Abstract
BACKGROUND:
Heart transplantation remains the last treatment option for patients with end-stage cardiac disease. Such diseases include ischemic cardiomyopathy, nonischemic cardiomyopathy and other conditions such as arrhythmogenic right ventricular dysplasia, cardiac sarcoidosis and cardiac amyloidosis.
OBJECTIVE:
To review the changes that have occurred over time in the etiology of heart disease in patients requiring heart transplantation, and to compare the clinical and histological diagnoses of explanted hearts from patients with progressive cardiac disease.
METHODS:
The pathological findings of 296 surgically excised hearts over a 20-year period (January 1987 to July 2006) at one institution were examined. Patients were separated into groups based on year of heart transplantation. The tissue was examined to determine the underlying cardiac pathology leading to congestive heart failure. Patient records were reviewed for preoperative clinical diagnoses and other relevant data, including pretransplant endomyocardial biopsy (EMB) results, information regarding left ventricular assist devices and, finally, evidence of disease recurrence in the grafted heart.
RESULTS:
A shift in the underlying etiology was found in patients who underwent heart transplantation from 1992 to 1996, and 1997 to 2001. Between 1987 and 1997, the majority of transplant cases consisted of ischemic cardiomyopathies. From 1997 to 2001, the majority of patients had nonischemic cardiomyopathies, and this trend continued to 2006. A majority of patients with ischemic and hypertrophic cardiomyopathy were diagnosed correctly (96.5% and 82%, respectively) before transplantation. Most patients diagnosed post-transplant with lymphocytic (viral, 15%), hypersensitive/eosinophilic (25%) and giant cell (100%) myocarditis, arrhythmogenic right ventricle dysplasia (100%), cardiac sarcoidosis (83%) and iron overload toxicity-associated cardiomyopathy (100%) had been misdiagnosed in pre-transplantation investigations. Investigations before transplantation did not include an EMB. Of all 296 patients, 51 patients (17%) were misdiagnosed. Excluding the patients with ischemic cardiomyopathy, 46 of 152 patients (30%) were misdiagnosed before transplantation.
CONCLUSIONS:
Although cardiac transplantation is a viable treatment option for patients with a variety of cardiac diseases, accurate diagnosis of patients before transplantation remains a priority. Accurate diagnosis of particular diseases (sarcoidosis, myocarditis, iron toxicity-associated cardiomyopathy and others) allows for proper treatment before transplantation, which may slow down disease progression and improve patient outcomes. Furthermore, it is important to accurately diagnose patients with diseases such as sarcoidosis, amyloidosis and particular types of myocarditis because these can readily recur in the grafted heart. The risk for recurrence must be known to practitioners and, most importantly, to the patient. We strongly recommend the use of EMB if a nonischemic cardiomyopathy is suspected, because the results may alter the diagnosis and modify the treatment strategy.
Keywords: Congestive heart failure, Endomyocardial biopsy, Heart transplant
Abstract
HISTORIQUE :
La greffe du cœur demeure la dernière possibilité de traitement pour les patients atteints d’une maladie cardiaque en phase terminale. Ces maladies incluent la myocardiopathie ischémique, la myocardiopathie non ischémique et d’autres maladies comme la dysplasie arythmogène du ventricule droit, la sarcoïdose cardiaque et l’amyloïdose cardiaque.
OBJECTIF :
Examiner les changements qui se sont produits au fil du temps dans l’étiologie de la maladie cardiaque chez les patients ayant besoin d’une greffe cardiaque et comparer les diagnostics cliniques et histologiques des cœurs explantés de patients atteints d’une maladie cardiaque évolutive.
MÉTHODOLOGIE :
Les auteurs ont examiné les résultats pathologiques de 296 cœurs excisés par voie chirurgicale pendant une période de 20 ans (janvier 1987 à juillet 2006) dans un établissement. Les patients ont été divisés en groupes selon l’année de la greffe cardiaque. Les auteurs ont examiné les tissus pour déterminer la pathologie cardiaque sous-jacente responsable de l’insuffisance cardiaque congestive. Ils ont examiné les dossiers des patients afin de connaître leur diagnostic clinique préopératoire et d’obtenir d’autres données pertinentes, y compris les résultats de la biopsie endomyocardique (BEM) avant la greffe, l’information au sujet des dispositifs d’assistance du ventricule gauche et, enfin, les observations de récurrence de la maladie dans le cœur greffé.
RÉSULTATS :
Les auteurs ont observé un virage dans l’étiologie sous-jacente chez les patients qui ont subi une greffe du cœur entre 1992 et 1996 et entre 1997 et 2001. Entre 1987 et 1997, la majorité des greffes étaient imputables à une myocardiopathie ischémique. De 1997 à 2001, la majorité des patients avaient une myocardiopathie non ischémique, et cette tendance s’est maintenue jusqu’en 2006. La majorité des patients atteints d’une myocardiopathie ischémique ou hypertrophique étaient bien diagnostiqués (96,5 % et 82 %, respectivement) avant la greffe. La plupart des patients diagnostiqués après la greffe étaient atteints d’une myocardiopathie lymphocytaire (virale, 15 %), hypersensible ou à éosinophiles (25 %) ou à cellules géantes (100 %), d’une myocardite, d’une dysplasie arythmogène du ventricule droit (100 %), d’une sarcoïdose cardiaque (83 %) et d’une myocardiopathie par surcharge en fer touchant le cœur (100 %), qui avaient été mal diagnostiqués lors des explorations précédant la greffe. Ces explorations n’incluaient pas de BEM. Des 296 patients, 51 (17 %) étaient mal diagnostiqués. Si on exclut les patients atteints d’une myocardiopathie ischémique, 46 des 152 patients (30 %) étaient mal diagnostiqués avant la greffe.
