Abstract
Purpose/Objective
Our current understanding of intra-fraction pancreatic tumor motion due to respiration is quite limited. In this study, we characterized pancreatic tumor motion and evaluated the application of several radiotherapy motion management strategies.
Materials/Methods
17 patients with unresectable pancreatic cancer were enrolled in a prospective IRB-approved study and imaged during shallow free-breathing using cine MRI on a 3T scanner. Tumors borders were agreed upon by a radiation oncologist and an abdominal MRI radiologist. Tumor motion and correlation with the potential surrogates of the diaphragm and abdominal wall were assessed. This data was also used to evaluate PTV margin construction, respiratory gating, and 4-dimensional treatment planning for pancreatic tumors.
Results
Tumor borders moved much more than expected. To provide 99% geometric coverage, margins of 20mm inferiorly, 10mm anteriorly, 7 mm superiorly, and 4 mm posteriorly are required. Tumor position correlated poorly with diaphragm and abdominal wall position, with patient-level Pearson correlation coefficients of −0.18 to 0.43. Sensitivity and specificity of gating with these surrogates was also poor, at 53–68%, with overall error of 35–38%, suggesting that the tumor may be underdosed and normal tissues overdosed.
Conclusions
Motion of pancreatic tumor borders is highly variable between patients and larger than expected. There is substantial deformation with breathing, and tumor border position does not correlate well with abdominal wall or diaphragmatic position. Current motion management strategies may not account fully for tumor motion and should be used with caution.
Keywords: breathing, organ motion, pancreas, gating, tracking
Introduction
We are moving from 3-dimensional radiotherapy toward 4-dimensional radiotherapy. As we make this transition, we must use caution, since our understanding of tumor motion is still quite limited. As treatments become increasingly conformal, without a full understanding of tumor motion, there is a danger of underdosing targets. In this study, we aimed to characterize intra-fraction pancreatic tumor motion due to breathing and evaluate several potential motion management strategies.
Radiotherapy is an integral part of treatment for unresectable pancreatic cancer. In a phase III randomized trial run by the Gastrointestinal Tumor Study Group (GITSG), chemotherapy alone was compared to chemotherapy with concurrent radiotherapy. The addition of 54 Gy radiotherapy improved median overall survival from 7 to 10 months.1 However, local progression is common due to persistent disease. Indeed, it is unrealistic to think that such a moderate dose of 54 Gy would be adequate to eradicate an aggressive adenocarcinoma. Unfortunately, the pancreas is surrounded by sensitive organs at risk (OARs) including the duodenum, stomach, small intestine, kidneys, and spinal cord. The duodenum, which is often immediately adjacent to pancreatic tumors, is most commonly the dose-limiting organ, as gastrointestinal bleeding increases in frequency above 54 Gy. Treatment tolerability is also an issue with standard large radiotherapy fields.
With the close proximity of OARs, IMRT is being increasingly utilized in the treatment of this disease. Recently, Ben-Josef, et al reported their initial results using IMRT for dose escalation with concurrent capecitabine for pancreatic cancer.2 Two targets were defined: the gross tumor volume or tumor bed, and the draining lymph nodes. The gross tumor or resection bed received 45–55 Gy in 25 fractions, while the draining nodes received 45 Gy. Although the report included only 15 patients, the results were quite encouraging, with only 7% grade 3 gastrointestinal toxicity. The next step in the refinement of radiation therapy in this disease is to ensure the adequacy of target coverage during highly conformal radiation therapy, taking motion into consideration. One advantage of traditional large fields is that they likely continue to cover targets through respiratory and other movements. With highly conformal therapy using tight margins, unless the extent of motion is known, there is a danger of inadequately treating the extremes of the targets.
Currently, pancreatic motion is poorly understood. Two small series have been published using implanted radio-opaque fiducials and fluoroscopy. Murphy et al at Stanford reported the results of one patient who had three 2-mm gold fiducials sutured into the tumor at the time of exploratory laparotomy as part of an aborted Whipple procedure.3 The patient was imaged fluoroscopically for one minute each in the antero-posterior (AP) and lateral directions to assess tumor motion during respiration. The maximal cranial-caudal (CC) movement was found to be 6mm with breathing, and the lateral deviation 1mm with aortic pulsation. Gierga, et al at Massachusetts General Hospital reported a study of six patients who also underwent invasive marker placement and were observed fluoroscopically for 30 seconds each in the AP and lateral dimensions.4 The range of CC maximum motion was 6.5–18 mm, with an average of 4.4–12 mm. Movement in the AP dimension was much smaller, with a range of maximum values of 6.0–8.7 mm and a range of average values of 2.5–6.9 mm. While these very small series have provided important initial data regarding pancreatic movement, fluoroscopy does not allow analysis of pancreatic organ deformation and the relationship to adjacent organs.
In a more advanced study, Bussels, et al in Leuven, Belgium, reported their data using dynamic MRI to quantify pancreatic motion.5 No fiducials were placed. Instead, they acquired one image every second for one minute in the axial and coronal planes. One reader contoured the pancreatic volume on each image, and the center of gravity on each frame was calculated. The movement over time of this center of gravity was analyzed in 12 patients. They found a larger degree of movement in the CC direction than reported by both the Stanford and Mass General groups, at 24mm ±16mm. A few limitations of this study include the use of the center of gravity as a convenient, but not very clinically relevant, focus of analysis, and the lack of information regarding organ deformation. Indeed, it is the motion of tumor borders, rather than a single or a few points in space, such as fiducials or a center of mass, that are most important when designing planning target volume (PTV) margins. It is inadequacy of coverage of these borders which could potentially lead to marginal misses in the era of highly conformal radiotherapy.
