Abstract
Time-based prospective memory (PM) has been found to be negatively affected by aging, possibly as a result of declining frontal lobe (FL) function. Despite a clear retrospective component to PM tasks, the medial temporal lobes (MTL) are thought to play only a secondary role in successful task completion. The present study investigated the role of the FLs and MTLs in time-based PM, as well as their involvement in clock monitoring, plan generation, and time estimation, each of which may play a role in the execution of time-based PM tasks. Based upon their scores on composite measures of FL and MTL function, 32 older adults were divided into four groups, and were then tested on a time-based laboratory PM task. Overall age effects were also assessed and each of the four groups was compared to a group of 32 younger adults. High-frontal functioning participants demonstrated better prospective memory than low-frontal functioning participants, and were not distinguishable from younger adults. Older adults with high-MTL scores performed significantly better than those with low-MTL scores, but only if they were also high in FL function. FL function, but not MTL function, predicted patterns of clock monitoring, quality of plans generated to assist in time-based PM performance, and the accuracy of time estimation. Again, on each of these measures the performance of the high-frontal group was equivalent to that of the younger adults. The results of this study suggest that it is not aging per se that disrupts PM performance, but it is instead primarily the diminished frontal function seen in a subset of older adults.
Keywords: Aging, Frontal lobes, Executive function, Prospective memory, Medial temporal lobes
Remembering to perform an action at a future point in time is a mnemonic ability that is particularly critical to daily living. In the last 10–12 years, this “remembering to remember,” referred to as prospective memory (PM), has begun to receive considerable research attention. PM is traditionally divided into two forms: event-based and time-based. An example of event-based prospective memory is remembering to deposit a check when seeing the bank on your way home. In this case, the bank serves as an environmental cue that prompts retrieval of an intended action, depositing a check. An example of time-based prospective memory is remembering that you have a 3:00 appointment. In this instance, there may be no environmental cue, but instead time alone serves as the cue to perform a previously formed intention, meeting the appointment. The implications of PM failure can be dramatic under some conditions, especially for older adults for whom medication adherence, for example, is often an important part of daily living (Insel, Morrow, Brewer, & Figueredo, 2006; Park, Morrel, Frieske, & Kincaid, 1992).
Prospective memory differs from retrospective memory (RM) in a number of ways. Although PM possesses a RM component—clearly the intention must be retained in memory—, it also incorporates cognitive processes not necessarily required in tasks of RM. For instance, in tasks involving PM, subjects are engaged in some ongoing activity and may have to search or monitor the environment for the presence of a cue. Upon recognizing the appropriate cue, people must then interrupt their performance of the ongoing task in order to successfully complete the intended action. In contrast, RM demands no such mental gymnastics. Tulving (1983) made the point that in tasks of RM, participants are put into a “retrieval mode” in which they are explicitly instructed to remember the contents of a previously learned list, the speaker of a sentence, or the order in which a set of numbers was presented. In tasks of PM, subjects are not cued to remember previously presented information, but are required to recognize a cue and recall its associated intention without any prompting, and then carry out the intended action. Craik (1986) refers to such subject-directed operations as “self-initiated processing.”
According to Craik (1986), memory accuracy in older adults is determined jointly by the processing requirements of a particular task and by the amount of “environmental support” available. He suggests that memory tasks are ordered hierarchically with procedural memory at the bottom of the hierarchy, providing the greatest environmental support and requiring the least self-initiated processing, and PM at the top, offering the least environmental support and consequently requiring the most self-initiated processing. According to this theory, older adults should have the most difficulty and perform most poorly relative to younger adults on tests of PM. This pattern of results has not always been obtained, however (see McDaniel, Einstein, & Jacoby, 2008). For example, studies of PM in natural environments have often found better performance in older than in young adults (Phillips, Henry, & Martin, 2008; Rendell &Craik, 2000). In this case, the real-world context may provide considerable environmental support, unlike the laboratory tasks, and non-cognitive factors such as motivation may play a role. In addition, a recent meta-analysis (Henry, MacLeod, Phillips, & Crawford, 2004) indicated that age-related performance differences were significantly greater in tasks of RM, particularly free recall, than in tasks of PM (but see Zeintl, Kliegel, & Hofer, 2007). Similarly, in a meta-analysis of event-based PM, Kliegel, Jäger, and Phillips (2008) reported effect sizes which were less dramatic than those seen for RM. Finally, several laboratory studies have found no age differences in some event-based PM tasks (e.g., Einstein & McDaniel, 1990). Although these findings appear incongruent with Craik’s self-initiated processing view, it may be that not all PM paradigms place equivalent demands on self-initiated processing. For example, as discussed by McDaniel, Einstein, & Rendell (2008); see also Einstein et al., 2005), when prospective memory cues are embedded in the primary task and are thus in focal attention, retrieval of the intended action may occur rather automatically with little demand on search or monitoring processes, and so age differences will be less likely. However, Kliegel, Jäger, and Phillips (2008) reported reliable age differences in focal tasks as well as in non-focal tasks, although effects sizes were greater when cues were non-focal. Overall, the majority of laboratory studies have shown that younger adults outperform older adults in PM tasks (Einstein, McDaniel, Richardson, Guynn, & Cunfer, 1995; Einstein, Smith, McDaniel, & Shaw, 1997; Maylor, 1996; McDaniel, Einstein, Stout, & Morgan, 2003; Park, Hertzog, Kidder, Morrell, & Mayhorn, 1997), and are generally consistent with Craik’s view that age-related deficits are observed to the extent that the PM task requires self-initiated processing.
Time-based tasks are particularly likely to fall into this category. While requiring many of the same processes as event-based tasks (e.g., recalling an association between intention and cue, dividing attention between an ongoing task and the PM task), time-based tasks may also place greater monitoring demands on the rememberer. Because there are no external cues to direct responding, one must frequently monitor time in order to determine the appropriateness of performing an intended action (Harris & Wilkins, 1982). Time monitoring thus may involve continual interruption of ongoing activities, and is likely to be entirely self-initiated. In contrast, tasks of event-based PM require interruption and inhibition only when specific cues prompt the intended action. Thus, time-based tasks usually place greater demands on self-initiated monitoring, and not surprisingly, have elicited the greatest and most consistent age-related differences in PM (d’Ydewalle, Bouckaert, & Brunfaut, 2001; Einstein et al., 1995; Jäger & Kliegel, 2008).
Much of the self-initiated processing required in PM tasks is believed to be heavily dependent upon the frontal lobes. For instance, PM tasks might include forming a strong association between a cue and an intention, maintaining the intention over a delay, dividing attention between tasks, monitoring the environment for a cue, and interrupting and inhibiting ongoing activities, all of which have been shown to be impaired in frontal lobe patients (Fuster, 1997; Stuss & Benson, 1984). The possibility that the operations involved in self-initiated processing are dependent upon the frontal lobes is critical in light of findings that suggest an age-related decline in frontal lobe function (Fuster, 1997; West, 1996). Support for a selective decline in frontal lobe function has come from a variety of sources. Morphological studies have found disproportionate volume loss in the prefrontal cortex relative to other regions of the brain (Haug & Eggers, 1991; Raz et al., 2005; Van Petten et al., 2004). Functional changes have also been reported. In a PET study, Leenders et al. (1990), observed significant age-related decreases in rCBF in prefrontal cortex over and above that seen in other areas, and numerous fMRI studies have suggested age-related differences in prefrontal activations across a variety of tasks (for review, see Dennis & Cabeza, 2008). Neuropsychological data have also implicated age-related decline in frontal function (Brickman et al., 2006; Daigneault, Braun, & Whitaker, 1992; Spencer & Raz, 1994).
