Abstract
BACKGROUND:
Myotonic dystrophy type 1 (DM1) has been associated with an increased risk of sudden death, either by heart block or malignant ventricular arrhythmias. Identifying patients at risk remains difficult and no consensus has been reached regarding the best approach for follow-up and prevention of sudden death.
OBJECTIVES:
To identify noninvasive clinical and electrocardiographic predictors of adverse cardiac events in patients with DM1.
METHODS:
Clinical and serial electrocardiographic data on 428 patients with a DNA-proven diagnosis of DM1, followed during a mean period of 11.7 years, were reviewed. Variables associated with adverse cardiac events were identified.
RESULTS:
Eleven patients (2.6%) experienced sudden death and 13 (3.0%) required implantation of a pacemaker. On univariate analysis, adverse events were associated with advancing age, prolongation of the PR, QRS and corrected QT (QTc) intervals, as well as the degree of neuromuscular impairment. No such relationship was found with the extent of genetic anomaly (number of cytosine-thymine-guanine repeats). However, multivariate analysis using Cox proportional hazards models showed that only baseline PR and QTc intervals were significantly linked to the end points of sudden death or pacemaker implantation; the age-adjusted RR was 3.7 (95% CI 1.5 to 8.6) if baseline PR was 200 ms or longer (P=0.003), and 3.0 (95% CI 1.0 to 8.8) if the baseline QTc was 450 ms or longer (P=0.047).
CONCLUSIONS:
In a large unselected cohort of 428 patients with DM1, the cumulative incidence of sudden death was relatively low, and the delayed conduction on surface electrocardiogram was found to be potentially helpful for identifying patients at risk for sudden death or pacemaker implantation.
Keywords: Electrocardiography, Epidemiology, Mortality, Pacemakers, Sudden death
Abstract
HISTORIQUE :
La dystrophie myotonique de type 1 (DM1) s’associe à un accroissement du risque de mort subite, que ce soit par blocage cardiaque ou arythmies ventriculaires malignes. Il reste difficile de dépister les patients à risque et on n’est pas parvenu à un consensus au sujet de la meilleure démarche de suivi et de prévention de la mort subite.
OBJECTIFS :
Déterminer les prédicteurs cliniques et électrocardiographiques non effractifs d’événements cardiaques indésirables chez des patients atteints de DM1.
MÉTHODOLOGIE :
Les auteurs ont analysé les données électrocardiographiques cliniques et sérielles de 428 patients ayant un diagnostic de DM1 corroboré par ADN, suivis pendant une période moyenne de 11,7 ans. Ils ont repéré les variables associées à des événements cardiaques indésirables.
RÉSULTATS :
Onze patients (2,6 %) ont péri d’une mort subite et 13 (3,0 %) ont dû se faire implanter un stimulateur cardiaque. D’après l’analyse univariée, les événements indésirables s’associaient au vieillissement, à la prolongation des intervalles PR, QRS et QT corrigés (QTc), ainsi qu’au degré d’atteinte neuromusculaire. On n’a découvert aucun lien de ce genre avec l’étendue de l’anomalie génétique (nombre de répétitions cytosine-thymine-guanine). Cependant, l’analyse multivariée faisant appel aux hasards proportionnels de Cox a révélé que seuls les intervalles PR et QTc de départ étaient liés de manière significative aux paramètres ultimes de mort subite ou d’installation d’un stimulateur cardiaque. Le RT rajusté selon l’âge correspondait à 3,7 (95 % IC 1,5 à 8,6) si l’intervalle PR de départ était de 200 ms ou plus (P=0,003) et à 3,0 (95 % IC 1,0 à 8,8) si l’intervalle QTc de départ était de 450 ms ou plus (P=0,047).
CONCLUSIONS :
Dans une vaste cohorte non sélectionnée de 428 patients atteints de DM1, l’incidence cumulative de mort subite était relativement faible et la conduction retardée à l’électrocardiogramme de surface pouvait être utile pour repérer les patients vulnérables à une mort subite ou à l’installation d’un stimulateur cardiaque.
