Abstract
We have selected piperaquine (PQ) and lumefantrine (LM) resistant Plasmodium berghei ANKA parasite lines in mice by drug pressure. Effective doses that reduce parasitaemia by 90% (ED90) of PQ and LM against the parent line were 3.52 and 3.93 mg/kg, respectively. After drug pressure (more than 27 passages), the selected parasite lines had PQ and LM resistance indexes (I90) [ED90 of resistant line/ED90 of parent line] of 68.86 and 63.55, respectively. After growing them in the absence of drug for 10 passages and cryo-preserving them at −80 °C for at least 2 months, the resistance phenotypes remained stable. Cross-resistance studies showed that the PQ-resistant line was highly resistant to LM, while the LM-resistant line remained sensitive to PQ. Thus, if the mechanism of resistance is similar in P. berghei and Plasmodium falciparum, the use of LM (as part of Coartem®) should not select for PQ resistance.
Keywords: Malaria, Plasmodium berghei, Drug resistance, Cross-resistance, Lumefantrine, Piperaquine
1. Introduction
Malaria is a global public health priority. The control of malaria is hampered by the rapid selection of parasites resistant to antimalarials. Indeed, there is no single antimalarial in clinical use to which the parasite has not yet developed resistance (Nzila, 2006; White, 2004). Current international strategies for treatment depend on the use of combinations of drugs that include artemisinin compounds. Although this strategy is designed to reduce the chance of resistance emerging, there is considerable concern that this will inevitably happen.
Studies devoted to understanding factors that promote the selection of resistance of Plasmodium falciparum to antimalarials have demonstrated that drug elimination profile in the body is one of the key parameters that determine the emergence and selection of resistance (Nzila et al., 2000; Watkins and Mosobo, 1993). When drugs are used in combination, a mismatch between their half-lives can have a substantial impact on the evolution of drug resistance. If one drug is rapidly eliminated, the other drug persists alone and new infections are exposed to sub-therapeutic level of drugs, a fact that promotes the development of resistance (Hastings, 2004).
For instance, the combination of lumefantrine (LM) and artemether (ATM), known as Coartem® has become the first line of treatment of malaria in many African countries, including Kenya (Davis et al., 2005b; Kokwaro et al., 2007; Mutabingwa, 2005; Nosten and White, 2007). Emerging reports indicate that the use of LM in (Coartem®) selects for parasites that show an increased tolerance to Coartem® and these parasites select for wild type genotype in, or show increased copy number of pfmdr1, a gene associated with chloroquine (CQ) and mefloquine (MFQ) resistance (Dokomajilar et al., 2006; Sisowath et al., 2007, 2005). Thus, there is now concern that resistance to LM will be rapidly selected (Hastings and Ward, 2005; Humphreys et al., 2007; Sisowath et al., 2007).
Piperaquine has been combined with dihydroartemisinin (DHA), the drug known as Artekin®. It has undergone successful clinical evaluation in Africa and Asia (Ashley et al., 2004, 2005; Davis et al., 2005a; Denis et al., 2002; Karema et al., 2006; Karunajeewa et al., 2004). Piperaquine has been used as monotherapy for the treatment of malaria infections for several years (in the 80s and early in the 90s) in China. However, when used alone, there was rapid selection of resistance in vitro, from 18% in the 1980s to 98% in 1990s (Fan et al., 1998; Yang et al., 1999, 1992; Zhang et al., 1987). This in vitro-resistance was followed inevitably by the emergence of in vivo resistance (Davis et al., 2005b; Guan et al., 1983; Pang et al., 1989).
In both combinations, the artemisinin derivatives (ATM and DHA) and the main components (PQ and LM) have different pharmacokinetic properties. Indeed, like most artemisinin-based compounds, DHA and ATM are short acting drugs, with a half-lives of less than 2 h (Navaratnam et al., 2000; White et al., 1999). On the other hand, PQ and LM have long half-lives, around 4–6 and 15–20 days, respectively (Ahmed et al., 2008; Hai et al., 2008; Kokwaro et al., 2007; Tarning et al., 2008). Under these circumstances, the selective pressure for resistance would be primarily exerted by the LM and PQ, leading to a rapid selection of PQ and LM resistance when the drug combinations will come into widespread use. In the case of Coartem®, a rapid emergence of parasite tolerant to LM have been reported following the use of Coartem® (Dokomajilar et al., 2006; Sisowath et al., 2007, 2005).
Thus, if strategies are to be devised to extend the useful therapeutic lifetime of Coartem® and Artekin®, there is a need to understand the mechanisms of PQ and LM resistance. However, to date, there are no well established and characterized PQ- and LM-resistant P. falciparum strains, which could be used to study the mechanism of drug resistance.
Here we report the selection of stable LM- and PQ-resistant Plasmodium berghei ANKA strains by continuous PQ and LM pressure in vivo. We also report the activity of the antimalarial drugs chloroquine (CQ), amodiaquine (AQ), LM and DHA against the backdrop of this LM and PQ resistance. These strains represent valuable tools to study the mechanisms of LM and PQ resistance.
2. Materials and methods
2.1. Parasites, hosts and test compounds
To select PQ resistance, we used a transgenic ANKA strain of P. berghei expressing Green Fluorescent Protein (GFP), resistant to pyrimethamine obtained from the MR4 repository (MRA-865, MR4, ATCC® Manassas, Virginia), while a P. berghei ANKA strain expressing GFP-Luciferase fusion, (MRA-868, MR4, ATCC® Manassas, Virginia) obtained from Dr. C.J. Janse of Center of Infectious Diseases Leiden University Medical Center, Netherlands was employed to induce LM resistance. Male, random-bred Swiss albino mice (20 ± 2 g), were each infected intraperitoneally with donor blood containing approximately 2 × 107 parasite red blood cells (PRBC) in 0.2 ml inoculum. However, during the first 4 passages of selection of PQ resistance, female NMRI mice were used and were infected intravenously. Infection was assessed by microscopic estimation of the proportion of infected erythrocytes in Giemsa-stained thin smears made from tail-vein blood.