To test the hypothesis that PM is dependent on frontal function, McDaniel, Glisky, Rubin, Guynn, and Routhieaux (1999) administered an event-based PM task to older adults who had been categorized as possessing high- or low-frontal function according to their performance on a battery of neuropsychological tests (Glisky, Polster, & Routhieaux, 1995). A general knowledge/trivia test served as the ongoing activity in this study, while the PM task required participants to press a particular computer key whenever the word “president” appeared in one of the trivia questions. Results of the study showed that the high-frontal group significantly out-performed the low-frontal group, suggesting that the frontal lobes are involved in the execution of event-based PM tasks.
Martin, Kliegel, and McDaniel (2003) conducted a study designed to address further the role of frontally mediated processes in a variety of PM tasks. The authors of this study, like McDaniel et al. (1999), used a composite measure of frontal/executive function. Four PM tests, requiring increasing amounts of frontal input, were administered; the Remembering-a-Belonging subtest of the Rivermead Behavioral Memory Test (RBMT; Wilson, Cockburn, & Baddeley, 1985), an event-based PM test, a time-based PM test, and a complex, multitask PM test (MTPM; Kliegel, McDaniel, & Einstein, 2000). Age differences were revealed on all but the RBMT task, and the magnitude of age differences paralleled the hypothesized frontal/executive function involvement in tasks of PM, with age effects greatest for the MTPM, followed by event- and time-based tasks. Furthermore, in older adults executive function was significantly correlated with performance on all three tasks. These findings further support the claim that the decline in frontal function often associated with aging plays a role in PM performance, particularly as the need for self-initiated processing increases.
In addition to the frontally mediated processes of dividing attention, associating a cue with an intention, interrupting an ongoing task, and monitoring the environment for a cue, PM may also involve planning, a skill that has been thought to depend on the frontal lobes (Lezak, 1982; Shallice, 1982). Many everyday PM tasks, such as preparing a meal for instance, consist of a complex set of actions, the order of which must be planned (Craik & Bialystok, 2006). To simulate these kinds of complex tasks in the laboratory, Kliegel et al. (2000; see also Kliegel, Mackinlay, & Jäger, 2008) used a modified version of the six elements task (Shallice & Burgess, 1991), which required participants to perform six different tasks in a limited time according to an arbitrary set of rules. They had to initiate performance on these tasks in the midst of filling out a personal information questionnaire, self-initiate switches between tasks, and remember to ask the experimenter to return a personal belonging at the end of the entire experiment. People were also asked to generate a plan for completing the six elements task. Results indicated that younger adults generated more elaborate plans and were more likely to initiate them than were older adults. PM performance was also better among young adults, as younger adults started significantly more sub-tasks than did older adults. Finally, the results of regression analyses indicated that more elaborate plans and greater inhibitory capabilities, as indicated by performance on Stroop tasks, were associated with better PM execution. More direct evidence for the involvement of the frontal lobes in event-based PM comes from neuroimaging studies (e.g., Burgess, Scott, & Frith, 2003; Okuda et al., 2007; Simons, Schölvink, Gilbert, Frith, & Burgess, 2006), as well as from ERP studies (e.g., West & Covell, 2001).
Although numerous findings suggest frontal lobe involvement in tasks of event-based PM, far fewer studies have investigated the specific roles played by the frontal lobes in time-based PM, and results have been inconsistent. For example, in the time-based task in the Martin et al. (2003) study, executive function accounted for the majority of performance variance, over and above that resulting from age, supporting the hypothesized frontal lobe involvement in time-based PM. Similarly, in a study conducted by Kerns and Price (2001), control subjects significantly outperformed children with ADHD on a task of time-based PM, suggesting that the frontal lobe deficits associated with ADHD played a role in the performance of the time-based task. Recent studies by Burgess and colleagues (for review, see Burgess et al., 2008) with frontal-lesion patients as well as evidence from neuroimaging studies of normal individuals (e.g., Okuda et al., 2007) have implicated rostral prefrontal cortex (BA 10), both medially and laterally, in time-based PM tasks, and suggest that this region is involved in attentional and executive control aspects of PM functions. However, Katai, Maruyama, Hashimoto, & Ikeda (2003) reported that Parkinson’s disease patients, who exhibit deficits on executive function tasks, performed poorly relative to controls on an event-based PM task, but their performance was equal to that of controls on a time-based task, findings that seem contrary to what would be expected in light of the increased self-initiated processing required on time-based tasks. In a more recent study of Parkinson’s patients, however (Costa, Peppe, Caltagirone, & Carlesimo, 2008), the opposite result was obtained, namely those with Parkinson’s disease were impaired in time-based but not event-based PM.
It may also be possible that time perception, which is thought to be frontally mediated (Picton, Stuss, Shallice, Alexander, & Gillingham, 2006; Rubia, 2008; Rubia & Smith, 2004), plays a role in both clock monitoring and time-based PM. However, although several have speculated about a relation between time perception and time-based PM (Block & Zakay, 2008; Graf & Grondin, 2008), we are not aware of any studies that have directly investigated this relationship.
So, although there is growing evidence that the frontal lobes are involved in the performance of both event-based and time-based PM tasks, their exact role remains uncertain. Thus, the primary aim of the current study was to investigate the role of the frontal lobes, as characterized by performance on neuropsychological tests, in the execution of a time-based PM task. We hypothesized that planning, clock monitoring, and time perception would all be important contributors to a time-based PM task and that these functions would depend on FL function. To explore these possibilities, we employed the frontal measure developed by Glisky et al. (1995) and utilized by McDaniel et al. (1999) in which older adults were classified as high or low in frontal function based on their performance on a battery of neuropsychological tests.
We were also interested in the role of the medial temporal lobes (MTL) in the execution of time-based PM tasks. In the McDaniel et al. study (1999), older adults were also categorized as high or low in MTL function (Glisky et al., 1995). Despite the fact that the need to remember the intention, which would require RM, suggests a clear rationale for expecting MTL involvement in a task of event-based PM, no significant relation was observed in that study. It is possible, however, that in many event-based tasks with a single response option, intentions are retrieved automatically in response to cues (McDaniel, Robinson-Riegler, & Einstein, 1998), thereby placing minimal demand on RM and MTL function. In a time-based task, however, where demands on the FLs are expected to be substantial, good MTL function may also be essential for the retention and retrieval of a task-appropriate response (Kliegel, Mackinlay, & Jäger, 2008), particularly when the intention is complex or when more than one response option is available. Though it is reasonable to expect MTL function to impact time-based PM performance, we are not aware of any studies that have explored the nature of MTL involvement in time-based PM (but see Smith & Bayen, 2006 for a discussion of the role of RM in PM). The secondary aim of the current study then, was to investigate the involvement of the MTLs in the successful completion of a time-based PM task. To that end, we utilized a second composite measure in which older adults were classified as high or low in MTL function based upon their performance on a battery of neuropsychological tests. Older adults were then selected for the present study based upon their performance on the two composite measures of FL and MTL function. Participants were given a time-based laboratory PM task in which they were required to monitor a clock and press one of two buttons in alternating order every 5 min.
