability changes occurred in response to astrocyte/ACM and shear stress are correlated with the changes in expression of tight and adherens junction proteins (e.g., ZO-1 and β-catenin, respectively). Immunofluorescent analysis of HBMEC exhibited ZO-1 and β-catenin expression around intercellular junctions (Fig.5a, b). Cell lysates from the HBMEC co-cultivated with ACM or exposed to shear stress cells were examined for the expression of ZO-1 and β-catenin by Western blot analysis. As shown in Fig 5c & d, ZO-1 expression was increased by 100% in HBMEC exposed to ACM when compared to the control (HBMEC without ACM). Similarly, cell lysates from shear exposed HBMEC also showed the up-regulation of ZO-1 by more than 50% (Fig.5e & f) when compared to the static control. These findings are similar to those of bovine retinal vascular endothelial cells, which showed increased barrier properties and ZO-1 expression in the presence of conditioned media from rat astrocytes [29]. In contrary, the adherens junction protein β-catenin of HBMEC did not show any significant changes in response to ACM and shear stress (Fig 5c & g, and e & h, respectively). These findings suggest that the observed decreased permeability changes with the ACM and shear exposed HBMEC may be related to increased expression of ZO-1.
Fig.5.
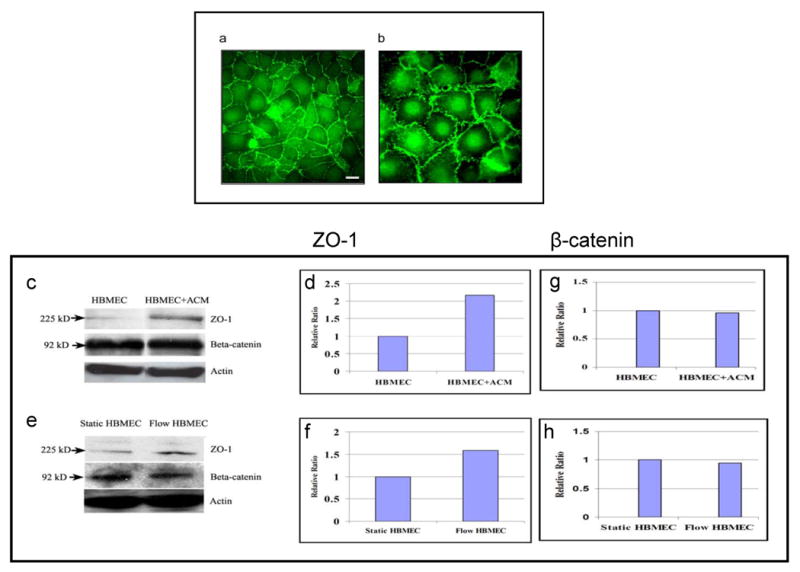
ACM and shear stress increases the expression of ZO-1 in HBMEC. (a) Immunofluorescence staining of tight junction protein (ZO-1) and (b) adherens junction protein (β-catenin). Western blot analysis of tight junction protein, ZO-1 and β-catenin from cell lysates of (c) HBMEC co-cultivated with ACM (e) HBMEC exposed to shear stress. (d, g) and (f, h) show relative expression ratio of the bands presented on (c) and (e) obtained using gel densitometer. Scale bar = 40 μm.
3. Discussion
The BBB is composed of a specialized microvascular endothelium and glial cell elements, including astrocytes and microglia that are in physical proximity to the endothelium and a basement membrane. All these elements contribute to maintaining the neural microenvironment. The BBB cells containing tight junctions serve to restrict and control the movement of substances between the systemic circulation and brain extracellular fluid and are characterized by a high TEER and low permeability [8,11, 14,18,49, 59]. BBB disruption leads to extravasation of plasma proteins into the brain through transcellular and paracellular routes [34,54,63]. Our understanding of the mechanisms that lead to the disruption of the BBB is limited. This is due, in part, to the lack of reliable in vitro models of BBB.
There is considerable body of evidence, in vitro and in vivo to indicate that astrocyte interaction with the cerebral endothelium helps determine the BBB function, morphology (i.e. tightness), and protein expression [3,6,12,58,81]. However, it remains unclear whether or not promotion of the endothelial barrier function by astrocytes requires a direct contact between astrocytes and endothelial cells. For example, contact co-cultivation of bovine adrenal capillary, aortic endothelial cells, rat brain microvascular endothelial cells with neonatal rat brain astrocytes increased the transendothelial electrical resistance and the expression of tight junctional protein such as occludin at endothelial cell junction [28]. A recent study showed that contact co-cultivation of astrocytes help bovine endothelial cells maintain the BBB properties by up-regulating the P-glycoprotein (P-gp) on the endothelial cells [27]. Since astrocytes do not express P-gp, this finding suggests that contact communication between the endothelial cells and astrocytes is necessary for the up-regulation of P-gp.