The animals were housed in experimental room in a standard Macrolon type II cages clearly labeled with experimental details at 22 °C and 60–70% relative humidity and fed on commercial rodent feed and water ad libitum.
CQ and AQ were purchased from Sigma Chemical Co. (Poole, UK). DHA, PQ and LM were gifts from Professor Steve Ward, Liverpool School of Tropical Medicine, Liverpool, UK. On the day of administration, the drug was freshly prepared by dissolving it in a vehicle consisting of 70% Tween-80 (d = 1.08 g/ml) and 30% ethanol (d = 0.81 g/ml) and subsequently diluted 10-fold with double distilled water.
2.2. Determination of 50% and 90% effective-dose level (ED50 and ED90)
Fifty percent and 90% effective doses (ED50 and ED90, respectively) were measured in a quantitative standard method ‘4-day test’ (4-DT), in which the parasites are exposed to four, daily, drug doses (Peters, 1975), except for the ED50 and ED90 of the parent strain and that of the line selected at the 4th passage of PQ pressure which were measured using the ‘1-day test’ (1-DT), in which the parasites are exposed to a single drug dose (Vennerstrom et al., 2004). The first 4 passages of PQ pressure were carried out at Swiss Tropical Institute (STI), Basel, Switzerland, using the 1-DT. However, experiments from the 4th passage of PQ pressure and the all LM pressure were carried out at the Kenya Medical Research Institute (KEMRI), Nairobi, Kenya, using the 4-DT. Drugs were administered by oral (po) route on day 1, (24 h post-infection) in the 1-DT or starting on the day 0, (4 h post-infection) and continuing for a total of four daily doses, days 0–3 (24, 48 and 72 h post-infection) in the 4-DT. Parasite count was estimated by microscopic examination of Giemsa-stained thin smears prepared from tail snips on day 3, 72 h post-infection in the 1-DT or on day 4, 96 h post-infection in the 4-DT. Percentage chemosuppression of each dose was then calculated as (A − B)/A] × 100], where A is the mean parasitaemia in the negative control group and B is the parasitaemia in the test group (Tona et al., 2001). ED50 and ED90 were estimated using a linear regression line.
2.3. Procedures for exerting drug-selection pressure and assessing the level of resistance
After inoculation (2 × 107 parasitized red blood cells contained in 0.2 ml inoculums) in 5 mice, on day zero (D0), mice were then orally treated once with the drug at concentration equivalent to ED99, 72 h post-infection (D3). Thereafter, parasitaemia was monitored until it reached 2–5%, when a mouse was selected for donation of PRBC to the next naive group of five mice. The parasites were exposed to increasing concentrations of PQ and LM by an ED99 factor of one in subsequent passages.
During the first 4 passages of PQ drug pressure, after parasite inoculation (D0), mice (a group of 5) were treated three times with the drug at concentration equivalent to ED99. The first treatment was carried out 72 h post-infection (D3). The second and third treatment followed on D6 (or 7) and 10. Drugs were administered orally in a volume of 0.01 ml per gram mouse. After the third treatment (D10), parasitaemias were monitored until they reached ⩾2% when a mouse was selected for donation of PRBC to the next naive group of five mice and subsequent steps were carried out as mentioned in the previous paragraph.
The level of resistance was evaluated at different intervals by measurement of ED50 and ED90 in the standard 4-DT or 1-DT which permits the calculation of an ‘index of resistance’, I50 and I90 (defined as the ratio of the ED50 or ED90 of the resistant line to that of the sensitive, parent line).
The I90 values were grouped into four categories, based on previous work (Merkli and Richle, 1980): (1) I90 = 1.0, sensitive, (2) I90 = 1.01–10.0, slight resistance, (3) I90 = 10.01–100.0, moderate resistance and (4) I90 > 100.0, high resistance.
2.4. Stability study
The stability of PQ and LM resistant line was evaluated by measuring drug responses after (i) making 10 drug free passages followed by measurement of ED90; (ii) freeze-thawing of parasites from −80 °C followed by measurement of ED90. Stable resistance was defined as the maintenance of the resistance phenotype when drug-selection pressure was removed for at least 10 passages in mice (Gervais et al., 1999).
2.5. Cross-resistance studies
The activity of CQ, AQ, LM and DHA against both drug sensitive and resistant lines (after 10 drug free passages) was assessed in the 4-DT. I90 was computed as the ratio of the ED90 of the resistant line to that of the sensitive, parent line. Cross-resistance was classified into three categories as previously described (Li, 1985; Li et al., 1985): I90 ⩽1.00 sensitive, I90 of 1.01–5.00 as slight cross-resistance, I90 of above 5.01 as high cross-resistance. Statistical analyses were carried out using the Student t-test (Minitab Inc. software, State College, PA, USA).
3. Results
The ED50 and ED90 of PQ against the parent line were 1.30 and 3.52 mg/kg, respectively. After 4 passages under PQ selective pressure (a total of 15 treatments), the PQ ED50 and ED90 increased to 160.28 and 262.59 mg/kg, respectively, yielding I50 of 123.29 and I90 of 74.70 (Table 1). However, after cryopreservation and revival of the parasite, this resistance decreased, with ED50 and ED90 of 7.50 and 21.90 mg/kg, respectively (Table 1).
Table 1.