Given the age differences that have been reported in the literature, we expected to find an age difference in the present study, such that young adults would successfully perform more PM tasks than would older adults. Furthermore, in light of the FL involvement found in the McDaniel et al. (1999) study and studies showing impaired PM performance in patients with FL lesions (e.g., Burgess et al., 2008; for review, see Kliegel, Jäger, Altgassen, & Shum, 2008) coupled with the increased demands of a time-based task, we anticipated that among older adults, participants characterized as high in FL function would outperform those participants characterized as low in FL function. More specifically, we hypothesized that participants in the low-FL group would have difficulty integrating cues with intentions, namely associating the 5-min clock intervals with the appropriate button, would have difficulty self-initiating clock monitoring activity, and would therefore monitor the clock less frequently and complete fewer PM tasks. Older adults with low-FL function would also be expected to have poorer time perception abilities and poorer plans for executing the PM task.
With regard to the medial temporal measure, we hypothesized that those participants characterized as high on the MTL measure might also execute more PM tasks, but in addition, make fewer button press errors than their low medial temporal counterparts. This hypothesis was based on the possibility that individuals in the low medial temporal group may have difficulty retrieving the details of the PM intention or may not remember which button to press. The requirement to press the two buttons in alternating order may have made the RM component of the task somewhat more challenging than in a single response situation. We also considered that MTL and FL function may interact. For example, if one has forgotten which button to press, initiating clock monitoring activity may not lead to a correct response.
1. Method
1.1. Participants
Thirty-two young adults were recruited from undergraduate psychology classes at the University of Arizona. Students were awarded class credit for participating in the experiment.
A total of 32 older adults (age 65 or older) were recruited from the subject pool of the Aging and Cognition Unit at the University of Arizona. All older participants were healthy, community-dwelling adults without dementia, depression or any history of drug or alcohol abuse. Participants were paid $8 per hour to take part in this study. All older adults had been previously categorized along two dimensions based on their performance on a battery of neuropsychological exams. Initial exploratory factor analyses of neuropsychological test performance of older adults (Glisky et al., 1995; Glisky, Rubin, & Davidson, 2001) and recent confirmatory factor analysis with a new sample of 227 older adults (see Glisky & Kong, 2008) revealed two separate neuropsychological components. The first factor is hypothesized to tap executive control processes that have been shown to be dependent on the frontal lobes; it will be referred to as the FL factor. Tests loading on the FL factor include the number of categories achieved on the modified Wisconsin Card Sorting Test (Hart, Kwentus, Wade, & Taylor, 1988), Mental Arithmetic from the Wechsler Adult Intelligence Scale — Revised (WAIS-R; Wechsler, 1981), Mental Control from the Wechsler Memory Scale — III (WMS-III; Wechsler, 1997), Backward Digit Span from the WMS — III, and the total number of words generated on the Controlled Oral Word Association Test (Benton & de Hamsher, 1976).
Neuropsychological tests loading on the second factor include Logical Memory I, Verbal Paired Associates I, Faces I (all from the WMS-III), Visual Paired Associates II (from the WMS-R, Wechsler, 1987), and Long-Delay Cued Recall from the California Verbal Learning Test (Dellis, Kramer, Kaplan, & Ober, 1987). The second factor taps basic memory processes that have been shown to be dependent on the medial temporal lobes; it will be referred to as the MTL factor.
The composite scores for both factors represent average z-scores relative to the normative population of 227 older adults. Variability attributable to age has been removed from these scores. Equal numbers of participants were placed into each of four cells created by crossing the two factors such that people were either above or below the mean on both factors, or above on one and below on the other. Characteristics of each group are presented in Table 1. Separate one-way analyses of variance (ANOVAs) indicated that there were no differences in age, education, or scores on the Mini-Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, 1975)as a function of neuropsychological group (all Fs ≤ 1.8).
Table 1.
Characteristics of older adults as a function of neuropsychological group.
| Variable | High-FL function |
Low-FL function |
||||||
|---|---|---|---|---|---|---|---|---|
| High-MTL function |
Low-MTL function |
High-MTL function |
Low-MTL function |
|||||
| M | SD | M | SD | M | SD | M | SD | |
| Age (years) | 74.88 | 5.2 | 76.43 | 3.3 | 72.88 | 4.2 | 71.14 | 4.9 |
| Education (years) | 16.38 | 1.3 | 15.86 | 3.2 | 15.5 | 2.1 | 15.29 | 2.8 |
| MMSE | 28.63 | 1.4 | 29.71 | .49 | 29.25 | 1.2 | 28.86 | 1.07 |
| FL scorea | .59 | 0.24 | .55 | .20 | -.73 | .39 | -.50 | .22 |
| MTL scorea | .77 | .33 | -.50 | .33 | .53 | .31 | -.27 | .22 |
Note: FL, frontal lobe; MTL, medial temporal lobe; MMSE, Mini-Mental Status Examination.
z scores (see text).
1.2. Materials
The ongoing, background task in this experiment was a multiple choice test of general knowledge and trivia. Three hundred and forty-four questions were selected from the McDaniel et al. (1999) study and were presented on a Gateway M280 Tablet PC with touch screen capabilities, using DMDX (Forster & Forster, 2003). Questions appeared in the center of the monitor. Beneath each question were four touch sensitive buttons corresponding to the multiple choice answers. Participants had a maximum of twelve seconds to respond to each question. Successive questions appeared as soon as an answer for a prior question was selected or 12 s had expired. Questions were presented in a different random order for each participant (see Fig. 1).
Fig. 1.
Sample slide showing placement of buttons “1” and “2,” as well as the clock icon which reveals the elapsed time when touched.
The PM task of this experiment required participants to press one of two touch sensitive buttons at 5-min intervals. For example, 5 min after the start of the multiple choice test participants were to touch the button labeled “1” using a stylus designed for use with the computer. Five minutes later, participants were to touch the button labeled “2” and 5 min after that, button “1.” The appropriate button to be touched alternated every 5 min throughout the course of the multiple choice test, for a total of eight button presses (40 min). The “1” and “2” buttons were located side by side in the upper right hand corner of the monitor. For time monitoring purposes, a clock icon was present in the upper right hand corner, just above the “1” and “2.” In order to see the elapsed time, participants needed to touch the clock icon with the stylus. The clock icon disappeared when it was touched, and appearing in its place was the elapsed time displayed in minutes and seconds for a duration of 2 s. DMDX recorded each occasion the clock icon was touched, providing a measure of clock monitoring.
1.3. Procedure
The experiment lasted approximately 1 h. After providing informed consent, participants were told that they would be completing a multiple choice test of general knowledge and trivia and that they would have a total of 12 s in which to make a response to each question. Also, they were told that a second task to perform was to press either the button labeled “1” or the button labeled “2” every 5 min in alternating fashion. Participants were instructed to press buttons “1” and “2” as close to the 5-min interval as possible. Additionally, participants were told that if they should happen to “miss” a 5-min interval (e.g., forget to press button “1” at 5 min), they should make the appropriate button press as soon as they remembered to do so. For example, participants were told that if they remembered to press button “1” at 8 min instead of at 5 min, they should press button “1” at 8 min, and then press button “2” at 10 min. Four separate practice trials were then given, providing people the opportunity to become acquainted with the clock and how to activate it, the qualities of the touch screen, including the pressure required to record a response, as well as the placement on the screen of the various buttons. A series of practice trivia questions were also presented so that participants developed a sense of the type of questions that they would see during the experiment. After participants had completed the practice trials, the experimenter answered any questions and participants were told that they could take a moment to consider how they should perform the task and to begin as soon as they were ready by pressing the “start” button located in the center of the screen. The multiple choice trivia questions, with the embedded PM task, then proceeded for 42 min after which participants were asked to describe the tasks involved (i.e., answering multiple choice questions; pressing buttons 1 and 2 at 5-min intervals). This served as a measure of retrospective memory. Participants were then asked to report and describe any strategy or plan that they may have developed to assist them in remembering to check the clock. If participants reported having developed a plan, they were then asked whether they developed a strategy to help them perform the PM tasks in the order described in the instructions (i.e., pressing button “1” or “2”). Plans were evaluated by two independent blind raters and were given a rating from 0 to 3, with 0 indicating no plan, and 3 indicating an optimal plan. This provided an experimental measure of planning.
