Abstract
c-Src is the normal human cellular protein homologue of the viral oncogene v-src. c-Src activity was reported recently to increase in CD40-activated human B lymphocytes, suggesting its involvement in proliferation. To elucidate the exact role of c-Src in this process, we investigated the effects of c-Src over-expression on normal B lymphocyte growth. B lymphocytes purified from human peripheral blood were infected with Ad5/F35 vector encoding either a constitutively active c-Src (c-Src/dominant-positive) or a dominant-negative c-Src (c-Src/DN). Little variation of B lymphocytes expansion could be observed between control enhanced yellow fluorescent protein and c-Src/dominant-positive-infected cells. In contrast, over-expression of c-Src/DN results in a 40% inhibition of B lymphocyte expansion. These results suggest that DN c-Src may compete with endogenous c-Src, resulting in partial inhibition of a transcriptional pathway involved in B lymphocyte proliferation. We demonstrate further that c-Src can phosphorylate signal transducer and activator of transcription 5b (STAT5b) on tyrosine 699 and that c-Src and STAT5b co-associate during B lymphocyte proliferation. These results confirm an important role for c-Src in the expansion of normal human B lymphocytes in vitro, in which c-Src may regulate STAT5b in the intracellular signalling pathway important for the proliferation of normal human B lymphocytes.
Keywords: adenovirus, cellular proliferation, c-Src, human B lymphocytes, phosphorylation, STAT5
Introduction
Normal human peripheral blood B lymphocytes can be expanded in vitro through a variety of different stimuli [1–6]. However, using a combination of CD40 stimulation in the presence of interleukin (IL)-2, IL-4 and IL-10 appears to be a good stimulus for expansion of human and other animal B lymphocytes in vitro[7–10], and also plays a significant role in the normal development and function of B lymphocytes [11–14] as well as in the propagation of malignant B lymphocytes [15]. The exact signalling pathways responsible for the induction and maintenance of B lymphocytes proliferation are unknown, but previous studies have implicated a role for the c-Src protein tyrosine kinase [9].
c-Src is a 60-kilodalton phosphoprotein (pp60c-src) that is encoded by the c-src gene, which is the normal human cellular homologue of the highly transforming v-src gene of the Rous sarcoma virus (reviewed in [16,17]). c-Src protein tyrosine kinase is ubiquitous in nature; however, it was described only recently to be present in both normal human T cells [18] and B cells [9]. The reason for this is due to previous investigators looking only at the expression of c-Src in resting human peripheral blood T or B cells [19,20]; however, c-Src kinase is found only in normal human T and B cells following activation [9,18]. In B lymphocytes, c-Src was inducible following stimulation with CD40 ligand (CD154) and to a lesser extent other stimuli and the expression and activation of c-Src correlated with the expansion of the B lymphocytes in culture [9]. These results suggested that c-Src may play a specific role in normal B lymphocyte proliferation.
Previous investigators have shown that signal transducers and activators of transcription (STATs) can be activated by the Src kinase [21,22]. Furthermore, it has been shown that Src co-associates with and phosphorylates STAT3 or a related STAT and this enhances the DNA-binding activity of the STAT [23]. In addition, Src kinase has been implicated in the activation of STAT5 and Src kinase was able to mediate growth pathways through activation of STAT5 in certain cancers [24,25]. These early findings have led to an interest in STATS as downstream transducers in Src family kinase-mediated tumorigenesis [26]. Recent interest has focused upon a role for c-Src in the regulation of STAT5b in transformation and certain malignancies [27–30]. Thus, there appears to be a direct relationship between c-Src and STATs, in particular STAT5b, whereby c-Src is able to phosphorylate and activate STATs and this then drives transformation and proliferation of cells. Moreover, in normal human B lymphocytes, over-expression studies of a constitutively active STAT5b in human B lymphocytes revealed an important role of this transcription factor in B lymphocyte proliferation [31].
In this report, we demonstrate that in normal human B lymphocyte proliferation, c-Src is activated and is then able to associate with STAT5b and phosphorylate this protein on Y699. Using a dominant-negative (DN) form of c-Src results in a loss of phosphorylation of co-associated STAT5b as well as a significant decrease in the proliferative potential of the B lymphocytes. This report provides the first evidence that, in the normal proliferation of human B lymphocytes, c-Src is necessary for the phosphorylation of STAT5b, which then mediates the proliferation of the B lymphocytes.
Material and methods
Human peripheral B lymphocytes
Blood samples were obtained from healthy individuals after informed consent and peripheral blood mononuclear cells were prepared by density centrifugation over Ficoll-Paque (Amersham/Biosciences, Baie D'Urfé, QC, Canada). B lymphocytes were purified by negative selection using the StemSep CD19 mixture according to the manufacturer's instructions (Stem Cell Technologies, Vancouver, BC, Canada). Purified human B lymphocytes were >95% CD19+, as determined by flow cytometry. Prior to adenoviral infection, B lymphocytes were activated and cultured for 3 days using SCM-7 membrane preparation expressing CD154, as described previously [32,33], in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% heat-inactivated ultra-low immunoglobulin (Ig)G fetal bovine serum, 10 µg/ml insulin, 5·5 µg/ml transferrin, 6·7 ng/ml sodium selenite, antibiotics (all from Invitrogen, Burlington, ON, Canada) and 100 U/ml IL-4 (R&D Systems, Minneapolis, MN, USA) and 50 U/ml IL-2 and 25 U/ml IL-10 (PeproTech, Rocky Hill, NJ, USA). Cell count and viability were evaluated in triplicate by Trypan blue dye exclusion. Cultured B lymphocytes were always >96% CD19+, and unless specified otherwise viability was >85%.