CONCLUSIONS :
Même si la greffe cardiaque est une possibilité de traitement viable pour les patients atteints de diverses maladies cardiaques, il est prioritaire d’obtenir le bon diagnostic avant la greffe. En effet, le bon diagnostic de maladies précises (sarcoïdose, myocardite, myocardiopathie par surcharge en fer et autres) permet d’administrer le bon traitement avant la greffe, ce qui peut ralentir l’évolution de la maladie et améliorer l’issue du patient. De plus, il est important de bien diagnostiquer les patients atteints de maladies comme la sarcoïdose, l’amyloïdose et des types précis de myocardite, qui peuvent récidiver rapidement dans le cœur greffé. Les praticiens et, surtout, les patients, doivent connaître ce risque de récurrence. Il est fortement recommandé de procéder à une BEM en cas de présomption de myocardiopathie non ischémique, car les résultats pourraient modifier le diagnostic et la stratégie de traitement.
Congestive heart failure (CHF) occurs when the heart is unable to sufficiently distribute blood to the organs in the body for cellular metabolism (1). In the 1980s and 1990s, the most common condition resulting in CHF in developing countries was coronary artery disease (CAD) (2,3). Patients with end-stage CHF are often treated with orthotopic heart transplantation. However, this treatment modality may not be beneficial for patients whose underlying pathology, including myocarditis, sarcoidosis and amyloidosis, may recur in the graft heart. All of the aforementioned pathologies, as well as nonischemic cardiomyopathies and arrhythmogenic right ventricle dysplasia, may be detected at endomyocardial biopsy (EMB). Because EMB is not typically indicated as a standard investigation when CAD is suspected as the cause of CHF, a biopsy should be undertaken in cases of nonischemic cardiomyopathy, to rule out other conditions (4). The present retrospective study aimed to identify shifts in the etiology of heart failure in patients coming for heart transplantation in the past 20 years, to identify the cause of CHF, to correlate the pathological diagnosis with the pretransplant clinical diagnosis and to determine whether EMB could be beneficial for patients waiting for transplantation.
METHODS
A review of the records at Toronto General Hospital (Toronto, Ontario) from January 1987 to July 2006 showed 296 cases of patients who received an orthotopic heart transplant. All specimens were excised at surgery, and all were fixed in 10% formalin, photographed and examined in detail by a senior cardiovascular pathologist (JB). Sections of surgically excised tissues were submitted for histology. All sections were paraffin-embedded and stained with hematoxylin and eosin, and Movat’s pentachrome stain. Staining for microorganisms (Gram stain for bacteria and Grocott’s methenamine silver for fungi) and special stains, such as congo red for cardiac amyloidosis and iron stains for iron toxicity-associated cardiomyopathy, were used when indicated (all stains were from Dako Diagnostics, Canada).
The etiology of heart failure was identified by detailed gross and histological analysis, and the failed hearts were categorized into different groups: ischemic cardiomyopathy and nonischemic cardiomyopathy (further categorized as idiopathic dilated [IDM] and hypertrophic [HCM]), arrhythmogenic right ventricular dysplasia (ARVD), myocarditis (viral, bacteria or hypersensitive/eosinophilic), cardiac amyloidosis, cardiac sarcoidosis, Fabry’s disease, valvular cardiomyopathy, iron toxicity-associated cardiomyopathy, congenital anomalies and others.
Patient records were reviewed and demographic data, clinical diagnosis based on investigations and EMB, and transplant information (including left ventricular assist device [LVAD]) were recorded.
RESULTS
Patient demographics
Two hundred ninety-six patients, including 224 men (76%) and 72 women (24%), were admitted for surgery for orthotopic heart transplantation. Patients had a mean (± SD) age of 48.46±11.58 years (range 20 to 75 years). Seventeen patients (6%) received an LVAD as a bridge to transplantation and 15 (5%) had a biopsy before transplantation. It should be noted that the LVAD was introduced at Toronto General Hospital in 2001.
Clinical diagnosis
The clinical diagnosis for each patient was determined and grouped into five-year intervals: 1987 to 1991 (n=19), 1992 to 1996 (n=87), 1997 to 2001 (n=85) and 2002 to 2006 (n=105) (Table 1). In the first two groups, a majority of patients (74% [n=14] and 71.6% [n=62], respectively) were clinically diagnosed with ischemic cardiomyopathy and required transplantation. Between 1997 and 2001, there were 35 cases (41.1%) of ischemic cardiomyopathy and 32 cases (37.6%) of IDM. Between 2002 and 2006, there was a decrease in heart transplant patients whose CHF was caused by ischemic damage from previous myocardial infarction and CAD. This last cohort had 29 patients (27%) with ischemic cardiomyopathy and 43 patients (40.9%) with IDM.
TABLE 1.