After reporting the use of any strategies, participants were asked to complete two time perception tasks. The first task was one of verbal estimation. Participants were informed that upon pressing the start button, a green rectangle would appear and would remain on the screen for a set amount of time, after which they should report in seconds how long the rectangle was present. The rectangle remained on the screen for 27 s. The second task was a production task in which participants were again asked to press a start button, prompting a green rectangle to appear in the center of the screen. In this task, however, participants were instructed to press the green rectangle after it had been on the screen for ten seconds. These two time perception tasks were selected because they have been used previously to study time perception in older adults (Craik & Hay, 1999), and were included in order to address the possibility that individual differences in time perception could have an effect on PM task completion and patterns of clock monitoring. In both time estimation tasks, participants were instructed not to attempt to keep time by tapping a foot or finger, or by counting aloud or subvocally. No practice trials were included for the time estimation tasks.
2. Results
2.1. Prospective memory task
For the time-based PM tasks, we recorded the number of times that each participant remembered to press the response keys. Given a total of eight possible responses, a PM score was computed with correct responses defined as those appropriate button presses (i.e., button 1 or button 2) occurring within a window of 15 s prior to and 15 s after the target time. Correct responses made within the allowable timeframe were given a value of 1. Those responses made outside of the established timeframe (i.e., more than 15 s before or after the target time) or out of order (i.e., button one is pressed when button two should be pressed) were given a value of 0. If, however, button 1 was pressed when button 2 should have been pressed, but the appropriate pattern of alternating button presses was maintained from that point forward, only the first out-of-order button press was considered incorrect, and given a value of zero. The mean proportion of correct responses as a function of neuropsychological group is shown in Table 2. A 2 (high- vs. low-FL function) × 2 (high- vs. low-MTL function) ANOVA indicated a significant main effect of frontal status with the high-FL group completing significantly more tasks than the low-FL group, F(1, 28) = 13.81, MSE = 1.13, p <.001. Though the medial temporal groups did not differ in the number of PM tasks completed (F < 1), there was an interaction between the two neuropsychological factors, F(1, 28) = 4.03, MSE = 5.19, p < .05. The interaction indicated that high-FL function differentially benefited those with high-MTL function, t(14) = 5.01, p < .001. Those with below average MTL function showed a much smaller and non-significant advantage of good FL function, t(14) = 1.04. Additionally, one might note that when FL function was low, performance was poor overall and did not differ for high- and low-MTL groups, t(14) = −1.12.
Table 2.
Mean (SD) proportion of PM tasks executed as a function of neuropsychological group
| Young adults | .79 (.28) | ||
|---|---|---|---|
| Older adults | |||
| MTL function | FL function |
||
| High | Low | Mean | |
| High | .80 (.21) | .22 (.25) | .51 (.23) |
| Low | .55 (35) | .38 (30) | .47 (32) |
| .67 (.28) | 30 (.27) | .49 (.28) | |
Note: FL, frontal lobe; MTL, medial temporal lobe.
There was also an overall significant main effect of age, such that younger adults significantly outperformed older adults, t(62) = 3.86, p < .001. This was true for all older adult subgroups (all ps < .05) except the high-FL/high-MTL group, which did not differ from the younger adults, t(38) = .07.
Mean number of errors for each error type is presented in Table 3 as a function of neuropsychological group. A measure of PM errors was constructed by combining omissions with responses that were made either too early or too late (i.e., before or after the allowable time frame) although they were correct button presses. A 2 (high- vs. low-FL function) × 2 (high- vs. low-MTL function) ANOVA indicated a significant main effect of frontal status with the low-FL group committing significantly more PM errors than the high-FL group, F(1, 28) = 5.06, MSE = 24.5, p < .05. There was no effect of medial temporal status, nor was there an interaction, Fs < 1. Incorrect button presses (whether they were made on time or early or late) served as a measure of RM errors. There was no main effect of frontal status, F(1, 28) = 3.05, nor was there an effect of medial temporal status, F(1, 28) = .56. However, there was an interaction, F(1, 28) = 3.98, MSE = 2.01, p < .05, indicating a trend toward fewer RM errors among the high-MTL group than among the low-MTL group, but only for those with high-FL function, t(14) = −1.78, p = .09. When FL function was below average, RM error rates did not differ between MTL groups, t(14) = .98.
Table 3.
Mean (SD) error rates as a function of neuropsychological group and error type.
| High-FL function |
Low-FL function |
|||
|---|---|---|---|---|
| High-MTL function M |
Low-MTL function M |
High-MTL function M |
Low-MTL function M |
|
| RM errors | .25 (.70) | 1.63 (2.07) | 2.13 (1.55) | 1.50 (.93) |
| PM errors (total) | 1.38 (1.10) | 1.88 (1.64) | 3.63 (3.07) | 3.14 (2.42) |
| Omissions | .13 (35) | .25 (.46) | 1.25 (2.82) | .88 (2.48) |
| Early | .13 (35) | .25 (.71) | .63 (1.06) | 1.13 (.99) |
| Late | 1.13 (.99) | 138 (1.07) | 1.75 (1.67) | 1.13 (1.46) |
| Total errors | 1.63 (1.69) | 3.51 (2.97) | 5.75 (2.25) | 4.63 (2.62) |
Note: FL, frontal lobe; MTL, medial temporal lobe.
2.2. Clock monitoring
There was no main effect of MTL function on any aspect of clock monitoring, nor did it interact with FL function, Fs < 1. All analyses in this section therefore focus only on differences between young adults and the two groups of older adults characterized by FL function. A comparison of the number of times subjects monitored the clock revealed a significant effect of age such that young adults (M = 40.34) monitored the clock significantly more often than older adults (M = 31.06), t(62) = 2.03, p < .05. Young adults and the low-FL group (M = 27.31) differed significantly, t(46) = 2.54, p < .02, whereas young adults and the high-FL group (M = 34.81) did not, t(46) = 1.08.
To explore the possibility that groups differed in their patterns of clock monitoring, we submitted clock checking to a 3 × 5 ANOVA, with 1-min intervals as the within-subjects variable, and group (low-FL, high-FL, young) as a between-subject variable. Fig. 2 presents clock monitoring patterns as a function of group. There was no main effect of group, F(2, 61) = 2.76, MSE = 65.79, p <.07. There was a main effect of interval, F(4, 244) = 162.80, MSE = 24.41, p < .001, that was modulated by a group by interval interaction, F(8, 244) = 5.29, MSE = 24.41, p < .001. Visual inspection of Fig. 2 suggests that young and high-FL groups monitored the clock similarly, showing a slight increase across the first four intervals followed by a dramatic increase in clock checking activity in the fifth interval. The low-FL group, however, showed a similar pattern across the first four intervals, but a much smaller increase in clock checking activity in the fifth interval. Two separate 2 × 5 analyses comparing the young group to the high-FL group and the high-FL group to the low-FL group confirmed these observations: There was no interaction when young were compared to the high-FL older group, F < 1, but a significant group by interval interaction when the high- and low-FL groups were compared, F(4, 120) = 4.85, MSE = 12.86, p < .005.
Fig. 2.