In contrast, non-contact co-cultivation of astrocytes has been shown to enhance the transendothelial resistance and decrease the transendothelial permeability in in vitro model of blood-brain barrier. [1,35,39,44,61,62]. Astrocytes do not make direct contact with endothelial cells in vivo, but rather, are separated from endothelial cells by an extracellular matrix. The ∼20 nm gap between adjacent astrocytes and endothelial cell is readily diffusible by various proteins, such as horseradish peroxidase [9]. Previously, it was shown that cultivation of human and bovine brain endothelial cells in the presence of ACM further reduced diffusion of FITC-BSA and sucrose permeability [56,62]. Moreover, the soluble factors present in the ACM exhibited the upregulation of HT7, UEA lectin binding sites and angiotensin receptors on the bovine and human adult brain endothelial cells that help to define the phenotype of brain endothelial cells [56]. These findings suggest that the influence of astrocytes may be mediated by a secreted factor(s) [62]. The conditioned medium derived from astrocytes has been shown to be capable of maintaining the BBB characteristics, suggesting that an astrocyte derived soluble factor is responsible for the endothelial cells to develop a BBB property [50,62]. We also showed that non-contact co-cultivation with HFA as well as ACM decreased the permeability (Fig.2) and increased TEER of HBMEC (Fig.3a.b), suggesting that soluble factor(s) present in the ACM is likely to contribute to the enhanced barrier properties of HBMEC.
Several reports on aortic endothelial cells suggest that the application of shear stress induces morphological and functional changes. The modulation is mediated in part by the regulation of genes encoding for many proteins including vasoactive substances, growth factors and proto-oncogenes [15,33,47,60], affecting cell structure and function in endothelium [21,48]. Recent studies have shown that shear stress affected the brain endothelial cell surface [20,43,70]. In our flow model system, the comparison between static vs flow systems using the same size snapwell exhibited significantly greater reduction in the permeability of dextran molecules with the flow system (Fig.4a), suggesting that laminar shear stress enhances the barrier properties of HBMEC monolayer. In contrast, shear stress was shown to increase the dextran permeability and decrease the TEER in bovine aortic endothelial cells [55].
Since we observed the decreased permeability of HBMEC with shear stress when compared to the static cultivation, we next examined the effects of astrocyte/ACM on the barrier properties of HBMEC monolayer using our flow system (Fig.1). Co-cultivations of rat astrocytes and rat glioma cells with rat brain endothelial cells and bovine aortic endothelial cells have been shown to increase the resistance in the presence of shear stress when compared to the endothelial cells alone grown on the hollow fiber apparatus [43,70]. In our shear stress model, non-contact co-cultivation of HBMEC with HFA/ACM also exhibited a decrease in permeability, supporting the roles of HFA/ACM and shear stress in maintaining/exhibiting the barrier properties of HBMEC. Earlier it has been reported that non-contact co-cultivation of bovine and human brain endothelial cells with rat brain astrocytes and conditioned medium enhances the barrier properties [56,62]. Similarly, co-cultivation of bovine, human and rat brain endothelial cells with rat brain astrocytes and C6 astroglioma in the hollow fiber apparatus also increased the resistance, suggesting that both shear stress and glial cells contribute to the maintenance of barrier properties of endothelial cells [70].
Junction complex in the brain microvascular endothelial cells comprises tight and adherens junctions [22,64]. Tight junctions in general, fulfill the two functions. First, they contribute to the maintenance of cell polarity [77,79], and second they seal the gap between adjacent cells and prevent uncontrolled paracellular exchange of small and macromolecules [66,77]. However, the sealing properties of tight junctions vary between endothelial cells of different locations. The mechanisms regulating cell junction organization in endothelial cells are incompletely understood. Several possibilities, such as changes in the expression level, phosphorylation status and distribution pattern of junctional proteins, have been proposed [41,42,51]. The tight junction consists of three integral membrane proteins, namely claudin, occludin and junction adhesion molecules, and a number of cytoplasmic accessory proteins including ZO-1, ZO-2 and ZO-3, and cingulin [4]. ZO and occludin molecules are shown to be primary regulatory proteins of tight junction that modulate BBB permeability [4]. Increased numbers of tight junctions in the presence of astrocytes or astrocyte CM have been demonstrated by the presence of inward ZO-1 expression [29, 44, 62]. We found that ZO-1 expression of HBMEC was increased in the presence of ACM (Fig. 5 c) and in response to shear stress (Fig. 5e). Moreover, penetration of low molecular weight molecule across the monolayer of HBMEC was decreased, suggesting that increased expression of ZO-1 can be correlated with enhanced barrier properties. Similarly, non contact co-cultivation of retinal endothelial cells with rat brain astrocytes significantly increased the ZO-1 content and also increased the resistance [29], suggesting that up-regulation of ZO-1 can be correlated with enhanced barrier properties. In contrast, we showed that β-catenin, which is part of adherens junction complex did not exhibit any significant changes in the presence of ACM and in response to shear stress. These findings suggest the differential responses of tight and adherens junction proteins in our HBMEC in response to ACM and shear stress.
