PREAMBLE
In 2004, the American College of Cardiology (ACC) and American Heart Association (AHA) task force on practice guidelines undertook a comprehensive rewriting of the ST elevation myocardial infarction (STEMI) guidelines (1). The Canadian Cardiovascular Society (CCS) collaborated on these guidelines and a working group from the CCS provided a Canadian perspective and adaptation (2).
In late 2007, a focused update of the ACC/AHA 2004 STEMI guidelines was published to address new evidence from recent publications and presentations at major scientific meetings, consistent with the ACC/AHA practice guidelines committee’s desire to continue maintaining the existing guidelines at the highest scientific level (3). This process was also completed in collaboration with the CCS, with involvement from Dr Paul Armstrong on the writing group committee as well as through independent review (RC Welsh).
A Canadian working group (CWG) was formed under the auspice of the CCS to provide interpretation and, where appropriate, adaptation of the focused guidelines update to fit the specific geographical and health economic issues in the Canadian health care system. The CWG was selected to provide adequate representation from major geographical regions within the nation, both community and academic thought leadership, representation from interventional and noninterventional cardiologists, as well as the addition of an emergency medicine physician with emergency medical services (EMS) leadership in prehospital care.
INTRODUCTION
Due to the arduous task of reviewing all available information regarding the management of STEMI patients and obtaining consensus from a group of leading experts, guidelines are frequently outdated soon after their formal publication. In an attempt to manage these issues, the ACC/AHA task force on practice guidelines has created a focused update process to revise existing guidelines. The approach to this task is well described elsewhere but, in brief, involves a review of key peer-reviewed publications not included in the full guidelines and a review of late-breaking clinical trial presentations at major academic cardiology meetings, including the congresses of the ACC, AHA and European Society of Cardiology (3). The focused update and full guidelines are intended to assist health care providers and clinical decision makers by describing a range of generally acceptable approaches for the diagnosis, management and prevention of specific diseases or conditions. They attempt to define practices that meet the needs of most patients and circumstances. The ultimate judgment regarding care of a particular patient must be made by the health care provider and patient in light of all the circumstances presented by both that patient and the existing health care system.
Consistent with the available evidence, the majority of the 2007 focused update content is related to new information about analgesics, utilization of beta-blockers, and fibrinolysis-conjunctive antithrombotic and antiplatelet agents. Further evidence regarding the approach to mechanical cointervention following reperfusion with fibrinolysis were also incorporated, addressing facilitated percutaneous coronary intervention (PCI), rescue PCI and the use of coronary angiography in stable patients following fibrinolysis. Despite a great attempt to stay up to date on current evidence, several key trials in STEMI patients have been published or presented since the 2007 focused update was completed.
It is recognized that the implementation of guidelines must consider the quality and availability of expertise in the area in which care is being provided, and must be specific to the Canadian health care system. The present CWG document will focus on a practical summary of the 2007 focused update of the ACC/AHA STEMI guidelines, with directed comments when appropriate and further discussion regarding the notion of first medical contact, systems approaches to STEMI care (a topic in which Canada has provided leadership), appropriate transfer of remote patients to tertiary care PCI centres for primary, rescue and elective PCI, and the importance of continuous quality improvement programs within the Canadian health care environment. The CWG does not intend for the present document to cover the spectrum of issues related to STEMI. For that, we refer our colleagues to the complete 2004 ACC/AHA STEMI guidelines and the 2007 focused update to which we are providing the present perspective (1,3).
KEY HIGHLIGHTS FROM THE 2007 ACC/AHA FOCUSED UPDATE OF STEMI GUIDELINES
The following seven points summarize the key highlights that were presented in the 2007 ACC/AHA focused update of STEMI guidelines. When appropriate, the CWG has provided a perspective or adaptation (identified in italics).
With the exception of acetylsalicylic acid (ASA), both nonselective and cyclooxygenase-2-selective nonsteroidal anti-inflammatory drugs have been associated with increased risk of mortality, reinfarction, hypertension, heart failure and myocardial rupture (3). Therefore, patients presenting with STEMI who are routinely taking nonsteroidal anti-inflammatory agents should immediately discontinue taking these drugs. For those requiring ongoing therapy for pain, ASA and/or morphine sulphate are appropriate alternatives.
Early aggressive beta-blocker therapy (intravenous and oral) was not associated with clinical benefit but was actually associated with increased risk when delivered to a broad spectrum of STEMI patients within the ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) II (4). Evidence still supports the initiation of oral beta-blocker therapies within the first 24 h from diagnosis in patients who do not have signs of heart failure, low output states, increased risk for cardiogenic shock or other relative contraindications for beta-blockers (first-, second- or third-degree heart block, active asthma or reactive airway disease). Beta-blockers should be initiated at low to moderate doses and titrated consistent with patient stability, heart rate and blood pressure response. Intravenous beta-blockers maintain clinical utility in selected patient populations, especially those with ongoing myocardial ischemia associated with significant hypertension and in the absence of high-risk features for congestive heart failure or cardiogenic shock.
Systems goals for the treatment of STEMI include achieving a time to reperfusion (measured from first medical contact) of 90 min for primary PCI and 30 min for fibrinolysis, both of which represent the longest time that should be considered, rather than the ideal time (3,5). STEMI patients presenting to a hospital that is incapable of achieving primary PCI within 90 min of first medical contact should administer fibrinolysis within 30 min with a proviso that those with absolute or relative contraindications to fibrinolytic therapy may appropriately be referred for primary PCI, accepting longer delays (although intervention should occur as rapidly as possible). The update discouraged a strategy of routine immediate cardiac catheterization following administration of fibrinolysis except for patients with cardiogenic shock, severe congestive heart failure and/or pulmonary edema, or hemodynamic compromising ventricular arrhythmias refractory to medical therapy. However, this point will be further discussed in the context of recent scientific presentations.
