Abstract
Muramyl dipeptide (MDP) is the minimal active fragment of peptidoglycan of the cell wall of Gram-positive bacteria, with potential beneficial effects as a vaccine adjuvant. Peptidoglycans and MDP are recognized by the intracellular receptor NOD2 (nucleotide-binding oligomerization domain 2), leading to production of proinflammatory cytokines. In the present study, it is shown that, despite stimulatory effects on isolated human mononuclear cells, MDP does not stimulate production of tumour necrosis factor-α, interleukin-1β or interleukin-6 in a whole-blood assay. However, MDP retains synergistic effects on lipopolysaccharide-induced cytokines in whole blood. Screening tests of NOD2 function based on whole-blood stimulation should therefore employ strategies based on the synergistic effects of MDP on Toll-like receptor-induced cytokine production. Plasma was not responsible for the inhibition of MDP in whole blood. The inhibition of MDP stimulation was dependent upon cellular components, with erythrocyte-derived haemoglobin and neutrophils collaborating in the inhibition of MDP effects in whole blood.
Keywords: cytokine, haemoglobin, neutrophil, NOD2, peptidoglycan
Introduction
Muramyl dipeptide (MDP) is the minimal biologically active fragment of peptidoglycan of the cell wall of Gram-positive bacteria [1]. MDP is recognized by leucocytes and other cells of humans and animals, and this recognition leads to production of proinflammatory cytokines such as interleukin (IL)-1β, tumour necrosis factor (TNF)-α and IL-6 [1,2]. Recent studies have demonstrated that the recognition receptor of MDP is the nucleotide-binding oligomerization domain 2 (NOD2), an intracellular receptor of the NACHT-LRR (NLR) family [3]. Mutations in the LRR recognition domain of NOD2 have been associated with Crohn's disease [4,5], while mutation in its NOD domain leads to Blau syndrome [6].
The NOD2 engagement induces dendritic cell maturation [7], and both peptidoglycans and MDP have long been known to be potent vaccine adjuvants [8]. In addition, testing the cytokine production capacity of cells from patients with various diseases, especially in whole-blood cytokine assays, is an increasingly used diagnostic tool [9]. Therefore, interest in the cytokine-stimulating capacity of MDP is of great potential clinical importance. When stimulated with MDP, differences in cytokine production may be expected when either isolated peripheral blood mononuclear cells (PBMC) or whole-blood assays are used. Little is known about these differences (if any), and the blood components that are capable of modulating cytokine production induced by NOD2 engagement by MDP.
The aim of the present study was to assess cytokine production induced by MDP either in isolated PBMC or in whole-blood assays. Because NOD2 is an important amplification signal for Toll-like receptor (TLR)-induced cytokine production [10,11], we have investigated the effects of MDP alone and its amplification effects on TLR-4 signalling. The effects of blood components (plasma, haemoglobin, neutrophils) on MDP-induced cytokines were also assessed.
Materials and methods
Reagents
Synthetic MDP was obtained from Calbiochem (San Diego, CA, USA). Lipopolysaccharide (LPS) from Escherichia coli strain O55:B5 was purchased from Sigma Chemical Co. (St Louis, MO, USA) and repurified as described previously [12].
Volunteers
The study was approved by the Combined Colorado Investigational Review Board, and each blood donor gave informed consent. Blood was harvested from the antecubital vein of eight healthy male volunteers, aged between 17 and 61 years. None of the volunteers experienced a recent infection, and they had no inflammatory diseases. None of the volunteers used cyclooxgenase inhibitors 2 weeks prior to the study.
Whole-blood cultures
Heparinized blood was diluted 1:1, 1:5 or 1:10 in RPMI-1640 and stimulated with the various stimuli for 24 h at 37°C in a total volume of 1 ml in 24-well plates. The stimuli used were LPS (1 ng/ml), MDP (10 µg/ml; this concentration was chosen to reach a stimulatory effect but also to maintain pathophysiologically relevant concentrations) or a combination of MDP and LPS. After 24 h stimulation, the supernatants were collected and stored at −80°C until cytokine measurement. In separate experiments, the whole cellular blood fraction was washed gently twice in sterile phosphate-buffered saline (PBS) (10 min, 500 g). The plasma component was reconstituted by culture medium and cells were stimulated with the various stimuli as described above.