Myotonic dystrophy type 1 (DM1) is the most common form of adult dystrophy. The reported worldwide prevalence is estimated to be 2.1 to 14.3 per 100,000 population. In the Saguenay Lac-Saint-Jean (SLSJ) region of Quebec, where a common ancestor couple has been identified, the prevalence reaches 189 per 100,000 population (1). DM1 is a multisystemic autosomal dominant disorder with variable expression resulting from an unstable cytosine-thymine-guanine (CTG)-repeat expansion in the 3′ untranslated region of the myotonin kinase gene on chromosome 19q13.3 (2). Its clinical picture includes ptosis and weakness of the facial, jaw and anterior neck muscles, weakness of the limbs progressing from distal to proximal limbs, myotonia, as well as involvement of other systems. Cardiac arrhythmias and conduction defects in patients with DM1 have been recognized for many years (3–6). In more recent years, cardiac structural and functional anomalies have become more readily identified using different imaging techniques. In some cases, correlations have been made between the severity of these anomalies and the degree of electrophysiological disturbances (7–9).
Whereas pneumonia and respiratory insufficiency are the most common cause of death in patients with severe weakness, sudden death has been reported to be more frequent in DM1 patients than in the general population (10,11). Sudden death in DM1 patients has traditionally been associated with conduction defects and heart block, but malignant ventricular arrhythmias have been identified and may contribute significantly to those deaths, complicating the task of developing a strategy to prevent sudden death in patients with DM1 (12–14). Such a strategy may involve the use of devices such as standard pacemakers or implantable cardioverter-defibrillators (ICDs). However, it is obvious that a better understanding of the evolution of conduction disturbances and of the pathophysiology of sudden death in DM1 is needed to identify high-risk patients who will benefit the most from invasive therapies.
Many studies have been performed to find variables that could help predict which patients are at increased risk for an adverse cardiac event (15–23). These studies, using different approaches, usually showed that advancing age and severity of neurological impairment, and a higher degree of conduction disturbance, assessed either by surface electrocardiogram (ECG) or by electrophysiological study, correlate with a worse outcome. However, there are conflicting results concerning the relationship between the extent of CTG repeat length and the severity of cardiac involvement. Currently, no clear consensus has been reached regarding the best way to investigate, follow-up on and treat cardiac involvement in DM1.
The rationale for the present study was to take advantage of the large cohort of patients with DM1 followed by the Neuromuscular Clinic, Carrefour de la santé (Saguenay, Quebec) to find easily identifiable predictors of adverse cardiac events. These predictors could eventually be used in the early selection of patients for prophylactic pacemaker implantation or invasive electrophysiological investigation, and in doing so, prevent sudden death of some patients.
METHODS
Charts of all patients followed at the Neuromuscular Clinic, Carrefour de la santé, with a diagnosis of myotonic dystrophy were reviewed. Of those, 428 patients with a DNA-proven diagnosis of DM1 were retained for the present study. Charts provided demographic and clinical data for each patient. Serial ECGs obtained during routine follow-up visits were reviewed to obtain ECG data pertinent to the study. Electrophysiological studies were rarely used at the time of the study, ambulatory monitoring was not systematically performed and results were not collected in the database. The primary end point for analysis was a composite of sudden death or pacemaker placement.
When available, death certificates, hospital charts and autopsy reports were reviewed to assess the cause of death. Sudden death was defined as death by a natural cause, occurring within 1 h of the onset of symptoms or unwitnessed death of a person known to be healthy and alive within 24 h in the absence of any other obvious cause.
Statistical analyses were conducted using SPSS software (SPSS Inc, USA). Kaplan-Meier survival curves were used to estimate the outcome experience according to a patient’s baseline characteristics. Log-rank tests were used to determine differences in outcome distributions. Cox proportional hazards models were used to take into account the simultaneous effect of age and other predictor variables of outcome identified by univariate analysis, and to estimate the age-adjusted RRs of sudden death or pacemaker need as well as 95% CIs. Rates of increase in the width of PR and QRS intervals between groups were compared using the nonparametric Mann-Whitney U test.