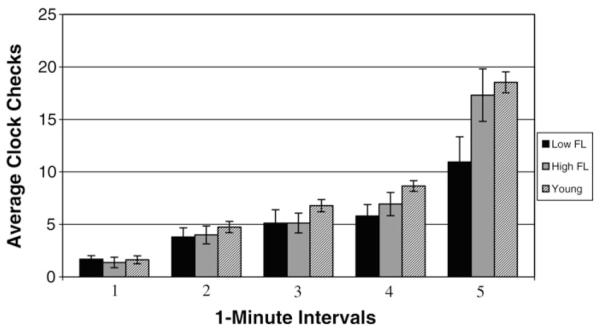
Clock monitoring as a function of group and interval.
Further exploration of the differences in clock monitoring between the high- and low-FL groups was conducted using clock interval as an ordered variable for a polynomial trend analysis, with frontal status as a between-subjects variable. When only the first four intervals were included, there was a significant linear trend of clock monitoring, Flinear(1,30) = 51.37, MSE = 7.73, p < .001, but no interaction with group, and no significant higher-order effects of interval. When the fifth interval was included in the analysis, there was both a significant linear trend, Flinear(1,30) = 66.86, MSE = 36.61, p < .001, and a significant quadratic trend, Fquadratic(1,30) = 46.07, MSE = 36.61, p < .001, as well as a significant group by int erval interaction, Fquadratic(1,30) = 4.48, MSE = 36.61, p < .05, indicating that the two groups differed only in the fifth interval.
There was also a significant relation between clock monitoring in the fifth interval and PM, r =.74, p < .001, among older adults, such that greater clock monitoring in the fifth interval resulted in better PM performance.
2.3. Plans
Participants reported different plans for remembering the PM task, particularly with respect to which button should be pressed. Plans were scored on a 0–3 scale: Having no plan received a score of 0, trying to remember which button was last pressed received a score of 1, using one’s fingers to remember which button was last pressed received a score of 2, associating the pressing of button 1 with times ending in 5 and the pressing of button 2 with times ending in 0 received a score of 3. Plans were scaled according to the relative likelihood that they would be effective. It was determined a priori that associating button presses 1 and 2 with times ending in 5 and 0 respectively would be optimal for successful performance. Relying on alternating fingers may be an adequate plan, but is often poorly executed and prone to error. Data for plans are presented in Table 4 as a function of group. A 2 (high- vs. low-FL function) × 2 (high- vs. low-MTL function) ANOVA revealed a significant main effect of frontal status, with the high-FL group reporting significantly better plans than the low-FL group, F(1, 28) = 10.30, MSE = .78, p < .005. There was no main effect of medial temporal status, F(1, 28) = 2.6, p > .11, nor was there an interaction, F(1, 28) = 4.02, p > .05. Only the low-FL group was impaired relative to the young, t(46) = 4.68, p <.001.
Table 4.
Mean (SD) plan rating as a function of group.
| Young adults | 2.5 (.88) | |
|---|---|---|
| Older adults | ||
| MTL function | FL function |
|
| High | Low | |
| High | 2.75 (.46) | 1.13 (1.25) |
| Low | 1.63 (.92) | 1.25 (.71) |
Note: FL, frontal lobe; MTL, medial temporal lobe.
There was also a positive correlation between plan quality and PM performance, r = .53, p < .01, as well as a negative correlation between plan quality and button press errors, r = −.38, p < .05, among older adults.
2.4. Time perception
Means and standard deviations for the time perception data are presented in Table 5. There were no effects of MTL function on either of the measures, nor did it interact with FL function. There were large differences in variances across groups in verbal time estimation and so non-parametric tests were used to analyze these data. A Mann—Whitney test of verbal time estimation (correct time = 27 s), revealed a significant difference between the high-FL group (Mdn = 26 s) and the low-FL group (Mdn =35s), U = 82.5, p < .05, whereby low-FL functioning older adults produced significantly longer and less accurate time estimates than high-FL functioning older adults. No overall age effect emerged, U = 419.00, p > .11, although the young (Mdn = 28 s) performed significantly more accurately than the low-FL group, U = 159.5, p < .05, whereas there was no difference between the young and the high-FL group. Spearman’s correlation coefficients indicated non-significant correlations between verbal time estimation and PM performance, rs = −.23, p > .05, and between verbal time estimation and clock monitoring, rs = −.16, p > .05.
Table 5.
Mean (SD) time perception as a function of group.
| Time estimation (27 s) | Time production (10 s) | |
|---|---|---|
| Young adults | 28.44 (8.78) | 10.32 (3.45) |
| Older adults | ||
| High-FL | 29.00 (9.22) | 9.64 (2.70) |
| Low-FL | 59.12(69.13) | 8.21(2.86) |
Note: FL, frontal lobe.
Analysis of the time production task revealed no significant effect of frontal status, F(1, 28) = 2.09, and no overall effect of age, t(62) = 1.76, p > .08. However, a significant difference did emerge between young adults (M = 10.32 s) and the low-FL group (M = 8.21 s), t(46) = 2.10, p < .05, whereby young adults made more accurate time estimations than the low-FL group, whereas the young and the high-FL group did not differ (M = 9.64), t(46) = .68, p > .05. Performance on the production task did not correlate with either PM performance, r =.18, p > .05, or with clock monitoring, r = .05, p > .05.
2.5. Question answering
The number of questions answered on the background task was also assessed (see Table 6). The high-FL group answered significantly more questions correctly than the low-FL group, F(1, 28) = 5.79, MSE = .03, p < .05. There was no effect of medial temporal status, F(1, 28) = 1.1, nor was there an interaction, F(1, 28) = 1.3. There was a reverse effect of age, in that older adults (M =.48) answered significantly more questions correctly than did young adults (M = .36), t(62) = −6.98, p < .001. However, both the high-FL group and the low-FL group answered significantly more questions correctly than young adults, t(46) = 8.16, p< .001 for the high-FL group, and t(46) = −4.41, p < .001 for the low-FL group. Thus, the proportion of questions answered correctly cannot account for the poorer PM performance of the low-FL group. In fact, there was no correlation (r = −.06) between question-answering accuracy and PM performance.
Table 6.
Question answering as a function of group.
| Group | Dependent measure |
|
|---|---|---|
| Mean # of questions answered (SD) | Proportion correct | |
| FL function | ||
| High | 298 (33.70) | .51 (.07) |
| Low | 266 (27.80) | .45 (.08) |
| MTL function | ||
| High | 282 (31.32) | .49 (.09) |
| Low | 281 (38.50) | .47 (.07) |
| Young | 290 (35.20) | .36 (.06) |
Note: FL, frontal lobe; MTL, medial temporal lobe.
However, the high-FL group and the young adults attempted significantly more questions than the low-FL group, t(28) = 2.95, p <.01 and t(46) = 2.36, p < .03 respectively, but did not differ from each other, t(46) = −.80.
2.6. Regression analysis
Zero-order correlations between PM performance and the other variables investigated in this study are shown in Table 7. Because the FL factor was related to PM performance as well as to other predictor variables—clock monitoring in the 5th interval, planning, and verbal time estimation—we conducted a simultaneous regression analysis to identify more precisely the nature of the relation between these variables and PM task completion. This analysis revealed that strategic clock monitoring accounted for 55% of the variance, and the FL factor accounted for an additional 11% of the variance. The other variables did not account for any additional variance.
Table 7.
Correlations between predictor variables and prospective memory (PM) among older adults.
| Variable | Prospective memory (PM) |
|---|---|
| Clock monitoring (5th interval) | .74** |
| FL factor | .55** |
| Plan quality | .53** |
| Time estimation | −.23 |
Note: FL, frontal lobe.
p < .01 (two-tailed).