The PBMC isolation and stimulation
Blood was collected in heparinized tubes (sodium heparin: final concentration 20 U/ml; Elkins-Sinn, Cherry Hill, NJ, USA). Isolation of PBMC was performed as described elsewhere [13], with minor modifications. The PBMC fraction was obtained by density centrifugation of blood diluted 1:1 in pyrogen-free saline over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). Cells were washed twice in saline and suspended in culture medium (RPMI-1640) supplemented with gentamicin 10 µg/ml, L-glutamine 10 mM and pyruvate 10 mM. The cells were counted in a Coulter counter (Coulter Electronics, Hialeah, FL, USA) and the number was adjusted to 5 × 106 cells/ml. A total of 5 × 105 MNC in a 100 µl volume was added to round-bottomed 96-well plates (Greiner) and incubated with either 100 µl of culture medium (negative control) or the various stimuli: LPS (1 ng/ml), MDP (10 µg/ml) or a combination of MDP and LPS.
In additional experiments, the role of the various blood components on cytokine production induced in PBMC by MDP were also investigated. The neutrophil fraction was collected after centrifugation through Ficoll Hypaque. The erythrocytes were lysed in lysis buffer for 15 min in the dark at room temperature. Afterwards, neutrophils were centrifuged at 200 g for 5 min and washed in sterile PBS. They were resuspended in RPMI-1640 at a final concentration of 5 × 106 cells/ml. Haemoglobin was obtained by adding sterile distilled water (1:1) to the erythrocyte fraction obtained after Ficoll-Paque centrifugation. After 15 min incubation, the mixture was centrifuged at 1000g for 10 min, and the supernatant was collected as a source of free haemoglobin.
A total of 5 × 105 PBMC was incubated in 96-well plates with the stimulus in the absence or presence of non-lysed erythrocytes, free haemoglobin or neutrophils. After stimulation with MDP, LPS or MDP/LPS for 24 h at 37°C, the supernatants were collected for cytokine measurements.
Cytokine measurements
The TNF, IL-1β and IL-6 concentrations were measured by electrochemiluminescence, as described previously [14].
Statistical analysis
Data were expressed as means ± standard errors of the mean of experiments performed with cells isolated from eight volunteers. Differences between data obtained with the various stimuli in the same volunteers were analysed with the paired Student's t-test. Each time, stimulations were compared with two different stimuli for the production of cytokines.
Results
Stimulation of whole blood by MDP
Previous studies have shown that MDP stimulates cytokine production in human isolated PBMC both alone and in synergism with TLR ligands such as LPS [10,11]. When whole blood was stimulated with MDP alone, no cytokine production was observed (Fig. 1), although similar amounts of MDP stimulated TNF or IL-1β production in isolated PBMC (Fig. 2a and b). However, MDP amplified moderately the release of IL-1β and TNF induced by LPS in whole blood (Fig. 1a and b).
Fig. 1.
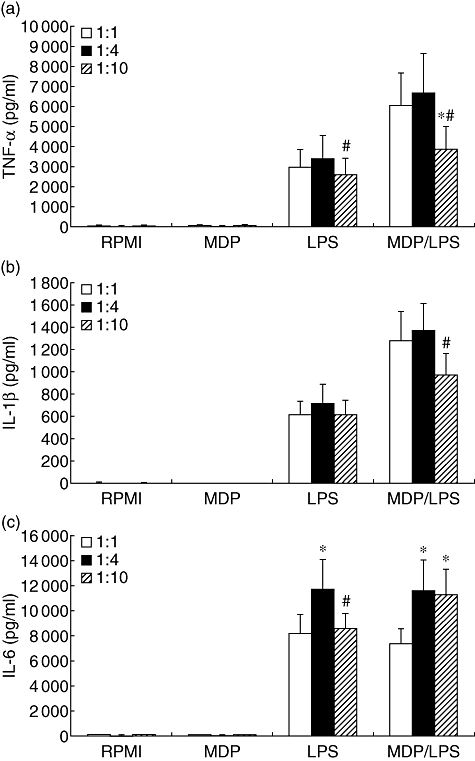
Stimulation of cytokine production in whole blood. Freshly harvested whole blood was diluted 1:1, 1:5 or 1:10 in RPMI-1640 and stimulated with muramyl dipeptide (MDP) (10 µg/ml), lipopolysaccharide (LPS) (1 ng/ml) or a combination of MDP and LPS for 24 h at 37°C. After 24 h stimulation the supernatants were collected and stored at −80°C until cytokine measurements. Data are presented as means ± standard error of the mean of eight volunteers. *P < 0·05 in comparison with 1:1 whole-blood dilution; #P < 0·05 in comparison with stimulation with 1:4 whole-blood dilution.