RESULTS
Baseline characteristics of the 428 patients included in the present population-based study are shown in Table 1. During a mean follow-up period of 11.7 years (range 0.02 to 23.5 years), 11 patients (2.6%) experienced sudden death and 14 (3.3%) required placement of a permanent pacemaker for clinically recognized indications. In most patients, such indications were symptomatic bradycardia secondary to atrioventricular (AV) block or sick sinus syndrome. Only two patients received prophylactic pacing after an electrophysiological study showed infrahissian conduction delay. Both had left fascicular block associated with right bundle branch block. Interestingly, they developed complete AV block within a few years of pacemaker placement. No patients were resuscitated from sudden cardiac death and none received an ICD. Overall, death occurred in 61 patients (14%) during the study period
TABLE 1.
Baseline characteristics of 428 patients with myotonic dystrophy type 1
| Age, years, mean (range) | 33 (2 to 81) |
| Male, n (%) | 192 (45) |
| Cytosine-thymine-guanine repeats, n (%) | |
| <200 | 59 (13.8) |
| 200 to 400 | 55 (12.9) |
| 401 to 850 | 92 (21.5) |
| 851 to 1100 | 78 (18.2) |
| 1101 to 1500 | 94 (21.9) |
| >1500 | 50 (11.7) |
| Koch classification, n (%) | |
| Infantile | 72 (16.8) |
| Early adult | 157 (36.7) |
| Adult | 111 (25.9) |
| Mild or late form | 56 (13.1) |
| Unknown | 32 (7.5) |
| Degree of neuromuscular impairment, n (%) | |
| None | 49 (11.4) |
| Mild | 228 (53.3) |
| Moderate to severe | 139 (32.5) |
| Unknown | 12 (2.8) |
| Mean PR interval, ms | 176 |
| Mean QRS interval, ms | 97 |
| Mean corrected QT, ms | 420 |
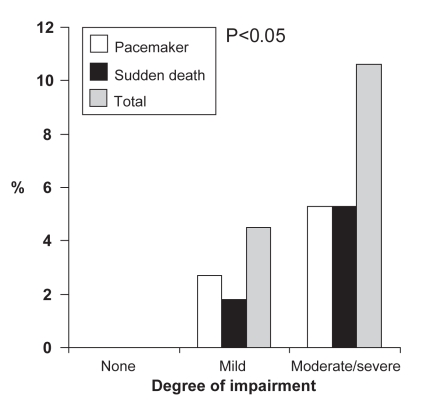
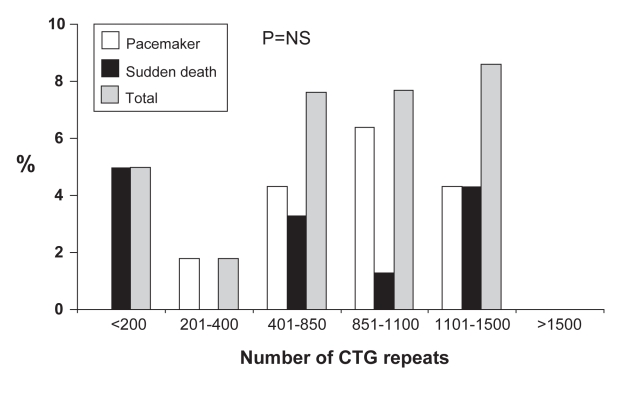
Univariate analysis using Kaplan-Meier survival curves showed that age (older than 32 years), PR (200 ms or longer), QRS (120 ms or longer), corrected QT (QTc; 450 ms or longer) and severity of neuromuscular impairment were predictive of sudden death or pacemaker placement. As shown in Figure 1, the number of both events increases with the severity of neurological impairment, with none occurring in the subgroup of patients with no clinical signs of myotonia. This relationship was not found with increasing numbers of CTG repeats (Figure 2).
Figure 1).