Adenoviral vector production
Ad5/F35 vectors were generated as described previously [32] by in vivo recombination in Escherichia coli BJ5183 cells between pAdenoVator transfer plasmids and pAdEasy-1/F35 adenoviral genome (a generous gift from Dr X. Fan, University of Lund) using the AdenoVator™ Vector system (QBiogene, Carlsbad, CA, USA). Transfer plasmids containing the cytomegalovirus (CMV) promoter/enhancer with a β-globin/IgG chimeric intron (CMVi) were purchased from QBiogene. For enhanced yellow fluorescent protein (EYFP) control Ad5/F35 vectors, EYFP from p internal ribosome entry site-EYFP (Clontech, Palo Alto, CA, USA) was cloned into the transfer plasmids described above. For c-src expression Ad5/F35 vectors, an expression cassette encoding EYFP under the control of the mouse phosphoglycerate kinase (PGK) (described in [32]) was first cloned into the CMVi transfer plasmid, and c-src cDNA were then cloned into the CMVi expression cassette. cDNA encoding dominant-positive (DP) and DN c-src were generated from full-length human cDNA [9] and mutants generated by oligonucleotide-site-directed mutagenesis to create Y530F (DP) and K298M-Y530F (DN) full-length mutant forms. Recombinant Ad5/F35 vectors were transfected into QBI-293A cells using standard calcium phosphate transfection, and recombinant viruses were plaque-purified. Viruses were then amplified by transduction of large HEK293 cell cultures (QBiogene) and purified as described [33]. The viral titres were determined by the tissue culture infectious dose (TCID50) method following the manufacturer's instructions (QBiogene). Adenoviral infection of cells were performed as described previously for B lymphocytes [32,33]. Briefly, cells were washed in IMDM and incubated (10 × 106 cells/ml) in 200 µl of IMDM containing virus at a multiplicity of infection = 500 into 24-well culture plates. After 1 h at 37°C and 10% CO2, volumes were adjusted to 1 ml of their respective culture medium. Cells were incubated further at 37°C and 10% CO2 and reinfected each 96 h to maintain transgene expression. At different times (as indicated) after transduction, the cells were recovered and subjected to analysis.
Real-time quantitative–polymerase chain reaction
Total RNA was extracted from 1 × 106 infected cells using the Absolutely RNA extraction kit (Stratagene, La Jolla, CA, USA). One µg of RNA was used to make cDNA with the Moloney murine leukaemia virus (M-MLV) reverse transcriptase (Invitrogen). Quantitative–polymerase chain reaction (Q–PCR) reactions were performed with the full velocity SYBR green Q–PCR master mix according to the manufacturer's instructions and the Mx3005P Q–PCR system (Stratagene). For each time-point, the average of three replicate reactions was calculated. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control for normalization. The following sequences of primers were used for Q–PCR of src-5′: 5′-GGC TAC ATC CCC AGC AAC TA-3′; 3′: 5′-TGA GAG GCA GTA GGC ACC TT-3′ primers for STAT5b 5′: 5′-GCG TTA TAT GGC CAG CAT TT-3; 3′: 5′-TCT GGA GCT GTG TGG CAT AG-3′ and primers for the GAPDH mRNA: 5′-ATG CAA CGG ATT TGG TCG TAT-3′; 5′-TCT CGC TCC TGG AAG ATG GTG-3′. The specificity of the primers was verified by melting curve analysis. The comparative Ct method was used to calculate the relative expression of mRNA normalized with the housekeeping gene control (GAPDH) and fold expression of the transcripts were expressed relatively to transcripts level in EYFP-infected B lymphocytes.
Immune complex kinase assay
In vitro immune complex kinase assay was performed after immunoprecipitation of c-Src, as described previously [9]. Briefly, 150 µg of total protein from radioimmunoprecipitation assay (RIPA) buffer lysed B lymphocytes at various times in culture was immunoprecipitated using anti-c-Src monoclonal 327 (Calbiochem, San Diego, CA, USA) that had first been attached to Protein A/G-Sepharose beads (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Following immunoprecipitation, the beads were washed and resuspended in kinase buffer, incubated with 10 µCi γ32P-ATP (ICN Biomedicals, Irvine, CA, USA) and 2 µg rabbit muscle enolase (Sigma, Oakville, ON, Canada). After 10 min incubation at 37°C, reduced sample buffer was added, the mixture was boiled for 10 min and the entire sample loaded onto 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) for separation of the proteins. The 32P-labelled proteins were electrotransferred to Immobilon membranes (Millipore Corp., Bedford, MA, USA) and radiolabelled proteins visualized by autoradiography.
Western immunobloting
Twenty µg of total protein from RIPA lysed transduced B lymphocytes were separated on an 8% SDS-PAGE and transferred electrophoretically to Immobilon membranes. Membranes were blocked for 1 h at room temperature with 5% skimmed milk powder and 1% polyvinylpyrrolidone (Sigma) in Tris-buffered saline (TBS) (10 mM Tris, 100 mM Nacl, pH 7·5). Membranes were then incubated with primary antibodies, c-Src monoclonal GD11 (Upstate Cell Signaling Solutions, Charlottesville, VA, USA), STAT5a/b rabbit polyclonal sc-835 (Santa Cruz Biotechnology) and phosphoStat5a/b (Tyr 699) monoclonal 14H2 (Cell Signaling, Danvers, MA, USA). The blots were washed in TBS and then incubated with secondary antibodies conjugated to horseradish peroxidase and enhanced chemiluminescence was performed to visualize the proteins (Amersham Pharmacia Biotech). PhosphoSTAT5a/b, STAT5a/b, c-Src and actin were probed in this sequence on the same blot between stripping with Restore Western Blot Stripping Buffer (Pierce, Rockford, IL, USA). Actin was detected with the A-2066 rabbit anti-actin antibody (Sigma).