The chemical nature of the glial cell-produced signal(s) in induction and maintenance of the barrier properties of HBMEC is unclear. Several candidate molecules have been identified, such as TGF β [7,76], GDNF [36,78] and bFGF [69], which can up-regulate the barrier properties by increasing the resistance and decreasing the paracellular permeability in brain endothelial, cells. Several lines of evidence suggest that the enhancement of endothelial barrier properties by astrocytes/ACM and shear stress may be related to TGF-β1 [19,28,76,80]. For example, astrocyte regulation of endothelial cell function has been shown to be mediated in part by astrocyte-derived TGF-β1 and TGF-β1 was shown to be present in ACM collected from rat astrocytes [23,28,76]. In addition, TGF-β1 expression is shown to be increased when human umbilical vein and bovine aortic arterial endothelial cells are exposed to shear stress [19,80]. In our preliminary experiments, treatment of HBMEC with TGF- β1 at a physiological relevant concentration (1 ng/ml) exhibited an increased resistance of the HBMEC monolayer (data not shown). Studies are in progress to determine whether the barrier properties of HBMEC observed with HFA/ACM and shear stress are related to TGF β-1.
In summary, co-cultivation of HBMEC with HFA/ACM and HBMEC exposed to shear stress decreased the permeability and increased the resistance, and co-culitvation with HFA/ACM and shear stress were additive in decreasing the permeability of HBMEC. Western blot analysis of the HBMEC exposed to ACM and shear stress showed the increased expression of ZO-1, whereas β-catenin did not show any significant changes. We speculate that the observed changes of the HBMEC permeability in response to co-culture with HFA/ACM and shear stress may be mediated through tight junction protein(s) and studies are in progress to elucidate the mechanisms.
4. Experimental Procedures
4.1. Materials
Alexa fluor® 488 goat anti-mouse IgG, Alexa fluor® 568 goat anti-rabbit IgG, were purchased from Molecular Probes Inc. (Eugene OR). Monoclonal anti-ZO-1 antibody and polyclonal anti-β-catenin antibody were obtained from Zymed laboratories (San Francisco, CA). Rabbit-anti-actin antibody, protease inhibitors, Tween 20, Propidium iodide and Bovine Serum Albumin (BSA) were obtained from Sigma (St. Louis, MO). Horseradish peroxidase secondary antibody was purchased from Cell Signalling Technology (Beverly, MA). Medium 199, Modified Eagle's Medium (MEM), penicillin and streptomycin, trypsin, Hanks Balanced Salt Solution (HBSS) and SDSPAGE gels were purchased from Invitrogen Corporation (Carlsbad, CA). Heat inactivated fetal bovine serum (FBS) was purchased from Omega scientific Inc. (Tarzena, CA), and Nu Serum was from BD Biosciences (Bedford, MA). L-glutamine was purchased from Irvine Scientific (Santa Ana, CA). 6.5 mm transwell inserts and 12 mm snapwell inserts (0.4 μm pore size) were obtained from Corning Incorporated (Corning, NY). Pronectin-F was purchased from Deepwater Chemicals (Woodward, OK). M-PER extraction buffer was purchased from Pierce (Rockford, IL). Protein assay reagent and peristaltic pump fittings kit were purchased from Bio-Rad (Hercules, CA). PVDF membranes and sterile disposable vacuum filtration system with 0.22 _m Millipore Express Plus Membrane were obtained from Millipore (Bedford, MA). ECL Plus detection system and Hyperfilm™ were obtained from Amersham (Piscataway, NJ). Endohm™ (TEER measurement chambers), EVOM™ (Epithelial Volt ohmmeter), silicone tubing and Peri-Star peristaltic pump were obtained from World Precision Instruments (Sarasota, FL). Nalgene reusable in-line filter holder was purchased from Fisher-Scientific (Newark, DE). ECIS culture-ware™ (8W10E) was purchased from Applied Biophysics Inc. (Troy, NY).