Percentage of pacemaker implantations or sudden deaths according to severity of neuromuscular impairment
Figure 2).
Percentage of pacemaker implantations or sudden deaths according to the number of cytosine-thymine-guanine (CTG) repeats. NS Not significant
However, multivariate analysis using Cox proportional hazards models showed that only two variables (PR and QTc) remained significant. The age-adjusted RR of sudden death or pacemaker placement was 3.7 (95% CI 1.5 to 8.6) if the baseline PR was 200 ms or longer, and 3.0 (95% CI 1.0 to 8.8) if the baseline QTc was 450 ms or longer.
A significant relationship between a more rapid increase in the QRS interval and the occurrence of sudden death or pacemaker implantation was identified. The median rate of increase in the QRS interval was 2.26 ms/year (range −14.00 ms/year to 20.00 ms/year) for those with an adverse cardiac event and only 0.37 ms/year (range −1.30 ms/years to 8.93 ms/year) for the rest of the cohort (P=0.006). However, this difference was mostly driven by pacemaker implantation rather than sudden death. No correlation between the rate of increase in the width of PR and QRS intervals over the years, and the degree of neuromuscular impairment or number of CTG repeats were identified.
DISCUSSION
Sudden death was responsible for 18% of all deaths in our cohort, occurring at an average rate of 0.25% per year with a cumulative incidence of 2.6% for a mean period of 11.7 years. This was slightly higher than a previously reported rate of 0.1% to 0.2% per year for the general population (24), especially considering that the mean age in the present cohort was only 33 years. However, this rate remains quite low compared with the rates of 0.4% to 2% per year that can be extracted from other series (11,15,19,25). This can be explained by the fact that our cohort was derived from a systematic ascertainment and follow-up program among family members of patients diagnosed with DM1. By doing so, the present study includes a number of lower-risk subjects with very mild disease who might otherwise have gone unrecognized and are probably not included in other series. The higher rate of sudden death among subjects with more severe neurological impairment (approximately 0.5% per year) and the absence of events in those with no signs of myotonia support this assumption.
It is our opinion that the rate of events described in our cohort represents a reliable estimate of the true natural history of unselected patients with DM1. The presence of a single referral neuromuscular centre for the entire SLSJ region, the lack of migration of the DM1 population living in a semi-isolated area and a systematic familial ascertainment and follow-up program at the Neuromuscular Clinic allowed us to proceed to population-based studies of the many clinical characteristics of this disease including cardiac risks, without introducing significant selection bias. Because we were able to link our population to a common ancestor couple, we often receive comments that it might represent a different subgroup of patients whose clinical behaviour could not be extrapolated to other subjects with DM1. We do not support this point of view and believe that our findings are applicable to any patient with DM1.
First, we must remember that this is a genetically transmitted disease and, by definition, it is normal to have familial clustering even over many centuries. In that sense, we can hypothesize that patients from other series are also genetically linked to some degree. Second, we cannot assume that our population represents a distinct clinical entity. The age at onset, the muscular involvement, the clinical phenotypes, the distribution of CTG expansions, the genotype-phenotype correlations, the age at death and the causes of death observed in the DM1 population of the SLSJ region (10,26–33) are similar to other published cohorts of DM1 patients, suggesting a comparable disease severity spectrum.
The results of the present study, like those of other published studies, emphasize the fact that patients with more severe neurological impairment are at greatest risk for adverse cardiac events. Interestingly, in our cohort, the magnitude of the genetic anomaly did not correlate with the risk of a cardiac event.
Although such a correlation between the extent of CTG repeats and the degree of cardiac involvement has been described in some series, others have shown conflicting results (9,17,18,25,34–39). Of note is the study by Lazarus et al (17); using precise invasive electrophysiological measurements, the study was unable to show such a relationship between conduction delays and the extent of CTG repeats. These differences among studies likely indicate that the conduction system is influenced by many other factors, some possibly still unidentified, that interact and create an unpredictable evolution of the conduction anomalies. The presence of conduction delay on baseline and serial ECG was helpful in identyfying patients at risk for future adverse cardiac events.