Fig. 2.
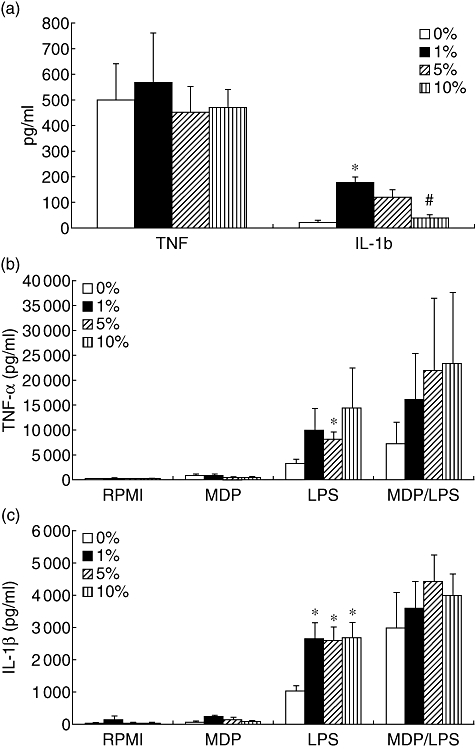
The effect of plasma on muramyl dipeptide (MDP)-induced production of cytokines. Isolated peripheral blood mononuclear cells were stimulated either with MDP alone (a–c) or with combinations of MDP and lipopolysaccharide (b,c). Plasma was added in various concentrations, as depicted. After 24 h stimulation the supernatants were collected and stored at −80°C until cytokine measurements. Data are presented as means ± standard error of the mean of cells harvested from eight volunteers. *P < 0·05 in comparison with stimulation with 0% plasma; #P < 0·05 in comparison with stimulation with 1% plasma.
The effect of plasma fraction on MDP-induced cytokine production
Various components of plasma have been demonstrated to modulate cytokine production induced by TLR ligands. The presence of plasma in the culture medium of PBMC amplified the MDP-induced IL-1β but not TNF (Fig. 2a). Moreover, plasma also potentiated the synergism between the MDP/NOD2 and LPS/TLR-4 pathways (Fig. 2b and c).
This demonstrates that the plasma component is not responsible for inhibiting the MDP-stimulatory action seen in whole blood. This assumption is supported by experiments performed in washed blood, in which the plasma component was reconstituted by culture medium, and which showed no stimulatory effects of MDP alone, similar to that in whole-blood assays (Fig. 3). This suggests that cellular components in the blood inhibit the effects of MDP.
Fig. 3.
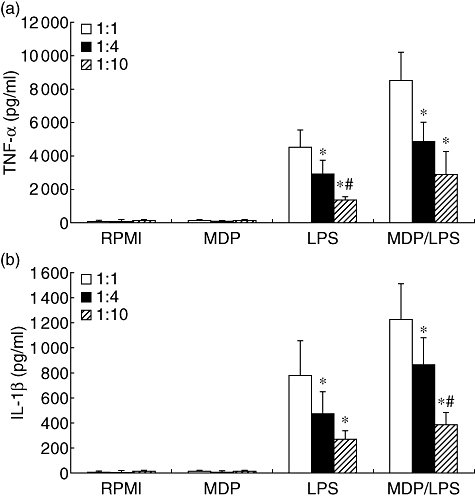
The effect of plasma reconstitution with culture medium on muramyl dipeptide (MDP) stimulation of whole blood. Whole-blood fraction was washed gently twice in sterile phosphate-buffered saline (10 min, 500 g). The plasma component was reconstituted by culture medium (RPMI-1640) and cells were stimulated with MDP (10 µg/ml), lipopolysaccharide (LPS) (1 ng/ml), or a combination of MDP and LPS for 24 h at 37°C. After 24 h stimulation the supernatants were collected and stored at −80°C until cytokine measurements. Data are presented as means ± standard error of the mean of cells harvested from six volunteers. *P < 0·05 in comparison with stimulation with 1:1 diluted washed blood; #P < 0·05 in comparison with stimulation with 1:4 diluted washed blood.