Prevalance of clinical diagnosis between 1987 and 2006 (n=296)
| Year | Clinical diagnosis | n (%) |
|---|---|---|
| 1987–1991, n=19 | Ischemic cardiomyopathy | 14 (74) |
| Idiopathic dilated cardiomyopathy | 3 (16) | |
| Valvular cardiomyopathy | 1 (5) | |
| Tumour (sarcoma) | 1 (5) | |
| 1992–1996, n=87 | Ischemic cardiomyopathy | 62 (71.6) |
| Idiopathic dilated cardiomyopathy | 16 (18.4) | |
| Congenital anomaly | 3 (3.4) | |
| Hypertrophic cardiomyopathy | 2 (2.2) | |
| Fabry’s disease | 1 (1.1) | |
| Viral myocarditis | 1 (1.1) | |
| Valvular cardiomyopathy | 1 (1.1) | |
| Hypersensitive/eosinophilic cardiomyopathy | 1 (1.1) | |
| 1997–2001, n=85 | Ischemic cardiomyopathy | 35 (41.1) |
| Idiopathic dilated cardiomyopathy | 32 (37.6) | |
| Hypertrophic cardiomyopathy | 6 (7) | |
| Congenital anomaly | 4 (4.7) | |
| Eisenmenger’s syndrome | 2 (2.4) | |
| Doxorubicin hydrochloride-induced cardiomyopathy | 2 (2.4) | |
| Valvular cardiomyopathy | 1 (1.2) | |
| Viral myocarditis | 1 (1.2) | |
| Postpartum cardiomyopathy | 1 (1.2) | |
| Cardiac amyloidosis | 1 (1.2) | |
| 2002–2006, n=105 | Idiopathic dilated cardiomyopathy | 43 (40.9) |
| Ischemic cardiomyopathy | 29 (27) | |
| Hypertrophic cardiomyopathy | 7 (7) | |
| Eisenmenger’s syndrome | 6 (6) | |
| Doxorubicin hydrochloride-induced toxicity | 4 (3.8) | |
| Viral myocarditis | 4 (3.8) | |
| Valvular cardiomyopathy | 3 (2.8) | |
| Congenital cardiac anomaly | 3 (2.8) | |
| Cardiac amyloidosis | 2 (1.9) | |
| Hypersensitive/eosinophilic myocarditis | 1 (1) | |
| Postpartum cardiomyopathy | 1 (1) | |
| Infiltrative cardiomyopathy | 1 (1) | |
| Becker’s MD cardiomyopathy | 1 (1) |
MD Muscular dystrophy
Pathological findings
Pathological diagnosis was determined by gross and detailed histological analysis in all cases. One-hundred forty-four patients (49%) had ischemic cardiomyopathy, 53 (17.9%) had IDM, 20 (7%) had active myocarditis (likely viral), 17 (6%) had HCM, 12 (4%) had ARVD, 10 (3%) had congenital anomalies, eight (2.7%) had Eisenmenger’s syndrome, six (2%) had cardiac sarcoidosis, six (2%) demonstrated doxorubicin hydrochloride-induced cardiomyopathy, five (1.6%) had a valvular cardiomyopathy, four (1.3%) had hypersensitive/eosinophilic myocarditis, three (1%) had cardiac amyloidosis, two (0.7%) had Fabry’s disease, and one each (0.3%) had postpartum cardiomyopathy, mitochondrial myopathy, giant cell myocarditis, a tumour (sarcoma), Becker’s muscular dystrophy cardiomyopathy and iron toxicity-associated cardiomyopathy. Images of these various pathologies can be seen in Figures 1 to 5. Each patient’s pathological (post-transplant) diagnosis was correlated with their clinical (pretransplant) diagnosis (Table 2). Fifty-one patients (17%) were found to be misdiagnosed. Excluding the patients with ischemic cardiomyopathy, as this cohort was not indicated for EMB, 46 of 152 patients (30%) were clinically misdiagnosed. Of the 94 patients diagnosed with IDM (diagnosis of exclusion) pretransplant, 52 (55.3%) were correctly diagnosed with IDM, 20 (21%) had active myocarditis and 26 (27.6%) had dilated cardiomyopathy secondary to another pathology (cardiac amyloidosis, cardiac sarcoidosis, giant cell and hypersensitive/eosinophilic myocarditis, and ARVD) found post-transplant.
Figure 1).