4.2 Cell lines and culture conditions
Primary HBMEC from adult human brain were isolated and characterized as previously described [72]. Briefly, the HBMEC were cultured in medium 199 and supplemented with 10% (vol/vol) heat inactivated fetal bovine serum (FBS), Nu Serum (10%), 100 U/ml penicillin and 100 μg/ml streptomycin. Confluent HBMEC were lifted with 0.05% trypsin-0.5 mM EDTA solution, and resuspended in fresh medium 199. HFA were cultured in MEM medium containing 2 mM L-glutamine and 10% FBS as described previously (HFA medium) [25]. Rat smooth muscle cell (SMC), human astrocytoma (SW1783), and L15 medium were obtained from ATCC (Manassas, VA). SMC were cultured in the HFA culture medium and astrocytoma cultured in the L15 medium.
4.3 Preparation of conditioned medium
To obtain the conditioned medium, HFA were grown on the 75-cm culture flask until confluency. Thereafter, every 24 hours the old medium was collected as ACM and replaced with fresh HFA medium. ACM was sterile filtered and stored at -70 C until use. Rat SMC were grown in the same HFA medium and the conditioned medium from smooth muscle (SMC CM) was also collected like ACM. In the same way, conditioned medium from astroglioma cells using L15 medium was also collected.
4.4 Static co-cultivation of HBMEC and HFA/ACM
Frozen stocks (passage number 13-15) of HBMEC were thawed and cultured on the 75cm culture flasks. For the experiments, medium 199 supplemented with 10% FBS, 10% Nu serum and L-glutamine (2 mM) were used. 2×10 cells were seeded onto the 6.5mm transwell inserts (0.4 μm pore size) coated with Pronectin-F (10 μg/ml). Frozen stocks of HFA, passage number 6-10, were thawed and cultured in MEM supplemented with 10% (vol/vol) heat-inactivated FBS, L-glutamine (2 mM), 100 U/ml penicillin and 100 μg/ml of streptomycin as described previously [25]. Confluent HFA was detached using the 0.05% trypsin-0.5 mM EDTA solution and resuspended with HFA medium. 2×10 HFA were seeded onto the Pronectin-F coated 24 well plate. Once the HBMEC became confluent, the transwells containing HBMEC were transferred to 24 well plate containing confluent astrocytes. Fresh 250 μl of medium 199 was added to the upper chamber of the transwell inserts and 1 ml of HFA medium was added into the lower chamber of the 24 well plate. In separate experiments, astrocytes were replaced with ACM, for which1:1 ratio of ACM and HFA medium was used. HBMEC and HFA or ACM were allowed to grow for 5 more days. Fresh medium was provided every other day for both upper and lower chambers. On day 5, the permeability assays were performed as described below.
4.5 Preparation of the flow system
In vitro flow chambers were fabricated in collaboration with World Precision Instruments (Sarasota FL). The dynamic in vitro flow system consists of a transparent chamber, made of thick plastic having hollow space, in which a snapwell insert with 12 mm diameter can be housed, that separates the upper from lower chamber. The attachment of the upper and lower chamber was sealed by Teflon O-ring and was tightened with the stainless steel screws. As shown in the figure 1, the upper chamber has the inlet and outlet ports through which multiple individual chambers are connected to the speed-controllable peristaltic pump with the gas-permeable, platinum-cured silicone tubes that run through the medium 199 reservoir and back to the chambers. The medium passing through the upper chamber applies the shear stress on the confluent HBMEC growing on the snapwell membrane. The in-line filter holder with 0.22-μm filters was placed between the chamber and the medium reservoir to remove the dead cells floating from the system. The whole setup was kept inside the temperature-controlled, water-jacketed CO2 incubator. The flow chamber, gas permeable platinum-cured silicone tubes, in-line filters and accessories used for the flow system were sterilized by ethylene oxide.
Before applying the shear stress, 5×10 HBMEC were seeded on the Pronectin-F coated 12-mm snapwell inserts and allowed to attach and grow in a static condition. At the same time, HFA were seeded on the 8-mm glass cover slips that fit onto the bottom of the flow chamber. After 24-36 hours, the endothelial cells were transferred to the flow chamber and subjected to fluid shear stress 0.5 dyne/cm2. Once the HBMEC reached confluence (based on the microscopic observation), the 8-mm cover slips with HFA were transferred to the lower chamber of the flow system. In other experiments, ACM was added instead of HFA. The HBMEC and HFA or HBMEC and ACM were allowed to grow for 5 days and subjected to physiologically relevant shear stress 1-2 dyne/cm2. The medium 199 was replaced with the fresh medium every 24 hours. The fluid shear stress strength was calculated according to the formulae: τ =6μQ/a2b, where μ is the viscosity of the perfusate, Q is flow volume (ml/s), a and b is cross sectional dimension of the flow path in centimeters [68].