In this study, we had two aims: to characterize the motion of tumor borders, and to evaluate the application of several motion management strategies including PTV expansions, respiratory gating, and 4-dimensional treatment planning with blurred dose distributions, reflecting the distribution of tumor position over time. We used cine MRI to address these issues.
Methods and Materials
Patients and scan parameters
17 patients with unresectable pancreatic cancer were enrolled in an IRB-approved, prospective imaging trial. Cine MRI was obtained during shallow free-breathing on a 3-Tesla scanner, using a 2D balanced fast field echo sequence. 3 images were obtained per second for a minute in sagittal and coronal planes through the tumor, set by a radiologist and/or radiation oncologist. Scans were obtained prior to and during chemoradiation (at 27–30Gy).
Tumor delineation and quantification of motion
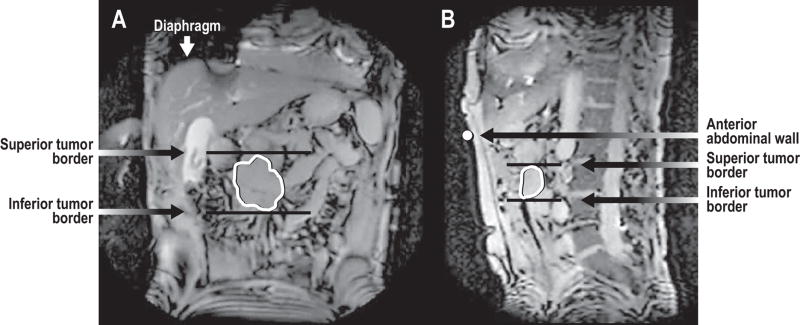
Tumor borders were agreed upon by a radiation oncologist (M.F.) and an abdominal MRI radiologist (S.A.). Motion of tumor borders was quantified using an in-house functional imaging analysis tools (FIAT) program for dynamic image analysis to measure the distance from the end-exhale position, which was the most superior and anterior. Motion of potential surrogates was also quantified. (Figure 1)
Figure 1.
In panel a, the tumor is contoured on a coronal image. The superior and inferior tumor borders are marked, as is the diaphragm. In panel b, in addition to the tumor contour and superior and inferior borders, the abdominal wall is marked.
Statistical analysis
All statistical analyses were performed in SAS v. 9.1. To determine the inhale and exhale positions of superior-inferior and anterior-posterior tumor motion of each cine MRI, nonparametric cluster analysis6 was applied to the absolute location of the feature (e.g., anterior tumor location) and cine MRI time click. This automatically identified clusters of measurements that were similar in location and ordered and contiguous in time. The mean location was calculated for each cluster, as was the duration in time. It remained to identify each cluster of observations as inhale, exhale, or intermediate: if a cluster was surrounded by two clusters both with larger mean positions, it was identified as an exhale, if it was surrounded by two clusters with smaller mean positions, it was identified as an inhale, otherwise, it was intermediate. The position of each inhale and exhale cluster was set equal to the cluster mean, and the duration of the inhale and exhale was set equal to the difference between the minimum and maximum times of the cluster.
Margins for geometric coverage of tumor motion were calculated from differences between symmetric percentiles of location along the axes of motion; for instance, the 90% margin equaled the difference of the 95th and 5th percentiles of location.
Gating was evaluated by correlation of tumor borders with a reference location on the anterior surface of the patient (abdominal wall marker, see figure 1). The gating boundary on each axis was determined by the point 10% of the distance from mean exhale to mean inhale. The sensitivity of gating for a given feature was determined by pF+A/pF, where pF+A equals the proportion of time the abdominal mark was below the abdominal gating boundary and the feature was below the feature gating boundary, and pF is the proportion of time the feature was below the feature gating boundary, irrespective of the position of the abdominal mark. Specificity is defined by comparing, in a similar fashion, the proportions of time the feature and the abdominal mark are above the gating boundary.
