Abstract
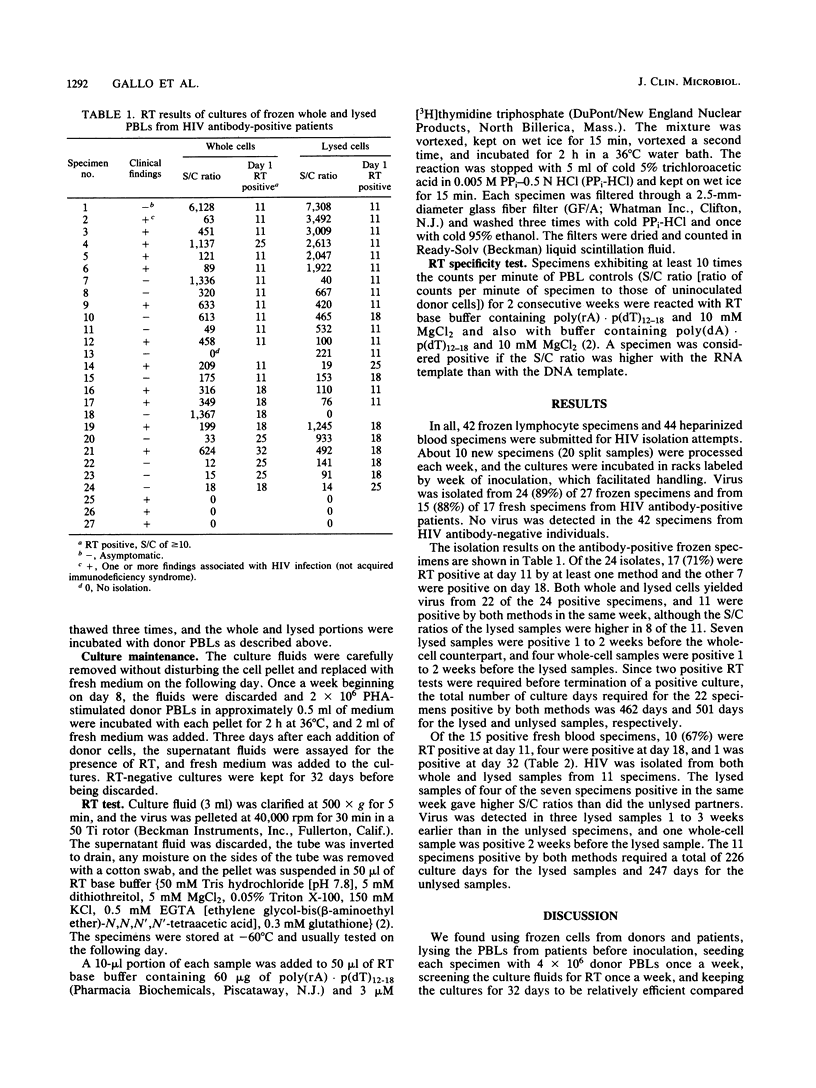
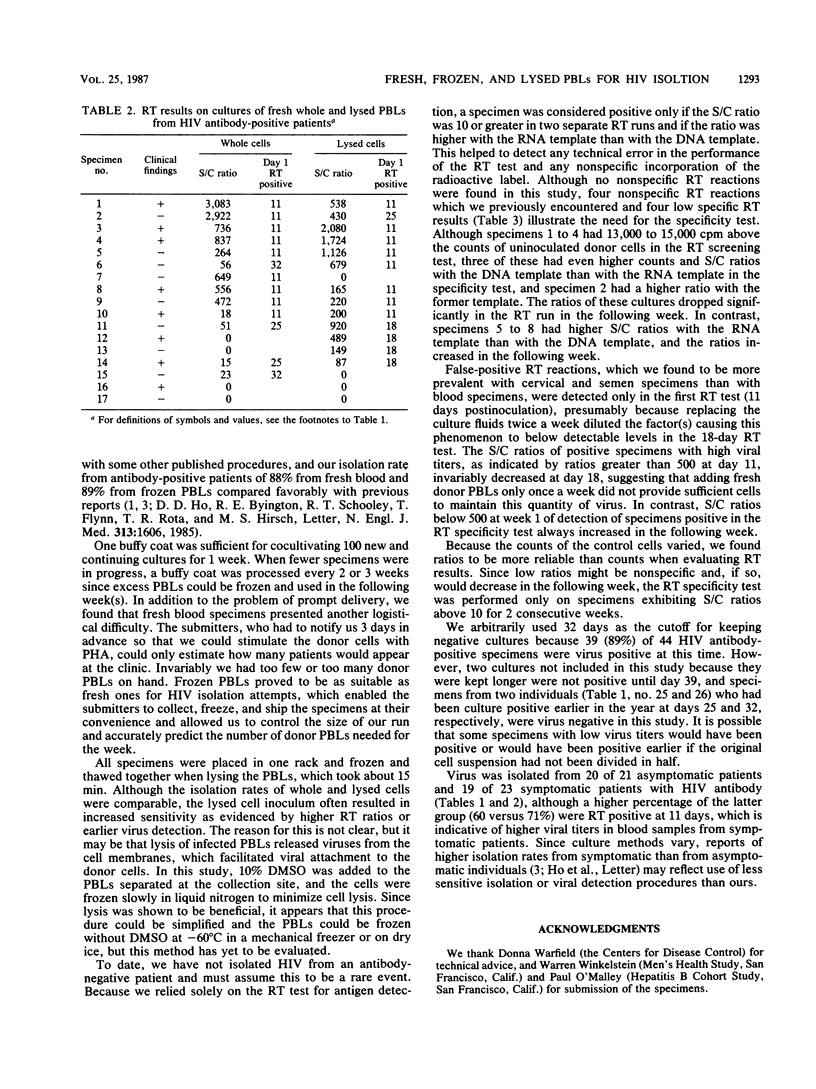
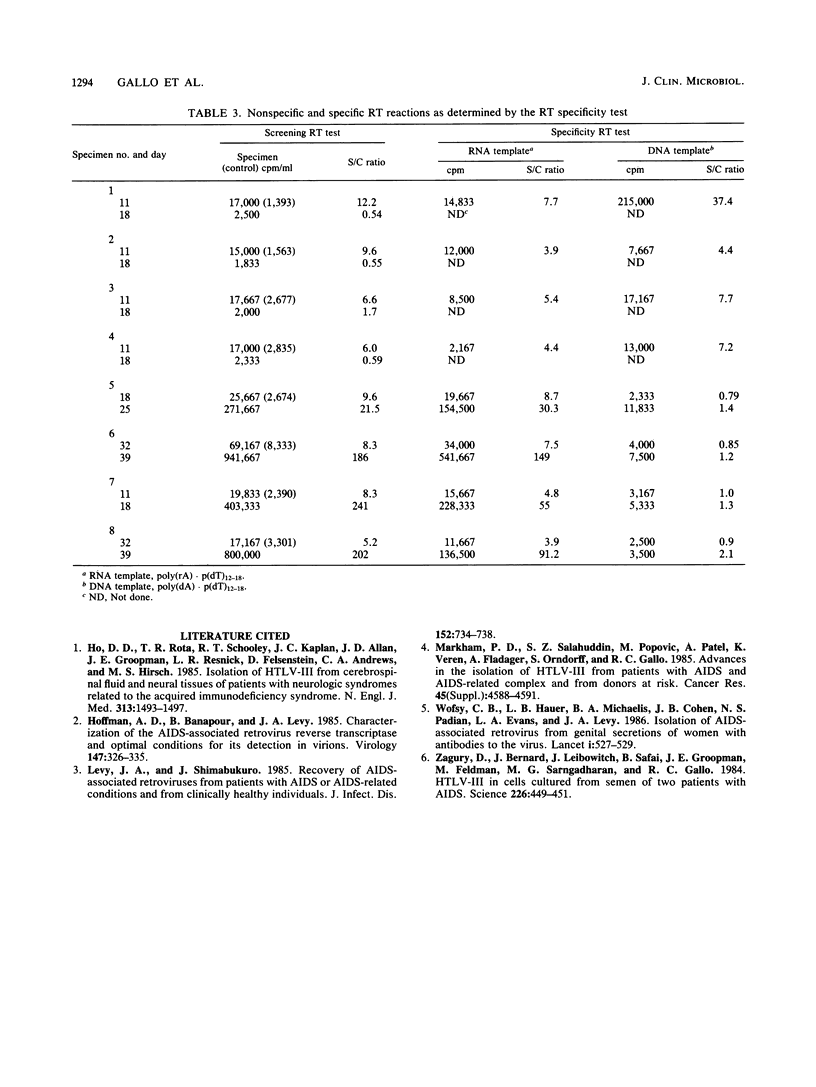
Heparinized blood specimens (n = 44) and frozen peripheral blood lymphocyte (PBL) specimens (n = 42) were used to evaluate the effects of lysis on human immunodeficiency virus (HIV) isolation. In the two respective groups, 17 and 27 specimens were HIV antibody positive. In the first group there were 8 and in the second group there were 25 that were symptomatic and were classified as indicating an acquired immunodeficiency syndrome-related condition or a pre-acquired immunodeficiency syndrome-related condition by the Centers for Disease Control definition. One-half of the cells from each specimen were frozen and thawed three times before cocultivation with uninfected lymphocytes, and the isolation rates from whole and lysed cells were compared. HIV was isolated from 15 (88%) of 17 fresh specimens and from 24 (89%) of 27 frozen PBLs from HIV antibody-positive patients, and lysis had no overall effect on the isolation rate, which suggested that frozen PBLs were as suitable as fresh blood for HIV isolation attempts and that it was not necessary to maintain cell integrity when submitting PBL samples. Of 21 asymptomatic patients, 20 were culture positive, and of 23 symptomatic patients, 19 were culture positive. Specimens from the 42 antibody-negative individuals were culture negative.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985 Oct;152(4):734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- Wofsy C. B., Cohen J. B., Hauer L. B., Padian N. S., Michaelis B. A., Evans L. A., Levy J. A. Isolation of AIDS-associated retrovirus from genital secretions of women with antibodies to the virus. Lancet. 1986 Mar 8;1(8480):527–529. doi: 10.1016/s0140-6736(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Leibowitch J., Safai B., Groopman J. E., Feldman M., Sarngadharan M. G., Gallo R. C. HTLV-III in cells cultured from semen of two patients with AIDS. Science. 1984 Oct 26;226(4673):449–451. doi: 10.1126/science.6208607. [DOI] [PubMed] [Google Scholar]