Co-immunopreprecipitation of c-Src and STAT5
c-Src was immunoprecipitated from transduced B lymphocytes (5 × 106 cells) as described in the immune complex kinase assay section. The entire immunoprecipitate was resolved on an 8% SDS-PAGE and electrotransferred to Immobilon membranes. PhosphoSTAT5a/b, STAT5a/b and c-Src were then detected by Western immunoblotting.
Phosphorylation of STAT5b by c-Src
The DB cells (a human B cell lymphoma) that were shown not to express endogenous c-Src (Fig. 5), but to express STAT5b, were used in an immune complex kinase assay; 3 × 106 DB cells were lysed in RIPA. The lysate immunoprecipitated with 2 µg anti-STAT5b (C-17; Santa Cruz Biotechnology) pre-bound to protein A/G PLUS Agarose beads. The immunoprecipiate was incubated in kinase buffer containing 10 µCi γ32P-ATP (MP Biomedical, Santa Ana, CA, USA) and 2 µl (6 units) human recombinant c-Src (p60c-src; Upstate Biotechnolgy, Lake Placid, NY, USA). After 10 min incubation at 37°C a reduced sample buffer was added, the mixture boiled for 10 min and the entire sample loaded onto 12% SDS-PAGE for separation of the proteins. The 32P-labelled proteins were electrotransferred to Immobilon-P membranes (Millipore Corp.) and radiolabelled proteins visualized by autoradiography. The membrane was probed using anti-STAT5b(C-17) and then stripped and re-probed using anti-pSTAT5b(Tyr 699) and anti-Src (clone GD11; Upstate Biotechnology).
Fig. 5.
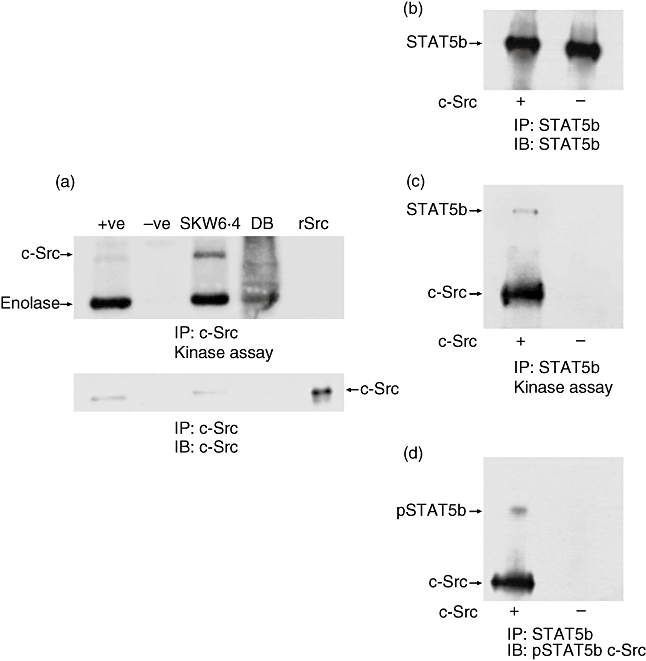
Signal transducer and activator of transcription 5b (STAT5b) is phosphorylated on tyrosine 699 by c-Src. DB cells that do not contain c-Src kinase were used to immunoprecipitate STAT5b followed by addition of human recombinant c-Src (rSrc) in a kinase assay. (a) 50 × 106 DB cells and 5 × 106 SKW 6·4 control cells were immunoprecipitated with anti-c-Src (monoclonal antibody 327) bound to protein A-Sepharose beads and a kinase assay performed with rabbit muscle enolase as exogenous substrate (a: top part); +ve: rSrc and −ve: just anti-c-Src bound to protein A-Sepharose without lysate. Thre lower part of the figure shows a Western blot using anti-c-Src (GD11). (b) 5 × 106 DB cells were used to immunoprecipiate STAT5b followed by a kinase assay using rSrc without rabbit muscle enolase. A Western blot was performed showing that STAT5b was immunoprecipitated. (c) The kinase results of the same experiment show phosphorylation of only the STAT5b and the autophosphorylation of the rSrc. (d) The membrane was stripped and reprobed using anti-pSTAT5b and anti-c-Src, to control for the Western blot.
Results
c-Src over-expression increases B lymphocyte proliferation
We have reported previously that c-Src may be involved in the proliferation of human B lymphocytes [9]. Therefore, to determine whether the expression of c-Src has any direct effect on the proliferation of B lymphocytes, we examined the effects of c-Src over-expression on their proliferation. For this purpose we first prepared two Ad5/F35 adenoviral expression vectors encoding c-Src-mutants, as we have demonstrated previously that these types of vectors are highly efficient for gene expression in human B lymphocytes [32,33]. We used a constitutive active form of c-Src (c-src DP) in which the tyrosine in position 530 was mutated to phenylalanine (Y530F) [34], and a kinase dead c-Src where the adenosine 5′-triphosphate (ATP) binding site at the lysine at position 298 is mutated to methionine (L298M) [9]. The tyrosine in position 530 was also mutated to phenylalanine in c-Src DN to ensure that the regulatory C-terminal tyrosine of c-Src could not be phosphorylated by other tyrosine kinases such as Csk and, thus, inhibit normal c-Src substrate binding to its SH2 and SH3 domains. This L298M/Y530F double mutant is expected to compete successfully for normal c-Src substrates, and being kinase-dead thus acts as a true DN form of c-Src. cDNA were each cloned into a Ad5/F35 virus containing the EYFP as a reporter gene, and Ad5/F35 virus containing only EYFP was used as control.