Patients undergoing reperfusion with fibrinolysis should receive anticoagulation therapy for a minimum of 48 h and, preferably, for the duration of the index hospitalization, with evidence supporting the use of enoxaparin administered according to the EnoXaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment (EXTRACT) – Thrombolysis in Myocardial Infarction 25 (EXTRACT-TIMI 25) study protocol (6). Although based on a modest sample, anti-Xa (pharmacokinetic) data support the requirement to administer supplemental intravenous enoxaparin (0.3 mg/kg to 0.5 mg/kg) in conjunction with rescue or urgent PCI in patients who receive fibrinolysis with subcutaneous enoxaparin only (7); an issue that is especially relevant to those older than 75 years of age in whom the intravenous enoxaparin bolus is excluded, consistent with the EXTRACT protocol and guideline recommendations (3,6). Fondaparinux is an alternative to unfractionated heparin in STEMI patients but its use requires caution in those undergoing PCI and should not be used for patients in whom primary PCI is planned (3).
In STEMI patients undergoing reperfusion with fibrinolysis, evidence supports the acute administration of ASA as well as clopidogrel delivered as a 300 mg loading dose in patients younger than 75 years of age and only 75 mg in those 75 years of age and older (8,9). Although information regarding the optimal duration of therapy in the STEMI population is absent, based on extrapolation of data from non-ST elevation acute coronary syndromes and PCI patients, it is expected that further benefit would be obtained through administration of dual antiplatelet therapy over the long term, probably for a duration of one year (3).
For all post-PCI STEMI patients who underwent intracoronary stenting, ASA 162 mg/day to 325 mg/day should be given for at least one month with bare metal stents, three months with sirolimus-eluting stents and six months with paclitaxel-eluting stents. After this, ASA should be continued at a dose of 75 mg/day to 162 mg/day over the long term (3). Recognizing current Canadian practise and the lack of data assessing the safety and efficacy of low-dose ASA (ie, 81 mg/day), the Canadian Association of Interventional Cardiology and CCS joint statement on drug-eluting stents recommends ASA 81 mg/day to 325 mg/day indefinitely (10). In all patients who receive a drug-eluting stent, clopidogrel 75 mg should be administered once per day for 12 months if patients are not at high risk for bleeding. Patients receiving a bare metal stent should be given clopidogrel for a minimum of one month and, ideally, up to 12 months unless the patient is at increased risk for bleeding, at which point, two weeks would be acceptable.
Aggressive lifestyle modification, risk factor management and cardiac rehabilitation should be promoted in all patients following STEMI. Formal smoking cessation programs should be encouraged in the hospital, and every tobacco user and family member should be advised to quit during every visit to a health care provider.
TIME TO TREATMENT IN STEMI: WHEN DOES THE ‘STOP WATCH’ START?
Pathophysiological animal models and a wealth of clinical data demonstrate definitively that time to effective reperfusion is a key modulator of outcomes in STEMI (11). Recognizing the unfortunate reality that the majority of lost opportunities occur due to patient delay in seeking medical assistance, optimal treatment is achieved if sustained reperfusion occurs within 1 h of coronary occlusion, with decaying benefit as the time delay progresses. In an attempt to maximize care, systems approaches to reduce treatment delay have become a major focus in Canadian health care regions.
To improve systems of care and minimize treatment delays, knowledge of local realities regarding components of delay and actions to minimize any excess delays is required. Because guidelines and available evidence suggest and support using time-to-treatment information to guide the choice of reperfusion therapy, it is essential to establish common definitions of time points.
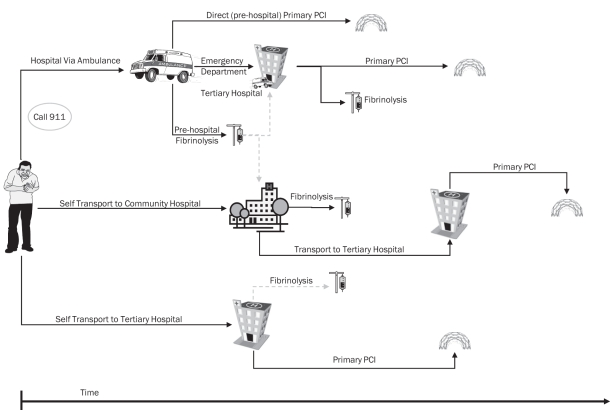
The point of first medical contact provides the first opportunity for health care professionals and the health care system to intervene, and is the most logical and consistent point to start tracking time to treatment. To fully optimize care, all segments of time must be tracked and reviewed with the expectation to further enhance care. For patients who self-present to the hospital, the starting point for time to treatment is first contact with a person from the health care team (nurse or physician, triage or registration clerk). For patients who activate the prehospital emergency medical system, the starting point for time to treatment is arrival of the prehospital care provider (emergency medical technician). Although there has been substantial progress that is expected to continue in the years ahead, it is recognized that not all jurisdictions in Canada currently have the capacity to diagnose, treat and/or triage STEMI patients in the prehospital environment. Tracking inclusive time to treatment, including the prehospital environment, across the nation will provide a strong impetus for funding agencies to provide appropriate prehospital resources, thereby improving care to STEMI patients within our country. Therefore, the CWG recommends that the first physical contact of the patient with medical personnel, including prehospital care providers, is the point when the system indicator of time to treatment starts (Figure 1).
Figure 1).