Selection of piperaquine resistance in Plasmodium berghei GFP ANKA strain using serial technique. Data are presented as effective doses that reduce parasitaemia by 50% and 90% (ED50, ED90) and as 50% and 90% indexes of resistance (I50 and I90, defined as the ratio of the ED50 or ED90 of the resistant line to that of the parent strain).
| Passage no. | ED50 (mg/kg) | (I50) | ED90 (mg/kg) | (I90) |
|---|---|---|---|---|
| Parent | 1.30 | 1 | 3.52 | 1 |
| 4th | 160.28 | 123.29 | 262.59 | 74.70 |
| (6 months cryopreservation) (5th passage) | 7.50 | 5.77 | 21.90 | 6.22 |
| 9th | 21.40 | 16.46 | 64.50 | 18.32 |
| 17th | 122.00 | 93.85 | 194.00 | 55.11 |
| 27th | 168.08 | 129.29 | 242.38 | 68.86 |
| Drug free passages | ||||
| 5th | 185.27 | 142.52 | 283.71 | 80.60 |
| 10th | 191.46 | 147.28 | 294.98 | 83.80 |
| 27th passage line after 4 months cryopreservation | 110.03 | 84.64 | 223.15 | 63.39 |
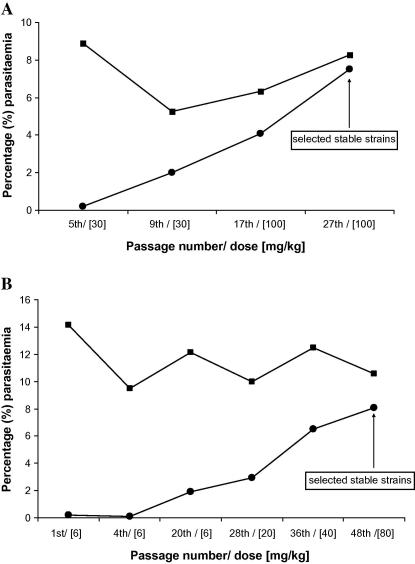
When exposed to further 23 passages (27th passage) of selection pressure, parasites regained the resistant phenotype and reached a high level of resistance with I50 and I90 of 129.29 and 68.86, respectively (Table 1). Fig. 1A shows the changing response of the P. berghei ANKA to PQ in the course of PQ drug pressure. After the 5th passage under PQ pressure, a dose of 30 mg/kg (>8 times higher the ED90 of the parent strain) suppressed the bulk of parasitaemia, indeed treated mice had parasitaemia of 0.22% only, compared to almost 9% of the untreated group. Thereafter, PQ resistance arose quite rapidly from the 9th passage. Infected mice treated with 30 mg/kg could yield parasitaemia of 2% at the 9th passage, and at 17th passage, parasitaemia reached 4% after a higher dose, 100 mg/kg. The continuous PQ pressure to 27th passage allowed the selection of parasite lines that reached 7.5% (parasitaemia almost as high as the control [8.26%]), after treated mice with 100 mg/kg, a clear indication of the rise in resistance. This resistant phenotype was stable and these parasite lines were scored as PQ-resistant strains (Table 1 and Fig. 1A).
Fig. 1.
Development of parasitaemia in the treated (●) and untreated mice (■) at different levels during the selection of piperaquine (A) and lumefantrine (B) resistant Plasmodium berghei GFP (for PQ) and GFP-Luciferase (for LM) ANKA strains in mice. Parasitaemias were assessed after 4 days post-infection (in both control and treated groups) and mice were treated using a 4-day test (4-DT, see Materials and methods).
Results pertaining to selection of LM resistance are summarised in Table 2. ED50 and ED90 of the parent line were 1.67 and 3.93 mg/kg, respectively. The continuous LM pressure after 48 consecutive passages led to the selection of a parasite line yielding ED50 of 140.15 and ED90 of 249.75 corresponding to an I50 and I90 of 83.92 and 63.55, respectively. Such values of the I50/90 indicate that the parasite developed resistance to the drug.
Table 2.
Selection of lumefantrine resistance in Plasmodium berghei GFP-Luciferase ANKA strain using serial technique. Data are presented as effective doses that reduce parasitaemia by 50% and 90% (ED50, ED90) and as 50% and 90% indexes of resistance (I50 and I90, defined as the ratio of the ED50 or ED90 of the resistant line to that of the parent strain).
| Passage no. | ED50 (mg/kg) | (I50) | ED90 (mg/kg) | (I90) |
|---|---|---|---|---|
| Parent | 1.67 | 1 | 3.93 | 1 |
| 4th | 1.34 | 0.80 | 3.49 | 0.89 |
| 12th | 1.58 | 0.95 | 3.25 | 0.83 |
| 20th | 2.96 | 1.77 | 5.25 | 1.34 |
| 28th | 9.76 | 5.84 | 25.50 | 6.49 |
| 36th | 42.50 | 25.33 | 69.70 | 17.74 |
| 48th | 140.15 | 83.92 | 249.75 | 63.55 |
| Drug free passages | ||||
| 10th | 133.17 | 79.74 | 256.21 | 65.19 |
| 48th line after 2 months cryopreservation | 116.34 | 69.66 | 204.58 | 52.06 |
Like with PQ, the first 5 passages were not associated with increase in LM resistance. At the 5th passage, a dose 6 mg/kg allowed a parasitaemia of 0.08% only (Fig. 1B) However, at the 20th passage, at the same dose (6 mg/kg), parasite grew and reached 2% parasitaemia, a clear indication of emergence of resistance. This resistance increased further with the number of passages. At 28th, a dose of 10 mg/kg did not prevent parasitaemia to reach 4.1%, and at 36th, a higher parasitaemia of 6.5% was reached at a dose of 40 mg/kg. The higher level of resistance was observed at 48th passage, with mice harboring parasitaemia of 8% when treated with 80 mg/kg. During all 48 passages, parasitaemias in the untreated controls remained steady, ranging between 9% and 12%, a confirmation of dramatic rise in LM resistance (Fig. 1B). At this point (48th passage), a stable LM-resistant strain was selected (Fig. 1B, Table 2).
