Abstract
Analogs of the potent antifungal agent, bengazole A, were prepared and evaluated against Candida spp. in both microbroth dilution and disk diffusion assays.
Keywords: antifungal, oxazole, azole, marine natural product
Bengazoles A (1) and B (2) are homologous bis-oxazole natural products first reported by Crews and coworkers from the sponge Jaspis sp. with anthelmintic activity against the nematode Nippostrongulus braziliensis.1
In 1990 we re-isolated 1 and 2, together with five additional homologs, bengazoles C-G (3a-e), from Jaspis sp. collected on the Great Barrier Reef,2 during a bioassay-guided screening of marine organisms for antifungal activity and found both compounds exhibited exceptionally potent in vitro activity against Candida albicans (e.g., MIC 1 μg/mL, C. albicans ATCC 14503),3 a pathogenic fungus that is responsible for systemic infections in immunocompromised individuals, including AIDS patients. The absolute stereostructure of 1 was determined using a combination of chemical degradation, synthesis of model compounds and comparisons of the CD spectra of perbenzoyl derivatives of both models and the natural product.2 Only total syntheses of bengazoles have been reported; bengazole A (1) by our group in 19994 and, more recently, 1 and 2 by Ley,5 and ‘bengazole Z’ (4) by Shioiri.6 The bis-oxazole, digonazole from J. digonoxea and its homologs,1b lack the C10 acyloxy group of 1–3,7 and have not been made or evaluated.
The mechanism of action of 1 is unknown and only limited of structure-activity relationships (SAR) are available.7 The clinically important ‘azole’ antifungal drugs (e.g., fluconazole (5), miconazole and voriconazole) inhibit 14-α-demethylase in yeast by tight binding of one of the 1,2,4-triazole rings to the Fe-heme active site.8 A cursory comparison of the structures of 1 and 4 suggested one of the oxazole rings in 1 might play a similar role, although this is not certain and other structural elements also appear to be important.
Bengazole 1 exhibits ergosterol-dependent in vitro activity against C. albicans similar to amphotericin B (AmB). When grown on agar plates containing Sabouraud media and defined concentrations of ergosterol or cholesterol, C. albicans showed diminished susceptibility that was dose-dependent upon 1, but inversely dependent upon the sterol concentration.9 Conversely, the activity of miconazole, exhibited no ergosterol-dependence.9b Interestingly, incorporation of cholesterol or synthetic ent-cholesterol9c in the media elicited dose-dependent, differential suppression of susceptibility of C. albicans towards AmB, but no enantiospecific differences were observed with (−)- and (+)-cholesterol and 2.9a
Here, we report the synthesis of new bengazole A analogs and an advanced SAR study of the new and known compounds that reveal the importance of structural features in 1 for anti-Candida activity; a long-hydrophobic side-chain, the polyol side chain, and at least one oxazole in the heterocyclic core.
The fatty acyl side chain of 1 is important for antifungal activity. We observed that 1 was prone to spontaneous solvolysis in the presence of protic solvents, or more rapidly, by ammoniolysis (NH3, MeOH).2 The des-acyl product, ‘bengazole Z’ (4),10 showed no anti-Candida activity in disk diffusion assays.
In order to examine the role of the heterocyclic core of 1, analogs were prepared with replacement of the polyol side-chain with a simple 1-hydroxy-1-butyl group (the parent, 1,3-oxazole (6) is too volatile, b.p. 69–70°C, for bioassay).
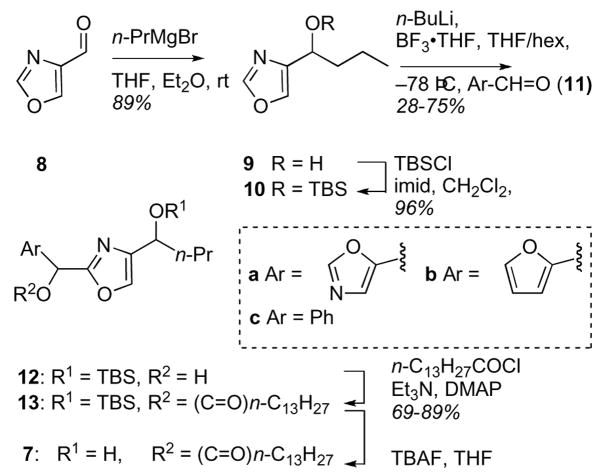
Compounds 7a-c were prepared by a variant of the ‘oxazole grafting’ approach employed for the synthesis of 1 (Scheme 1).4,11 Aldehyde 8 (prepared by DIBAL reduction of ethyl oxazole-4-carboxylate) was treated with n-PrMgBr and the product 9 was protected as the silyl ether 10 (TBSCl, imidazole, THF). Deprotonation of 10 using the Vedejs’ protocol12 (i. −78 °C, THF/hexanes, ii. BH3•THF), followed by separate additions of three aldehydes (oxazole-5-carboxaldehyde, 11a,13 furfural, 11b and benzaldehyde, 11c), gave 2,4-substituted oxazole adducts 12a-c.14
Scheme 1.