2.7. Retrospective memory
As a measure of retrospective memory, participants were asked to describe the tasks involved in the experiment. All participants correctly reported that they were to answer multiple choice questions and make alternating button presses at 5-min intervals. The MTL factor did not predict performance differences among older adults in any of these measures, Fs<1.
3. Discussion
The present study demonstrated a relation between neuropsychological function and the execution of time-based PM, thereby building upon previous findings regarding the role of frontal/executive function in PM. Older adults characterized as possessing high executive function, as determined by performance on a set of neuropsychological tests, executed significantly more PM tasks than did older adults characterized as possessing low executive function and made significantly fewer PM errors. These results are consistent with theoretical notions regarding the role of the frontal lobes in the performance of time-based PM tasks. They are also in line with recent findings with patient populations (Burgess et al., 2008; Kliegel, Jäger, Altgassen, et al., 2008; Troyer & Murphy, 2007), as well as with results of imaging work (Okuda et al., 2007), which have suggested a prominent role for the frontal lobes in time-based PM. Although the neuropsychological tests provide only an indirect measure of frontal function, the FL factor score has previously been found to be predictive of both source memory (Glisky et al., 1995, 2001; Glisky & Kong, 2008) and event-based PM (McDaniel et al., 1999), both of which have been associated more directly with frontal functioning (Bisiacchi, 1996; Glisky, 1996; Janowsky, Shimamura, & Squire, 1989; Senkfor & Van Petten, 1998; Swick, Senkfor, & Van Petten, 2006). The present study thus provides evidence that time-based PM depends on at least some of the same executive processes that have been implicated in source memory and in many event-based PM tasks.
One process that appears to be involved is monitoring. PM requires monitoring of the environment for a cue or stimulus that might signal the appropriateness of performing a previously formed intention. It is possible that low-frontal lobe function is associated with less frequent monitoring of the environment or in this case, monitoring of the clock. This, in fact, was the case. More importantly, however, was the differential pattern of clock monitoring across groups. Young people and older adults with high-frontal function monitored the clock significantly more often during the 1min intervals just prior to target times than adults with low-frontal function.1 This pattern suggests that the frontal lobes may be particularly involved in strategic monitoring, whereby individuals with high-frontal function initiate increased monitoring in the critical period just prior to the target time. If this were the case, we might also expect to find that older adults with high-FL scores have better time perception abilities than those with low-FL scores. Although this finding was obtained in the verbal estimation task, consistent with previous findings that time estimation is a frontally mediated process (Picton et al., 2006; Rubia, 2008; Rubia & Smith, 2004), older adults with low-FL function gave verbal time estimates that significantly overestimated the amount of time that had passed. One might therefore have expected the low-FL group to check the clock sooner than the other groups, but this did not happen.
However, the time estimation tasks in the present study required participants to estimate time in the absence of a secondary task, whereas in the PM task, participants were engaged in an additional cognitive task (i.e., answering trivia questions). Previous studies (Craik & Hay, 1999; Hertzog, Touron, & Hines, 2007) have shown that older adults underestimate the passage of time when engaged in attentionally demanding cognitive tasks. This might be especially likely for those with poor attentional control (i.e., the low-FL group). Given the engaging and attentionally demanding nature of the trivia questions in the present study, low-FL participants might have underestimated the amount of time that had passed and consequently failed to increase their clock monitoring activity in the 5th interval. The lack of correlation between performance on the verbal estimation task and performance on the PM task may therefore be attributable to the fact that there was no ongoing activity during the verbal estimation task.
Although a significant difference between the high- and low-FL groups was revealed in the verbal estimation task but not the time production task, the pattern of performance across groups was similar in both tasks. Younger adults made more accurate estimations and productions than the low-FL group, but the young and the high-FL group did not differ. This pattern of performance suggests that frontal status impacted performance of both the time estimation and time production tasks, although the small differences and relatively large variances on the production task may have made it difficult to obtain significant differences between the high- and low-FL groups.
A second process thought to be implicated in PM involves the development and initiation of plans that can assist in the execution of previously formed intentions (Kliegel et al., 2000; Kliegel, Mackinlay, & Jäger, 2008; Mäntylä, 1996), processes likely associated with frontal function. In the present experiment, older adults in the high-FL group reported higher-rated plans for remembering the order in which to press buttons, and successfully performed more PM tasks than did those in the low-FL group. However, the FL groups did not differ in the number of button pressing errors. This finding, considered along with the relation between higher-rated plans and more successful PM performance, suggests that having a well-developed plan increased one’s awareness of the impending arrival of the target time, thereby contributing to successful PM task completion. Remembering which button to press may have depended more on retrospective memory, which did not differ significantly across FL groups in this study.
Alternatively, the plan could have played a role in another process believed to be important in PM: the binding of the cue (in this case, the clock) to the specific intended action at encoding (in this case, the button press). This is an especially attractive possibility given previous findings that the FL composite score, used to characterize frontal function in the present study (and in McDaniel et al., 1999), has previously been associated with source memory performance in older adults (Glisky & Kong, 2008; Glisky et al., 1995, 2001), such that those with low-FL scores performed more poorly than those with high-FL scores. A plausible explanation of those findings is that the frontal lobes are involved in integrating the content and context of an experience during initial encoding, a process that has been associated with the frontal lobes, particularly BA 10 (Mitchell, Johnson, Raye, & D’Esposito, 2000; Prabhakaran, Narayanan, Zhao, & Gabrieli, 2000; for further discussion see Glisky & Kong, 2008). This same integration function may be involved in the integration of cue and intention, which may be critical for successful PM.
Gollwitzer (1999) reported a technique designed to bind a cue to an intended action, which he referred to as “implementation intentions”. Implementation intentions rely upon the formation of an association at encoding between an intention and a specific aspect of the contextual environment (e.g., time, place) in which the intention is likely to be implemented (e.g., “If situation X occurs, then I will do Y”). Implementation instructions have been shown to result in enhanced PM performance in older adults and in frontal lobe patients (Lengfelder & Gollwitzer, 2001; Liu & Park, 2004). Gollwitzer (1999) proposed that implementation intentions are effective because they result in heightened accessibility to, or apprehension of the cue, as well as in automatic retrieval of the intention when the cue occurs, thereby reducing the need to monitor the environment. Although Cohen and Gollwitzer (2008) have suggested that implementation intentions are always event-based, in the present study the presence of the clock icon on the screen may have served as the cue for the intended action. High-frontal older adults and young adults may have constructed their own implementation intentions in which the plan for remembering which button to press resulted in good integration of the cue and the intended action (e.g., “If the elapsed time on the clock ends in a 5, press 1; if it ends in a 0, press 2.”), thereby contributing to their better PM performance. Low-frontal functioning older adults, on the other hand, having formed less effective plans, may also not have developed implementation intentions and therefore were not able to benefit from heightened accessibility of the cue (i.e., the clock) or automatic intention retrieval. They thus failed to initiate the PM task at all on several occasions.
An alternative but related way to interpret the failure of the low-FL group to initiate the PM task is in terms of goal maintenance theory (Braver & West, 2008), which contends that a fundamental function of the prefrontal cortex is the representation and maintenance of goal information over some extended period. According to this view, older adults with low-frontal function should have difficulty with tasks in which goals must be maintained over time, as is necessary in time-based PM. Failure to maintain the goal in an accessible state would in this case result in a failure to initiate clock monitoring at the appropriate time and in fewer PM tasks being executed. The fact that PM failures are not an all-or-none phenomenon but instead occur rather sporadically throughout the course of the experiment, suggest that the PM goal is not forgotten but drops temporarily below some threshold level of activation (see also Schmitter-Edgecombe & Wright, 2004).