We believe that the prolongation of the QTc interval is mostly accounted for by a widening of the QRS interval and represents delayed conduction rather than a true repolarization anomaly and does not represent a form of long QT syndrome.
Clinicians who are involved in the follow-up of patients with DM1 should be aware that the presence of a clearly prolonged PR or QRST interval, especially if rapidly increasing, need to be taken seriously, emphasizing the need for regular ECG assessement, especially in patients with a more significant neuromuscular impairment. However, the management of patients with delayed conduction remains controversial.
In 2002, the American College of Cardiology/American Heart Association/North American Society for Pacing and Electrophysiology Task Force updated the 1998 guidelines for pacemaker implantation and, for the first time, introduced specific indications for neuromuscular diseases, recognizing the unpredictable evolution of AV conduction disease in those conditions. A new recommendation supported the use of prophylactic pacing in DM1 patients with first-degree AV block (40). However, this recommendation was of the class IIb level, reflecting the lack of randomized trials to support this approach.
Some centres currently advocate the use of prophylactic pacemakers in DM1 patients with an HV interval longer than 70 ms (15) and, while some abnormal ECG patterns are highly suggestive of delayed infrahissian conduction, a normal surface ECG does not rule out abnormal conduction or other forms of cardiac involvement. It is also important to remember that patients with a normal ECG, as well as subjects with a properly functioning pacemaker, have died suddenly, reflecting the presence of a different mechanism of sudden death such as malignant ventricular arrhythmias. Electrophysiological testing is more reliable in identifying delayed infrahissian conduction and potentially malignant ventricular arythmia. However, its role in the regular follow-up of patients with DM1 remains uncertain.
Nearly 500 patients with clinical or DNA-proven DM1 are currently followed at our clinic, the majority of them having a normal or only slightly abnormal surface ECG. We find it difficult and do not recommend routinely submitting all those patients to invasive electrophysiological testing, especially in the face of a relatively low risk of sudden death in the overall cohort. Too many questions remain unanswered. Among them, if the first electrophysiological study shows normal conduction, how should we follow the patient afterward, given the unpredictable nature of conduction anomalies in DM1 patients? The approach to inducible ventricular arrhythmia in asymptomatic patients is also not clear, and the place of ICDs in this situation has not been defined and could eventually raise some ethical questions, particularly in patients with severe neurological deficit who are at greatest risk.
In this situation, our current approach is to obtain a 12-lead ECG each year and to consider prophylactic pacing in patients with more advanced conduction disturbances such as right bundle branch block associated with left fascicular block, or bundle branch block with a significant increase in PR interval, especially if there is evidence of worsening conduction over time. When doubt exists, patients are referred for electrophysiological testing and, generally, they are determined to have prolonged HV intervals.
To maximize the potential cost effectiveness of this empirical approach, we advocate placing a simple ventricular demand pacemaker programmed at a low back-up frequency to avoid interfering with spontaneous rhythm and to minimize the potentially deleterious effect of permanent pacing from the right ventricular apex on cardiac function.
We believe that this approach remains consistent with current available data and recommendations (40–42).
CONCLUSION
In our large cohort of unselected patients with DM1, we observed that the cumulative incidence of sudden death was relatively low. We also showed that the prolongation of PR and QRST intervals, when present, is a marker of more severe cardiac involvement, and should be followed carefully, because those patients are at increased risk for an adverse cardiac event. Subjects with more severe neurological impairment also are at greater risk. According to current recommendations, prophylactic pacing can be considered in such patients, while recognizing that some might present with malignant ventricular arythmia rather than heart block. The eventual place of ICD in those subjects is certainly not defined at this time. More information about the exact mechanism of sudden death in those patients is required before a better and less empirical approach can be designed to prevent it.