The effect of cellular fractions on MDP-induced cytokine production
Adding either free haemoglobin or neutrophils inhibited cytokine production stimulated in PBMC by MDP alone (Fig. 4a and b), while adding intact erythrocytes had no effect. In contrast to MDP, only neutrophils were able to inhibit LPS-induced cytokine production significantly (P < 0·05, Fig. 4c and d). The synergistic effect of MDP on LPS-induced cytokine production was not influenced by either erythrocytes or free haemoglobin, while MDP/LPS cytokine production was decreased strongly by neutrophils because of their effects on both MDP and LPS (P < 0·05, Fig. 4c and d).
Fig. 4.
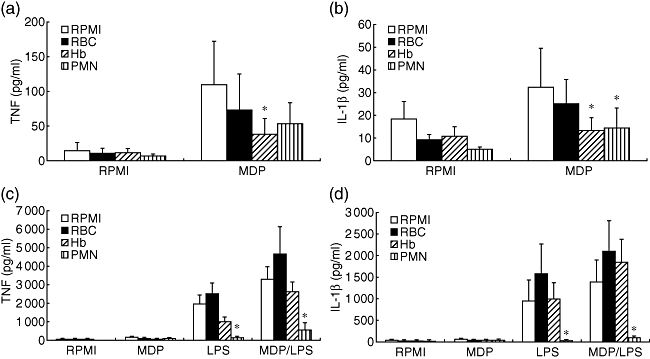
The effects of cellular components on muramyl dipeptide (MDP)-stimulation of cytokines. Isolated peripheral blood mononuclear cells were stimulated either with MDP alone (a,b) or with combinations of MDP and lipopolysaccharide (c,d). The various cellular components: erythrocytes [red blood cells (RBC)], free haemoglobin (Hb) or polymorphonuclear neutrophils (PMN), were added to the assay. After 24 h stimulation the supernatants were collected and stored at −80°C until cytokine measurements. Data are presented as means ± standard error of the mean of cells harvested from eight volunteers. *P < 0·05 in comparison with stimulation with RPMI-1640.
Discussion
The first aim of our study was to compare cytokine stimulation induced by MDP in isolated PBMC and whole-blood assays. The potential importance of MDP as a vaccine adjuvant, as well as the role of its receptor NOD2 in Crohn's disease and Blau syndrome [4–6], has prompted justifiable interest in its mechanisms of cell stimulation. While much knowledge has been gained in the stimulation of isolated mononuclear cells (either monocytes or macrophages) by MDP, very little is known about the capacity of MDP to stimulate cytokine production in whole blood. Surprisingly, despite stimulation of cytokine production in PBMC assays, as also known previously [1,15], MDP was unable to induce cytokine production in whole blood. However, MDP was still able to amplify the LPS-dependent cytokine release moderately. This suggests that while blood components potently neutralize MDP so that it is unable to stimulate cytokines on its own, the residual MDP activity is still able to synergize with the LPS/TLR-4 pathway for induction of cytokines. It is known that MDP is significantly less potent than LPS for cytokine induction and thus possibly more susceptible to inhibitory effects. Conversely, MDP (especially in low concentrations) has strong synergistic effects on TLR-induced cytokine production [10,11], which explains the synergism with LPS in whole blood. This finding implies that a whole-blood assay designed to assess the MDP/NOD2 pathway should include MDP/LPS stimulation, not MDP alone.
The stimulation of whole blood by bacterial stimuli, in this case MDP, is important because it is closer to the natural situation: bacterial components are released during infection in blood and not in isolated cells. In addition, rapid screening of the function of certain stimulatory pathways can be performed much more easily in whole-blood assays than in isolated cell populations. One such assay is used in tuberculosis diagnosis [9], and this methodology has invaluable importance in field studies in resource-poor settings [16].