Assessing time to treatment from point of first medical contact. This figure schematically presents reperfusion options and their associated time to treatment measured from first medical contact. In patients who activate the prehospital emergency medical system, treatment delays can be reduced through the use of prehospital fibrinolysis or direct triage to a cardiac catheterization tertiary care centre that is capable of delivering timely primary percutaneous coronary intervention (PCI). In patients who self-transport to a tertiary care hospital emergency department, rapid delivery of primary PCI is the treatment of choice, with fibrinolysis maintained as an alternative. In patients who self-transport to a community hospital, transfer for primary PCI has been employed, although fibrinolysis may be considered reasonable because challenges remain in achieving timely primary PCI. In all situations, first medical contact (either arrival of the ambulance at the scene or patient arrival at the hospital) is the point from which the treatment is tracked
THE CANADIAN PERSPECTIVE ON THE SYSTEMS APPROACH TO STEMI MANAGEMENT
During the past 10 years, especially in the past five years, many regions in North America have invested significant financial and intellectual energy in developing systems and protocolized approaches to the treatment of STEMI. Canada has been at the forefront of this movement with established regional approaches in major urban centres, albeit based on various reperfusion strategies (12–14). Recognizing that a substantial amount of debate continues, the current ACC/AHA STEMI guidelines define 60 min as the acceptable PCI-related delay (determined by estimating the time from first medical contact to first balloon inflation [primary PCI] minus the time from first medical contact to initiation of fibrinolysis). Receiving primary PCI within this time frame remains difficult or impossible for many patients within Canada. This reality confirms the need to continue to develop mechanisms to expedite reperfusion and to consider integrating both pharmacological and mechanical forms to optimize individual patient care.
Patients presenting directly to Canadian PCI centres should undergo primary PCI with a first medical contact to balloon time of 90 min, regardless of the time of day or day of the week. The creation of regional cardiac destination hospitals, facilities capable of rapid cardiac catheterization and primary PCI delivered by experienced interventional cardiologists, has been proposed as a way to improve outcomes in STEMI patients (15,16). The use of prehospital EMS has been associated with expedited evaluation in the emergency department (ED), wider use of acute reperfusion therapy, and earlier pharmacological or mechanical reperfusion (17–22). A prehospital paramedic-based diagnosis with the triage of patients directly to a centre capable of expedited primary PCI has been successfully employed within the Canadian health care environment. Evidence supports bypassing the ED by direct transfer of patients diagnosed before hospitalization to the catheterization laboratory, which has been demonstrated to reduce time to treatment by 30 min to 50 min (12,13,23–26). Assessment and stabilization in the ED or coronary care unit may be appropriate in specific situations including when the prehospital diagnosis is in doubt, the catheterization laboratory staff is not readily available (off-hours or committed to another procedure) and when a patient requires urgent resuscitation due to electrical or hemodynamic instability.
Prehospital fibrinolysis is a growing and feasible option nationally, particularly in centres without timely access to cardiac catheterization facilities (14,27). Canadian and international experiences have demonstrated the capability of nonphysician paramedic staff to deliver prehospital care for STEMI patients (24). In England and Wales, where nonphysician paramedic staff deliver prehospital care (similar to Canada), 28 of the 31 ambulance services now give fibrinolytic treatment to patients before they reach the hospital (28). Fully integrated, advanced prehospital management of STEMI has not achieved widespread implementation in Canada (29,30). Such programs, which are based on individualized patient triage using available evidence outlined by current guidelines, allow the administration of pharmacological reperfusion in situations in which mechanical reperfusion is not feasible within the appropriate time frame of 60 min. This opportunity is especially relevant when the catheterization laboratory is occupied, in periods of high traffic in urban regions or during adverse weather conditions (which are common throughout many populous regions), and when hospital overcrowding has led to diversions of emergency room patients. In patients with contraindications to fibrinolysis or those with high-risk clinical characteristics such as hypotension, cardiogenic shock or pulmonary edema, direct triage to a prehospital-activated cardiac catheterization laboratory expedites mechanical reperfusion. Additionally, a fully integrated system may minimize the need for ‘lights and sirens’ transportation, which has been linked to vehicular accidents and excess risk to patients, ambulance personnel and innocent bystanders (31,32).
Risk stratification for selecting mode of reperfusion: primary PCI or fibrinolysis
According to the ACC/AHA guidelines, the optimal reperfusion therapy is best determined by assessing the time from symptom onset to first medical contact, the estimated baseline patient risk, location and extent of myocardial ischemia and the predicted PCI-related delay measured as the interval between initiation of fibrinolysis and the first balloon inflation (1). Within a specific health care system, the decision is further influenced by regional staff, resources and hospital protocols, as well as physician and patient bias. It is possible to develop reperfusion algorithms for STEMI patients diagnosed at non-PCI centres to transfer high-risk patients for primary PCI and administer fibrinolysis to low-risk patients; a strategy that is supported by evidence (33). The advantage of using a risk-stratified reperfusion algorithm is that it allocates limited cardiac catheterization laboratory and EMS resources to those patients most likely to derive benefit from primary PCI. The potential disadvantage of this approach is that it adds to the complexity of decision-making in the ED, which may delay treatment (34).