4.6 Permeability assay
In the case of in vitro flow chamber system 12-mm snapwell insert-containing HBMEC was removed from the flow chamber and transferred to the 6-well plate filled with 1.5 ml of HBSS without phenol red. The HBMEC were washed with medium 199. The final volume of the medium inside the snapwell was 300 μl. 150 μl of the medium (total volume 300 μl) was removed and replaced with 150 μl of HBSS containing 0.5 mg/ml of fluorescent-conjugated dextran (70,000 Da) and 0.5 mg/ml propidium iodide (668 Da). The plates were kept in temperature controlled CO2 chamber at 37°C. After 60 minutes of incubation, 50-μl aliquots from the bottom of the 12 mm snapwell insert were collected and transferred to the 96-well plate. Fluorescent intensity of the assays were measured using the plate reader, Fluoroskan Ascent FL, having two different filters for fluorescent dextran and propidium iodide (Thermo Electron Corporation, Franklin, MA). The graphs were made using Microsoft Excel. For permeability assays using 6.5-mm transwell inserts, 0.5-mg/ml fluorescent-conjugated dextran and propidium iodide in a total volume of 100 μl was added. The absolute fluorescence intensity was used after background subtraction. HBMEC grown alone on the transwell or snapwell insert were used as a control.
4.7 Measurement of transendothelial electrical resistance (TEER)
To measure TEER across the 6.5 mm transwell, we used Endohm-EVOM measuring system (World Precision Instruments). Resistance was measured using an assembly containing current passing and voltage measuring electrodes. The resistance of blank inserts was subtracted and the results were calculated per cm of the membrane. The resistance readings were taken every 24 hours.
In addition, a new special ECIS™ (Electric Cell-substrate Impedance Sensing) Model 1600R Morphological Biosensor (Applied Biophysics) was used to measure the real-time TEER during exposure to ACM and SMC-CM. HBMEC were directly seeded on a collagen-coated eight-well gold electrode array in medium 199 supplemented with 10% FBS and Nu serum. Each well had one active electrode (250-μm diameter) and a large counter electrode. Both electrodes were connected to a phase-sensitive lock-in amplifier. The electrodes were fed with a constant current of 1μA supplied by a 1-V, 4,000-Hz AC signal. Each well contained 400 μl of the medium. Initial resistance of electrodes in medium 199 was 1400-1500Ω. After reaching maximal, steady-state readings of TEER that meant maximal confluence, cells were additionally incubated overnight in low-serum (0.5% FBS) medium 199, and then ACM and SMC-CM-induced changes in resistance of endothelial monolayers were monitored. The conditioned medium was mixed in an equal volume (1:1) with the fresh medium.
4.8 Immunofluorescent staining of cell junctional proteins
Primary HBMEC grown on cover slips were fixed in 4% paraformaldehyde for 20 min at room temperature. The cells were washed three times with PBS for 5 min each. Cells were then permeabilized with 0.2% Triton X-100 in PBS for 3 minutes and again washed three times with PBS. Then, the cells were blocked for 1 hr in a PBS solution containing 5% BSA (Sigma). This was followed by overnight incubation at 4°C with 1:200 dilution of the primary ZO-1 and β-catenin antibody. The cells were washed three times in PBS, 5 min each, and incubated for 2 h at room temperature with secondary antibodies (Alexa fluor® 488-conjugated anti-goat and Alexa fluor® 568 conjugated anti-rabbit antibodies) diluted 1:500 in PBS/BSA. Cover slips with the cells were again washed three times in PBS before being mounted onto the stage of a fluorescent microscope. Normal rabbit IgG, mouse IgG, and goat IgG were used as negative controls, which did not show any fluorescence increase above background.
4.9 Gel electrophoresis and Immunoblotting
Total cell lysates were obtained from HBMEC grown on the transwell or snapwell inserts under the flow and static conditions as described previously. Briefly, HBMEC rinsed with ice-cold PBS and lysed with MPER extraction buffer plus cocktail of protease inhibitors (20 mM Tris pH, 8.0, 150 mM NaCl, 1 mM DTT, 1% sodium deoxycholate, 0.5% SDS, 1% nonidet P40, 2 μg/ml aprotinin, 5 μg/ml leupeptin, 0.5 mM phenylmethylsulfonyl fluoride and 10 mM sodium fluoride). Protein concentrations were determined with a protein assay reagent (Bio-Rad). For each condition, 10 μg protein was separated on 10% SDS-PAGE gel in an Xcell Surelock™ Electrophoresis Cell (Invitrogen) and transferred to PVDF membranes. The membranes were blocked for 1 hr at room temperature with 5% non-fat dry milk, and 0.05% Tween 20 in PBS, and subsequently incubated with the primary antibody against monoclonal ZO-1, polyclonal β-catenin and rabbit-anti-actin in 5% BSA and 0.05% Tween 20 for overnight at 4°C with shaking. The membranes were washed three times with 0.1% Tween 20 in PBS for 10 min each. The membranes were incubated with anti-mouse and anti-rabbit horseradish peroxidase secondary antibody (1:2000) for 1 hr at room temperature, and then washed as above. Antibody-antigen complexes were visualized using the ECL Plus detection system and detected on Hyperfilm™. The bands were normalized against their respective controls using Software Quantity One, version 4.2.2 (Bio-Rad, Hercules, CA).