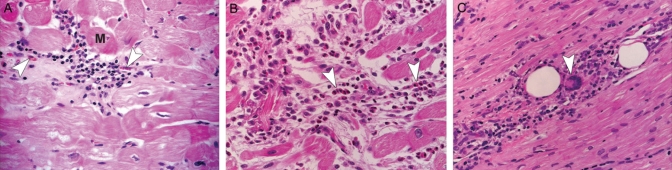
Histological sections from patients with myocarditis. A Viral myocarditis. A predominantly lymphocyte infiltrate is seen between the myofibrils. The myocyte (M) has irregular contours suggesting an active myocarditis. Macrophages (arrowheads) are also seen among the inflammatory infiltrate (hematoxylin and eosin stain, original magnification ×40). B Eosinophilic myocarditis. A heavy infiltrate of inflammatory cells, predominately eosinophils (arrowheads) with macrophages and some mononuclear cells. These clusters of cells are found in areas of damaged myocardium (hematoxylin and eosin stain, original magnification ×40). C Giant cell myocarditis. Lymphocytes, occasional plasma cells and giant cells (arrowhead) are found in areas of damaged myocytes (hematoxylin and eosin stain, original magnification ×40)
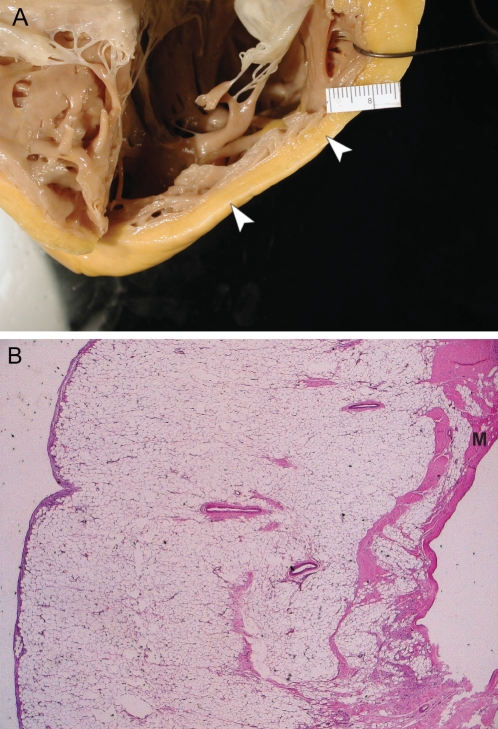
Figure 5).
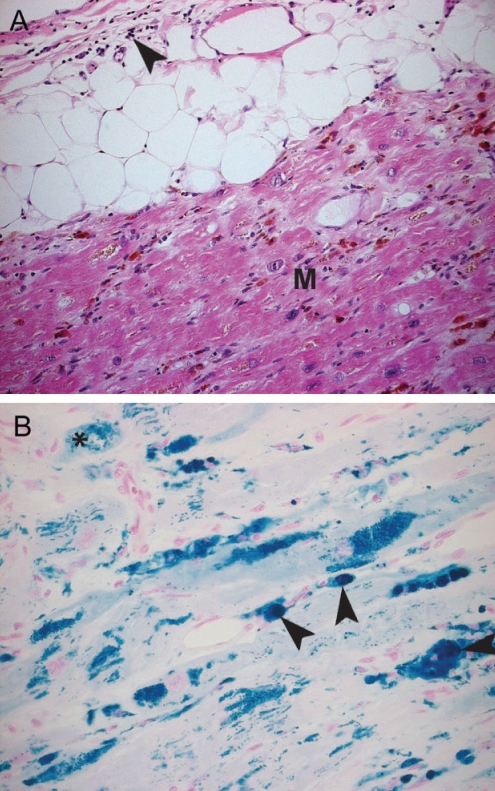
Iron toxicity-associated cardiomyopathy. Histological findings of a patient with hemochromatosis. A A macrophage and lymphocytic infiltrate (arrowhead) is seen in the epicardium. The myocardium (M) contains iron deposits (staining brown). There is a decrease in myocytes in this layer with increasing areas of fibrosis (hematoxylin and eosin stain, original magnification ×20). B Areas of intracellular iron deposition can be seen in two distinct patterns: within the mitochondria (asterisk) alone and within the cells as the mitochondrial cells have lysed (white arrowhead). Iron deposition has also involved the endothelial cells of the vasculature (black arrowheads) (Prussian blue stain, original magnification ×40)
TABLE 2.
Clinicopathological correlation of patients with orthotopic heart transplantation (n=296)
| Pathological diagnosis | n (%) | Clinical diagnosis | n (%)* |
|---|---|---|---|
| Ischemic cardiomyopathy | 144 (49) | Ischemic cardiomyopathy | 140 (97.2) |
| Idiopathic dilated cardiomyopathy | 2 (1.3) | ||
| Hypersensitive/eosinophilic myocarditis | 1 (0.7) | ||
| Valvular cardiomyopathy | 1 (0.7) | ||
| Idiopathic dilated cardiomyopathy | 52 (17.5) | Idiopathic dilated cardiomyopathy | 50 (96.1) |
| Viral myocarditis | 2 (3.9) | ||
| Viral myocarditis | 20 (7) | Idiopathic dilated cardiomyopathy | 17 (85) |
| Viral myocarditis | 3 (15) | ||
| Hypertrophic cardiomyopathy | 17 (6) | Hypertrophic cardiomyopathy | 14 (82) |
| Idiopathic dilated cardiomyopathy | 3 (18) | ||
| Arrhythmogenic right ventricular dysplasia | 12 (4) | Idiopathic dilated cardiomyopathy | 10 (83) |
| Viral myocarditis | 1 (8.5) | ||
| Valvular cardiomyopathy | 1 (8.5) | ||
| Congenital defect | 10 (3) | Congenital defect | 10 (100) |
| Eisenmenger’s syndrome | 8 (2.7) | Eisenmenger’s syndrome | 8 (100) |
| Cardiac sarcoidosis | 6 (2) | Idiopathic dilated cardiomyopathy | 5 (83) |
| Infiltrative cardiomyopathy | 1 (17) | ||
| Doxorubicin hydrochloride-induced cardiomyopathy | 6 (2) | Doxorubicin hydrochloride-induced cardiomyopathy | 6 (100) |
| Valvular cardiomyopathy | 5 (1.6) | Valvular cardiomyopathy | 4 (80) |
| Idiopathic dilated cardiomyopathy | 1 (20) | ||
| Hypersensitive/eosinophilic myocarditis | 4 (1.3) | Idiopathic dilated cardiomyopathy | 3 (75) |
| Hypersensitive/eosinophilic myocarditis | 1 (25) | ||
| Cardiac amyloidosis | 3 (1) | Cardiac amyloidosis | 3 (100) |
| Fabry’s disease | 2 (0.7) | Fabry’s disease | 1 (50) |
| Hypertrophic cardiomyopathy | 1 (50) | ||
| Postpartum cardiomyopathy | 2 (0.7) | Postpartum cardiomyopathy | 2 (100) |
| Mitochondrial myopathy | 1 (0.3) | Idiopathic dilated cardiomyopathy | 1 (100) |
| Giant cell myocarditis | 1 (0.3) | Idiopathic dilated cardiomyopathy | 1 (100) |
| Tumour (sarcoma) | 1 (0.3) | Tumour (sarcoma) | 1 (100) |
| Becker’s MD cardiomyopathy | 1 (0.3) | Becker’s MD cardiomyopathy | 1 (100) |
| Iron toxicity-associated cardiomyopathy | 1 (0.3) | Idiopathic dilated cardiomyopathy | 1 (100) |