The risk of death and other adverse cardiac events for patients with STEMI can be estimated based on a number of clinical characteristics assessed at the time of presentation, including age, comorbid conditions, hemodynamic status, body weight, electrocardiographic findings and symptom duration (35–37). Several studies have shown that the benefit of primary PCI over fibrinolysis is greatest in high-risk patients, whereas low-risk patients have similar outcomes with fibrinolysis and primary PCI. In the 2004 ACC/AHA STEMI practice guidelines, an invasive strategy was recommended for patients with specific high-risk characteristics (cardiogenic shock, Killip class of 3 or higher, symptom duration of more than 3 h) (38). Recognizing the impact of treatment delays on patients sent for primary PCI is greatest for high-risk patients (39); patients with cardiogenic shock or contraindications to fibrinolysis clearly benefit from rapid transfers to PCI centres (40). Analyses from the National Registry of Myocardial Infarction suggest that specific patient subgroups (ie, symptoms for longer than 2 h, 65 years of age and older) may benefit from primary PCI, even when the procedure cannot be performed within a PCI-related delay of 60 min (41).
Role of cardiac catheterization after fibrinolysis
The 2007 focused update of the ACC/AHA STEMI guidelines recommended rescue PCI for patients with persistent ischemic symptoms and/or persistent ST segment elevation, determined as a less than 50% reduction in ST elevation on a 90 min electrocardiogram following fibrinolysis (with a moderate to large area of myocardium at risk). Immediate angiography for patients with cardiogenic shock, severe congestive heart failure or hemodynamically compromising ventricular arrhythmia is a class I recommendation. However, the role and optimal timing of coronary angiography with the intent to perform PCI (or emergency coronary artery bypass graft surgery) following successful fibrinolysis in hemodynamically stable patients remains unclear, and was given a class IIb recommendation in the guidelines update. Studies performed before the era of coronary stenting, thienopyridines and glycoprotein IIb/IIIa antagonists failed to show benefit from routine early PCI after successful fibrinolysis, and showed higher rates of bleeding and emergency bypass surgery (42,43). More recent studies have suggested that routine early PCI using coronary stents and contemporary pharmacotherapy may be safe and beneficial after fibrinolysis (42,44).
The Combined Angioplasty and Pharmacological Intervention versus Thrombolysis ALone in Acute Myocardial Infarction (CAPITAL-AMI) study, performed in Ottawa (Ontario), randomly assigned 170 STEMI patients to either fibrinolysis alone or ‘tenecteplase-facilitated’ PCI within a median of 104 min after random assignment and approximately 99 min after fibrinolysis (45). The group undergoing routine early PCI had significantly lower rates of recurrent ischemia, a trend toward less reinfarction and no excessive bleeding.
The Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) study further investigated the strategy of facilitated PCI (46). This trial had three treatment arms, including half-dose reteplase and abciximab, early treatment with abciximab before PCI and in-catheterization laboratory administration of abciximab in conjunction with primary PCI. Consistent with the Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) trial (47), which tested the concept of full-dose fibrinolysis-facilitated PCI, FINESSE failed to demonstrate an advantage of half-dose fibrinolysis combined with abciximab in conjunction with urgent PCI. Furthermore, despite previous small investigations suggesting a possible benefit of early abciximab administration before PCI, there was no clear benefit for those patients who received early administration of abciximab versus those receiving this agent in the catheterization laboratory at the time of primary PCI (46,48–50).
The Which Early ST elevation myocardial infarction therapy (WEST) study was performed in four Canadian cities, and randomly assigned 304 STEMI patients to fibrinolysis alone, fibrinolysis with a pharmacoinvasive strategy including predefined criteria for rescue PCI and/or routine PCI within 24 h, or primary PCI (27). The primary efficacy end point was similar among the three groups. Secondary analysis demonstrated decreased risk of death or reinfarction for primary PCI compared with standard care (fibrinolysis). The pharmacoinvasive approach was similar to primary PCI, with no difference in death or reinfarction, suggesting possible benefit of a dedicated rescue strategy and/or early cardiac catheterization following fibrinolysis.
Preliminary results for the Trial of Routine ANgioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI) study were recently presented and the study design was previously reported (51). Briefly, approximately 1000 STEMI patients with high-risk characteristics (either anterior ST segment elevation or inferior ST segment elevation with tachycardia, hypotension, heart failure, right ventricular involvement or anterior ST depression) were treated with tenecteplase at non-PCI centres and randomly assigned to a pharmacoinvasive strategy (PCI within 6 h of fibrinolysis, regardless of reperfusion status) or ‘standard treatment’ (early transfer only for failed fibrinolysis or hemodynamic instability). Cardiac catheterization beyond 24 h and within two weeks was recommended for patients in the ‘standard treatment’ who did not require early transfer. At 30 days, the pharmacoinvasive strategy was associated with a significant reduction in the primary end point (10.6% versus 16.6% [preliminary results]; death, reinfarction, recurrent ischemia, congestive heart failure, shock) and no difference in major bleeding complications.
Canadian geography and health care resources: Their impact on STEMI patient management
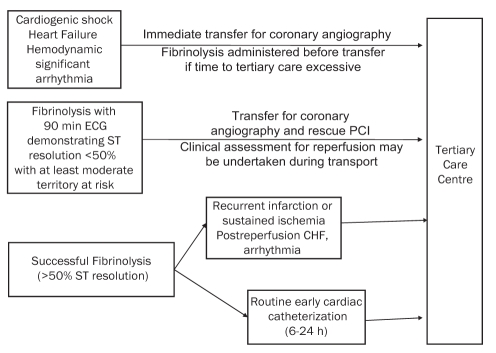
Although the majority of Canadians live in urban or semi-urban regions, the realities of geography within Canada, as well as many other nations, necessitate reperfusion strategies whereby patients who are incapable of receiving timely mechanical reperfusion receive expedited fibrinolysis followed by risk stratification to determine the urgency of transport to a tertiary care centre for mechanical cointervention. This pharmacoinvasive strategy is recommended with the transfer of STEMI patients for rescue PCI, or in high-risk individuals, even if pharmacological reperfusion is successful. Figure 2 outlines suggestions regarding patient selection and timing to identify patients who should be considered for transfer from rural areas to an urban centre (ie, close proximity to a tertiary care PCI centre). Clearly, these are recommendations, and individual patient assessments given by onsite physicians, with appropriate communication and discussion with potential receiving cardiologists in a tertiary care centre, are warranted.
