Abstract
Common variable immunodeficiencies (CVID) are a heterogeneous group of antibody deficiency disorders complicated by autoimmune, lymphoproliferative and/or granulomatous manifestations, suggesting variations in immunoregulation. We sought to quantify regulatory CD4 T cells (Treg cells) in the blood of CVID patients and to correlate the frequency with clinical manifestations and classification subgroups. Blood samples from 99 CVID patients in Freiburg, London and Sydney, who had been phenotyped clinically and stratified according to their memory B cell phenotype (Freiburg and Paris classification schemes), were analysed for the proportion of Treg cells, defined either as CD25+/forkhead box P3 (FoxP3)+, CD25+/CD127low/FoxP3+ or CD25+/CD127low CD4+ T cells, and results compared with 49 healthy controls. Irrespective of the phenotype used to define them, there was a significant decrease in the Treg cell proportion in patients with granulomatous disease and immune cytopenias. This allowed the definition of a subgroup of CVID patients with abnormally low Treg cells, which had a higher rate of these two manifestations as well as autoimmune disease in general. There was also a significant reduction in the proportion of Treg cells in the Freiburg group Ia compared with other CVID patients and controls, but there were no differences between the Paris groups. The reduction in Treg cells in subsets of CVID patients may be relevant to their clinical manifestations, and may contribute to our understanding of the pathogenesis of CVID complications.
Keywords: autoimmunity, common variable immunodeficiency, granuloma, regulatory T cells
Introduction
The common variable immunodeficiency diseases (CVID) comprise a heterogeneous group of immunological disorders characterized by low serum immunoglobulin (Ig)G along with a decrease in serum levels of IgA and/or IgM, defective specific antibody production and an increased susceptibility to bacterial infections [1]. With an estimated prevalence of approximately one in 25 000, CVID is the most common symptomatic primary immunodeficiency. It may manifest at any age; however, two-thirds of patients are adults at the time of diagnosis [1,2]. In addition, CVID patients may develop splenomegaly (∼30%), a variety of autoimmune diseases (∼20%), chronic inflammatory disorders such as granulomatous disease (∼10%) and less frequently malignancies (non-Hodgkin's lymphoma or gastric cancer) [1,3–5].
In the majority of CVID patients there is a failure of B cell differentiation which alters peripheral blood B cell subsets, in 75% of cases with a loss of class-switched memory B cells [5,6]. Current classifications, such as the Freiburg [5] and the Paris [7] schemes, are based on such changes. In addition, several abnormalities in T cell-mediated immunity have been demonstrated in CVID patients, including reduced T cell numbers, loss of CD4+ naive T cells, abnormal T cell receptor signalling, cytokine production, CD40 ligand expression, proliferative responses and T helper or T suppressor function [8].
Naturally occurring regulatory T cells (Treg cells), defined by surface expression of CD4 and CD25 in combination with intracellular expression of the forkhead box transcription factor (FoxP3), are arguably the best-characterized subset of Treg cells, and act to suppress pathological immune responses such as autoimmunity, but are also responsible for control of immune responses to foreign antigens [9]. Central Treg cells develop in the thymus and constitute a variable percentage of CD4+ T cells in the human circulation; some studies suggest that they make up 5–10% of peripheral blood lymphocytes, although only 2–3% may in fact be functionally regulatory [9]. Low expression of the interleukin (IL)-7 receptor alpha chain (CD127) on CD4+/CD25+ T cells has been proposed as a reliable marker of Treg cells and correlates well with FoxP3 expression [10].
The immune dysregulation present in CVID patients extends beyond B cell dysfunction, as demonstrated by the development of autoimmune and chronic inflammatory disorders, posing questions as to whether alterations in Treg cell function might contribute to disease pathogenesis. Fevang et al. demonstrated a significant decrease in the proportions of Treg cells in 26 CVID patients, particularly marked in those with evidence of chronic inflammation [11]. To verify and extend their findings, we quantified Treg cells in a large group of CVID patients combining three different cohorts (Freiburg, London and Sydney). We compared the frequency of naturally occurring Treg cells in CVID patients stratified on the basis of three cardinal clinical manifestations, lymphoproliferation, autoimmune disease (particularly autoimmune cytopenias) and granulomatous disease, and by the Freiburg and Paris CVID classifications.
Methods
Patients and healthy controls
Ninety-nine patients (aged 44 ± 14 years; mean ± standard deviation, range 18–75 years) were recruited from three cohorts of CVID patients in Freiburg (37), London (30) and Sydney (32) under approved protocols at respective sites. Forty-nine healthy donors (aged 37 ± 10 years, range 25–59) in Freiburg (17), London (15) and Sydney (17) served as controls. A diagnosis of CVID was based on International Union of Immunological Societies diagnostic criteria [12]. In all centres, patients with fewer than 1% B cells in peripheral blood were excluded. Informed consent was obtained from all patients. The clinical characteristics and CVID patient classifications are summarized in Table 1.
Table 1.