Synthesis of truncated 2,4-disubstitued oxazole analogs of 1.
Diastereomeric mixtures of the secondary carbinols 12a-c were acylated in good yield (myristoyl chloride, Et3N, DMAP) to give the long-chain esters 13a-c that were deprotected (TBAF, THF) to afford bengazole A analogs 7a-c. This small library, 7a-c, 12a-c and 13a-c, was tested in paper disk diffusion assays against Candida albicans (96–489, a patient clinical isolate, and ATCC 14503), C. glabrata and C. krusei. To our surprise, none were active at doses of up to 300 μg/disk. In order to determine the properties of mono-oxazole analogs of 1, selected 5- and 2,5-substituted oxazoles (Scheme 2) were tested in disk diffusion assays against five species of Candida including fluconazole-resistant C. glabrata, C. krusei and C. albicans (Table 1).
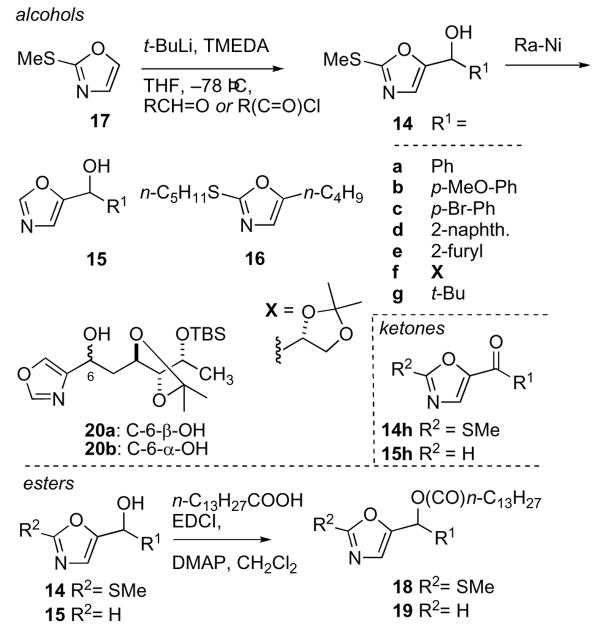
Scheme 2.
2-Methylthio-, 5- and 2,5-disubstituted oxazoles. See Ref. 15 for 14a-g, and 15a,d,g, Ref. 4 for 18a:18b, and Ref. 15b. for 16.
Table 1.
Anti-Candida activity of analogs of 1: disk diffusion assays.