Results
Range of tumor border motion
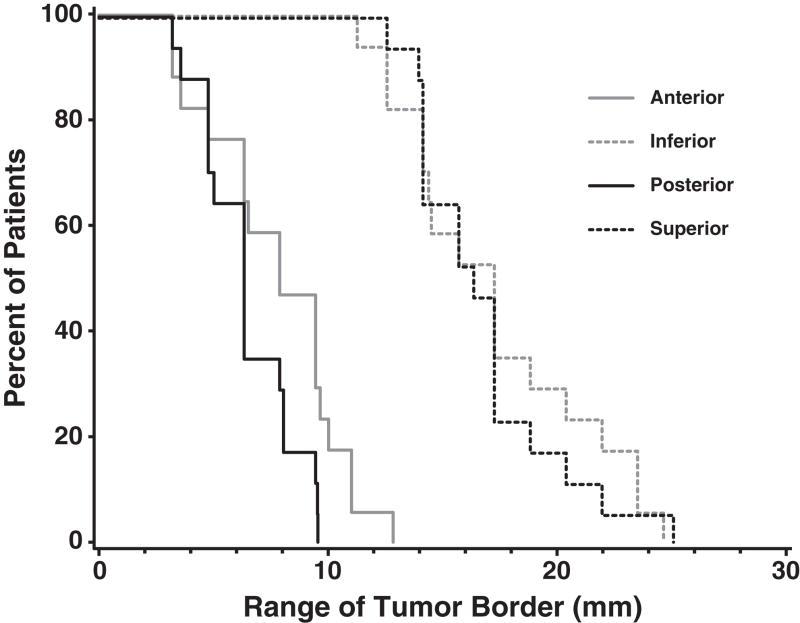
The amplitude of tumor border motion was larger than expected (Table 1). As measured from the most extreme end-exhale state, the superior and inferior borders moved an average of 20 mm, with a range of 13–42mm for the superior border and 13–38mm for the inferior border. The amplitude of motion of the anterior and posterior borders was smaller, at 8 and 6 mm, with ranges of 3–13mm and 3–9mm, respectively. Lateral border motion was negligible. Figure 2 shows that all patients had a greater than 10mm range of motion of both superior and inferior borders, with most patients exhibiting between 15 and 20 mm of motion of these borders. In 2 patients, the superior border moved more than 20 mm, and in 4 patients, the inferior border moved a similar magnitude. For the anterior and posterior borders, the minimum magnitude of motion was 2mm, with only 3 patients exhibiting more than 10mm motion of the anterior tumor border.
Table 1.
Range of motion of tumor borders and potential surrogates
| Mean ± SD in mm | Range in mm | |
|---|---|---|
| Superior Border | 20 ± 10 | 13–42 |
| Inferior Border | 20 ± 8 | 13–38 |
| Anterior Border | 8 ± 3 | 3–13 |
| Posterior Border | 6 ± 2 | 3–9 |
| Diaphragm | 16 ± 9 | 9–27 |
| Abdominal Wall | 9.6 ± 4.0 | 4.7–18.7 |
Figure 2.
Cumulative histogram of range of tumor border motion.
Tumor deformation
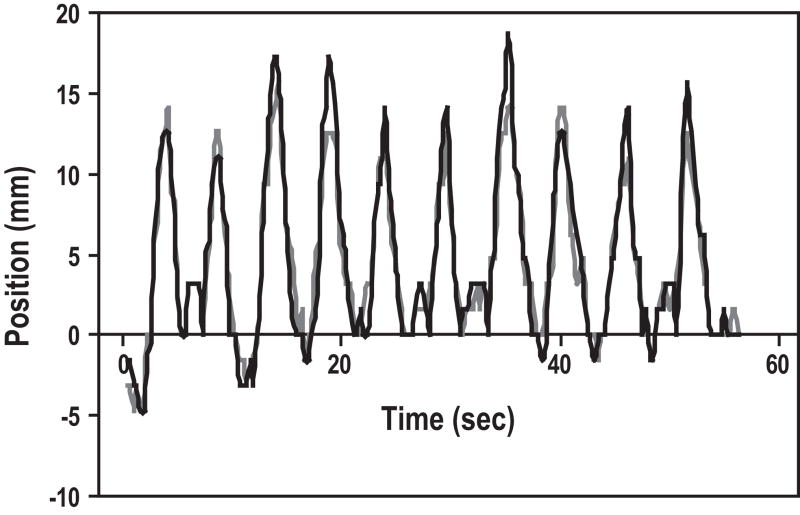
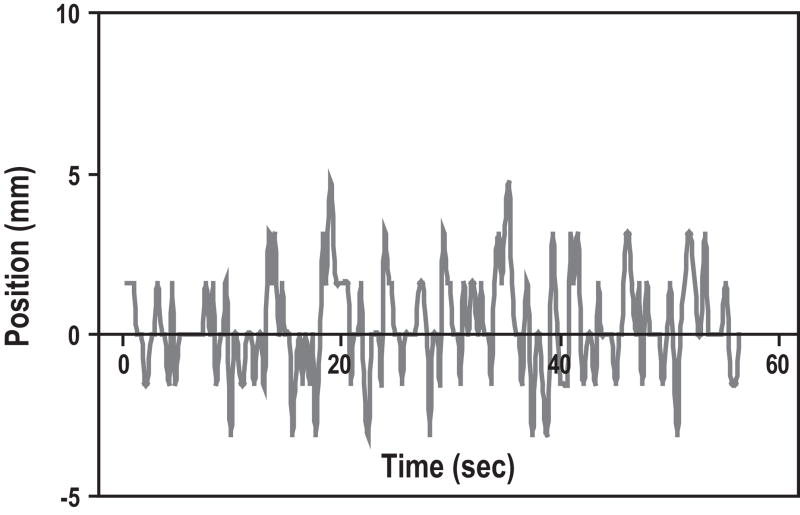
It was apparent by watching the cine MRI images that the pancreas deformed with respiration. Rather than moving as a solid block with fixed borders, there appeared to be compression and stretching of the tumors. Figure 3 is an example of a typical tracing of the superior and inferior tumor border positions over time. The general respiratory pattern is apparent, and there appears to be reasonable general correlation of tumor borders. However, the peaks of the tracings are often discordant, with the inferior border moving up to 5mm more than the superior in some breathing cycles, and the superior border moving a few mm more than the inferior border in others. Figure 4 quantitates these differences, which represent deformation of the tumor. Pearson correlation coefficients (r) were calculated for each patient between superior and inferior tumor borders, and between anterior and posterior tumor borders (Table 2). Some patients displayed poor correlation between tumor border positions; the average r for anterior versus posterior borders was 0.21, and the minimum r on that axis was negative (−0.31), suggesting poor predictability as well as tumor deformation.