The inefficient stimulation of blood by MDP raises the question of which blood components inhibit the MDP activity. Plasma components are known to modulate cytokine release induced by TLR ligands such as LPS, with both stimulatory and inhibitory effects: the LPS-binding protein stimulates cytokine production by forming a shuttle between LPS micellae and the TLR-4/CD14 complex [17], while lipoproteins in the plasma bind and neutralize circulating LPS [18]. When plasma was added to the medium during stimulation of PBMC with MDP, a significant increase in cytokine release was observed. Only IL-1β production (but not TNF) induced by MDP alone seemed to be inhibited at high plasma concentrations (10%) compared with low concentrations (1%), but not compared with stimulation in the absence of plasma. For the other stimuli, including LPS or LPS + MDP, a stimulatory effect of plasma was observed, and this known phenomenon is due most probably to plasma factors such as LPS-binding protein or soluble CD14. This implies that plasma facilitates cell stimulation by MDP, and that plasma components are not responsible for the inhibitory effects on MDP in whole blood. This conclusion was strengthened by the absence of MDP-induced cytokine release when plasma was removed and reconstituted with culture medium in the whole-blood stimulation assay.
The lack of inhibitory effects of plasma on MDP-induced cytokines suggested that a cellular or cell-derived component from the blood is responsible for the down-regulation of MDP effects in whole blood. One of the obvious candidates is the red blood cell. Although non-damaged erythrocytes do not alter LPS/TLR-4 cytokine production [19], both oxidatively modified erythrocytes [19] and erythrocyte-derived free haemoglobin [20,21] augment cytokine release from human monocytes by bacterial stimuli such as LPS. Similar to the absence of effects on TLR-2 or TLR-4, intact erythrocytes did not influence MDP- or MDP/LPS-induced cytokine production. In contrast to earlier studies that have suggested stimulatory effects of free haemoglobin on LPS-induced cytokine production, in our experiments haemoglobin moderately inhibited the induction of cytokines induced by MDP (Fig. 4), which can partly explain the effects observed in whole-blood assays. The relatively high amounts of free haemoglobin used are not to be found physiologically, but are relevant for whole-blood stimulation assays and certain pathological events where high levels of haemolysis exist.
However, the most impressive effect on MDP stimulation was observed when purified neutrophils were added to the culture. When we added neutrophils to the MDP/monocyte cultures, a significant decrease in cytokine production was observed. Similarly, the MDP/LPS synergistic release of cytokines was inhibited strongly by the presence of neutrophils. Although neutrophils can be stimulated by bacterial components, they can also internalize and neutralize them. An example of the latter effect is neutralization of LPS activity by the neutrophil-derived bactericidal-permeability increasing protein [22]. Thus, a significant decrease in cytokine release was measured when adding neutrophils to LPS/monocyte cultures. Interestingly, while MDP is the minimal active motif of peptidoglycans that can stimulate cytokine production in mononuclear cells, it does not stimulate either cytokine production [23] or metabolic activation [24] in neutrophils, while peptidoglycans are capable of eliciting these effects.
In conclusion, in the present study we describe the lack of stimulatory effects of MDP alone in a whole-blood assay. Screening tests of NOD2 function based on whole-blood stimulation should employ strategies based on the synergistic effects of MDP on TLR-induced cytokine production. The inhibition of MDP stimulation in whole blood is dependent upon cellular components and not by plasma. Free haemoglobin and neutrophils collaborate in the inhibition of MDP effects in whole blood.
Acknowledgments
This work was supported by the NIH grants AI-15614, HL-68743 and CA046934 to C. A. D. M. G. N. was supported by a Vidi Grant from the Netherlands Organization for Scientific Research.
Disclosure
No potential conflicts of interest are present for any of the authors of the paper.