| Zones of inhibition. mm @ 300μg/diska | |||||
|---|---|---|---|---|---|
| Cmpd. | C. albicansc | C. glabrata | C. krusei | C. albicansd,e | C. albicanse |
| 1b | 50 | 15 | 35 | 15 | 50 |
| 14a | 10 | 0 | 9 | 9 | 9 |
| 14b | 8 | 7.5 | 0 | 0 | 7.5 |
| 14c | 13 | 10 | 9 | 12 | 17 |
| 14d | 7.5 | 0 | 6.5 | 8 | 9 |
| 14e | 8 | 7.5 | 7.5 | 0 | 8 |
| 14f | 8 | 7 | 0 | 0 | 7 |
| 14g | 0 | 0 | 0 | 0 | 0 |
| 14h | 8 | 7 | 11 | 10 | 12 |
| 15a | 10 | 0 | 9 | 12 | 12 |
| 15d | 10 | 0 | 7.5 | 8 | 8 |
| 15g | 0 | 0 | 0 | 0 | 0 |
| 15h | 9 | 10 | 8 | 12 | – |
| 16 | 8 | 0 | 7 | 0 | – |
| 18a,c,g | 0 | 0 | 0 | 0 | 0 |
| 19a,g | 0 | 0 | 0 | 0 | 0 |
| 20a | 10 | 0 | 9 | 0 | 0 |
| 20b | 8 | 0 | 0 | 0 | 0 |
| AmBf | 22 | 19 | 15 | 12 | 23 |
The preparation of alcohols 14a-g, 15a,d,g, ketones 14h and 15h, and 2-methylthio-5-butyl-oxazole (16), starting with 2-methylthiooxazole (17),16 have been reported previously15 and summarized in Scheme 2. Selected alcohols 14 and 15 were O-acylated (EDCI, DMAP, n-C13H27COOH) group to give the new myristate esters 18 and 19. When assayed against fungi (Table 1), all compounds were found to be less potent that 1 or AmB. The most active compounds were 14c (9–17 mm zones of inhibition) and ketones 14h (8–12 mm) and 15h (9–12 mm). 2-Methylthio-5-oxazolyl analog 14a appeared to be slightly less active than its 5-monosubstituted counterpart 15a. Surprisingly, myristate esters 18a,c,g and 19a,g lost activity compared to their active parent alcohols, suggesting the activities of the compounds were limited by solubility. This contrasts with 1 where the fatty acyl chain is required for activity. Intermediates 20a and 20b, employed in the synthesis of 1,4 (Table 1) showed modest activity (9–10 mm) although diastereomer 20a, matching the configuration of 1, was slightly more active with a susceptibility profile similar to that of 1.
The most active compounds retained antifungal activity when tested in microbroth dilution assays (Table 2, e.g. 14c, MIC = 4 μg/mL against C. albicans and C. krusei), but again, myristate esters 18c,d and 19d were inactive.
Table 2.
Anti-Candida activity of analogs of 1: minimum inhibitory concentrations (MIC’s).a
| Cmpd. | C. albicansc | C. kruseib,d | C. albicanse |
|---|---|---|---|
| 1 | 1 | – | 1 |
| 14c | 4 | 4 | 8 |
| 14d | 8 | 16 | 8 |
| 15d | >64 | >64 | 32 |
| 18c | >64 | >64 | >64 |
| 18d | >64 | >64 | >64 |
| 19d | >64 | >64 | >64 |
| AmB | 0.50 | 0.25 | 0.50 |
RPMI media, 37 °C, 24 h incubation.
48 h incubation.
See caption in Table 1 for strain identification.
Clearly, the antifungal activity of 1 is not accounted for by simple analogs with one or two oxazole rings alone. At the very least, activity was correlated with the presence of a 5-monosubstituted oxazole (Scheme 2). 2,4-Disubstituted oxazolyl carbinol analogs, including their long-chain esters, were inactive. Interestingly, the closest heterocyclic analog, 7a, to bengazole A (1), was inactive suggesting abrogration of biological activity upon replacement of the hydrophilic polyol side chain of 1 with a truncated n-propyl carbinol. The fatty acyloxy chain at C-10 potentiates activity only when the native polyol chain is present (e.g. 1) since highly lipophilic 18a,c,g and 19a,g are inactive.
Although lipophilicity plays a role in activity of bengazole analogs, not all the effects can be explained by simple logP considerations, and subtle effects appear to be conferred by both the hydrophilic and fatty acyl chains.
In summary, several analogs of bengazole A (1) were prepared, containing one or two 1,3-oxazole rings, and modified side chains. Good activity was observed in some compounds but none were more potent than 1.
Partial recovery of susceptibility of Candida strains was observed in some 5-monosubstituted oxazole analogs. These findings will guide further development of antifungal analogs of 1, particularly modifications of the lipophilic acyl and hydrophilic polyol side-chains.18
Figure 1.