Figure 3.
Tracing of superior (gray) and inferior (black) tumor border positions over time in a typical patient.
Figure 4.
Difference between superior and inferior tumor borders over time. Differences up to 5mm suggest tumor deformation.
Table 2.
Pearson correlation coefficient between different tumor features. The correlation coefficient was calculated for each of the 17 subjects; the table presents the mean, minimum and maximum of the 17 correlation coefficients for the three pairs of features.
| Correlation Coefficient by Subject | |||
|---|---|---|---|
| Feature | Mean | Minimum | Maximum |
| Superior vs Inferior Coronal | 0.69 | 0.38 | 0.95 |
| Superior vs Inferior Sagittal | 0.72 | 0.13 | 0.96 |
| Anterior vs Posterior | 0.21 | −0.31 | 0.74 |
Evaluation of diaphragm and abdominal wall as surrogates for tumor position
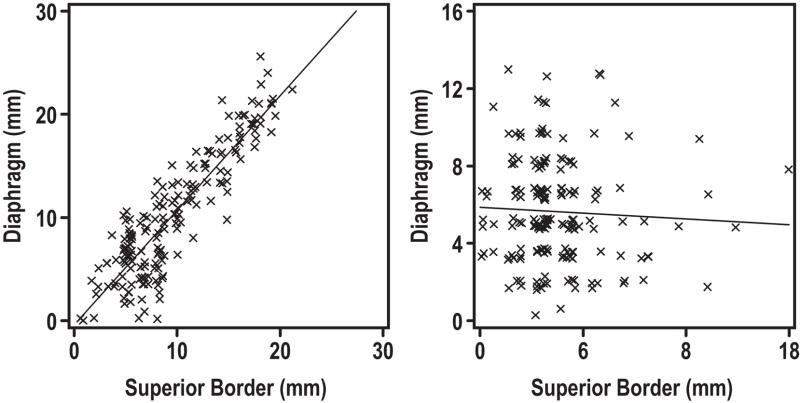
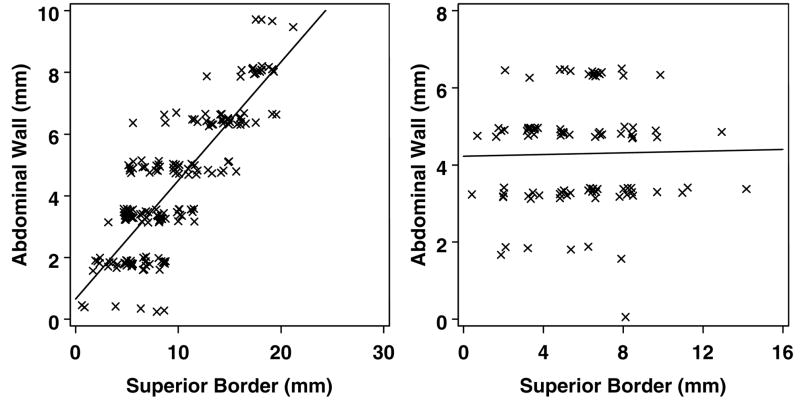
The mean amplitude of diaphragm motion in the cranial-caudal dimension was 16 mm, with a standard deviation of 9 mm, and range from 9–27 mm (Table 1). This suggests that diaphragm motion is not equal to, and may be smaller than, tumor border motion, both in mean amplitude of motion and overall range of motion. Therefore, if the diaphragm is used as a surrogate, tumor motion may be underestimated. In addition to comparing range of motion of the diaphragm with those of tumor borders, we also evaluated the correlation of tumor position to that of the diaphragm. Despite a qualitative agreement, we were unable to find a stable correlation between the diaphragm position and that of any tumor border. Figure 5a shows an example of a scan with a good correlation between the diaphragm and superior tumor border position. However, this was a rare finding, and the vast majority of scans looked more like figure 5b, so that patient-level Pearson correlation coefficients were from −0.18 to 0.37, with a mean of 0.2. Analysis of the potential correlation between the abdominal wall and superior tumor border position gave similar results, with a few scans exhibiting good correlation, but the majority showing poor correlation (figure 6). Patient-level Pearson correlation coefficients were from −0.12 to 0.43, with a mean of 0.18. All other tumor borders also demonstrated poor correlation with the abdominal wall and diaphragm.
Figure 5.
Figure 5a and b. Poor correlation between tumor borders and the diaphragm.
Figure 6.
Figure 6a and b. Poor correlation between tumor borders and the abdominal wall.