These high values of PQ and LM resistance indices led us to test the stability of the resistant phenotypes. We maintained these two resistant strains in absence of the drug pressure for 10 passages (at least 2 months) and then assessed in vivo activity of the drugs. The resulting PQ I50 and I90 remained high, with values of 147.28 and 83.80, respectively, and those of the LM were 79.74 for I50 and 65.19 for I90, a clear indication of the stability of the resistant phenotype. To further check this stability, we cryo-preserved these parasite lines for 2 and 4 months for LM and PQ, respectively. Upon revival, the analysis of the drug activity showed PQ I50 and I90 indexes of 84.64 and 63.39, respectively, and those for LM as 69.66 and 52.06, respectively. These values are slightly lower than those obtained before cryopreservation. However, they remained high, 50–80 times higher than those of the parent lines, a further indication of the stability of the phenotype (Tables 1 and 2).
We also tested the extent to which resistance to PQ and LM affect the activity of other antimalarial drugs, a phenomenon known as cross-resistance, and the results are summarized in Tables 3 and 4. Against the PQ-resistant line, the activity of AQ, CQ and DHA decreased significantly by a factor of 3–7 (p < 0.01 at least) (Table 3), an indication of the existence of slight cross-resistance of PQ with AQ, CQ and DHA. Surprisingly, the highest level of cross-resistance was recorded with LM, with its activity decreasing 97-fold against this PQ-resistant parasite line (p < 0.0001).
Table 3.
Response of piperaquine resistant Plasmodium berghei GFP ANKA line to amodiaquine (AQ), chloroquine (CQ), lumefantrine (LM) and dihydroartemisinin (DHA). Results are presented as effective doses that reduce parasitaemia by 90% (ED90) and as 90% indexes of resistance (I90, defined as the ratio of the ED90 of the resistant line to that of the sensitive, parent strain).
| Antimalarial drug | ED90⁎ (mg/kg) |
Index of resistance (I90) | |
|---|---|---|---|
| Parent strain | Resistant line | ||
| AQ | 3.72 | 13.48§ | 3.62 |
| LM | 2.52 | 245.06¶ | 97.25 |
| CQ | 3.57 | 26.24‡ | 7.35 |
| DHA | 4.08 | 12.06§ | 2.96 |
Differences between parent and resistant lines were significant according to Student’s t-test.
p < 0.01.
p < 0.001.
p < 0.0001.
Table 4.
Response of lumefantrine resistant Plasmodium berghei GFP-Luciferase ANKA line to amodiaquine (AQ), piperaquine (PQ), chloroquine (CQ) and dihydroartemisinin (DHA). Results are presented as effective doses that reduce parasitaemia by 90% (ED90) and as 90% indexes of resistance (I90, defined as the ratio of the ED90 of the resistant line to that of the sensitive, parent strain).
| Antimalarial drug | ED90⁎(mg/kg) |
Index of resistance (I90) | |
|---|---|---|---|
| Parent strain | Resistant line | ||
| AQ | 4.29 | 4.53† | 1.06 |
| PQ | 3.70 | 3.37† | 0.91 |
| CQ | 4.47 | 7.22¶ | 1.62 |
| DHA | 6.69 | 35.86‡ | 5.36 |
Differences between parent and resistant lines were analyzed by Student’s t-test.
p > 0.05 (insignificant).
Significant at p < 0.05.
Significant at p < 0.0001.
Overall, the LM-resistant parasite line retained relative susceptibility to the 4-aminoquinolines, AQ and PQ (Table 4). Indeed, AQ activity did not change (I90 of 1.06), and more interestingly, this parasite line remained susceptible to the bisquinoline PQ (I90 of 0.91). However, a significant decrease in activity was observed with the aminoquinoline CQ, with an I90 of 1.62 (p < 0.0001), and the endoperoxide DHA, with an I90 of 5.36 (p < 0.05). Thus the selection of LM resistance is associated with a decrease in CQ and DHA activity and the retention of AQ and PQ susceptibility.
4. Discussion
Our study shows that PQ and LM resistance in P. berghei ANKA can be selected within 18 months of continuous drug pressure. To the best of our knowledge, this is the first report of the selection of stable PQ- and LM-resistant strains in murine malaria following drug pressure. A PQ-resistant P. berghei strain had been selected in 5 months of selection pressure, but when the drug was removed, the strain reversed to sensitive phenotype (Li, 1985; Li et al., 1985), and a stable phenotype was observed only after mouse–mosquito–mouse passages (Li et al., 1985).
Two approaches by other laboratories have been used to select resistant murine malaria parasites: the 2% relapse technique (2% RT) in which a single and high drug dose is administered at the time of each passage (Peters and Robinson, 1999) and the serial technique (ST), in which drug dose is gradually increased after each passage (Peters, 1999; Peters and Robinson, 1999).
Using 2% RT, a number of phenotypes stably resistant to pyronaridine, amodiaquine, atovaquone and tafenoquine have been selected in P. berghei (Peters and Robinson, 1992, 1999, 2000; Peters et al., 2003) and tafenoquine in Plasmodium yoelii and Plasmodium chabaudi (Peters et al., 2003). However, using this method, stable resistance to sulfadoxine/pyrimethamine in P. berghei and artemisinin in P. yoelii could not be selected (Peters, 1999; Peters and Robinson, 1999). On the other hand, the ST approach has allowed the establishment of strains stably resistant to various antimalarials, including atovaquone in P. berghei (Syafruddin et al., 1999) and mefloquine in P. chabaudi, (Cravo et al., 2003), artemisinin in P. chabaudi (Afonso et al., 2006), halofantrine in P. yoelii (Singh and Puri, 2000) and arteether in P. vinckei (Puri and Chandra, 2006). Though failure to select stable resistance to piperaquine, chloroquine and primaquine in P. berghei has been reported (Li, 1985; Peters, 1999; Peters et al., 2003), overall, the ST approach has proven to be more efficient to select for stably resistant strains than 2% RT (Afonso et al., 2006; Cravo et al., 2003; Puri and Chandra, 2006). Using this approach, we have, for the first time, successfully established stable PQ- and LM-resistant P. berghei strains within 12–18 months of drug pressure.