Acknowledgments
This study was supported by NIH grants to Prof. KS. Kim and American Heart Association, SDG (0435177N) to YV Kim.
Footnotes
Neuroscience classification codes: Cellular and Molecular Biology, Blood-brain barrier.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Venkatraman Siddharthan, Division of Pediatric Infectious Diseases, Johns Hopkins University, School of Medicine, Baltimore, MD 21287.
Yuri V. Kim, Division of Pediatric Infectious Diseases, Johns Hopkins University, School of Medicine, Baltimore, MD 21287.
Suyi Liu, World Precision Instruments Inc., 175 Sarasota Center Blvd, Sarasota FL 34240 U.S.A..
Kwang Sik Kim, Division of Pediatric Infectious Diseases, Johns Hopkins University, School of Medicine, Baltimore, MD 21287.
References
- 1.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- 3.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 4.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Bauer HC, Bauer H. Neural induction of the blood-brain barrier: still an enigma. Cell Mol Neurobiol. 2000;20:13–28. doi: 10.1023/A:1006939825857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck DW, Vinters HV, Hart MN, Cancilla PA. Glial cells influence polarity of the blood-brain barrier. J Neuropathol Exp Neurol. 1984;43:219–224. doi: 10.1097/00005072-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Behzadian MA, Wang XL, Jiang B, Caldwell RB. Angiostatic role of astrocytes: suppression of vascular endothelial cell growth by TGF-beta and other inhibitory factor(s) Glia. 1995;15:480–490. doi: 10.1002/glia.440150411. [DOI] [PubMed] [Google Scholar]
- 8.Brightman MW, Kadota Y. Nonpermeable and permeable vessels of the brain. NIDA Res Monogr. 1992;120:87–107. [PubMed] [Google Scholar]
- 9.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brightman MW, Zis K, Anders J. Morphology of cerebral endothelium and astrocytes as determinants of the neuronal microenvironment. Acta Neuropathol Suppl (Berl) 1983;8:21–33. doi: 10.1007/978-3-642-68970-3_2. [DOI] [PubMed] [Google Scholar]
- 11.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancilla PA, Frommes SP, Kahn LE, DeBault LE. Regeneration of cerebral microvessels: a morphologic and histochemical study after local freeze-injury. Lab Invest. 1979;40:74–82. [PubMed] [Google Scholar]
- 13.Cecchelli R, Dehouck B, Descamps L, Fenart L, Buee-Scherrer VV, Duhem C, Lundquist S, Rentfel M, Torpier G, Dehouck MP. In vitro model for evaluating drug transport across the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:165–178. doi: 10.1016/s0169-409x(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 14.Cereijido M, Gonzalez-Mariscal L, Contreras RG, Gallardo JM, Garcia-Villegas R, Valdes J. The making of a tight junction. J Cell Sci Suppl. 1993;17:127–132. doi: 10.1242/jcs.1993.supplement_17.18. [DOI] [PubMed] [Google Scholar]
- 15.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 16.Citi S, Amorosi A, Franconi F, Giotti A, Zampi G. Cingulin, a specific protein component of tight junctions, is expressed in normal and neoplastic human epithelial tissues. Am J Pathol. 1991;138:781–789. [PMC free article] [PubMed] [Google Scholar]
- 17.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 18.Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241:49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- 19.Cucina A, Sterpetti AV, Borrelli V, Pagliei S, Cavallaro A, D'Angelo LS. Shear stress induces transforming growth factor-beta 1 release by arterial endothelial cells. Surgery. 1998;123:212–217. [PubMed] [Google Scholar]
- 20.Cucullo L, McAllister MS, Kight K, Krizanac-Bengez L, Marroni M, Mayberg MR, Stanness KA, Janigro D. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002;951:243–254. doi: 10.1016/s0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- 21.Davies PF, Tripathi SC. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res. 1993;72:239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- 22.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 23.Dhandapani KM, Mahesh VB, Brann DW. Astrocytes and brain function: implications for reproduction. Exp Biol Med (Maywood) 2003;228:253–260. doi: 10.1177/153537020322800303. [DOI] [PubMed] [Google Scholar]
- 24.Dodane V, Kachar B. Identification of isoforms of G proteins and PKC that colocalize with tight junctions. J Membr Biol. 1996;149:199–209. doi: 10.1007/s002329900020. [DOI] [PubMed] [Google Scholar]
- 25.Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 26.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaillard PJ, van der Sandt IC, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, Breimer DD. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood-brain barrier. Pharm Res. 2000;17:1198–1205. doi: 10.1023/a:1026406528530. [DOI] [PubMed] [Google Scholar]