Clinical Applications: Construction of margins to account for motion
We calculated the margins required to account for motion of each tumor border, if applied to the clinical target volume at the end exhale state (Table 3). Inferior and anterior expansions were therefore determined by motion, while the superior and posterior margins were determined by the reproducibility of the end exhale state. In order to provide 99% geometric coverage, margins of 20mm inferiorly, 10mm anteriorly, 7 mm superiorly, and 4 mm posteriorly were required. If the goal is only 95% or 90% coverage, expansions are smaller, especially in the inferior direction.
Table 3.
Margins required to provide geometric coverage in the face of tumor motion.
| 99% coverage (mm) | 95% coverage (mm) | 90% coverage (mm) | |
|---|---|---|---|
| Inferior | 20 | 14 | 13 |
| Anterior | 10 | 8 | 8 |
| Superior | 7 | 5 | 4 |
| Posterior | 4 | 3 | 2 |
Clinical Applications: Tumor tracking for respiratory gating or beam tracking
As part of our evaluation of diaphragm and abdominal wall as surrogates for tumor position, we simulated respiratory gating scenarios and calculated the sensitivity and specificity of gating, as applied to each tumor border. We chose to gate to 10% of the range of motion of the surrogate, since this is a common threshold in clinical practice. Sensitivity was defined as the proportion of time the beam was on when it should have been to treat the tumor, and specificity as the proportion of time the beam was off when it should have been to spare normal tissues. Table 4 illustrates the results for the simulation as applied to the inferior tumor border. The sensitivity and specificity of using the abdominal wall as a surrogate for gating were only 57% and 63%, respectively, with an accuracy of 62%. If the diaphragm was used, sensitivity and specificity were similarly low at 53% and 68%, respectively, with an accuracy of 65%. Calculations for the superior, anterior, and posterior tumor borders also gave low sensitivity and specificity, and high error rates.
Table 4.
Sensitivity, specificity, and accuracy of gating based on 10% range of tumor motion between exhale and inhale for various potential surrogates for the position of the inferior tumor border. Accuracy is defined as the surrogate within 10% of the distance from exhale to inhale while the tumor border is also within that range.
| Feature | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| Abdominal Wall | 57 | 63 | 62 |
| Diaphragm | 53 | 68 | 65 |
| Inferior Coronal | 43.3 | 57.5 | 52.3 |
| Inferior Sagittal | 59.3 | 63.5 | 62.4 |
| Superior Coronal | 42.3 | 57.0 | 51.9 |
| Superior Sagittal | 61.5 | 65.5 | 64.3 |
| Anterior | 47.4 | 58.3 | 53.2 |
| Posterior | 50.0 | 65.4 | 57.1 |
Clinical Applications: 4-dimensional treatment planning, blurred dose distributions
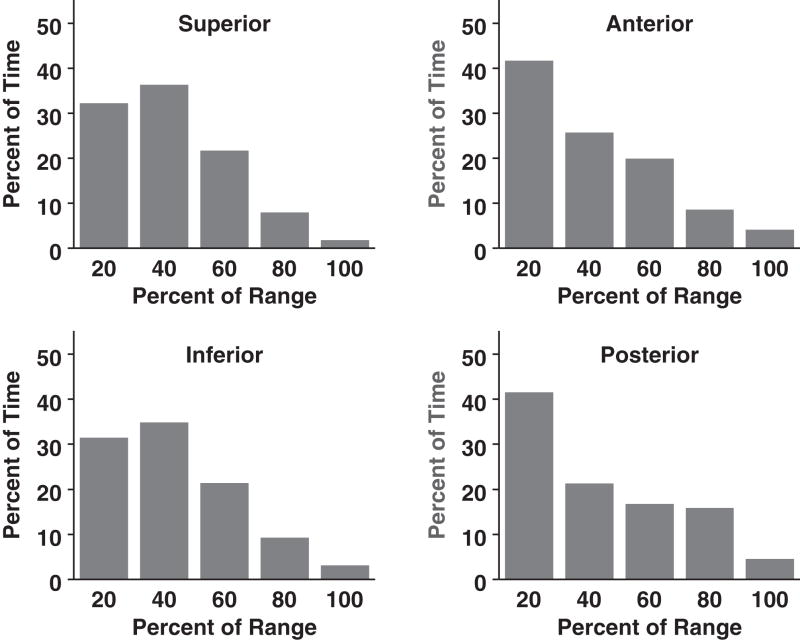
Rather than adding a margin to account for motion and treating this expanded target with full dose, it may be possible to match a dose distribution to the frequency distribution of tumor position. Therefore, if the tumor spends only a small fraction of time at the extremes of the range of motion, then those areas may not need to receive full dose.7 In order to determine how to scale dose, we need to understand the frequency distribution of tumor position and be assured of its reproducibility. Figure 7 illustrates the frequency distributions of tumor border position in our study. The x axis is the distance from the end exhale position divided into 20% range intervals. The y axis is the percent time spent in each position. As expected, tumors spent the majority of time toward the exhale portion of the breathing cycle. Although the range of motion could be 20mm or more in the superior and inferior dimensions, the fraction of time spent at the extremes was quite small, less than 5%.
Figure 7.
Temporal distribution of tumor border position.
Comparison between pre-treatment and intra-treatment scans
If a treatment plan is constructed based on a model of patient breathing, the variability of the breathing pattern must be understood. We calculated the percent time the tumors spent within specified ranges from end-exhale and compared these values between the first and second scan for each patient. There was poor correlation in each range assessed, including 95% of the range of motion (Table 5).