Interestingly, after cryopreservation of both PQ and LM-resistant strains, a decrease in ED50/90 was recorded upon revival of the strains. This is common, it indicates that some of the mechanisms of resistance are the result of epigenetic changes such as gene amplification, protein over expression and protein modifications. However, if resistance is well established, the degree of ED50/90 decrease is small, the strains remained resistant to the drugs, as our data show.
Evaluation of cross-resistance patterns revealed that PQ and AQ retain potency against the LM resistant parasite line. LM is an arylaminoalcohol closely related to mefloquine (MQ), halofantrine and pyronaridine (Schlitzer, 2008). PQ, AQ and CQ are 4-aminoquinoline derivatives, and are likely to share a similar mechanism of action (Raynes, 1999). Resistance to CQ and AQ in P. falciparum is reported to be inversely correlated with resistance to arylaminoalcohols (Duraisingh and Cowman, 2005), and the selection of the resistance to arylamino-alcohol MQ results in an increase in CQ sensitivity (Cowman et al., 1994; Peel et al., 1993). In our experiments, LM resistance was not associated with a decrease in PQ efficacy. Similarly, the efficacy of the 4-aminoquinolines AQ and CQ did not change or only slightly decreased against the LM-resistant strain. Assuming that the mechanism of LM resistance is similar in P. falciparum and P. berghei, these results would suggest a high efficacy of PQ against LM-resistant strains in P. falciparum.
It is very interesting to note the activity of LM against PQ-resistant line decreased by 97-fold, a rate which is even higher than its activity against the LM-resistant parasite line selected after 2 years of LM pressure (I90 of 64). Thus, the selection of PQ resistance is associated with a higher level of LM resistance, while, as discussed earlier, the selection of LM resistance is associated with PQ susceptibility. This demonstrates that two different LM-resistance phenotypes exist. The first phenotype is associated with PQ resistance, while the second is associated with PQ susceptibility. Assuming that the same pattern prevails in P. falciparum, the use of either drug could be associated with resistance or susceptibility to the other. For instance, currently, Coartem® is being used to treat malaria, thus the selection of resistance to LM could be associated with susceptibility to PQ (component of Artekin®). While if Artekin® is first used, resistance to this drug may render Coartem® ineffective.
Our data show significant 3- and 5-fold decreases in dihydroartemisinin activity against PQ- and LM-resistant strains, respectively, an indication of the existence of a slight cross-resistance between LM and artemisinin, and PQ and artemisinin, in P. berghei. In P. falciparum, resistance to the arylamino-alcohol mefloquine, as the result of the increase copy number of pfmdr1, is associated with a decrease in activity of artemisinin derivatives (Nelson et al., 2005; Pickard et al., 2003; Price et al., 1999), thus a similar phenomenon may prevail in P. berghei with the arylamino-alcohol LM. We report cross-resistance between PQ and artemisinin in P. berghei, in agreement with previous work (Li, 1985; Li et al., 1985).
Thus, if the mechanism of LM and PQ resistance is similar in P. berghei and P. falciparum, the selection of LM and PQ resistance would be associated with a reduced artemisinin derivative efficacy, compromising the potential of artemisinin-based combinations. Consequently, there is an urgent need to clarify the mechanism of LM and PQ resistance and establish the extent of cross-resistance between these important antimalarials. The existence of cross-resistances to chemically and mechanistically unrelated drugs suggests the likely involvement of changes in drug accumulation, i.e. a ‘multi-drug resistance’ phenotype.
The LM and PQ resistant parasite lines have been selected so as to study the mechanism of drug resistance in P. berghei and use this information as platform to explore the resistance mechanism in P. falciparum. In the latter species, reports indicate that the use of LM + ATM (Coartem®) selects for 2 haplotypes at 86Y-184Y-1246Y and 86Y-184F-1246D of pfmdr1, a gene associated with changes in susceptibility to chloroquine (Dokomajilar et al., 2006; Humphreys et al., 2007; Sisowath et al., 2007, 2005). The copy number of pfmdr1 has also been reported to increase with the use of LM in field isolates in Thailand (Price et al., 2006), and a decrease in copy number was found to heighten in vitro lumefantrine susceptibility in laboratory selected parasites (Duraisingh and Cowman, 2005; Sidhu et al., 2006). These observations indicate that pfmdr1 will likely contribute to LM resistance, but the full definition of the mechanism of resistance remains to be elucidated since overall, Coartem® retains sensitivity against CQ-resistant isolates. In P. berghei and P. chabaudi, amplification of the pfmdr1 orthologue is associated with mefloquine resistance (Carlton et al., 2001) as in P. falciparum (Cowman et al., 1994; Peel, 2001; Sidhu et al., 2005). Thus, pfmdr1 could also be involved in LM resistance in P. berghei.
PQ is a bis-chloroquine derivative (Davis et al., 2005a; Raynes, 1999). Thus one could expect that PQ and CQ would share the same mode of action and perhaps a similar mechanism of resistance. However, PQ remains active against CQ-resistant isolates (Basco and Ringwald, 2003), clearly indicating that though PQ is closely related to CQ, these two drugs have different mechanisms of resistance. To date, no gene or candidate gene has been associated with PQ resistance in P. falciparum. Thus, further analysis of this PQ resistant P. berghei line could provide insight into the mechanism of PQ resistance.
However, we are aware that mechanism of resistance in P. falciparum and murine Plasmodium species may be different. For instance, the mechanisms of resistance to CQ are different in P. falciparum and in murine malaria and there is still a debate whether those of artemisinin derivatives will be similar (Afonso et al., 2006; Carlton et al., 2001; Hunt et al., 2007, 2004a,b). However, for drugs such as mefloquine, antifolates, and atovaquone, similar mechanisms of resistance have been reported (Carlton et al., 2001). Thus, the use of murine malaria could provide critical information on the mechanisms of resistance to PQ and LM.