- 28.Garcia CM, Darland DC, Massingham LJ, D'Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res. 2004;152:25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Gardner TW, Lieth E, Khin SA, Barber AJ, Bonsall DJ, Lesher T, Rice K, Brennan WA., Jr Astrocytes increase barrier properties and ZO-1 expression in retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 1997;38:2423–2427. [PubMed] [Google Scholar]
- 30.Gordon EL, Danielsson PE, Nguyen TS, Winn HR. A comparison of primary cultures of rat cerebral microvascular endothelial cells to rat aortic endothelial cells. In Vitro Cell Dev Biol. 1991;27A:312–326. doi: 10.1007/BF02630909. [DOI] [PubMed] [Google Scholar]
- 31.Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci U S A. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh HJ, Li NQ, Frangos JA. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991;260:H642–646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- 34.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 35.Hurst RD, Fritz IB. Properties of an immortalised vascular endothelial/glioma cell co-culture model of the blood-brain barrier. J Cell Physiol. 1996;167:81–88. doi: 10.1002/(SICI)1097-4652(199604)167:1<81::AID-JCP9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, Sawada N. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem Biophys Res Commun. 1999;261:108–112. doi: 10.1006/bbrc.1999.0992. [DOI] [PubMed] [Google Scholar]
- 37.Isobe I, Watanabe T, Yotsuyanagi T, Hazemoto N, Yamagata K, Ueki T, Nakanishi K, Asai K, Kato T. Astrocytic contributions to blood-brain barrier (BBB) formation by endothelial cells: a possible use of aortic endothelial cell for in vitro BBB model. Neurochem Int. 1996;28:523–533. doi: 10.1016/0197-0186(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 38.Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE- cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1143–1153. doi: 10.1152/ajplung.00305.2003. [DOI] [PubMed] [Google Scholar]
- 39.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 40.Keon BH, Schafer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kevil CG, Okayama N, Trocha SD, Kalogeris TJ, Coe LL, Specian RD, Davis CP, Alexander JS. Expression of zonula occludens and adherens junctional proteins in human venous and arterial endothelial cells: role of occludin in endothelial solute barriers. Microcirculation. 1998;5:197–210. [PubMed] [Google Scholar]
- 42.Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VEcadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol. 2003;285:L434–442. doi: 10.1152/ajplung.00075.2003. [DOI] [PubMed] [Google Scholar]
- 43.Krizanac-Bengez L, Kapural M, Parkinson F, Cucullo L, Hossain M, Mayberg MR, Janigro D. Effects of transient loss of shear stress on blood-brain barrier endothelium: role of nitric oxide and IL-6. Brain Res. 2003;977:239–246. doi: 10.1016/s0006-8993(03)02689-1. [DOI] [PubMed] [Google Scholar]
- 44.Kuchler-Bopp S, Delaunoy JP, Artault JC, Zaepfel M, Dietrich JB. Astrocytes induce several blood-brain barrier properties in non-neural endothelial cells. Neuroreport. 1999;10:1347–1353. doi: 10.1097/00001756-199904260-00035. [DOI] [PubMed] [Google Scholar]
- 45.Laterra J, Guerin C, Goldstein GW. Astrocytes induce neural microvascular endothelial cells to form capillary-like structures in vitro. J Cell Physiol. 1990;144:204–215. doi: 10.1002/jcp.1041440205. [DOI] [PubMed] [Google Scholar]
- 46.Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 47.Malek AM, Gibbons GH, Dzau VJ, Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest. 1993;92:2013–2021. doi: 10.1172/JCI116796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malek AM, Izumo S. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertens. 1994;12:989–999. [PubMed] [Google Scholar]
- 49.Mandel LJ, Doctor RB, Bacallao R. ATP depletion: a novel method to study junctional properties in epithelial tissues. II. Internalization of Na+,K(+)-ATPase and Ecadherin. J Cell Sci. 1994;107(Pt 12):3315–3324. doi: 10.1242/jcs.107.12.3315. [DOI] [PubMed] [Google Scholar]
- 50.Neuhaus J, Risau W, Wolburg H. Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann N Y Acad Sci. 1991;633:578–580. doi: 10.1111/j.1749-6632.1991.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 51.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 52.Pekny M, Stanness KA, Eliasson C, Betsholtz C, Janigro D. Impaired induction of blood-brain barrier properties in aortic endothelial cells by astrocytes from GFAP-deficient mice. Glia. 1998;22:390–400. [PubMed] [Google Scholar]