Footnotes
FUNDING: The present study was supported by grants awarded by the Canadian Institutes for Health Research (CIHR) Neuromuscular Research Partnership Program (#MOP-49556) and by ECOGENE-21, a research program in community genetics and genomics supported by the Canada Research Chairs Program and by the CIHR (CIHR/Community Alliances for Health Research program grant #CAR43283).
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare regarding the present paper.
REFERENCES
- 1.Mathieu J, DeBraekeleer M, Prévost C. Genealogical reconstruction of myotonic dystrophy in the Saguenay-Lac-Saint-Jean area (Quebec, Canada) Neurology. 1990;40:839–42. doi: 10.1212/wnl.40.5.839. [DOI] [PubMed] [Google Scholar]
- 2.Harley HG, Brook JD, Rundle SA, et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992;355:545–6. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- 3.Harper PS. Myotonic Dystrophy. 2nd edn. London: WB Saunders; 1989. [Google Scholar]
- 4.Pelargonio G, Dello Russo A, Sanna T, De Martino G, Belloci F. Myotonic dystrophy and the heart. Heart. 2002;88:665–70. doi: 10.1136/heart.88.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips MF, Harper PS. Cardiac disease in myotonic dystrophy. Cardiovasc Res. 1997;33:13–22. doi: 10.1016/s0008-6363(96)00163-0. [DOI] [PubMed] [Google Scholar]
- 6.Finsterer J, Stöllberger C, Blazek G, Spahits E. Cardiac involvement in myotonic dystrophy, Becker muscular dystrophy and mitochondrial myopathy: A five-year follow-up. Can J Cardiol. 2001;10:1061–9. [PubMed] [Google Scholar]
- 7.De Ambroggi L, Raisaro A, Marchiano V, Radice S, Meola G. Cardiac involvement in patients with myotonic dystrophy: Characteristic features of magnetic resonance imaging. Eur Heart J. 1995;16:1007–10. doi: 10.1093/oxfordjournals.eurheartj.a061011. [DOI] [PubMed] [Google Scholar]
- 8.Vignaux O, Lazarus A, Varin J, et al. Right ventricular MR abnormalities in myotonic dystrophy and relationship with intracardiac electrophysiologic test findings: Initial results. Radiology. 2002;224:231–5. doi: 10.1148/radiol.2241010986. [DOI] [PubMed] [Google Scholar]
- 9.Bhakta D, Lowe MR, Groh WJ. Prevalence of structural cardiac abnormalities in patients with myotonic dystrophy type 1. Am Heart J. 2004;147:224–7. doi: 10.1016/j.ahj.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu J, Allard P, Potvin L, Prévost C, Bégin P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology. 1999;52:1658–62. doi: 10.1212/wnl.52.8.1658. [DOI] [PubMed] [Google Scholar]
- 11.deDie-Smulders CEM, Höweler CJ, Thijs C, et al. Age and causes of death in adult onset myotonic dystrophy. Brain. 1998;121:1557–63. doi: 10.1093/brain/121.8.1557. [DOI] [PubMed] [Google Scholar]
- 12.Merino JL, Carmona JR, Fernandez-Lozano I, Peinado R, Basrerra N, Sobrino JA. Mechanisms of sustained ventricular tachycardia in myotonic dystrophy. Circulation. 1998;98:541–6. doi: 10.1161/01.cir.98.6.541. [DOI] [PubMed] [Google Scholar]
- 13.Grigg LE, Chan W, Mond HG, Vohra JK, Downey WF. Ventricular tachycardia and sudden death in myotonic dystrophy, electrophysiologic and pathologic features. J Am Coll Cardiol. 1985;6:254–6. doi: 10.1016/s0735-1097(85)80286-2. [DOI] [PubMed] [Google Scholar]