The percentage of clinical diagnoses is based on the number of cases that were correctly diagnosed by pathology. MD Muscular dystrophy
Pretransplantation biopsy
Fifteen patients (5%) were biopsied before transplantation, and one other patient had multiple biopsies to monitor graft rejection from a previous heart transplant. Of these 15 biopsies, eight (53%) were in patients with IDM and one (6.7%) was in a patient with postpartum cardiomyopathy; all nine biopsies were performed to rule out myocarditis. Two biopsies (13%) were diagnostic for HCM and one (6.7%) for Fabry’s disease. One (6.7%) sarcoid patient was biopsied and confirmed to have an infiltrative cardiomyopathy, and one (6.7%) patient with ARVD was diagnosed with IDM. Finally, one patient with active hypersensitive/eosinophilic myocarditis had an inadequate sample. His biopsy was inconclusive.
Recurrence of disease post-transplant
Two patients, one with cardiac amyloidosis and the other with cardiac sarcoidosis, presented with recurrent amyloidosis and tuberculosis (TB), respectively. Postoperative right ventricular EMB six years post-transplantation in the first patient detected amyloid deposits in the graft heart. The second patient presented five months after transplantation with a one-week history of productive cough and fever. Pleural biopsy was diagnostic for TB.
DISCUSSION
CHF remains an international concern; 80% of men and 70% of women younger than 65 years of age with CHF will die within eight years after diagnosis (5). Despite the poor prognosis, the incidence of CHF from 1950 to 2000 has not changed drastically (6,7). Randomized controlled trials between 1986 and 1997 found that CAD was the main etiology (found in 70% of cases) leading to CHF (3). These findings are consistent with a clinical diagnosis for patients transplanted between 1987 and 1996, but from 1997 onward, nonischemic cardiomyopathies (IDM and HCM) became the predominant etiology for CHF necessitating transplant, and were present in 48.1% (n=41) of the present study’s cases from 1997 to 2006. These findings are consistent with other published studies based in North America (Nebraska and Texas, USA) from Bortman et al (8) and Waller et al (9). Fifty-seven per cent (n=112, 1988 to 1993) and 51% (n=92, 1993 to 1997) of their patients, respectively, were diagnosed with ischemic cardiomyopathy pretransplant. Pretransplant diagnosis in an Italian study by Angelini et al (10) had different findings. The authors studied 257 patients between 1985 and 1994. Dilated cardiomyopathy was diagnosed in 49% of their patient cohort, and ischemic cardiomyopathy was diagnosed in 33.8% of their patients. Their results vary greatly from our study and those discussed previously. These observations may be due to a combination of both genetic and environmental factors.
This shift in etiology in our study may be accounted for by an increase in the incidence of nonischemic cardiomyopathy alone, a decrease in incidence of ischemic cardiomyopathy, or both. It may also be accounted for by the improved pharmacological agents available to treat CHF (11), which allow patients with nonischemic cardiomyopathy to survive longer and progress to end-stage disease, thus requiring transplantation. Concurrently, pharmacological agents given to patients early following myocardial infarction, the advent of interventional cardiology, as well as improved surgical technique may also contribute to this shift in etiology, effectively decreasing the number of patients with ischemic cardiomyopathy requiring transplantation. All of these reasons may account for the shift in etiology for heart transplantation.
In our cohort, 96.5% of patients (139 of 144) with cardiomyopathy due to ischemic conditions were diagnosed correctly clinically. Cases with ischemic damage are typically diagnosed by patient history, physical examination and investigations such as echocardiography and chest x-ray (12); EMB is generally not indicated for diagnosis (4).