Normal human peripheral blood B lymphocytes were grown in the CD40 culture system and infected with the various adenoviruses. As revealed by EYFP expression, more than 70% of B lymphocytes expressed adenovirus-transferred cDNAs as early as 48 h post-infection, thus B lymphocytes were harvested and c-Src expression was analysed by Q–PCR, Western blot and kinase assay. As shown in Fig. 1a, c-src transcripts were increased by 54- and 26-fold in c-SrcDP and c-SrcDN transduced cells, respectively, compared with empty EYFP-vector (mock) transduced cells. Consequently, a higher amount of c-Src proteins was detected in c-Src transduced B lymphocytes (Fig. 1b). c-Src were immunoprecipitated from transfected B lymphocytes and kinase activity was assayed on the exogenous substrate enolase. As shown in Fig. 1c, a significant increase in kinase activity was observed in c-Src DP-transduced cells compared with mock-transduced cells, as shown by the increase in phosphorylated enolase. In contrast, a significant decrease in kinase activity was observed in c-Src DN-transduced cells compared with mock-transduced cells. Moreover, autophosphorylation of c-Src was increased in c-Src DP-transduced cells, whereas it was decreased in c-Src DN-transduced cells.
Fig. 1.
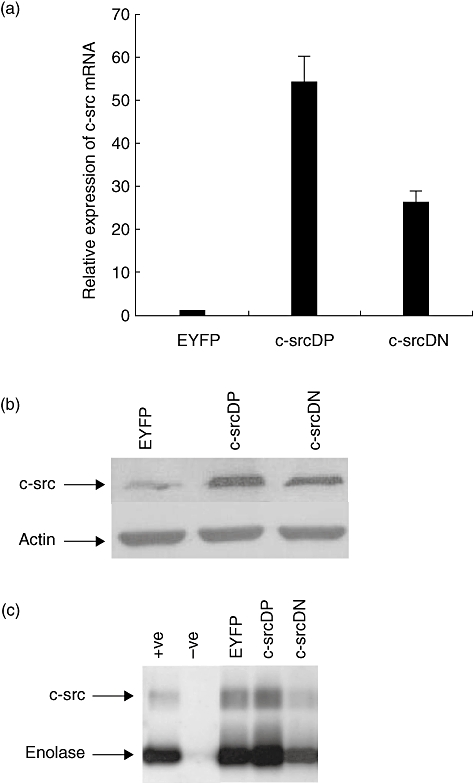
c-Src expression in transduced human B lymphocytes. Normal human B lymphocytes were infected with recombinant adenovirus encoding enhanced yellow fluorescent protein (EYFP), c-src dominant-positive (DP) or c-src dominant-negative (DN) and cultured in the CD40/CD154 system. Expression of c-Src was analysed 48 h post-infection. (a) c-src mRNA levels were determined by quantitative competitive–polymerase chain reaction (Q–PCR). Fold expression of the transcripts was expressed relative to transcripts level in Ad5/F35-EYFP-infected B lymphocytes. All data represent the means of three independent experiments; standard deviations are indicated. (b) c-Src protein levels were determined by Western blot analysis with anti-c-Src clone GD11 as described in Materials and methods. Actin staining was used as loading control. (c) c-Src kinase activity in transduced human B lymphocytes (3 × 106 cell equivalents per lane) was performed as described in Materials and methods. 32P-labelled proteins were visualized by autoradiography. Recombinant human c-Src was used as positive control (+ve) and antibody coated beads without lysate was the negative control (−ve). Arrows indicate the position of autophosphorylated c-Src and tyrosine phosphorylated enolase.
We next examined whether over-expression of c-Src had any affect on the proliferation of human B lymphocytes over an extended period of time. Purified human peripheral B lymphocytes were infected with the different adenovirus vectors and maintained in culture for 19 days in the CD40 system. B lymphocytes were reinfected every 4th day, as we observed previously that these manipulations are required to maintain a similar level of expression of adenoviral vector transferred cDNA (data not shown). No differences in cellular expansion between EYFP and c-src adenovirus vector transduced cells was observed during the first 7 days post-infection (Fig. 2). Expansion factors and rate were shown to be similar (Fig. 2). Thereafter, we observed a slight increase of cellular expansion in c-src DP transduced cells versus mock transduced cells (Fig. 2), the difference being more evident at day 19 post-infection, with a increase of 13% (Fig. 2). These results suggest that B lymphocyte proliferation induced by endogenous c-Src was already at a maximum rate of expansion, which cannot be enhanced further by supplemental exogenous c-Src. In contrast, over-expression of c-Src DN results in a partial inhibition of B lymphocytes expansion starting at day 9 post-infection (Fig. 2). At day 19 post-infection the expansion of c-src DN transduced B lymphocytes was reduced by 40% compared with mock transduced cells. These results are in accord with our previous observation in which c-src inhibitor reduced normal human B lymphocyte proliferation [9]. Moreover, our present results suggest that DN c-Src may compete with endogenous c-Src, resulting in a partial inhibition of a transcriptional pathway involved in B lymphocyte proliferation.