Table 5.
Correlation between first and second scans of proportion of time subjects spend above 20%, 40%, 60%, 80%, 90% or 95% of full inhale
| 20% | 40% | 60% | 80% | 90% | 95% | |
|---|---|---|---|---|---|---|
| Anterior Tumor Border | 0.58* | 0.32 | −0.21 | 0.01 | 0.27 | 0.27 |
| Inferior Tumor Border | −0.24 | 0.23 | −0.21 | 0.24 | −0.18 | −0.21 |
| Posterior Tumor Border | 0.40 | 0.31 | 0.12 | 0.16 | 0.06 | −0.08 |
| Superior Tumor Border | −0.05 | 0.30 | 0.50 | −0.19 | 0.24 | −0.15 |
p<0.05.
Discussion
The outcome after definitive chemoradiation for unresectable pancreatic cancer is dismal. Traditional radiation fields are large and cover much of the upper abdomen. This has resulted in poor tolerance of treatment, leading to delivery of inadequately low doses of radiation. The dose administered to the pancreatic tumors has been limited by sensitive adjacent structures in the upper abdomen including the small bowel, liver, spinal cord, and kidneys. Using forward-planned highly conformal therapy techniques or inverse-planned IMRT, the dose distribution can be tailored to exclude much of the volume of these sensitive adjacent organs.2, 8 However, this also increases the potential for geographical miss, especially at the margins of the targets. It is then of utmost importance that targets are accurately delineated and their motion understood and accounted for.
The main finding of this study is that motion of the borders of pancreatic tumors is highly variable between patients and is generally larger than previously thought and accounted for during treatments with patients freely breathing. Our results are more in line with those from Leuven,5 and less so with Stanford3 and Massachusetts General Hospital,4 showing a relatively large range of motion with shallow free breathing. Part of this difference may be due to placement of fiducials in stable vs. unstable portions of the tumor by these groups, as portions near the center of tumors or large vessel trunks such as the celiac axis may exhibit less motion than the periphery of tumors. This could also be explained by patient variability in breathing, which would underscore the importance of investigating simple and effective methods of coaching to increase reproducibility of the breathing pattern.9, 10 The periphery of the tumors is the most sensitive to marginal miss during radiotherapy, and its motion should therefore be better understood and considered. This study is the first, to our knowledge, to characterize the motion of the borders of tumors rather the position of a single point such as the centroid or a handful of implanted fiducials.
Our study also provides important data for rational expansion of the CTV for treatment planning. Margins to account for motion of pancreatic tumors vary by institution and range from 1cm to 2cm or more. This study suggests that small 1cm margins may not provide complete geometric coverage. To attain this, margins must be quite large, and likely would be poorly tolerated by patients, especially with intensified concurrent chemotherapy. Our group recently found that PTV volume was a determinant of GI toxicity, with a higher risk of GI toxicity for patients with PTV>260cc.11 The addition of a few millimeters to the expansion may increase the likelihood of grade 3 or higher toxicities. Still, it is not clear whether full geometric coverage is necessary. Several groups have studied the dosimetric effects of setup variation and target motion. Lujan, et al found that for small movements of liver tumors, even if they were frequent, there was less than a fraction of underdosage over the course of a patient’s treatment.12 Craig, et al also demonstrated only a small difference in the treatment of prostate cancer, even with hypofractionation.13 If this is the case, it may be reasonable to aim for 99%, 95%, or even 90% geometric coverage rather than 100%. Since, as we have shown, tumors typically spend only a small fraction of time in the most extremes of the range, reducing the expected coverage by only a small amount could reduce margins by several millimeters with minimal dosimetric consequences. These dosimetric studies should be conducted, especially with more modern 4-dimensional imaging techniques, as they could lead to improved normal tissue sparing and increased treatment tolerability or dose escalation.
Gating and tracking are alternative respiratory management strategies. With respiratory gating, the radiation beam is turned on when the target is believed to be in the field and off when it is believed out of the field. With tracking, the radiation field putatively moves with the target. Both have allowed for construction of smaller PTVs. Some groups are using internal fiducials to track tumors, while others are using external surrogates for tumor position. The group in Hokkaido, Japan, has the most experience for treating liver and lung cancers, with respiratory gating to internal fiducials. 14, 15 Stanford has also developed a program for stereotactic radiosurgery for pancreatic tumors, with gating based on internal markers.16
Using fiducials implanted in tumors is not commonly practiced, since this involves an invasive, potentially risky procedure. The majority of centers are using external surrogates, most commonly the abdominal wall, as a commercial system, the Real-Time Position Management (RPM) system by Varian, is available.17, 18 If a perfect surrogate existed for tumor position, then tracking this surrogate would allow for a radiation beam to be triggered on when the tumor is in the field and off when the tumor is out of the field. Theoretically, this would allow for construction of smaller margins to spare more normal tissue and would not require invasive placement of internal markers. However, our study suggests that the abdominal wall cannot accurately be used as a surrogate for tumor border position, as the sensitivity and specificity are quite poor. This is the first study to evaluate these measures as applied to surrogates for actual tumor position rather than implanted fiducials. Further studies should be conducted prior to widespread usage of gating based on the abdominal wall as a surrogate for tumor position. The poor sensitivity and specificity and high error rate we found for this suggests that it can lead to both underdosage of tumor and overdosage of normal tissues.