A number of variables thus seem to have influenced successful PM performance. The regression analysis showed, not surprisingly, that clock monitoring in the critical final minute before the target time, accounted for the greatest share of the variance. The relation between frontal function and clock monitoring, however, suggests that strategic clock monitoring was at least partly dependent on good frontal function. In addition, frontal function contributed variance over and above that captured by clock monitoring. This additional contribution may reflect the finding that those with good frontal function developed better plans perhaps through the establishment of implementation intentions, which may have helped to integrate the cue and the PM response. High-FL older adults may also have been more likely to maintain the overall PM goal throughout the task.
Finally, the benefits of good frontal function in the time-based PM task appear also to depend on retrospective memory. Although at the end of the experiment all older adults were able to recall the tasks that they were to perform, the interaction between the FL factor and the MTL factor in PM performance and in RM errors suggests an RM component. So, even if the intention is initiated (i.e., in the high-FL group), people with poorer memory function might have more difficulty remembering the details of the task, and thus fail to press the correct button. If the intention is not initiated, however (i.e., in the low-FL group), MTL function will be of little consequence.
In summary, the present study has added to our understanding of frontal lobe involvement in tasks of time-based PM. The findings of this study are in line with theoretical notions concerning a general role for frontal function in PM, and also provide insight into some of the specific processes for which the frontal lobes are critically important. The present findings suggest that although strategic clock monitoring, plan development, and time estimation all affect PM performance, it is frontal function that drives each of those, and in turn, predicts PM performance. Furthermore, the finding that high-FL older adults did not differ from young adults in PM performance suggests that declining PM is not an unavoidable consequence of aging, but is instead primarily the product of diminished frontal function.
Acknowledgments
This research was supported by National Institute on Aging Grant AG14792 as well as by a grant from the Social and Behavioral Sciences Research Institute at the University of Arizona. We would like to thank Drs. Lee Ryan and Cyma Van Petten for their valuable insight and assistance with this project, as well as Dr. Jonathon Forster for his assistance in programming.
Footnotes
A similar pattern of clock monitoring in a time-based prospective memory task has also been demonstrated in children (Mäntylä, Carelli, & Forman, 2007).
References
- Benton AL, de Hamsher KS. Multilingual Aphasia Examination Manual. University of Iowa; Iowa City: 1976. [Google Scholar]
- Bisiacchi PS. The neuropsychological approach in the study of prospective memory. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Erlbaum; Mahwah, NJ: 1996. pp. 297–318. [Google Scholar]
- Block RA, Zakay D. Prospective memory involves time estimation and memory processes. In: Glicksohn J, Myslobodsky MS, editors. Timing the future: The case for a time-based prospective memory. World Scientific; New Jersey: 2008. pp. 25–50. [Google Scholar]
- Braver TS, West R. Working memory, executive control, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed Psychology Press; New York: 2008. pp. 311–372. [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, et al. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ, Okuda J, Schölvinck, Simons JS. On the role of rostral prefrontal cortex (area 10) in prospective memory. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory. Erlbaum; New York: 2008. pp. 235–260. [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: A lateral vs. medial dissociation. Neuropsychologia. 2003;41:906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Cohen A-L, Gollwitzer PM. The cost of remembering to remember: Cognitive load and implementation intentions influence ongoing task performance. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory. Erlbaum; New York: 2008. pp. 367–390. [Google Scholar]
- Costa A, Peppe A, Caltagirone C, Carlesimo GA. Prospective memory impairment in individuals with Parkinson’s disease. Neuropsychology. 2008;22:283–292. doi: 10.1037/0894-4105.22.3.283. [DOI] [PubMed] [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Klix F, Hagendorf H, editors. Human memory and cognitive capabilities: Mechanisms and performances. Elsevier Science Publishers, B.V.; North-Holland: 1986. pp. 409–422. [Google Scholar]
- Craik FIM, Bialystok E. Planning and task management in older adults: Cooking breakfast. Memory & Cognition. 2006;34:1236–1249. doi: 10.3758/bf03193268. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Hay JF. Aging and judgments of duration: Effects of task complexity and method of estimation. Perception and Psychophysics. 1999;61:549–560. doi: 10.3758/bf03211972. [DOI] [PubMed] [Google Scholar]
- Daigneault S, Braun CMJ, Whitaker HA. Early effects of normal aging on perseverative and non-perseverative prefrontal measures. Psychology and Aging. 1992;5:148–151. [Google Scholar]
- Dellis DC, Kramer J, Kaplan E, Ober BA. The California Verbal Learning Test. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed Psychology Press; New York: 2008. pp. 1–54. [Google Scholar]
- d’Ydewalle G, Bouckaert D, Brunfaut E. Age-related differences and complexity of ongoing activities in time- and event-based prospective memory. The American Journal of Psychology. 2001;114:411–423. [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Richardson SL, Guynn MJ, Cunfer AR. Ageing and prospective memory: Examining the influence of self-initiated processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:996–1007. doi: 10.1037//0278-7393.21.4.996. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, et al. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General. 2005;1314:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Einstein GO, Smith RE, McDaniel MA, Shaw P. Aging and prospective memory: The influence of increased task demands at encoding and retrieval. Psychology and Aging. 1997;12:479–488. doi: 10.1037//0882-7974.12.3.479. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SF, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forster KI, Forster JC. DMDX: A Windows display program with millisecond accuracy. Behavior Research Methods, Instruments, & Computers. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. Lippincott-Raven; Philadelphia, PA: 1997. [Google Scholar]
- Glisky EL. Prospective memory and the frontal lobes. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Erlbaum; Mahwah, NJ: 1996. pp. 249–266. [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:809–822. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54:493–503. [Google Scholar]
- Graf P, Grondin S. Time perception and time-based prospective memory. In: Glicksohn J, Myslobodsky MS, editors. Timing the future: The case for a time-based prospective memory. World Scientific; New Jersey: 2008. pp. 1–24. [Google Scholar]
- Harris JE, Wilkins AJ. Remembering to do things: A theoretical frame-work and an illustrative experiment. Human Learning. 1982;1:123–136. [Google Scholar]
- Hart RP, Kwentus JA, Wade JB, Taylor JR. Modified Wisconsin Card Sorting Test in elderly normal, depressed and demented patients. Clinical Neuropsychologist. 1988;2:49–56. [Google Scholar]
- Haug H, Eggers R. Morphology of the human cortex cerebri and corpus striatum during aging. Neurobiology of Aging. 1991;12:336–338. doi: 10.1016/0197-4580(91)90013-a. [DOI] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychology and Aging. 2004;19:27–39. doi: 10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Touron DR, Hines JC. Does a time-monitoring deficit influence older adults’ delayed retrieval shift during skill acquisition? Psychology and Aging. 2007;22:607–624. doi: 10.1037/0882-7974.22.3.607. [DOI] [PubMed] [Google Scholar]
- Insel K, Morrow D, Brewer B, Figueredo A. Executive function, working memory, and medication adherence among older adults. Journal of Gerontology: Psychological Sciences. 2006;61B:P102–P107. doi: 10.1093/geronb/61.2.p102. [DOI] [PubMed] [Google Scholar]