Fig. 2.
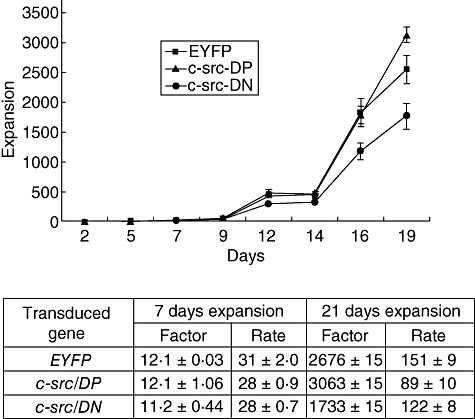
Expansion of human B lymphocytes following adenoviral transduction. Normal human B lymphocytes were infected with recombinant adenovirus encoding enhanced yellow fluorescent protein (EYFP), c-src dominant-positive (DP) or c-src dominant-negative (DN) and cultured in the CD40/CD154 system for 19 days. Cell counts were evaluated in triplicate by Trypan blue dye exclusion every 2 days and total expansion was determined. Data shown are representative of three experiments, performed with B lymphocytes isolated from different donors. Expansion factor and rate are indicated.
c-Src interacts with and activates STAT5b
Previous reports have indicated a relationship between c-Src and STATs, including STAT5b in the proliferation of several types of tumour cells (i.e. squamous carcinoma, human breast cancer cell [24,27–30]), particularly following epidermal growth factor stimulation. Therefore, we addressed whether a relationship between c-Src and STAT5b exists in CD40-stimulated human B lymphocytes. First, we analysed the expression of STAT5b expression in EYFP and c-src transduced human B lymphocytes by Q–PCR. As shown in Fig. 3, no variation of STAT5b mRNA expression was observed. Consistent with this finding, we did not observe any variation of either STAT5a or STAT5b protein (Fig. 4a) or their phosphorylation levels in the mock and mutant c-Src transduced lymphocytes. Next, to determine if c-Src might interact with STAT5 in B lymphocytes, we performed co-immunoprecipitation studies from mock and c-src transduced B lymphocytes. We found that c-Src forms a complex mainly with STAT5b, whereas only a limited amount of STAT5a was found to be associated with c-Src (Fig. 4b). We have found that c-Src forms a complex mainly with STAT5b, whereas very low levels of STAT5a were found to be associated with c-Src (Fig. 4b); however, the total quantity of STAT5a detected in our lysate was also very low compared with STAT5b. Furthermore, we did not observe significant variations in the level of STAT5b associated with c-Src in mock- or c-Src-transduced B lymphocytes. In contrast, phosphoSTAT5a was more abundant than phosphoSTAT5b in anti-c-Src immunoprecipitates. In addition, a higher level of immunoprecipitated phosphoSTAT5b was detected in cells over-expressing the DP form of c-Src, whereas the level of phosphoSTAT5b associated with the DN form of c-Src was comparable to that of mock-transduced cells (Fig. 4b). No significant variations in phosphoSTAT5a associated with c-Src were observed. Quantification by densitometry analysis revealed a 20% increase of phosphoSTAT5b associated with DP c-Src. In contrast, with DN c-Src, densitometry analysis revealed a 20% decrease of phophoSTAT5b compared with mock transduced cells and a 35% decrease compared with c-SrcDP transduced cells. The fact that a small amount of phosphoSTAT5b was found to be associated to c-Src isolated from c-srcDN transduced cells may actually be from the endogenous, wild-type c-Src. These results suggest that c-Src may activate STAT5b by phosphorylation of its tyrosine 699 and that this activation takes place once c-Src associates with STAT5b. To support this hypothesis, we isolated STAT5b from a human B lymphocyte line that lacks endogenous c-Src, and analysed whether it can be phosphorylated by exogenous c-Src. STAT5b was isolated by immunoprecipitation from the DB cell line, which does not express c-Src (Fig. 5a), thus avoiding any tyrosine phosphorylation contributed by endogenous, co-associating c-Src. Monoclonal antibody (mAb) 327 was raised originally against v-src, and although comparative testing of several anti-Src antibodies performed in our laboratory has shown that mAb 327 is by far the best immunoprecipitating antibody, it is probably not 100% c-Src specific and probably cross-reacts with other src-family kinases. The slight enolase phosphorylation in DB cells is due probably to some cross-reactivity of the anti-Src 327 with other src family members found in DB cells. In particular, Fig. 5a shows another phosphorylated protein of slightly lower molecular weight than c-Src, which could correspond to autophosphorylated Lyn or Lck kinase (p56), and which would be capable of phosphorylating enolase. Interestingly, this lower molecular weight protein has not been detected in previous immune complex kinase assays reported herein and, therefore, might represent either Lyn or Lck kinase or another kinase recognized by mAb 327 that is found specifically in DB cells, but not in activated normal B cells.
Fig. 3.
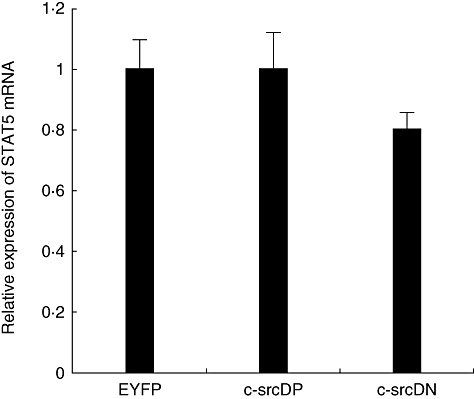
Signal transducer and activator of transcription 5b (STAT5b) mRNA expression in transduced human B lymphocytes. Normal human B lymphocytes were infected with recombinant adenovirus encoding enhanced yellow fluorescent protein (EYFP), c-src dominant-positive (DP) or c-src dominant-negative (DN) and cultured in the CD40/CD154 system. Expression of STAT5b was analysed 48 h post-infection. mRNA levels were determined by quantitative competitive–polymerase chain reaction (Q–PCR). Fold expression of the transcripts were expressed relative to transcripts level in Ad5/F35-EYFP infected B lymphocytes. All data represent the means of three independent experiments; standard deviations are indicated.