References
- 1.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 2.Inohara N, Ogura Y, Nunez G. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr Opin Microbiol. 2002;5:76–80. doi: 10.1016/s1369-5274(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 3.Uehara A, Yang S, Fujimoto Y, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7:53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 4.Hugot J-P, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 5.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 6.Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 7.Kramer M, Netea MG, de Jong DJ, Kullberg BJ, Adema GJ. Impaired dendritic cell function in Crohn's disease patients with NOD2 3020insC mutation. J Leukoc Biol. 2006 doi: 10.1189/jlb.0805484. [DOI] [PubMed] [Google Scholar]
- 8.Jolles P, Migliore-Samour D, Maral R, Floc’h F, Werner GH. Low molecular weight water-soluble peptidoglycans as adjuvants and immunostimulants. Z Immunitatsforsch Exp Klin Immunol. 1975;149:331–40. [PubMed] [Google Scholar]
- 9.Mazurek GH, LoBue PA, Daley CL, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740–7. doi: 10.1001/jama.286.14.1740. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, Ferwerda G, De Jong DJ, et al. NOD2 modulates specific Toll-like receptor pathways for the induction of cytokine release. J Immunol. 2005;174:6518–23. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 11.van Heel DA, Ghosh S, Hunt K, et al. Muramyl dipeptide and Toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet. 2005;365:1794–6. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 12.Hirschfeld M, Weis JJ, Toshchakov V, et al. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–82. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endres S, Ghorbani R, Lonnemann G, Van der Meer JWM, Dinarello CA. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin Immunol Immunopathol. 1988;49:424–38. doi: 10.1016/0090-1229(88)90130-4. [DOI] [PubMed] [Google Scholar]
- 14.Netea MG, Azam T, Ferwerda G, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–14. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netea MG, Ferwerda G, de Jong DJ, et al. The frameshift mutation in Nod2 results in unresponsiveness not only to Nod2- but also Nod1-activating peptidoglycan agonists. J Biol Chem. 2005;280:35859–67. doi: 10.1074/jbc.M504924200. [DOI] [PubMed] [Google Scholar]
- 16.Van Crevel R, van der Ven-Jongekrijg J, Netea MG, de Lange W, Kullberg BJ, Van der Meer JWM. Disease-specific ex vivo stimulation of whole blood for cytokine production: applications in the study of tuberculosis. J Immunol Methods. 1999;222:145–53. doi: 10.1016/s0022-1759(98)00192-6. [DOI] [PubMed] [Google Scholar]
- 17.Gallay P, Barras C, Tobias PS, Calandra T, Glauser MP, Heumann D. Lipopolysaccharide (LPS)-binding protein in human serum determines the tumor necrosis factor response of monocytes to LPS. J Infect Dis. 1994;170:1319–22. doi: 10.1093/infdis/170.5.1319. [DOI] [PubMed] [Google Scholar]
- 18.Parker TS, Levine DM, Chang JC, Laxer J, Coffin CC, Rubin AL. Reconstituted high-density lipoprotein neutralizes Gram-negative bacterial lipopolysaccharides in human whole blood. Infect Immun. 1995;63:253–8. doi: 10.1128/iai.63.1.253-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liese AM, Siddiqi MQ, Siegel JH, Denny T, Spolarics Z. Augmented TNF-alpha and IL-10 production by primed human monocytes following interaction with oxidatively modified autologous erythrocytes. J Leukoc Biol. 2001;70:289–96. [PubMed] [Google Scholar]
- 20.Carrillo EH, Gordon LE, Richardson JD, Polk HC., Jr Free hemoglobin enhances tumor necrosis factor-alpha production in isolated human monocytes. J Trauma. 2002;52:449–52. doi: 10.1097/00005373-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 21.McFaul SJ, Bowman PD, Villa VM, Gutierrez-Ibanez MJ, Johnson M, Smith D. Hemoglobin stimulates mononuclear leukocytes to release interleukin-8 and tumor necrosis factor alpha. Blood. 1994;84:3175–81. [PubMed] [Google Scholar]
- 22.Marra MN, Wilde CG, Griffith JE, Snable JL, Scott RW. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990;144:662–6. [PubMed] [Google Scholar]
- 23.Timmerman CP, Mattsson E, Martinez-Martinez L, et al. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–72. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Martinez L, Timmerman CP, Fleer A, Verhoef J. Chemiluminescence of human polymorphonuclear leucocytes after stimulation with whole cells and cell-wall components of Staphylococcus epidermidis. J Med Microbiol. 1993;39:196–203. doi: 10.1099/00222615-39-3-196. [DOI] [PubMed] [Google Scholar]