In summary, we have selected LM and PQ resistant lines of P. berghei. The stability of this phenotype indicates that mechanisms that underlie it are coded into the cell genome. Amplification of pfmdr1 has been associated with resistance to mefloquine, an amino-alcohol (Carlton et al., 2001). We hypothesise that the same could prevail in LM, which is also an amino-alcohol. Studies are underway to explore the mechanisms of resistance to LM and PQ.
Acknowledgments
We thank the director of the Kenya Medical Research Institute for permission to publish these data. This work was supported by a Global Health Research Award to A.B. and A.N. from the Irish Health Research Board (GHRA-06-03) and by the European and Developing Countries Clinical Trials Partnership (EDCTP). A.N. is an EDCTP Senior Fellow.
References
- Afonso A., Hunt P., Cheesman S., Alves A.C., Cunha C.V., do Rosario V., Cravo P. Malaria parasites can develop stable resistance to artemisinin but lack mutations in candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrobial Agents Chemotherapy. 2006;50:480–489. doi: 10.1128/AAC.50.2.480-489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T., Sharma P., Gautam A., Varshney B., Kothari M., Ganguly S., Moehrle J.J., Paliwal J., Saha N., Batra V. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. Journal of Clinical Pharmacology. 2008;48:166–175. doi: 10.1177/0091270007310384. [DOI] [PubMed] [Google Scholar]
- Ashley E.A., Krudsood S., Phaiphun L., Srivilairit S., McGready R., Leowattana W., Hutagalung R., Wilairatana P., Brockman A., Looareesuwan S., Nosten F., White N.J. Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. Journal of Infectious Diseases. 2004;190:1773–1782. doi: 10.1086/425015. [DOI] [PubMed] [Google Scholar]
- Ashley E.A., McGready R., Hutagalung R., Phaiphun L., Slight T., Proux S., Thwai K.L., Barends M., Looareesuwan S., White N.J., Nosten F. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clinical Infectious Diseases. 2005;41:425–432. doi: 10.1086/432011. [DOI] [PubMed] [Google Scholar]
- Basco L.K., Ringwald P. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrobial Agents Chemotherapy. 2003;47:1391–1394. doi: 10.1128/AAC.47.4.1391-1394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton J.M., Hayton K., Cravo P.V., Walliker D. Of mice and malaria mutants: unravelling the genetics of drug resistance using rodent malaria models. Trends in Parasitology. 2001;17:236–242. doi: 10.1016/s1471-4922(01)01899-2. [DOI] [PubMed] [Google Scholar]
- Cowman A.F., Galatis D., Thompson J.K. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proceedings of National Academy of Sciences USA. 1994;91:1143–1147. doi: 10.1073/pnas.91.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo P.V., Carlton J.M., Hunt P., Bisoni L., Padua R.A., Walliker D. Genetics of mefloquine resistance in the rodent malaria parasite Plasmodium chabaudi. Antimicrobial Agents Chemotherapy. 2003;47:709–718. doi: 10.1128/AAC.47.2.709-718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T.M., Hung T.Y., Sim I.K., Karunajeewa H.A., Ilett K.F. Piperaquine: a resurgent antimalarial drug. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- Davis T.M., Karunajeewa H.A., Ilett K.F. Artemisinin-based combination therapies for uncomplicated malaria. The Medical journal of Australia. 2005;182:181–185. doi: 10.5694/j.1326-5377.2005.tb06650.x. [DOI] [PubMed] [Google Scholar]
- Denis M.B., Davis T.M., Hewitt S., Incardona S., Nimol K., Fandeur T., Poravuth Y., Lim C., Socheat D. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clinical Infectious Diseases. 2002;35:1469–1476. doi: 10.1086/344647. [DOI] [PubMed] [Google Scholar]
- Dokomajilar C., Nsobya S.L., Greenhouse B., Rosenthal P.J., Dorsey G. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrobial Agents Chemotherapy. 2006;50:1893–1895. doi: 10.1128/AAC.50.5.1893-1895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh M.T., Cowman A.F. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Tropica. 2005;94:181–190. doi: 10.1016/j.actatropica.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Fan B., Zhao W., Ma X., Huang Z., Wen Y., Yang J., Yang Z. In vitro sensitivity of Plasmodium falciparum to chloroquine, piperaquine, pyronaridine and artesunate in Yuxi prefecture of Yunnan province. Chinese Journal of Parasitology and Parasitic Diseases. 1998;16:460–462. [PubMed] [Google Scholar]
- Gervais G.W., Trujillo K., Robinson B.L., Peters W., Serrano A.E. Plasmodium berghei: identification of an mdr-like gene associated with drug resistance. Experimental Parasitology. 1999;91:86–92. doi: 10.1006/expr.1999.4344. [DOI] [PubMed] [Google Scholar]
- Guan W.B., Huang W.J., Zhou Y.C., Pan W.Q. Effect of piperaquine and hydroxypiperaquine on a chloroquine-resistant strain of Plasmodium falciparum. Chinese Journal of Parasitology and Parasitic Diseases. 1983;1:88–90. [PubMed] [Google Scholar]
- Hai T.N., Hietala S.F., Van Huong N., Ashton M. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Tropica. 2008;107:145–149. doi: 10.1016/j.actatropica.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Hastings I.M. The origins of antimalarial drug resistance. Trends in Parasitology. 2004;20:512–518. doi: 10.1016/j.pt.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Hastings I.M., Ward S.A. Coartem (artemether-lumefantrine) in Africa: the beginning of the end? Journal of Infectious Diseases. 2005;192:1303–1304. doi: 10.1086/432554. [DOI] [PubMed] [Google Scholar]