Of the 94 patients clinically diagnosed with IDM, 44.6% (n=42) were incorrectly diagnosed; IDM was confirmed in only 52 of the 94 patients (55.3%) on morphological analysis. A diagnosis of IDM is one of exclusion, and is dependent on patient history and twodimensional (2D) echocardiography (2D echocardiography ejection fraction of less than 0.45% and/or a fractional shortening of less than 25%, and a left ventricular end diastolic dimension of more than 112% of the predicted value, corrected for age and body surface area) (13,14). Similarly, the diagnosis of HCM is dependent on a thorough history and by unexplained left ventricular hypertrophy on 2D echocardiography (15). Although EMB should be performed if there is a clinical suspicion for these cardiomyopathies (4), a majority of our patients were not biopsied before transplantation. Of the patients biopsied, two were diagnosed correctly with HCM, and one with Fabry’s disease, eight with ICM and one with postpartum cardiomyopathy were diagnosed correctly pretransplantation.
Conditions such as sarcoidosis, amyloidosis, hypersensitive/eosinophilic myocarditis and others should be ruled out by an EMB before transplantation (4). Five of six patients (83%) with sarcoidosis, three of four patients (75%) with hypersensitive/eosinophilic cardiomyopathy and one (100%) with iron toxicity-associated cardiomyopathy were incorrectly diagnosed before transplantation. Only one patient with cardiac sarcoidosis (suggestive) and one with hypersensitive/eosinophilic cardiomyopathy were biopsied, but in the latter case, the findings were not diagnostic due to insufficient sample size. These findings are concerning because patients incorrectly diagnosed did not receive either immune suppressive therapy or proper management (phlebotomy) in an attempt to offset or prevent disease progression. Although EMB may have poor diagnostic accuracy due to the area of the heart biopsied (sampling error), it remains a diagnostic tool for the clinician, and is the gold standard for diagnosis.
Moreover, patients with these diagnoses were unaware of the risk of disease recurrence in the donor heart or postoperative complications that may arise due to immune suppression therapy. Disease recurrence in the donor heart of patients with eosinophilic cardiomyopathy (16), sarcoidosis (17), giant cell myocarditis (18) and amyloid light-chain amyloidosis (19) have been revealed by post-transplantation EMB. Cantor et al (20) reported the recurrence of Fabry’s disease with cardiac involvement in one postoperative biopsy, but subsequent biopsies for the next year were negative and there was no clinical evidence of disease recurrence. Recurrence of Fabry’s disease in the graft heart has yet to be reported in the literature. In our cohort, one patient had recurrent cardiac amyloidosis and another patient developed active TB, likely due to the postoperative medical therapeutic course. The amyloid patient was properly diagnosed, but the patient with cardiac sarcoidosis was not biopsied before transplantation. It is likely that the patient was exposed to TB as a child, and post-transplant corticosteroids suppressed his immune system, predisposing the patient to active TB. Because sarcoidosis, giant cell myocarditis, hypersensitive/eosinophilic myocarditis and amyloid light-chain amyloidosis may recur in the donor heart, and latent infection may become active after postoperative immunosuppresion, proper diagnosis and complete medical history before transplantation is essential for appropriate medical management pre- and post-transplantation.
None of the 12 patients diagnosed with ARVD were biopsied before transplantation and therefore, they were misdiagnosed. Clinical diagnoses included IDM (n=10, 83%), viral myocarditis (n=1, 8.5%) and valvular cardiomyopathy (n=1, 8.5%). The diagnosis of ARVD is based on a complete patient history, physical examination, electrocardiogram and other diagnostic tests such as chest radiography, Holter monitoring, exercise stress tests, angiography and EMB (21). Although EMB is the preferred method of diagnosis, the sample is taken from the septum to minimize right ventricle perforation. Because morphological involvement of the septum is minimal, the biopsy has a specificity of 92% and a sensitivity of less than 20% (22). Because ARVD results in a dilated cardiomyopathy, it is likely that patients with ARVD who were not biopsied were misdiagnosed as having an IDM based on 2D echocardiography alone. If these patients were biopsied, misdiagnosis may still have occurred due to its poor diagnostic accuracy.
Sekiguchi and Konno (23) were the first to use an EMB for clinical diagnosis in 1963. Since then, there has been much debate about its use for diagnosis of cardiomyopathies. Issues of poor diagnostic accuracy and complications related to the EMB procedure have been reported (24). Complications of right ventricle biopsy in transplanted patients have been reported by Mason (25) and include right pneumothorax, air embolism, atrial arrhythmias, transient nerve palsies and paralysis, cardiac perforation and tamponade. Deckers et al (26) reported complications including arterial puncture, prolonged bleeding and vasovagal reaction in nontransplanted patients. Despite these complications, we believe that an EMB should be undertaken when there is clinical suspicion of an underlying disease that has a different therapeutic regimen, including viral, giant cell and hypersensitive/eosinophilic myocarditis, sarcoidosis, amyloidosis, neoplasia, iron toxicity-associated cardiomyopathy and arrhythmias (4). Ardehali et al (27) evaluated 845 patients, initially diagnosed with IDM, by performing an EMB in all of them to identify a missed diagnosis. In 264 (31%) of the patients, the final diagnosis after histological analysis from EMB differed from the initial diagnosis. Of these 264 patients, 196 (75%) diagnoses were changed based on biopsy alone and the other 68 (25%) were changed based on other diagnostic tests. The results were similar to those of our patients; excluding those with ischemic cardiomyopathy, 30% of our patients were misdiagnosed after microscopic examination of the heart post-transplant. This study, as well as ours, suggests that misdiagnosis occurs in a high percentage of cases, in the absence of EMB.