Figure 2).
Community ST elevation myocardial infarction patients requiring transfer to a tertiary care centre. Suggestions regarding appropriate transfer of patients to a tertiary care centre (region) following presentation to a community hospital (where primary percutaneous coronary intervention [PCI] cannot be achieved) are presented. In patients who present with (or develop) cardiogenic shock, heart failure or hemodynamically significant arrhythmia, immediate transfer to a centre capable of acute revascularization should occur. Patients should be transferred to a tertiary care centre after receiving fibrinolysis if they fail to achieve adequate reperfusion, as defined by a less than 50% ST segment resolution on a 90 min electrocardiogram (ECG) in association with at least a moderate territory of myocardium at risk. In patients who achieve successful reperfusion following fibrinolysis, preliminary evidence suggests there is benefit from routine cardiac catheterization and revascularization completed within 6 h to 24 h. In patients who develop recurrent infarction, sustained ischemia or high-risk features during convalescence, transfer to a tertiary care centre for cardiac catheterization and revascularization is appropriate. CHF Congestive heart failure
CHALLENGES FOR STEMI EMS, ED AND CARDIOLOGY SYSTEMS
Recent evidence from a large survey of 365 American hospitals demonstrated that the following six strategies were significantly associated with shorter door-to-balloon times: prehospital PCI activation (15.4 min time savings); emergency physicians activating the PCI laboratory (8.2 min savings); a single call to the central page operator to activate the laboratory (13.8 min savings); PCI staff arrival to the laboratory within 20 min (19 min savings); on-site cardiologist (14.6 min savings); and real time feedback between the ED and the cardiac catheterization laboratory (8.6 min time savings) (52–54). This led to the development of a major AHA initiative, which included structured core strategies to reduce the time to balloon inflation to less than 90 min in 75% of cases (55). The 2007 ACC/AHA STEMI-focused update suggested that national policies should be created for the treatment of patients with STEMI, and should be implemented in North America. This systems approach would be modelled after programs such as level I trauma systems, where STEMI patients are transported directly to designated centres. To balance this information, EMS, and emergency and cardiology stakeholders must evaluate the body of evidence from the public health literature that challenges the concept of regionalization of STEMI (5). For example, in a large American cohort of 158,831 acute myocardial infarction patients, STEMI patient survival improved with regional intensity of both invasive and medical management. In areas with higher rates of evidence-based medical management, there was no survival improvement associated with increased invasive treatment (56). Rathore et al (57) challenged that the benefits of STEMI care regionalization are not yet fully realized, and that a more substantial understanding of its benefits and consequences is required before its widespread implementation.
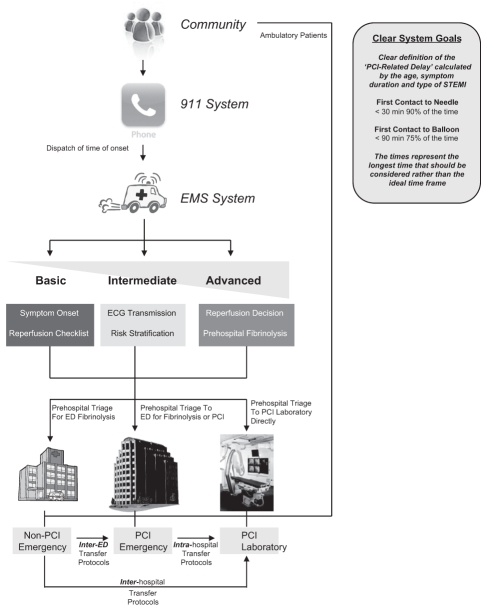
A regionalized approach to STEMI management requires appropriate prehospital EMS resources to achieve prompt prehospital diagnosis, triage and treatment, as well as to facilitate interinstitutional transportation for cardiac catheterization and repatriation to community hospitals (Figure 3). In the absence of specific recommendations, various approaches have been employed including using advanced EMS prehospital teams, basic life support emergency medical technician teams with or without nurse accompaniment and, in specific situations, physician presence during transportation. For repatriation, prehospital EMS are often unable or unwilling to provide transfer back to the original hospital in many regions, so private transfer services with nurse or physician accompaniment may be required, with an associated strain on health care resources and potential risk to society. Specifically, a rural region may only have a single ambulance unit available at any given time and, therefore, paramedic-based inter-hospital transportation may limit the health region’s ability to respond to the next EMS calls for extended periods of time. Similarly, if transportation requires an in-hospital acute care nurse or physician to facilitate transportation, it may significantly limit the region’s ability to respond to other medical conditions requiring emergency care. These problems are not limited to rural regions, but are also applicable to situations associated with ED overcrowding, when prolonged delays in ‘off-loading’ an ambulance patient may consume a significant proportion of EMS time. Within Canada, health care providers need to consider their local resources and environment to design and implement the most appropriate regional STEMI program.
Figure 3).