- 14.Cannom DS, Wyman GM, Goldreyer BN. Clinical and induced ventricular tachycardia in myotonic dystrophy. J Am Coll Cardiol. 1984;4:625–8. doi: 10.1016/s0735-1097(84)80112-6. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus A, Varin J, Babuty D, Anselme F, Coste J, Duboc D. Long-term follow-up of arrhythmias in patients with myotonic dystrophy treated by pacing: A multicenter diagnostic pacemaker study. J Am Coll Cardiol. 2002;40:1645–52. doi: 10.1016/s0735-1097(02)02339-2. [DOI] [PubMed] [Google Scholar]
- 16.Mammarella A, Paradiso M, Antonini G, et al. Natural history of cardiac involvement in myotonic dystrophy (Steinert’s disease): A 13-year follow-up study. Adv Ther. 2000;17:238–51. doi: 10.1007/BF02853163. [DOI] [PubMed] [Google Scholar]
- 17.Lazarus A, Varin J, Ounnoughene Z, et al. Relationships among electrophysiological findings and clinical status, heart function, and extent of DNA mutation in myotonic dystrophy. Circulation. 1999;99:1041–6. doi: 10.1161/01.cir.99.8.1041. [DOI] [PubMed] [Google Scholar]
- 18.Groh WJ, Lowe MR, Zipes DP. Severity of cardiac conduction involvement and arrhythmias in myotonic dystrophy type 1 corelates with age and CTG repeat length. J Cardiovasc Electrophysiol. 2002;13:444–8. doi: 10.1046/j.1540-8167.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 19.Nishioka SA, Martinelli Filho M, Marie S, Zatz M, Costa R.Myotonic dystrophy and heart disease: Behavior of arrhythmic events and conduction disturbances Arq Bras Cardiol 200584330–6.(Abst) [DOI] [PubMed] [Google Scholar]
- 20.Chebel S, Ben Hamda K, Boughammoura A, Frith Ayed M, Ben Farhat MH. Cardiac involvement in Steinert’s myotonic dystrophy. Rev Neurol (Paris) 2005;161:932–9. doi: 10.1016/s0035-3787(05)85156-2. [DOI] [PubMed] [Google Scholar]
- 21.Colleran JA, Hawley RJ, Pinnow EE, Kokkinos PF, Fletcher RD. Value of the electrocardiogram in determining cardiac events and mortality in myotonic dystrophy. Am J Cardiol. 1997;80:1494–7. doi: 10.1016/s0002-9149(97)00742-x. [DOI] [PubMed] [Google Scholar]
- 22.Babuty D, Fauchier L, Tena-Carbi D, et al. Is it possible to identify infrahissian cardiac conduction abnormalities in myotonic dystrophy by non-invasive methods? Heart. 1999;82:634–7. doi: 10.1136/hrt.82.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babuty D, Fauchier L, Tena-Carbi D, et al. Significance of late potentials in myotonic dystrophy. Am J Cardiol. 1999;84:1099–101. doi: 10.1016/s0002-9149(99)00510-x. [DOI] [PubMed] [Google Scholar]
- 24.Braunwald’s Heart Disease . A Textbook of Cardiovascular Medicine. 7th edn. Philadelphia: Elsevier Saunders; 2005. pp. 65–8. [Google Scholar]
- 25.Sabovic M, Medica I, Logar N, Mandic E, Zidar J, Peterlin B. Relation of CTG expansion and clinical variables to electrocardiogram conduction abnormalities and sudden death in patients with myotonic dystrophy. Neuromuscul Disord. 2003;13:822–6. doi: 10.1016/s0960-8966(03)00138-x. [DOI] [PubMed] [Google Scholar]
- 26.Mathieu J, DeBraekeleer M, Prévost C, Boily C. Myotonic dystrophy: Clinical assessment of muscular disability in an isolated population with presumed homogeneous mutation. Neurology. 1992;42:203–8. doi: 10.1212/wnl.42.1.203. [DOI] [PubMed] [Google Scholar]
- 27.Bégin P, Mathieu J, Almirall J, Grassino A. Relationship between chronic hypercapnia and inspiratory muscle weakness in myotonic dystrophy. Am J Resp Crit Care Med. 1997;156:133–9. doi: 10.1164/ajrccm.156.1.9509041. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu J, Boivin P, Meunier D, Gaudreault M, Bégin P. Assessment of a disease-specific impairment rating scale in myotonic dystrophy. Neurology. 2001;56:336–40. doi: 10.1212/wnl.56.3.336. [DOI] [PubMed] [Google Scholar]