In addition to poor correlation with the abdominal wall and diaphragm, tumor borders also exhibit poor correlation with each other. Instead of moving as a rigid body, pancreatic tumors exhibit deformation with breathing. Therefore, tracking 1 or 2 markers may not give an accurate indication of the position of tumor borders. Placement of multiple markers in the periphery of the tumor may help minimize the tracking problems caused by tumor deformation. Still, the duty cycle, or efficiency of the treatment can lead to problems with throughput in the clinic and patient discomfort, as it can significantly increase treatment times. Although a smaller gating window leads to a smaller treatment target when motion is considered, this must be balanced by practical considerations. We chose 10% of the range of motion (~2mm) for our gating simulation based on the Japanese experience in Hokkaido, who uses a 2mm gating window. This corresponds to a 20% duty cycle, which is also in line with recommendations by other groups.18–20 This study is the first to describe in detail the relationship between external markers and the borders of internal tumors. As the conformality of radiotherapy increases, so does the risk of marginal miss, especially if the borders of a moving target are not properly accounted for. Prior to mainstream utilization of gating and tracking for pancreatic and other cancers based on external surrogates, more work must be done to quantify the margin of error in the relationship of these surrogates to actual tumor border positions and account for this error in treatment planning and delivery.
While this study raises several important issues, it is limited by the nature of the imaging technique used. Our cine MRI technique acquired images in a single plane at a time. Although this allowed for rapid acquisition (3 images per second) and a good representation of motion in that plane, it potentially could have missed in- and out- of plane motion, which may have enhanced our perception of tumor deformation. To address this, we imaged 3 planes in both the sagittal and coronal orientations, but this information still did not allow us to completely rule out potential out of plane motion. Peristalsis could also have enhanced the perception of tumor deformation. We did not coach our patients prior to imaging, except that we suggested they breathe comfortably and quietly. Perhaps with coaching, variability between sessions would decrease.
An alternative to using respiratory gating to decrease treatment margins is to suspend breathing or use blurred dose distributions to match the frequency distributions of tumor positions during free breathing. In our institution, we suspend breathing during simulation and treatment using the active breathing control system (ABC), essentially eliminating respiratory motion. Margins added to the clinical target volume are only for setup variation and reproducibility of the breath-held state, and are minimal in comparison.21, 22 While this allows for construction of smaller PTVs and therefore can improve the therapeutic index, the system is complicated, and some patients cannot tolerate the breath holds. This has led us, and others, to investigate treatment plans which can be delivered during free breathing but still may lead to improved normal tissue sparing compared to a standard PTV plan. As our data indicate, tumors spend only a small fraction of time at the extremes of their ranges of motion. Therefore, full dose may not need to be delivered to those extremes. Rather, dose may be scaled in proportion to the time the tumor spends in each position. McShan, et al have described a new treatment planning method, multiple instance geometry approximation (MIGA), which allow for construction of a time-weighted model of tumor and normal tissue position, which can be used for IMRT optimization.23 A single plan, robust to target motion, delivered while a patient is free breathing, could maintain target coverage while improving normal tissue sparing.24, 25 This would accomplish the goal of improving the therapeutic ratio while minimizing treatment time for patient comfort and clinic throughput. Preliminary studies suggest that MIGA may be equivalent to ABC in allowing for dose escalation and normal tissue sparing in pancreatic cancer.26 However, prior to use in the clinic, methods to decrease the variability in breathing patterns must be refined to ensure adequate tumor coverage during fractionated radiotherapy.
In summary, we have demonstrated that motion of pancreatic tumors is quite complex, with deformation and high variability between patients. There is poor correlation between the tumor position and that of the abdominal wall and diaphragm, and poor reproducibility in breathing patterns. These findings should be considered when using small PTV margins or tracking or gating strategies.
Acknowledgments
Supported by NIH P01-59827 and a University of Michigan Cancer Center Grant.
Footnotes
Presented in part at the 49th Annual Meeting of the American Society of Therapeutic Radiology and Oncology, Los Angeles, CA, Oct. 28-Nov. 1, 2007.
Conflicts of Interest Notification. Actual or potential conflicts of interest do not exist.