Fig. 4.
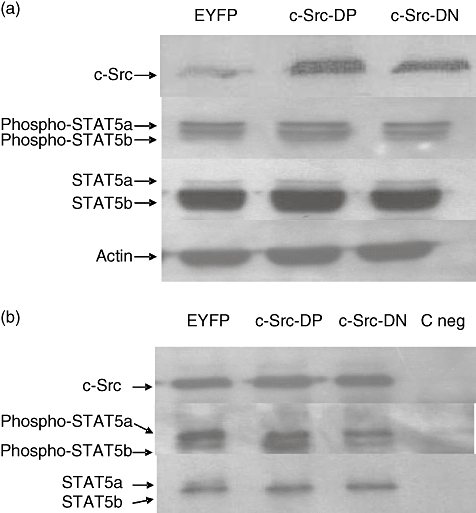
c-Src and signal transducer and activator of transcription 5b (STAT5b) association in human B lymphocytes. Normal human B lymphocytes were infected with recombinant adenovirus encoding enhanced yellow fluorescent protein (EYFP), c-src dominant-positive (DP) or c-src dominant-negative (DN) and cultured in the CD40/CD154 system. Cell pellets were collected 48 h post-infection and lysed in radioimmunoprecipitation assay (RIPA) lysis buffer. (a) c-Src and STAT5b protein levels were determined by Western blot analysis with anti-Src, anti-Stat5a/b and anti-phosphoStat5a/b antibodies. Actin staining was used as loading control. Representative result of one experiment of four are shown. (b) Proteins from 5 × 106 cell equivalents were immunoprecipitated using anti-c-Src (monoclonal antibody 327) bound to rabbit anti-mouse immunoglobulin (Ig)G-protein A Sepharose. Immunoprecipitates were analysed by Western blot with anti-c-Src, anti-Stat5a/b and anti-phosphoStat5a/b antibodies. Coated beads with an anti-SV40 large T antigen was used as negative control (C neg). Representative result of one experiment of three are shown.
Isolated STAT5b (Fig. 5b) was then subjected to a kinase assay using a human recombinant c-Src. As shown in Fig. 5c, STAT5b was phosphorylated, as was recombinant c-Src, known to autophosphorylate itself on Y419. Thus, this result indicates that c-Src can phosphorylate STAT5b. Furthermore, this phosphorylation of STAT5b occurred on tyrosine 699, as demonstrated by Western blot using an anti-phosphotyrosine 699 STAT5b antibody (Fig. 5d). These results show that STAT5b can be phosphorylated on tyrosine residue 699 by c-Src; thus, c-Src can activate STAT5b.
Discussion
Human B lymphocyte populations can be stimulated to proliferate in vitro through different receptors. For instance, CD40 activation through CD154 in the presence of IL-4 can induce proliferation and differentiation distinctively of peripheral human B lymphocyte subpopulations [7]. The mechanism by which normal human B lymphocytes proliferate to external signals is, however, not entirely clear. Although it is known that binding of CD154 to CD40 induces several signalling pathways, tyrosine phosphorylation appears to be a common denominator. Recently, we have shown that c-Src protein and activity increases and correlates with B lymphocyte activation and proliferation following CD40 stimulation [9]. These results suggested that c-Src may play a specific role in normal human B lymphocyte proliferation and led us to investigate the effect of c-Src over-expression on the proliferation of B lymphocytes.
We used the Ad55/F35 adenovirus expression system, which we have described previously as highly efficient for gene over-expression in normal human B lymphocytes [32,33], to over-express two mutant forms of c-Src, a constitutive active (DP) and a kinase-dead (DN) form of c-Src. Surprisingly, we did not observe a correlation between the amount of c-Src expression and proliferation in CD40-activated B lymphocytes. Indeed, whereas a 54-fold increase of c-src mRNA was achieved in c-SrcDP transduced B lymphocytes compared with mock transduced cells only a slight increase in the cell expansion was observed, and that only after 19 days of culture. These results demonstrate clearly that the relatively high level of endogenous c-Src expression following B lymphocyte activation [9] already induces a maximum rate of cell expansion, which cannot be enhanced further by supplemental exogenous c-Src. However, a significant strengthening of an involvement of c-Src in B lymphocyte proliferation comes from our observation that over-expression of a DN form of c-Src severely affects the expansion of the B lymphocytes. Taken together, these results suggest that, following CD40 activation of human B lymphocytes, c-Src is a definite player in the pathway leading to cellular proliferation. Indeed, c-Src may be an initial player that then activates a second factor by tyrosine phophorylation, and it is this second factor that then acts to increase proliferation.