- Humphreys G.S., Merinopoulos I., Ahmed J., Whitty C.J., Mutabingwa T.K., Sutherland C.J., Hallett R.L. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrobial Agents Chemotherapy. 2007;51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P., Afonso A., Creasey A., Culleton R., Sidhu A.B., Logan J., Valderramos S.G., McNae I., Cheesman S., do Rosario V., Carter R., Fidock D.A., Cravo P. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Molecular Microbiology. 2007;65:27–40. doi: 10.1111/j.1365-2958.2007.05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P., Cravo P.V., Donleavy P., Carlton J.M., Walliker D. Chloroquine resistance in Plasmodium chabaudi: are chloroquine-resistance transporter (crt) and multi-drug resistance (mdr1) orthologues involved? Molecular and Biochemical Parasitology. 2004;133:27–35. doi: 10.1016/j.molbiopara.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Hunt P., Martinelli A., Fawcett R., Carlton J., Carter R., Walliker D. Gene synteny and chloroquine resistance in Plasmodium chabaudi. Molecular and Biochemical Parasitology. 2004;136:157–164. doi: 10.1016/j.molbiopara.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Karema C., Fanello C.I., van Overmeir C., van Geertruyden J.P., van Doren W., Ngamije D., D’Alessandro U. Safety and efficacy of dihydroartemisinin/piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100:1105–1111. doi: 10.1016/j.trstmh.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Karunajeewa H., Lim C., Hung T.Y., Ilett K.F., Denis M.B., Socheat D., Davis T.M. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. British Journal of Clinical Pharmacology. 2004;57:93–99. doi: 10.1046/j.1365-2125.2003.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokwaro G., Mwai L., Nzila A. Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opinions in Pharmacotherapy. 2007;8:75–94. doi: 10.1517/14656566.8.1.75. [DOI] [PubMed] [Google Scholar]
- Li G.D. Development of a piperaquine-resistant line of Plasmodium berghei K 173 strain. Yao Xue Xue Bao. 1985;20:412–417. [PubMed] [Google Scholar]
- Li G.D., Qu F.Y., Chen L. Development of piperaquine-resistant line of Plasmodium berghei ANKA strain. Chinese Journal of Prevention and Treatment of Parasitic Diseases. 1985;3:189–192. [PubMed] [Google Scholar]
- Merkli B., Richle R.W. Studies on the resistance to single and combined antimalarials in the Plasmodium berghei mouse model. Acta Tropica. 1980;37:228–231. [PubMed] [Google Scholar]
- Mutabingwa T.K. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Tropica. 2005;95:305–315. doi: 10.1016/j.actatropica.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Navaratnam V., Mansor S.M., Sit N.W., Grace J., Li Q., Olliaro P. Pharmacokinetics of artemisinin-type compounds. Clinical Pharmacokinetic. 2000;39:255–270. doi: 10.2165/00003088-200039040-00002. [DOI] [PubMed] [Google Scholar]
- Nelson A.L., Purfield A., McDaniel P., Uthaimongkol N., Buathong N., Sriwichai S., Miller R.S., Wongsrichanalai C., Meshnick S.R. Pfmdr1 genotyping and in vivo mefloquine resistance on the Thai–Myanmar border. American Journal of Tropical Medicine Hygiene. 2005;72:586–592. [PubMed] [Google Scholar]
- Nosten F., White N.J. Artemisinin-based combination treatment of falciparum malaria. American Journal of Tropical Medicine Hygiene. 2007;77:181–192. [PubMed] [Google Scholar]
- Nzila A. Inhibitors of de novo folate enzymes in Plasmodium falciparum. Drug Discovery Today. 2006;11:939–944. doi: 10.1016/j.drudis.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Nzila A.M., Nduati E., Mberu E.K., Hopkins Sibley C., Monks S.A., Winstanley P.A., Watkins W.M. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate Pyrimethamine/Sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. Journal of Infectious Diseases. 2000;181:2023–2028. doi: 10.1086/315520. [DOI] [PubMed] [Google Scholar]
- Pang X.J., Li J., Fu S.G., Chen Q.Y., Chen H.Y., Su X.L. Two cases of piperaquine-resistant Plasmodium falciparum in Hainan. Chinese Journal of Prevention and Treatment of Parasitic Diseases. 1989;2:18. [Google Scholar]
- Peel S.A. The ABC transporter genes of Plasmodium falciparum and drug resistance. Drug Resistance Update. 2001;4:66–74. doi: 10.1054/drup.2001.0183. [DOI] [PubMed] [Google Scholar]
- Peel S.A., Merritt S.C., Handy J., Baric R.S. Derivation of highly mefloquine-resistant lines from Plasmodium falciparum in vitro. American Journal of Tropical Medicine Hygiene. 1993;48:385–397. doi: 10.4269/ajtmh.1993.48.385. [DOI] [PubMed] [Google Scholar]
- Peters W. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Annals of Tropical Medicine and Parasitology. 1975;69:155–171. [PubMed] [Google Scholar]
- Peters W. The chemotherapy of rodent malaria. LVII. Drug combinations to impede the selection of drug resistance, Part 1: which model is appropriate? Annals of Tropical Medicine and Parasitology. 1999;93:569–587. doi: 10.1080/00034989958087. [DOI] [PubMed] [Google Scholar]
- Peters W., Robinson B.L. The chemotherapy of rodent malaria. XLVII. Studies on pyronaridine and other Mannich base antimalarials. Annals of Tropical Medicine and Parasitology. 1992;86:455–465. doi: 10.1080/00034983.1992.11812694. [DOI] [PubMed] [Google Scholar]
- Peters W., Robinson B.L. The chemotherapy of rodent malaria. LVI. Studies on the development of resistance to natural and synthetic endoperoxides. Annals of Tropical Medicine and Parasitology. 1999;93:325–329. doi: 10.1080/00034989958320. [DOI] [PubMed] [Google Scholar]
- Peters W., Robinson B.L. The chemotherapy of rodent malaria. LVIII. Drug combinations to impede the selection of drug resistance, Part. 2: The new generation—artemisinin or artesunate with long-acting blood schizontocides. Annals of Tropical Medicine and Parasitology. 2000;94:23–35. doi: 10.1080/00034980057581. [DOI] [PubMed] [Google Scholar]