References
- 1.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 2.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Murphy MJ, Martin D, Whyte R, et al. The effectiveness of breath-holding to stabilize lung and pancreas tumors during radiosurgery. Int J Radiat Oncol Biol Phys. 2002;53:475–482. doi: 10.1016/s0360-3016(01)02822-x. [DOI] [PubMed] [Google Scholar]
- 4.Gierga DP, Chen GT, Kung JH, et al. Quantification of respiration-induced abdominal tumor motion and its impact on IMRT dose distributions. Int J Radiat Oncol Biol Phys. 2004;58:1584–1595. doi: 10.1016/j.ijrobp.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 5.Bussels B, Goethals L, Feron M, et al. Respiration-induced movement of the upper abdominal organs: a pitfall for the three-dimensional conformal radiation treatment of pancreatic cancer. Radiother Oncol. 2003;68:69–74. doi: 10.1016/s0167-8140(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 6.Gitman I. An Algorithm for Nonsupervised Pattern Classification. IEEE Transactions on Systems, Man, and Cybernetics, SMC. 1973:66–74. [Google Scholar]
- 7.Chan TC, Bortfeld T, Tsitsiklis JN. A robust approach to IMRT optimization. Phys Med Biol. 2006;51:2567–2583. doi: 10.1088/0031-9155/51/10/014. [DOI] [PubMed] [Google Scholar]
- 8.Spalding AC, Jee KW, Vineberg K, et al. Potential for dose-escalation and reduction of risk in pancreatic cancer using IMRT optimization with lexicographic ordering and gEUD-based cost functions. Med Phys. 2007;34:521–529. doi: 10.1118/1.2426403. [DOI] [PubMed] [Google Scholar]
- 9.George R, Chung TD, Vedam SS, et al. Audio-visual biofeedback for respiratory-gated radiotherapy: impact of audio instruction and audio-visual biofeedback on respiratory-gated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:924–933. doi: 10.1016/j.ijrobp.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Kini VR, Vedam SS, Keall PJ, et al. Patient training in respiratory-gated radiotherapy. Med Dosim. 2003;28:7–11. doi: 10.1016/S0958-3947(02)00136-X. [DOI] [PubMed] [Google Scholar]
- 11.Murphy JD, Adusumilli S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 12.Lujan AE, Larsen EW, Balter JM, et al. A method for incorporating organ motion due to breathing into 3D dose calculations. Med Phys. 1999;26:715–720. doi: 10.1118/1.598577. [DOI] [PubMed] [Google Scholar]
- 13.Craig T, Moiseenko V, Battista J, et al. The impact of geometric uncertainty on hypofractionated external beam radiation therapy of prostate cancer. International Journal of Radiation Oncology*Biology*Physics. 2003;57:833–842. doi: 10.1016/s0360-3016(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 14.Shirato H, Shimizu S, Kitamura K, et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int J Radiat Oncol Biol Phys. 2000;48:435–442. doi: 10.1016/s0360-3016(00)00625-8. [DOI] [PubMed] [Google Scholar]
- 15.Shirato H, Shimizu S, Kunieda T, et al. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1187–1195. doi: 10.1016/s0360-3016(00)00748-3. [DOI] [PubMed] [Google Scholar]
- 16.Schellenberg D, Columbo LA, Lee FL, et al. Phase II Trial of Gemcitabine and Single Fraction Stereotactic Body Radiotherapy (Trilogy) for the Treatment of Locally Advanced, Unresectable Adenocarcinoma of the Pancreas. International Journal of Radiation Oncology*Biology*Physics. 2007;69:S282–S283. [Google Scholar]
- 17.Keall P, Vedam S, George R, et al. The clinical implementation of respiratory-gated intensity-modulated radiotherapy. Med Dosim. 2006;31:152–162. doi: 10.1016/j.meddos.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Jiang SB. Technical aspects of image-guided respiration-gated radiation therapy. Med Dosim. 2006;31:141–151. doi: 10.1016/j.meddos.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi H, Sakuhara Y, Hige S, et al. Intercepting radiotherapy using a real-time tumor-tracking radiotherapy system for highly selected patients with hepatocellular carcinoma unresectable with other modalities. Int J Radiat Oncol Biol Phys. 2007;69:376–380. doi: 10.1016/j.ijrobp.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Berbeco RI, Nishioka S, Shirato H, et al. Residual motion of lung tumours in gated radiotherapy with external respiratory surrogates. Phys Med Biol. 2005;50:3655–3667. doi: 10.1088/0031-9155/50/16/001. [DOI] [PubMed] [Google Scholar]
- 21.Dawson LA, Brock KK, Kazanjian S, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 22.Koshani R, Balter JM, Hayman JA, et al. Short-term and long-term reproducibility of lung tumor position using active breathing control (ABC) International Journal of Radiation Oncology*Biology*Physics. 2006;65:1553–1559. doi: 10.1016/j.ijrobp.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 23.McShan DL, Kessler ML, Vineberg K, et al. Inverse plan optimization accounting for random geometric uncertainties with a multiple instance geometry approximation (MIGA) Med Phys. 2006;33:1510–1521. doi: 10.1118/1.2191016. [DOI] [PubMed] [Google Scholar]
- 24.Feng M, Vineberg KA, Lam KL, et al. Can We Replace PTV Expansions With a Model of Set-up Uncertainty in IMRT for Head and Neck Cancer? International Journal of Radiation Oncology*Biology*Physics. 2006;66:S102–S102. [Google Scholar]
- 25.Lin A, Moran JM, Marsh RB, et al. Evaluation of Multiple Instance Geometry Approximation (MIGA) in Inverse-Planned Optimization for Loco-Regional Breast Treatment. International Journal of Radiation Oncology*Biology*Physics. 2006;66:S102–S103. doi: 10.1016/j.ijrobp.2008.06.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng M, Oh KS, Vineberg KA, et al. Multiple Instance Geometry Approximation (MIGA) using Individualized Tumor Motion Data is Equivalent to Best Breath Hold Method in IMRT for Unresectable Pancreatic Cancer. International Journal of Radiation Oncology*Biology*Physics. 2008;72:S255–S255. [Google Scholar]