Several reports have demonstrated an essential role for c-Src kinase in mediating constitutive STAT5b activation in many human cancer cells [24,25,35–37]. In general, c-Src serves as an intermediate between tumorigenic protein-tyrosine kinases and STAT activation. In contrast to these reports, we demonstrate herein using co-immunoprecipitation studies that STAT5b interacts directly with c-Src. However, we did not observe differences in the amount of STAT5b associated with c-Src between the constitutively active c-src (DP) and kinase-dead c-Src (DN) used in this study. This, however, provides us with two clues on the nature of the STAT5b/c-Src interaction. First, kinase activity of c-Src is not required for the binding to STAT5b. Secondly, as reported previously, we can speculate that STAT5b can bind via its SH2 domain to the phosphotyrosine (Y419) of human c-Src. The only tyrosine that can be phosphorylated on DP and DN c-Src is Y419, as tyrosine 530 was mutated in both forms of c-Src that we used. Y419 is the autophosphorylation site used by c-Src and, although it is possible that other kinases can phosphorylate this site and, thus, explain the small amount of STAT5b detectable when using the c-Src DN samples, it is more likely that the small amount of STAT5b binding observed in the c-Src DN samples was due to residual endogenous wild-type activated c-Src. Indeed, it is likely that binding of STAT5b to an activated c-Src via pY419 is the normal interactive motif that leads to Src phosphorylation of Y699 of the STAT5b as has been described previously [38]. This tyrosine phosphorylation of STAT5b would result in activation and translocation of STAT5b to the nucleus, to act as a transcription factor [38]. Taken together, our findings that c-Src not only binds to STAT5b, but also activates this transcription factor by phosphorylation of its Y699, implicates this pathway's involvment in B lymphocyte proliferation [26]. This hypothesis is strengthened by the fact that both c-Src and STAT5b can be up-regulated in normal CD-40 activated human B lymphocytes by IL-2, IL-4 and IL-10 [9,39–41]. Moreover, over-expression of constitutively active STAT5b in normal human B lymphocytes increases their proliferation capacity [31]. This may indeed confirm that c-Src is an initial player in the pathway involved in B lymphocyte proliferation and activates STAT5b, which translocates to the nucleus, where it regulates transcription of additional factors required for B lymphocyte proliferation.
In summary, we demonstrate herein that c-Src, activated through stimulation of CD40 on the B lymphocytes, has a direct role in the activation of a signalling pathway involved in proliferation of normal human B lymphocytes. This is the first report of a co-association of c-Src and STAT5b in CD40-activated normal human B lymphocytes and provides evidence that c-Src can phosphorylate Y699 of STAT5b, thus activating this transcriptional regulator. These results implicate the importance of CD40 in the induction of c-Src, which can then act as an activator of STAT5b that leads ultimately to normal human B lymphocyte proliferation.
Acknowledgments
This study was supported in part by a grant from Bayer/Talecris/Canadian Blood services/Héma-Québec partnership Fund to D. J.; Maryse Proulx is supported by a scholar grant from the Natural Sciences and Engineering Research Council of Canada. We thank Dr X. Fan (Lund University) for providing the Ad5/F35-GFP adenovirus backbone and Nathalie Dussault for analysis of c-Src and STAT5b expression in the DB cell line. We are grateful to Dr Nicolas Pineault and Jean-François Leblanc for their comments on the manuscript. Finally, we are thankful to all participants of this study, and to Claudine Côté for co-ordination of blood sample collection.
Disclosure
The authors declare no competing financial interests.
References
- 1.Das T, Sa G, Ray PK. Mechanisms of protein A superantigen-induced signal transduction for proliferation of mouse B cell. Immunol Lett. 1999;70:43–51. doi: 10.1016/s0165-2478(99)00128-5. [DOI] [PubMed] [Google Scholar]
- 2.Falkoff RJ, Zhu LP, Fauci AS. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982;129:97–102. [PubMed] [Google Scholar]
- 3.Lee HK, Xia X, Choi YS. IL-4 blocks the up-regulation of IL-2 receptors induced by IL-2 in normal human B cells. J Immunol. 1990;144:3431–6. [PubMed] [Google Scholar]
- 4.Silverman GJ, Goodyear CS. A model B-cell superantigen and the immunobiology of B lymphocytes. Clin Immunol. 2002;102:117–34. doi: 10.1006/clim.2001.5143. [DOI] [PubMed] [Google Scholar]
- 5.Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003;102:1414–20. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald I, Knox KA, Gordon J. Stimulation of human B lymphocytes by phorbol esters reported to be selective in the protein kinase C isoforms they activate. Mol Immunol. 1994;31:671–4. doi: 10.1016/0161-5890(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 7.Fecteau JF, Néron S. CD40 stimulation of human peripheral B lymphocytes: distinct response from naïve and memory cells. J Immunol. 2003;171:4621–9. doi: 10.4049/jimmunol.171.9.4621. [DOI] [PubMed] [Google Scholar]
- 8.Neron S, Racine C, Roy A, Guerin M. Differential responses of human B-lymphocyte subpopulations to graded levels of CD40-CD154 interaction. Immunology. 2005;116:454–63. doi: 10.1111/j.1365-2567.2005.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neron S, Suck G, Ma XZ, et al. B cell proliferation following CD40 stimulation results in the expression and activation of Src protein tyrosine kinase. Int Immunol. 2006;18:375–87. doi: 10.1093/intimm/dxh377. [DOI] [PubMed] [Google Scholar]