Systems approach to ST elevation myocardial infarction (STEMI). ECG Electrocardiogram; ED Emergency department; EMS Emergency medical services; PCI Percutaneous coronary intervention
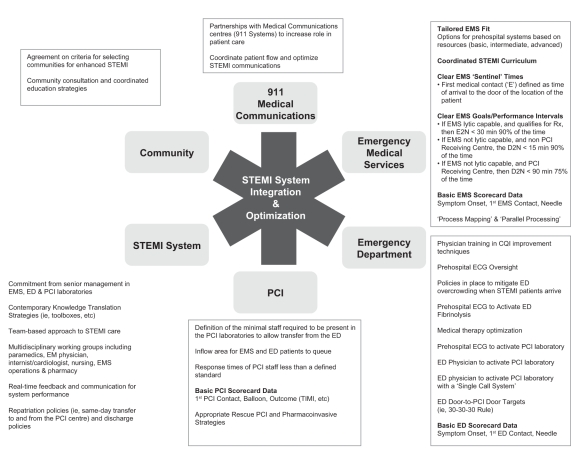
Establishing a dynamic partnership among patients, EMS, emergency medicine staff and cardiology staff, as alluded to in the 2007 ACC/AHA focused update, is feasible and practical in Canada. However, it requires a balanced and comprehensive set of strategies that are highlighted in Figure 4. Although expansive in scope, they reflect options for communities to ‘tailor fit’ their reperfusion strategies to their STEMI needs based on available resources and existing evidence.
Figure 4).
System requirements for ST elevation myocardial infarction (STEMI) care in Canada. CQI Continuous quality improvement; D2N Emergency door to needle (administration of fibrinolysis); E2N EMS arrival (first medical contact) to needle (administration of fibrinolysis); ECG Electrocardiogram; ED Emergency department; EM Emergency medicine; EMS Emergency medical services; PCI Percutaneous coronary intervention; Rx Prescription; TIMI Thrombolysis In Myocardial Infarction
CANADIAN REQUIREMENTS FOR CONTINUOUS QUALITY IMPROVEMENT
We recommend that continuous prospective registries evaluating the process and outcomes of STEMI care should be established at all institutions that provide STEMI care (including EMS). Measures should evaluate the quality of care provided by all health care professionals involved in STEMI management. Time of symptom onset, time of first medical contact and time of reperfusion should be monitored on an ongoing basis. From these basic key times, appropriate reperfusion intervals can be determined and reported. Key process outcomes, such as the proportion of STEMI patients who received reperfusion therapy and the proportion of timely delivered reperfusion therapy, should be collected. Moreover, discharge prescriptions, in-hospital mortality and in-hospital major complications, such as reinfarction, stroke and bleeding, may also be considered in regular reporting.
Participation in these quality control programs should be compulsory and results should be required for the accreditation process (similar to the American hospitals’ accreditation and the Joint Commission on Accreditation of Healthcare Organizations [JACHO]). The measures tracked should be consistent with existing national benchmarks. Although public disclosure of the results may not be entirely appropriate (the validity of results may be limited by a small number of cases at some institutions), hospitals and EMS should hold local regular meetings to compare their performance with the national benchmarks. Barriers to timely reperfusion therapy should be promptly identified and corrected.
SUMMARY
The present CCS CWG document has summarized key highlights from the 2007 focused update of the ACC/AHA STEMI guidelines and placed them within the context of the Canadian health care system. Discussion regarding national reporting of time to treatment initiated from first medical contact, recommendations for implementation of systems approaches to STEMI care, appropriate transfer of remote patients to tertiary care PCI centres for primary, rescue and elective PCI, and the importance of continuous quality improvement programs within the Canadian health care environment were emphasized. The CWG does not intend for this document to cover the spectrum of issues related to STEMI. For that, we refer our colleagues to the complete 2004 ACC/AHA STEMI guidelines and the 2007 focused update to which we have provided a perspective (1,3).
Acknowledgments
The CCS CWG acknowledges Drs E Cohen and J Kornder for providing an external review and comments on the present perspective.
REFERENCES
- 1.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong PW, Bogaty P, Buller CE, Dorian P, O’Neill BJ. The 2004 ACC/AHA Guidelines: A perspective and adaptation for Canada by the Canadian Cardiovascular Society Working Group. Can J Cardiol. 2004;20:1075–9. [PubMed] [Google Scholar]
- 3.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet. 2005;366:1622–32. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 5.Boden WE, Eagle K, Granger CB. Reperfusion strategies in acute ST-segment elevation myocardial infarction: A comprehensive review of contemporary management options. J Am Coll Cardiol. 2007;50:917–29. doi: 10.1016/j.jacc.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 6.Antman EM, Morrow DA, McCabe CH, et al. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med. 2006;354:1477–88. doi: 10.1056/NEJMoa060898. [DOI] [PubMed] [Google Scholar]
- 7.Welsh RC, Gordon P, Westerhout CM, O’Neill B, Buller CE, Armstrong PW. Anticoagulation after subcutaneous enoxaparin is time sensitive in STEMI patients treated with TNK: A WEST sub-study. Can J Cardiol. 2007;23(Suppl C):271C. doi: 10.1007/s11239-012-0697-7. (Abst) [DOI] [PubMed] [Google Scholar]