CONCLUSIONS
Orthotopic heart transplantation is a treatment modality reserved for patients at end-stage heart failure. In our series of 296 patients, the main etiology for heart transplantation in the 1980s and early 1990s was ischemic cardiomyopathies and later, in cases from the late 1990s to 2006, it was nonischemic cardiomyopathies (IDM and HCM). A majority of transplanted patients whose transplants were due to ischemic changes, IDM, HCM, congenital anomalies, Eisenmenger’s syndrome, drug toxicity-associated cardiomyopathy and cardiac amyloidosis, and were not biopsied, were diagnosed correctly. Patients with active viral or hypersensitive/eosinophilic myocarditis, ARVD, iron toxicity-associated cardiomyopathy and cardiac sarcoidosis, and were not biopsied, were misdiagnosed. Fifty-one patients (17%) were found to be misdiagnosed. Excluding the patients with ischemic cardiomyopathy, 46 patients (30%) were clinically misdiagnosed. We strongly recommend the use of EMB because it may alter the diagnosis and change the treatment strategy of the underlying pathology, and is well worth the nominal risk to each patient.
Figure 2).
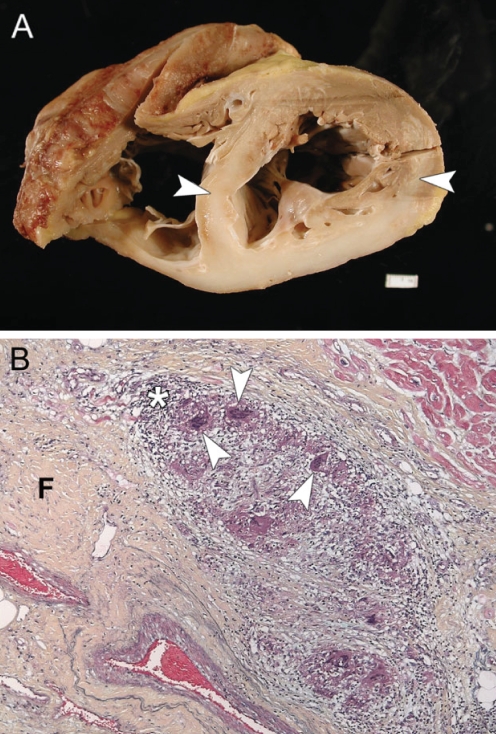
Cardiac sarcoidosis. A Transverse section of a heart from a patient clinically diagnosed with idiopathic dilated cardiomyopathy. The interventricular wall and left ventricular posterior wall show extensive transmural fibrosis (arrowheads). B Histological section of the interventricular septum. Multinucleated giant cells and granulomas (arrowheads) are found surrounded by a rim of mononuclear cells and macrophages (asterisk). The cells are found within areas of fibrosis and myocyte loss (F) (Movat’s pentachrome stain, original magnification ×10)
Figure 3).
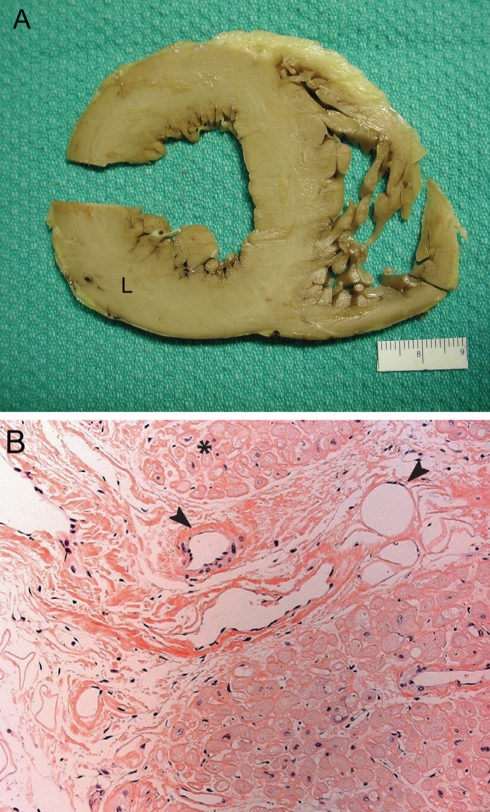
Cardiac amyloidosis. A Cross-section of a heart with amyloidosis, showing biventricular hypertrophy, predominately left-sided (L). The most remarkable feature on gross examination is the marked stiffening of the heart itself. B Amyloid deposits can be seen in nodules surrounding individual myocytes (asterisk), and also in a linear form surrounding blood vessels (arrowheads) (congo red stain, original magnification ×20)
Figure 4).