- 29.Brisson D, Tremblay M, Prévost C, Laberge C, Puymirat J, Mathieu J. Sibship stability of genotype and phenotype in myotonic dystrophy. Clin Genet. 2002;62:220–5. doi: 10.1034/j.1399-0004.2002.620306.x. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu J, Boivin H, Richards CL. Quantitative motor assessment in myotonic dystrophy. Can J Neurol Sci. 2003;30:129–36. doi: 10.1017/s0317167100053397. [DOI] [PubMed] [Google Scholar]
- 31.Laberge L, Bégin P, Montplaisir J, Mathieu J. Sleep complaints in patients with myotonic dystrophy. J Sleep Res. 2004;13:95–100. doi: 10.1111/j.1365-2869.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 32.Arsenault ME, Prévost C, Lescault A, Laberge C, Puymirat J, Mathieu J. Clinical characteristics of myotonic dystrophy type 1 patients with small CTG expansions. Neurology. 2006;66:1248–50. doi: 10.1212/01.wnl.0000208513.48550.08. [DOI] [PubMed] [Google Scholar]
- 33.Laberge L, Veillette S, Mathieu J, Auclair J, Perron M. The correlation of CTG repeat length with material and social deprivation in myotonic dystrophy. Clin Genet. 2007;71:59–66. doi: 10.1111/j.1399-0004.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 34.Antonini G, Giubilei F, Mammarella A, et al. Natural history of cardiac involvement in myotonic dystrophy: Correlation with CTG repeats. Neurology. 2000;55:1207–9. doi: 10.1212/wnl.55.8.1207. [DOI] [PubMed] [Google Scholar]
- 35.Clarke NRA, Kellion AD, Nixon J, Hilton-Jones D, Forfar JC. Does cytosine-thymine-guanine (CTG) expansion size predict cardiac events and electrocardiographic progression in myotonic dystrophy? Heart. 2001;86:411–6. doi: 10.1136/heart.86.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merlevede K, Vermander D, Theys P, Legius E, Ector H, Robberecht W. Cardiac involvement and CTG expansion in myotonic dystrophy. J Neurol. 2002;249:693–8. doi: 10.1007/s00415-002-0692-6. [DOI] [PubMed] [Google Scholar]
- 37.Melacini P, Villanova C, Menegazzo E, et al. Correlation between cardiac involvement and CTG trinucleotide repeat length in myotonic dystrophy. J Am Coll Cardiol. 1995;25:239–45. doi: 10.1016/0735-1097(94)00351-p. [DOI] [PubMed] [Google Scholar]
- 38.Finsterer J, Gharehbaghi-Schnell E, Stollberger C, Fheodoroff K, Seiser A. Relation of cardiac abnormalities and CTG-repeat size in myotonic dystrophy. Clin Genet. 2001;59:350–5. doi: 10.1034/j.1399-0004.2001.590509.x. [DOI] [PubMed] [Google Scholar]
- 39.Hardin BA, Lowe MR, Bhakta D, Groh WJ. Heart rate variability declines with increasing age and CTG repeat length in patients with myotonic dystrophy type 1. Ann Noninvasive Electrocardiol. 2003;8:227–32. doi: 10.1046/j.1542-474X.2003.08310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 Guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: Summary article. Circulation. 2002;106:2145–61. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 41.Hawley RJ, Colleran JA, Fletcher R, et al. Indications for cardiac pacemaker implantation in myotonic dystrophy. MedGenMed. 1999 Sep;7:E5. [PubMed] [Google Scholar]
- 42.Bushby K, Muntoni F, Bourke JP. 107th ENMC International Workshop: The management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th–9th June 2002, Naarden, the Netherlands. Neuromuscul Disord. 2003;13:166–72. doi: 10.1016/s0960-8966(02)00213-4. [DOI] [PubMed] [Google Scholar]