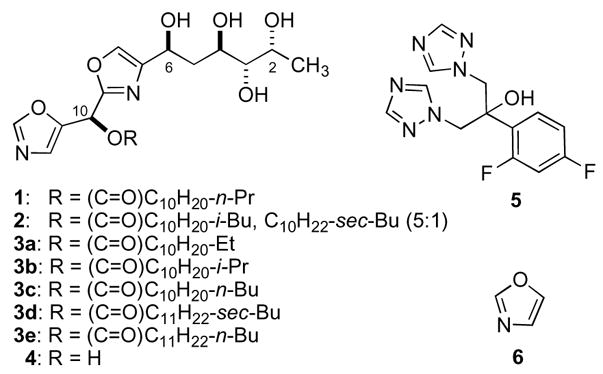
Bengazoles A–G (1–3), -Z (4), fluconazole (5) and oxazole (6)
Acknowledgments
We thank the Great Barrier Reef Authority for the permit to collect of Jaspis sp. This work was funded by the NIH (AI 039987).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.(a) Adamczeski M, Quiñoà E, Crews P. J Am Chem Soc. 1988;110:1598. [Google Scholar]; (b) Rodríguez J, Nieto RM, Crews P. J Nat Prod. 1993;56:2034. doi: 10.1021/np50102a002. [DOI] [PubMed] [Google Scholar]
- 2.Searle PA, Richter RK, Molinski TF. J Org Chem. 1996;61:4073. doi: 10.1021/jo952261a. [DOI] [PubMed] [Google Scholar]
- 3.Bengazole A (1) shows no antibacterial activity against gram-positive (Escherechia coli, Staphylococcus aureus) or gram-negative strains (Pseudomonas aeruginosa).
- 4.(a) Mulder RJ, Shafer CM, Molinski TF. J Org Chem. 1999;64:4995. doi: 10.1021/jo9906328. [DOI] [PubMed] [Google Scholar]; (b) Shafer CM, Molinski TF. Tetrahedron Lett. 1998;39:2903. [Google Scholar]
- 5.(a) Bull JA, Balskus EP, Horan RAJ, Langner M, Ley SV. Angew Chem Intl Ed. 2006;45:6714. doi: 10.1002/anie.200602050. [DOI] [PubMed] [Google Scholar]; (b) Bull JA, Balskus EP, Horan RAJ, Langner M, Ley SV. Chem Eur J. 2007;13:5515. doi: 10.1002/chem.200700033. [DOI] [PubMed] [Google Scholar]
- 6.Chittari P, Hamada Y, Shioiri T. Heterocycles. 2003;59:465. [Google Scholar]
- 7.Rudi A, Kashman Y, Benayahu Y, Schleyer M. J Nat Prod. 1994;57:829. doi: 10.1021/np50108a023. [DOI] [PubMed] [Google Scholar]
- 8.Trinci APJ, Ryley JF, editors. Mode of Action of Antifungal Agents. Cambridge; 1984. [Google Scholar]
- 9.(a) Richter RK, Mickus DE, Rychnovsky SD, Molinski TF. Bioorg Med Chem Lett. 2004;14:115. doi: 10.1016/j.bmcl.2003.10.018. [DOI] [PubMed] [Google Scholar]; (b) Molinski TF. J Nat Prod. 1993;56:1. doi: 10.1021/np50091a001. [DOI] [PubMed] [Google Scholar]; (c) Mickus DE, Levitt DG, Rychnovsky SD. J Am Chem Soc. 1992;114:359. [Google Scholar]
- 10.‘Bengazole Z’, a name coined by P. Crews for des-acyl 1, was found in methanol extracts of Jaspis sp. along with 1 and 2. See Ref. 1b.
- 11.Hodges JC, Patt WC, Connolly CJ. J Org Chem. 1991;56:449. [Google Scholar]
- 12.Vedejs E, Monahan SD. J Org Chem. 1996;61:5192. [Google Scholar]
- 13.(a) Kende AS, Kawamura K, DeVita RJ. J Am Chem Soc. 1990;112:4070. [Google Scholar]; (b) Schöllkopf U, Schröder R. Angew Chem, Int Ed. 1971;10:333. [Google Scholar]
- 14.Generally, the diastereomers (dr ~1:1, 1H NMR) we inseparable by TLC; no attempt was made to purify the isomers. All compounds gave satisfactory spectroscopic data.
- 15.(a) Shafer CM, Molinski TF. J Org Chem. 1998;63:551. doi: 10.1021/jo971410h. [DOI] [PubMed] [Google Scholar]; (b) Shafer CM. PhD Thesis. University of California; Davis: 1998. [Google Scholar]
- 16.Lacasse G, Muchowski JM. Can J Chem. 1972;50:3082. [Google Scholar]
- 17.Wong MYH, Gray GR. J Am Chem Soc. 1978;100:3548. [Google Scholar]
- 18.A recent report describes synthesis of ‘bengazole analogs’ and their antimicrobial activity. The compounds are actually based on 2,5-disubstitued furans; none of the analogs contain an oxazole ring. Kraus J, Kalkbrenner S, Schuster A, Obainoke A, Bracher F. Turk J Chem. 2008;32:125.