- Jäger T, Kliegel M. Time-based and event-based prospective memory across adulthood: Underlying mechanisms and differential costs on the ongoing task. The Journal of General Psychology. 2008;135:4–22. doi: 10.3200/GENP.135.1.4-22. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Katai S, Maruyama T, Hashimoto T, Ikeda S. Event based and time based prospective memory in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:704–709. doi: 10.1136/jnnp.74.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA, Price KJ. An investigation of prospective memory in children with ADHD. Child Neuropsychology. 2001;7:162–171. doi: 10.1076/chin.7.3.162.8744. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, Altgassen M, Shum D. Clinical neuropsychology of prospective memory. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory. Erlbaum; New York: 2008. pp. 283–308. [Google Scholar]
- Kliegel M, Jäger T, Phillips LH. Adult age differences in event-based prospective memory: A meta-analysis on the role of focal versus nonfocal cues. Psychology and Aging. 2008;23:203–208. doi: 10.1037/0882-7974.23.1.203. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Mackinlay R, Jäger T. A life span approach to the development of complex prospective memory. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory. Erlbaum; New York: 2008b. pp. 187–216. [Google Scholar]
- Kliegel M, Mackinlay R, Jäger T. Complex prospective memory: Development across the lifespan and the role of task interruption. Developmental Psychology. 2008d;44:612–617. doi: 10.1037/0012-1649.44.2.612. [DOI] [PubMed] [Google Scholar]
- Kliegel M, McDaniel MA, Einstein GO. Plan formation, retention, and execution in prospective memory: A new approach and age-related deficits. Memory and Cognition. 2000;28:1041–1049. doi: 10.3758/bf03209352. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJR, et al. Cerebral blood flow, blood volume, and oxygen utilization. Brain. 1990;113:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Lengfelder A, Gollwitzer PM. Reflective and reflexive action control in patients with frontal brain lesions. Neuropsychology. 2001;15:80–100. doi: 10.1037//0894-4105.15.1.80. [DOI] [PubMed] [Google Scholar]
- Lezak MD. The problems of assessing executive functions. International Journal of Psychology. 1982;17:281–297. [Google Scholar]
- Liu LL, Park DC. Aging and medication adherence: The use of automatic processes to achieve effortful things. Psychology and Aging. 2004;19:318–325. doi: 10.1037/0882-7974.19.2.318. [DOI] [PubMed] [Google Scholar]
- Mäntylä T. Activating actions and interrupting intentions: Mechanisms of retrieval sensitization in prospective memory. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Erlbaum; Mahwah, NJ: 1996. pp. 93–113. [Google Scholar]
- Mäntylä T, Carelli MG, Forman H. Time monitoring and executive functioning in children and adults. Journal of Experimental Child Psychology. 2007;96:1–19. doi: 10.1016/j.jecp.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Martin M, Kliegel M, McDaniel MA. The involvement of executive function in prospective memory performance of adults. International Journal of Psychology. 2003;38:195–206. [Google Scholar]
- Maylor EA. Age-related impairment in an event-based prospective memory task. Psychology and Aging. 1996;11:74–78. doi: 10.1037//0882-7974.11.1.74. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO, Jacoby LL. New considerations in aging and memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed Psychology Press; New York: 2008. pp. 251–310. [Google Scholar]
- McDaniel MA, Einstein GO, Rendell PG. The puzzle of inconsistent declines in prospective memory: A multiprocess explanation. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory. Erlbaum; New York: 2008. pp. 141–160. [Google Scholar]
- McDaniel MA, Einstein GO, Stout AC, Morgan Z. Aging and maintaining intentions over delays: Do it or lose it. Psychology and Aging. 2003;18:823–835. doi: 10.1037/0882-7974.18.4.823. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Glisky EL, Rubin SR, Guynn MJ, Routhieaux BC. Prospective memory: A neuropsychological study. Neuropsychology. 1999;13:103–110. doi: 10.1037//0894-4105.13.1.103. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Robinson-Riegler B, Einstein GO. Prospective remembering: Perceptually driven or conceptually-driven processes? Memory and Cognition. 1998;26:121–134. doi: 10.3758/bf03211375. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. FMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Yamadori A, Frith CD, et al. Differential involvement of regions of rostral prefrontal (Brodman area 10) in time- and event-based prospective memory. International Journal of Psychophysiology. 2007;64:233–246. doi: 10.1016/j.ijpsycho.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, Morrell RW, Mayhorn CB. Effect of age on event-based and time-based prospective memory. Psychology and Aging. 1997;12:314–327. doi: 10.1037//0882-7974.12.2.314. [DOI] [PubMed] [Google Scholar]
- Park DC, Morrel RW, Frieske D, Kincaid D. Medication adherence behaviors in older adults: Effects of external cognitive supports. Psychology and Aging. 1992;7:252–256. doi: 10.1037//0882-7974.7.2.252. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Henry JD, Martin M. Adult aging and prospective memory: The importance of ecological validity. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory. Erlbaum; New York: 2008. pp. 161–185. [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: Effects of frontal lesions. Neuropsychologia. 2006;44:1195–1209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JDE. Integration of diverse information in working memory within the frontal lobe. Nature Neuroscience. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue K, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rendell PG, Craik FIM. Virtual week and actual week: Age-related differences in prospective memory. Applied Cognitive Psychology. 2000;14:S43–S62. [Google Scholar]
- Rubia K. The neural correlates of timing functions. In: Glicksohn J, Myslobodsky MS, editors. Timing the future: The case for a time-based prospective memory. World Scientific; New Jersey: 2008. pp. 213–238. [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: A review. Acta Neurobiologiae Experimentalis. 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Wright MJ. Event-based prospective memory following severe closed-head injury. Neuropsychology. 2004;18:353–361. doi: 10.1037/0894-4105.18.2.353. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential study of source and item memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London, B. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Simons JS, Schölvink ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44:1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Smith RE, Bayen UJ. Source of adult age differences in event-based prospective memory: A multinomial modeling approach. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:623–635. doi: 10.1037/0278-7393.32.3.623. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Memory for facts, source and context: Can frontal lobe dysfunction explain age-related differences? Psychology and Aging. 1994;9:149–159. doi: 10.1037//0882-7974.9.1.149. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychological Bulletin. 1984;95:3–28. [PubMed] [Google Scholar]
- Swick D, Senkfor AJ, Van Petten C. Source memory retrieval is affected by aging and prefrontal lesions: Behavioral and ERP evidence. Brain Research. 2006;1107:161–176. doi: 10.1016/j.brainres.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ. Memory for intentions in amnestic mild cognitive impairment: Time- and event-based prospective memory. Journal of the International Neuropsychological Society. 2007;13:365–369. doi: 10.1017/S1355617707070452. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford University Press; New York: 1983. [Google Scholar]
- Van Petten C, Plante E, Davidson PSR, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: Relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale — Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale — Revised. Psychological Corporation; New York: 1987. [Google Scholar]
- Wechsler D. Wechsler Memory Scale — III manual. Psychological Corporation; San Antonio: 1997. [Google Scholar]
- West RL. An application of prefrontal cortex theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West R, Covell E. Effects of aging on age-related neural activity related to prospective memory. Neuroreport: For Rapid Communication of Neuroscience Research. 2001;12:2855–2858. doi: 10.1097/00001756-200109170-00020. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Cockburn J, Baddeley AD. The Rivermead Behavioral Memory Test. Thames Valley Test Company; Titchfield, UK: 1985. [Google Scholar]
- Zeintl M, Kliegel M, Hofer SM. The role of processing resources in age-related prospective and retrospective memory within old age. Psychology and Aging. 2007;22:826–834. doi: 10.1037/0882-7974.22.4.826. [DOI] [PubMed] [Google Scholar]