- 8.Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet. 2005;366:1607–21. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–89. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 10.Love MP, Schampaert E, Cohen EA, et al. The Canadian Association of Interventional Cardiology and the Canadian Cardiovascular Society joint statement on drug-eluting stents. Can J Cardiol. 2007;23:121–3. doi: 10.1016/s0828-282x(07)70731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 12.de Villiers JS, Anderson T, McMeekin JD, Leung RC, Traboulsi M. Expedited transfer for primary percutaneous coronary intervention: A program evaluation. CMAJ. 2007;176:1833–8. doi: 10.1503/cmaj.060902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le May MR, So DY, Dionne R, et al. A citywide protocol for primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2008;358:231–40. doi: 10.1056/NEJMoa073102. [DOI] [PubMed] [Google Scholar]
- 14.Welsh RC, Travers A, Senaratne M, Williams R, Armstrong PW. Feasibility and applicability of paramedic-based prehospital fibrinolysis in a large North American center. Am Heart J. 2006;152:1007–14. doi: 10.1016/j.ahj.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Califf RM, Faxon DP. Need for centers to care for patients with acute coronary syndromes. Circulation. 2003;107:1467–70. doi: 10.1161/01.cir.0000065160.19016.26. [DOI] [PubMed] [Google Scholar]
- 16.Henry TD, Atkins JM, Cunningham MS, et al. ST-segment elevation myocardial infarction: Recommendations on triage of patients to heart attack centers: Is it time for a national policy for the treatment of ST-segment elevation myocardial infarction? J Am Coll Cardiol. 2006;47:1339–45. doi: 10.1016/j.jacc.2005.05.101. [DOI] [PubMed] [Google Scholar]
- 17.Brown AL, Mann NC, Daya M, et al. Demographic, belief, and situational factors influencing the decision to utilize emergency medical services among chest pain patients. Rapid Early Action for Coronary Treatment (REACT) study. Circulation. 2000;102:173–8. doi: 10.1161/01.cir.102.2.173. [DOI] [PubMed] [Google Scholar]
- 18.Canto JG, Zalenski RJ, Ornato JP, et al. Use of emergency medical services in acute myocardial infarction and subsequent quality of care: Observations from the National Registry of Myocardial Infarction 2. Circulation. 2002;106:3018–23. doi: 10.1161/01.cir.0000041246.20352.03. [DOI] [PubMed] [Google Scholar]
- 19.Goff DC, Jr, Feldman HA, McGovern PG, et al. Prehospital delay in patients hospitalized with heart attack symptoms in the United States: The REACT trial. Rapid Early Action for Coronary Treatment (REACT) Study Group. Am Heart J. 1999;138:1046–57. doi: 10.1016/s0002-8703(99)70069-4. [DOI] [PubMed] [Google Scholar]
- 20.Hedges JR, Feldman HA, Bittner V, et al. Impact of community intervention to reduce patient delay time on use of reperfusion therapy for acute myocardial infarction: Rapid early action for coronary treatment (REACT) trial. REACT Study Group. Acad Emerg Med. 2000;7:862–72. doi: 10.1111/j.1553-2712.2000.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 21.Lambrew CT, Bowlby LJ, Rogers WJ, Chandra NC, Weaver WD. Factors influencing the time to thrombolysis in acute myocardial infarction. Time to Thrombolysis Substudy of the National Registry of Myocardial Infarction-1. Arch Intern Med. 1997;157:2577–82. [PubMed] [Google Scholar]
- 22.Swor R, Anderson W, Jackson R, Wilson A. Effects of EMS transportation on time to diagnosis and treatment of acute myocardial infarction in the emergency department. Prehosp Disaster Med. 1994;9:160–4. doi: 10.1017/s1049023x00041273. [DOI] [PubMed] [Google Scholar]
- 23.Dorsch MF, Greenwood JP, Priestley C, et al. Direct ambulance admission to the cardiac catheterization laboratory significantly reduces door-to-balloon times in primary percutaneous coronary intervention. Am Heart J. 2008;155:1054–8. doi: 10.1016/j.ahj.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Welsh RC, Chang W, Goldstein P, et al. Time to treatment and the impact of a physician on prehospital management of acute ST elevation myocardial infarction: Insights from the ASSENT-3 PLUS trial. Heart. 2005;91:1400–6. doi: 10.1136/hrt.2004.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afolabi BA, Novaro GM, Pinski SL, Fromkin KR, Bush HS. Use of the prehospital ECG improves door-to-balloon times in ST segment elevation myocardial infarction irrespective of time of day or day of week. Emerg Med J. 2007;24:588–91. doi: 10.1136/emj.2007.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swor R, Hegerberg S, Hugh-McNally A, Goldstein M, McEachin CC. Prehospital 12-lead ECG: Efficacy or effectiveness? Prehosp Emerg Care. 2006;10:374–7. doi: 10.1080/10903120600725876. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong PW. A comparison of pharmacologic therapy with/without timely coronary intervention vs primary percutaneous intervention early after ST-elevation myocardial infarction: The WEST (Which Early ST-elevation myocardial infarction Therapy) study. Eur Heart J. 2006;27:1530–8. doi: 10.1093/eurheartj/ehl088. [DOI] [PubMed] [Google Scholar]
- 28.Royal College of Physicians Fifth Public Report (2006) from the Myocardial Infarction National Audit Project (MINAP): How the NHS Manages Heart Attacks. <http://www.rcplondon.ac.uk/pubs/books/minap06/index.htm> (Version current at December 9, 2008).