- 10.Kothlow S, Morgenroth I, Tregaskes CA, Kaspers B, Young JR. CD40 ligand supports the long-term maintenance and differentiation of chicken B cells in culture. Dev Comp Immunol. 2008;32:1015–26. doi: 10.1016/j.dci.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–29. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov R, Aarts T, Hagenbeek A, Hol S, Ebeling S. B-cell expansion in the presence of the novel 293-CD40L–sCD40L cell line allows the generation of large numbers of efficient xenoantigen-free APC. Cytotherapy. 2005;7:62–73. doi: 10.1080/14653240510018055. [DOI] [PubMed] [Google Scholar]
- 13.Merluzzi S, D'Orlando O, Leonardi A, Vitale G, Pucillo C. TRAF2 and p38 are involved in B cells CD40-mediated APE/Ref-1 nuclear translocation: a novel pathway in B cell activation. Mol Immunol. 2008;45:76–86. doi: 10.1016/j.molimm.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 14.D'Orlando O, Gri G, Cattaruzzi G, et al. Outside inside signalling in CD40-mediated B cell activation. J Biol Regul Homeost Agents. 2007;21:49–62. [PubMed] [Google Scholar]
- 15.Willimott S, Baou M, Huf S, Deaglio S, Wagner SD. Regulation of CD38 in proliferating chronic lymphocytic leukemia cells stimulated with CD154 and interleukin-4. Haematologica. 2007;92:1359–66. doi: 10.3324/haematol.11340. [DOI] [PubMed] [Google Scholar]
- 16.Parsons JT, Weber MJ. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- 17.Brickell PM. The p60c-src family of protein-tyrosine kinases: structure, regulation, and function. Crit Rev Oncog. 1992;3:401–46. [PubMed] [Google Scholar]
- 18.Branch DR, Mills GB. pp60c-src expression is induced by activation of normal human T lymphocytes. J Immunol. 1995;154:3678–85. [PubMed] [Google Scholar]
- 19.Eiseman E, Bolen JB. src-related tyrosine protein kinases as signaling components in hematopoietic cells. Cancer Cells. 1990;2:303–10. [PubMed] [Google Scholar]
- 20.Tsygankov A, Bolen J. The Src family of tyrosine protein kinases in hemopoietic signal transduction. Stem Cells. 1993;11:371–80. doi: 10.1002/stem.5530110504. [DOI] [PubMed] [Google Scholar]
- 21.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–52. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao X, Tay A, Guy GR, Tan YH. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu CL, Meyer DJ, Campbell GS, et al. A-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–3. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 24.Xi S, Zhang Q, Dyer KF, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–83. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 25.Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM. STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem. 2003;278:1671–9. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- 26.Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23:8017–23. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- 27.Amorino GP, Deeble PD, Parsons SJ. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene. 2007;26:745–56. doi: 10.1038/sj.onc.1209814. [DOI] [PubMed] [Google Scholar]
- 28.Mirmohammadsadegh A, Hassan M, Bardenheuer W, et al. STAT5 phosphorylation in malignant melanoma is important for survival and is mediated through SRC and JAK1 kinases. J Invest Dermatol. 2006;126:2272–80. doi: 10.1038/sj.jid.5700385. [DOI] [PubMed] [Google Scholar]
- 29.Nagashima T, Maruyama T, Uchida H, et al. Activation of SRC kinase and phosphorylation of signal transducer and activator of transcription-5 are required for decidual transformation of human endometrial stromal cells. Endocrinology. 2008;149:1227–34. doi: 10.1210/en.2007-1217. [DOI] [PubMed] [Google Scholar]
- 30.Weaver AM, Silva CM. Modulation of signal transducer and activator of transcription 5b activity in breast cancer cells by mutation of tyrosines within the transactivation domain. Mol Endocrinol. 2006;20:2392–405. doi: 10.1210/me.2005-0418. [DOI] [PubMed] [Google Scholar]
- 31.Scheeren FA, Naspetti M, Diehl S, et al. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol. 2005;6:303–13. doi: 10.1038/ni1172. [DOI] [PubMed] [Google Scholar]
- 32.Cayer MP, Drouin M, Sea SP, et al. Comparison of promoter activities for efficient expression into human B cells and haematopoietic progenitors with adenovirus Ad5/F35. J Immunol Methods. 2007;322:118–27. doi: 10.1016/j.jim.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Jung D, Neron S, Drouin M, Jacques A. Efficient gene transfer into normal human B lymphocytes with the chimeric adenoviral vector Ad5/F35. J Immunol Methods. 2005;304:78–87. doi: 10.1016/j.jim.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Wallez Y, Cand F, Cruzalegui F, et al. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–77. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 35.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 36.Garcia R, Bowman TL, Niu G, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 37.Kabotyanski EB, Rosen JM. Signal transduction pathways regulated by prolactin and Src result in different conformations of activated Stat5b. J Biol Chem. 2003;278:17218–27. doi: 10.1074/jbc.M301578200. [DOI] [PubMed] [Google Scholar]
- 38.Okutani Y, Kitanaka A, Tanaka T, et al. Src directly tyrosine-phosphorylates STAT5 on its activation site and is involved in erythropoietin-induced signaling pathway. Oncogene. 2001;20:6643–50. doi: 10.1038/sj.onc.1204807. [DOI] [PubMed] [Google Scholar]
- 39.Lischke A, Moriggl R, Brandlein S, et al. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J Biol Chem. 1998;273:31222–9. doi: 10.1074/jbc.273.47.31222. [DOI] [PubMed] [Google Scholar]
- 40.Rolling C, Treton D, Pellegrini S, Galanaud P, Richard Y. IL4 and IL13 receptors share the gamma c chain and activate STAT6, STAT3 and STAT5 proteins in normal human B cells. FEBS Lett. 1996;393:53–6. doi: 10.1016/0014-5793(96)00835-6. [DOI] [PubMed] [Google Scholar]
- 41.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]