Arrhythmogenic right ventricle dysplasia (ARVD). A Gross image of a heart with ARVD. Note the dilated right ventricle and thin right ventricular wall, with fibrofatty tissue infiltrating the free wall of the right ventricle (arrowheads). B Microscopic findings include right ventricular myocyte (M) atrophy with infiltration of fatty or fibrofatty tissues (hematoxylin and eosin stain, original magnification ×1.6)
REFERENCES
- 1.Schoen FJ. The heart. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th edn. Philadelphia: Elsevier Saunders; 2005. p. 560. [Google Scholar]
- 2.Hare JM, Walford GD, Hruban RH, et al. Ischemic cardiomyopathy: Endomyocardial biopsy and ventriculographic evaluation of patients with congestive heart failure, dilated cardiomyopathy and coronary artery disease. J Am Coll Cardiol. 1992;20:1318–25. doi: 10.1016/0735-1097(92)90243-g. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: A manifestation of coronary artery disease. Circulation. 1998;97:282–9. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham KS, Veinot JP, Butany J. An approach to endomyocardial biopsy interpretation. J Clin Pathol. 2006;59:121–9. doi: 10.1136/jcp.2005.026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Heart Assocation Heart Disease and Stroke Statisitics – 2006 Update. Dallas: American Heart Association; 2006. [Google Scholar]
- 6.Levy D, Kenchaiah S, Larson MG, et al. Longterm trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 7.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 8.Bortman G, Sellanes M, Odell DS, et al. Discrepancy between pre-and post-transplant diagnosis of end-stage dilated cardiomyopathy. Am J Cardiol. 1994;74:921–4. doi: 10.1016/0002-9149(94)90587-8. [DOI] [PubMed] [Google Scholar]
- 9.Waller TA, Hiser WL, Capehart JE, et al. Comparison of clinical and morphologic cardiac findings in patients having cardiac transplantation for ischemic cardiomyopathy, idiopathic dilated cardiomyopathy, and dilated hypertrophic cardiomyopathy. Am J Cardiol. 1998;81:884–94. doi: 10.1016/s0002-9149(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 10.Angelini A, Boffa GM, Livi U, et al. Discordance between pre and post cardiac transplant diagnosis: Implications for pre- and postoperative decision making. Cardiovasc Pathol. 1999;8:17–23. doi: 10.1016/s1054-8807(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 11.Remme WJ, Swedberg K. Comprehensive guidelines for the diagnosis and treatment of chronic heart failure. Eur J Heart Failure. 2002;4:11–22. doi: 10.1016/s1388-9842(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 12.Ardehali H, Kasper EK, Baughman KL. Diagnostic approach to the patient with cardiomyopathy: Whom to biopsy. Am Heart J. 2005;149:7–12. doi: 10.1016/j.ahj.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–61. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- 14.Elliot P. Cardiomyopathy: Diagnosis and management of dilated cardiomyopathy. Heart. 2000;84:106–12. doi: 10.1136/heart.84.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho CY, Seidman CE. A contemporary approach to hypertrophic cardiomyopathy. Circulation. 2006;113:e858–62. doi: 10.1161/CIRCULATIONAHA.105.591982. [DOI] [PubMed] [Google Scholar]
- 16.di Gioia CRT, d’Amanti G, Grillo P, et al. Eosinophilic infiltration immediately following transplantation: Recurrent hypersensitivity reaction. Cardiovasc Pathol. 1999;8:297–99. doi: 10.1016/s1054-8807(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 17.Yager JEE, Hernandez AF, Steenbergen C, et al. Recurrence of cardiac sarcoidosis in a heart transplant recipient. J Heart Lung Transplant. 2005;24:1988–90. doi: 10.1016/j.healun.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Davies RA, Veinot JP, Smith S, et al. Giant cell myocarditis: Clinical presentation, bridge to transplantation with mechanical circulatory support, and long-term outcome. J Heart Lung Transplantation. 2002;21:674–9. doi: 10.1016/s1053-2498(02)00379-0. [DOI] [PubMed] [Google Scholar]
- 19.Dubrey SW, Burke MM, Khaghani A, et al. Long term results of heart transplantation in patients with amyloid heart disease. Heart. 2001;85:202–7. doi: 10.1136/heart.85.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantor WJ, Daly P, Iwanochko M, et al. Cardiac transplantation for Fabry’s disease. Can J Cardiol. 1998;14:81–4. [PubMed] [Google Scholar]
- 21.Anderson EL. Arrhythmogenic right ventricular dysplasia. Am Fam Physician. 2006;73:1391–8. [PubMed] [Google Scholar]
- 22.Iesaka Y, Hiroe M, Aonuma K, et al. Usefulness of electrophysiologic study and endomyocardial biopsy in differentiating arrhythmogenic right ventricular dysplasia from idiopathic right ventricular tachycardia. Heart Vessels Suppl. 1990;5:65–9. [PubMed] [Google Scholar]
- 23.Sekiguchi M, Konno S. Diagnosis and classification of primary myocardial disease with the aid of endomyocardial biopsy. Jpn Circ J. 1971;35:737–54. doi: 10.1253/jcj.35.737. [DOI] [PubMed] [Google Scholar]
- 24.Wu LA, Lapeyre AC, III, Cooper LT. Current role of endomyocardial biopsy in the management of dilated cardiomyopathy and myocarditis. Mayo Clin Proc. 2001;76:1030–8. doi: 10.4065/76.10.1030. [DOI] [PubMed] [Google Scholar]
- 25.Mason JW. Techniques for right and left ventricular endomyocardial biopsy. Am J Cardiol. 1978;41:887–92. doi: 10.1016/0002-9149(78)90729-4. [DOI] [PubMed] [Google Scholar]
- 26.Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: A seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol. 1992;19:43–7. doi: 10.1016/0735-1097(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 27.Ardehali H, Qasim A, Cappola T, et al. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J. 2004;147:919–23. doi: 10.1016/j.ahj.2003.09.020. [DOI] [PubMed] [Google Scholar]