- 29.Welsh RC, Ornato J, Armstrong PW. Prehospital management of acute ST-elevation myocardial infarction: A time for reappraisal in North America. Am Heart J. 2003;145:1–8. doi: 10.1067/mhj.2003.47. [DOI] [PubMed] [Google Scholar]
- 30.Welsh RC, Armstrong PW. It’s a matter of time: Contemporary pre-hospital management of acute ST elevation myocardial infarction. Heart. 2005;91:1524–6. doi: 10.1136/hrt.2004.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clawson JJ, Martin RL, Cady GA, Maio RF. The wake-effect – emergency vehicle-related collisions. Prehospital Disaster Med. 1997;12:274–7. [PubMed] [Google Scholar]
- 32.Custalow CB, Gravitz CS. Emergency medical vehicle collisions and potential for preventive intervention. Prehosp Emerg Care. 2004;8:175–84. doi: 10.1016/s1090-3127(03)00279-x. [DOI] [PubMed] [Google Scholar]
- 33.Thune JJ, Hoefsten DE, Lindholm MG, et al. Simple risk stratification at admission to identify patients with reduced mortality from primary angioplasty. Circulation. 2005;112:2017–21. doi: 10.1161/CIRCULATIONAHA.105.558676. [DOI] [PubMed] [Google Scholar]
- 34.Doorey A, Patel S, Reese C, et al. Dangers of delay of initiation of either thrombolysis or primary angioplasty in acute myocardial infarction with increasing use of primary angioplasty. Am J Cardiol. 1998;81:1173–7. doi: 10.1016/s0002-9149(98)00160-x. [DOI] [PubMed] [Google Scholar]
- 35.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–7. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 36.Morrow DA, Antman EM, Giugliano RP, et al. A simple risk index for rapid initial triage of patients with ST-elevation myocardial infarction: An InTIME II substudy. Lancet. 2001;358:1571–5. doi: 10.1016/S0140-6736(01)06649-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction: Results from an international trial of 41 021 patients. Circulation. 1995;91:1659–68. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 38.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:e82–292. [PubMed] [Google Scholar]
- 39.Brodie BR, Hansen C, Stuckey TD, et al. Door-to-balloon time with primary percutaneous coronary intervention for acute myocardial infarction impacts late cardiac mortality in high-risk patients and patients presenting early after the onset of symptoms. J Am Coll Cardiol. 2006;47:289–95. doi: 10.1016/j.jacc.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 40.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. N Engl J Med. 1999;341:625–34. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 41.Pinto DS, Kirtane AJ, Nallamothu BK, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: Implications when selecting a reperfusion strategy. Circulation. 2006;114:2019–25. doi: 10.1161/CIRCULATIONAHA.106.638353. [DOI] [PubMed] [Google Scholar]
- 42.Cantor WJ, Brunet F, Ziegler CP, Kiss A, Morrison LJ. Immediate angioplasty after thrombolysis: A systematic review. CMAJ. 2005;173:1473–81. doi: 10.1503/cmaj.045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collet JP, Montalescot G, Le MM, Borentain M, Gershlick A. Percutaneous coronary intervention after fibrinolysis: A multiple meta-analyses approach according to the type of strategy. J Am Coll Cardiol. 2006;48:1326–35. doi: 10.1016/j.jacc.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 44.Collet JP, Montalescot G, Le MM, Borentain M, Gershlick A. Percutaneous coronary intervention after fibrinolysis: A multiple meta-analyses approach according to the type of strategy. J Am Coll Cardiol. 2006;48:1326–35. doi: 10.1016/j.jacc.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 45.Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study) J Am Coll Cardiol. 2005;46:417–24. doi: 10.1016/j.jacc.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 46.Ellis SG, Tendera M, de Belder MA, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205–17. doi: 10.1056/NEJMoa0706816. [DOI] [PubMed] [Google Scholar]
- 47.Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): Randomised trial. Lancet. 2006;367:569–78. doi: 10.1016/S0140-6736(06)68147-6. [DOI] [PubMed] [Google Scholar]
- 48.Trial of abciximab with and without low-dose reteplase for acute myocardial infarction. Strategies for Patency Enhancement in the Emergency Department (SPEED) Group. Circulation. 2000;101:2788–94. doi: 10.1161/01.cir.101.24.2788. [DOI] [PubMed] [Google Scholar]
- 49.Maioli M, Bellandi F, Leoncini M, Toso A, Dabizzi RP. Randomized early versus late abciximab in acute myocardial infarction treated with primary coronary intervention (RELAx-AMI Trial) J Am Coll Cardiol. 2007;49:1517–24. doi: 10.1016/j.jacc.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 50.Montalescot G, Borentain M, Payot L, Collet JP, Thomas D. Early vs late administration of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction: A meta-analysis. JAMA. 2004;292:362–6. doi: 10.1001/jama.292.3.362. [DOI] [PubMed] [Google Scholar]
- 51.Cantor WJ, Fitchett D, Borgundvaag B, et al. Rationale and design of the Trial of Routine ANgioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI) Am Heart J. 2008;155:19–25. doi: 10.1016/j.ahj.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 52.Bradley EH, Roumanis SA, Radford MJ, et al. Achieving door-to-balloon times that meet quality guidelines: How do successful hospitals do it? J Am Coll Cardiol. 2005;46:1236–41. doi: 10.1016/j.jacc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Bradley EH, Curry LA, Webster TR, et al. Achieving rapid door-to-balloon times: How top hospitals improve complex clinical systems. Circulation. 2006;113:1079–85. doi: 10.1161/CIRCULATIONAHA.105.590133. [DOI] [PubMed] [Google Scholar]
- 54.Bradley EH, Herrin J, Wang Y, et al. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med. 2006;355:2308–20. doi: 10.1056/NEJMsa063117. [DOI] [PubMed] [Google Scholar]
- 55.American College of Cardiology Door to Balloon Initiative. <http://d2b.acc.org/> (Version current at December 9, 2008).
- 56.Stukel TA, Lucas FL, Wennberg DE. Long-term outcomes of regional variations in intensity of invasive vs medical management of Medicare patients with acute myocardial infarction. JAMA. 2005;293:1329–37. doi: 10.1001/jama.293.11.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rathore SS, Epstein AJ, Nallamothu BK, Krumholz HM. Regionalization of ST-segment elevation acute coronary syndromes care: Putting a national policy in proper perspective. J Am Coll Cardiol. 2006;47:1346–9. doi: 10.1016/j.jacc.2005.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]