- Peters W., Stewart L.B., Robinson B.L. The chemotherapy of rodent malaria. LXI. Drug combinations to impede the selection of drug resistance, part 4: the potential role of 8-aminoquinolines. Annals of Tropical Medicine and Parasitology. 2003;97:221–236. doi: 10.1179/000349803235001886. [DOI] [PubMed] [Google Scholar]
- Pickard A.L., Wongsrichanalai C., Purfield A., Kamwendo D., Emery K., Zalewski C., Kawamoto F., Miller R.S., Meshnick S.R. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrobial Agents Chemotherapy. 2003;47:2418–2423. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.N., Cassar C., Brockman A., Duraisingh M., van Vugt M., White N.J., Nosten F., Krishna S. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrobial Agents Chemotherapy. 1999;43:2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.N., Uhlemann A.C., van Vugt M., Brockman A., Hutagalung R., Nair S., Nash D., Singhasivanon P., Anderson T.J., Krishna S., White N.J., Nosten F. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clinical Infectious Diseases. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S.K., Chandra R. Plasmodium vinckei: selection of a strain exhibiting stable resistance to arteether. Experimental Parasitology. 2006;114:129–132. doi: 10.1016/j.exppara.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Raynes K. Bisquinoline antimalarials: their role in malaria chemotherapy. International Journal of Parasitology. 1999;29:367–379. doi: 10.1016/s0020-7519(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Schlitzer M. Antimalarial drugs—what is in use and what is in the pipeline. Archiv der Pharmazie. 2008;341:149–163. doi: 10.1002/ardp.200700184. [DOI] [PubMed] [Google Scholar]
- Sidhu A.B., Uhlemann A.C., Valderramos S.G., Valderramos J.C., Krishna S., Fidock D.A. Decreasing pfmdr1 copy number in plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. Journal of Infectious Diseases. 2006;194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A.B., Valderramos S.G., Fidock D.A. Pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Molecular Microbiology. 2005;57:913–926. doi: 10.1111/j.1365-2958.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- Singh N., Puri S.K. Modulation of halofantrine resistance after coadministration of halofantrine with diverse pharmacological agents in a rodent malaria model. Life Sciences. 2000;67:1345–1354. doi: 10.1016/s0024-3205(00)00728-1. [DOI] [PubMed] [Google Scholar]
- Sisowath C., Ferreira P.E., Bustamante L.Y., Dahlstrom S., Martensson A., Bjorkman A., Krishna S., Gil J.P. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Tropical Medicine and International Health. 2007;12:736–742. doi: 10.1111/j.1365-3156.2007.01843.x. [DOI] [PubMed] [Google Scholar]
- Sisowath C., Stromberg J., Martensson A., Msellem M., Obondo C., Bjorkman A., Gil J.P. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem) Journal of Infectious Diseases. 2005;191:1014–1017. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- Syafruddin D., Siregar J.E., Marzuki S. Mutations in the cytochrome b gene of Plasmodium berghei conferring resistance to atovaquone. Molecular and Biochemical Parasitology. 1999;104:185–194. doi: 10.1016/s0166-6851(99)00148-6. [DOI] [PubMed] [Google Scholar]
- Tarning J., Ashley E.A., Lindegardh N., Stepniewska K., Phaiphun L., Day N.P., McGready R., Ashton M., Nosten F., White N.J. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrobial Agents Chemotherapy. 2008;52:1052–1061. doi: 10.1128/AAC.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona L., Mesia K., Ngimbi N.P., Chrimwami B., Okond’ahoka, Cimanga K., de Bruyne T., Apers S., Hermans N., Totte J., Pieters L., Vlietinck A.J. In-vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Annals of Tropical Medicine and Parasitology. 2001;95:47–57. [PubMed] [Google Scholar]
- Vennerstrom J.L., Arbe-Barnes S., Brun R., Charman S.A., Chiu F.C., Chollet J., Dong Y., Dorn A., Hunziker D., Matile H., McIntosh K., Padmanilayam M., Santo Tomas J., Scheurer C., Scorneaux B., Tang Y., Urwyler H., Wittlin S., Charman W.N. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- Watkins W.M., Mosobo M. Treatment of Plasmodium falciparum malaria with pyrimethamine–sulfadoxine: selective pressure for resistance is a function of long elimination half-life. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87:75–78. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- White N.J. Antimalarial drug resistance. Journal of Clinical Investigation. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J., van Vugt M., Ezzet F. Clinical pharmacokinetic and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clinical pharmacokinetic. 1999;37:105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- Yang H., Liu D., Huang K., Yang Y., Yang P., Liao M., Zhang C. Assay of sensitivity of Plasmodium falciparum to chloroquine, amodiaquine, piperaquine, mefloquine and quinine in Yunnan province. Chinese Journal of Parasitology and Parasitic Diseases. 1999;17:43–45. [PubMed] [Google Scholar]
- Yang H.L., Yang P.F., Liu D.Q., Liu R.J., Dong Y., Zhang C.Y., Cao D.Q., He H. Sensitivity in vitro of Plasmodium falciparum to chloroquine, pyronaridine, artesunate and piperaquine in south Yunnan. Chinese Journal of Parasitology and Parasitic Diseases. 1992;10:198–200. [PubMed] [Google Scholar]
- Zhang K.Y., Zhou J.X., Wu Z., Huang Q.L. Susceptibility of Plasmodium falciparum to chloroquine, piperaquine, amodiaquine, mefloquine and quinine with in vitro microtechnique in Hainan Island. Chinese Journal of Parasitology and Parasitic Diseases. 1987;5:165–169. [PubMed] [Google Scholar]