- 53.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- 54.Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68:311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 55.Phelps JE, DePaola N. Spatial variations in endothelial barrier function in disturbed flows in vitro. Am J Physiol Heart Circ Physiol. 2000;278:H469–476. doi: 10.1152/ajpheart.2000.278.2.H469. [DOI] [PubMed] [Google Scholar]
- 56.Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- 57.Raub TJ. Signal transduction and glial cell modulation of cultured brain microvessel endothelial cell tight junctions. Am J Physiol. 1996;271:C495–503. doi: 10.1152/ajpcell.1996.271.2.C495. [DOI] [PubMed] [Google Scholar]
- 58.Raub TJ, Kuentzel SL, Sawada GA. Permeability of bovine brain microvessel endothelial cells in vitro: barrier tightening by a factor released from astroglioma cells. Exp Cell Res. 1992;199:330–340. doi: 10.1016/0014-4827(92)90442-b. [DOI] [PubMed] [Google Scholar]
- 59.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr, Gimbrone MA., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rist RJ, Romero IA, Chan MW, Couraud PO, Roux F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- 62.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 64.Ruffer C, Strey A, Janning A, Kim KS, Gerke V. Cell-cell junctions of dermal microvascular endothelial cells contain tight and adherens junction proteins in spatial proximity. Biochemistry. 2004;43:5360–5369. doi: 10.1021/bi035517c. [DOI] [PubMed] [Google Scholar]
- 65.Satoh H, Zhong Y, Isomura H, Saitoh M, Enomoto K, Sawada N, Mori M. Localization of 7H6 tight junction-associated antigen along the cell border of vascular endothelial cells correlates with paracellular barrier function against ions, large molecules, and cancer cells. Exp Cell Res. 1996;222:269–274. doi: 10.1006/excr.1996.0034. [DOI] [PubMed] [Google Scholar]
- 66.Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- 67.Seebach J, Dieterich P, Luo F, Schillers H, Vestweber D, Oberleithner H, Galla HJ, Schnittler HJ. Endothelial barrier function under laminar fluid shear stress. Lab Invest. 2000;80:1819–1831. doi: 10.1038/labinvest.3780193. [DOI] [PubMed] [Google Scholar]
- 68.Sill HW, Chang YS, Artman JR, Frangos JA, Hollis TM, Tarbell JM. Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am J Physiol. 1995;268:H535–543. doi: 10.1152/ajpheart.1995.268.2.H535. [DOI] [PubMed] [Google Scholar]
- 69.Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, Kato T. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 70.Stanness KA, Westrum LE, Fornaciari E, Mascagni P, Nelson JA, Stenglein SG, Myers T, Janigro D. Morphological and functional characterization of an in vitro blood-brain barrier model. Brain Res. 1997;771:329–342. doi: 10.1016/s0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- 71.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO 1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on beta mediates astrocyte-specific regulation of brain endothelial anticoagulant factors, human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 73.Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci U S A. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takada Y, Shinkai F, Kondo S, Yamamoto S, Tsuboi H, Korenaga R, Ando J. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun. 1994;205:1345–1352. doi: 10.1006/bbrc.1994.2813. [DOI] [PubMed] [Google Scholar]
- 75.Tontsch U, Bauer HC. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- 76.Tran ND, Correale J, Schreiber SS, Fisher M. Transforming growth factor. Stroke. 1999;30:1671–1678. doi: 10.1161/01.str.30.8.1671. [DOI] [PubMed] [Google Scholar]
- 77.Tsukita S, Furuse M, Itoh M. Molecular dissection of tight junctions. Cell Struct Funct. 1996;21:381–385. doi: 10.1247/csf.21.381. [DOI] [PubMed] [Google Scholar]
- 78.Utsumi H, Chiba H, Kamimura Y, Osanai M, Igarashi Y, Tobioka H, Mori M, Sawada N. Expression of GFRalpha-1, receptor for GDNF, in rat brain capillary during postnatal development of the BBB. Am J Physiol Cell Physiol. 2000;279:C361–368. doi: 10.1152/ajpcell.2000.279.2.C361. [DOI] [PubMed] [Google Scholar]
- 79.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 80.Wasserman SM, Topper JN. Adaptation of the endothelium to fluid flow: in vitro analyses of gene expression and in vivo implications. Vasc Med. 2004;9:35–45. doi: 10.1191/1358863x04vm521ra. [DOI] [PubMed] [Google Scholar]
- 81.Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, Farrell C, Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994;107(Pt 5):1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- 82.Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]