Clinical characteristics of the 99 CVID patients in the three cohorts, along with the Freiburg and Paris subgroup classifications. Chronic infections were defined as either (i) more than three infections per 6 months' observation period, justifying the need for prophylactic antibiotics; or (ii) chronic sinusitis or bronchitis (excluding bronchiectasis).
| Cohort | Chronic infections | Autoimmune phenomena | Granulomatous disease | Lymphoproliferation | Freiburg | Paris |
|---|---|---|---|---|---|---|
| European (Freiburg/London) (n = 67) | 33 (50%) | 20 (30%) | 17 (26%) | 26 (39%) | Ia17(25%) | MB033(49%) |
| Ib36(54%) | MB127(40%) | |||||
| II14(21%) | MB27(10%) | |||||
| Australian (Sydney) (n = 32) | Not assessed | 11 (31%) | 0 (0%) | 7 (22%) | Ia5(16%) | MB014(44%) |
| Ib10(31%) | MB17(22%) | |||||
| II17(53%) | MB211(34%) |
Cell separation and flow cytometry
In the two European centres, peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll density gradient and then stained with combinations of monoclonal antibodies. In the London cohort, Treg cells were identified by cell surface staining using peridinin chlorophyll protein-conjugated anti-CD4, phycoerythrin (PE)-conjugated anti-CD25 and fluorescein isothiocyanate (FITC)-conjugated anti-CD127 (all obtained from Becton-Dickinson, Oxford, UK). In the Freiburg cohort, Treg cells were identified by cell surface staining using anti-CD4-FITC, allophycocyanin (APC)-conjugated anti-CD25 and anti-CD127-PE (eBioscience, San Diego, CA, USA). In both European centres, intracellular staining was performed using anti-human FoxP3-APC (Clone PCH101; eBioscience) following the manufacturer's recommended intracellular staining protocol (http://www.ebioscience.com); the frequency of FoxP3+/CD25+ as a proportion of CD4 T cells was calculated, while in London, an additional analysis included gating on CD127low cells (Fig. 1a). In the pilot study comparing FoxP3 antibodies, staining with clone PCH101 was compared with 236A/E7 (eBioscience). In the Sydney cohort, Treg cell proportions were enumerated by surface staining with anti-CD4-FITC (Beckman-Coulter, Fullerton, CA, USA), PE-cyanine-5 (PE-Cy5)-conjugated CD25 and anti-CD127-PE (Immunotech, Marseille, France) using a whole blood lysis technique, and were defined as being CD25+/CD127low, expressed as a percentage of CD4 cells (Fig. 1b).
Fig. 1.
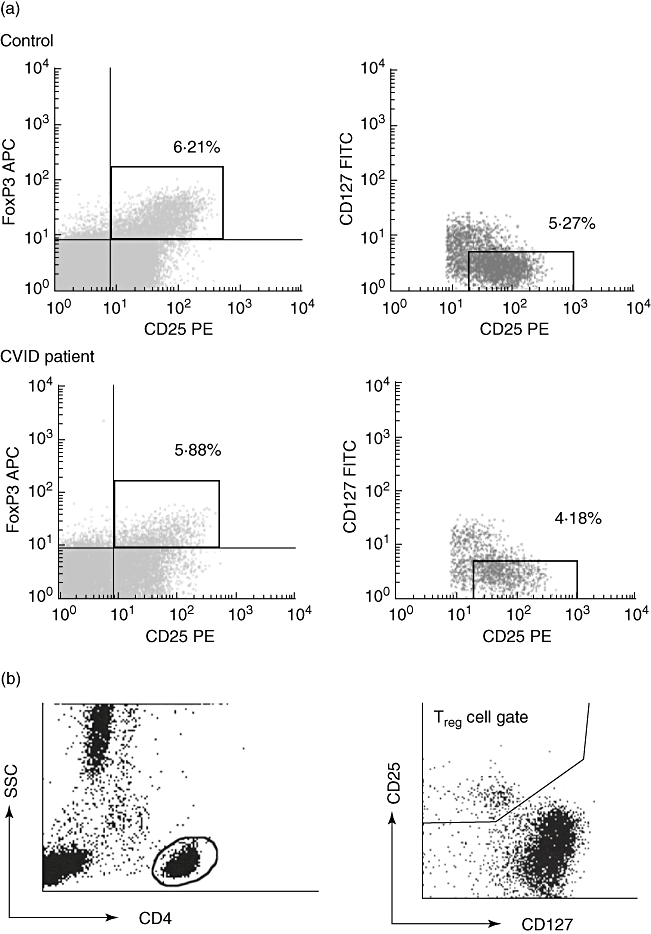
Gating strategy for the identification and phenotypic analysis of regulatory T cells (Treg) in healthy controls and common variable immunodeficiency (CVID) patients. (a) Representative plots from a control patient (upper plots) and a CVID patient (lower plots) based on fixed and permeabilized cells as used in the London and Freiburg cohorts. Analysis gates have been restricted to the lymphocyte population by means of their forward and side light-scatter characteristics, with subsequent gating on CD4+ lymphocytes to generate plots to enumerate CD25+/forkhead box P3+ cells (left panels); in the London cohort, subsequent gating excluded CD127 expressing cells (right panels). Percentages represent proportions of CD4 cells in the specified gate. (b) Analysis in fresh cells, as used in the Sydney cohort, with initial gating on CD4+ lymphocytes by scatter (left panel) followed by gating on CD25+/CD127low cells (right panel).
In the Freiburg cohort, stratification of patients into Freiburg and Paris classification groups was based on staining of PBMCs with the following antibodies, as published in Wehr et al.[13]: anti-CD27-FITC, anti-IgD-PE, PE-Cy7-conjugated anti-CD19, anti-IgM-PE-Cy5, anti-CD38-FITC, anti-CD21-PE, anti-CD19-PE-Cy7 and anti-IgM-PE-Cy5. In the London patients, the same combination of antibodies was used in a whole blood lysis technique as detailed in Ferry et al.[14]. For the Sydney cohort, a whole blood lysis technique was used to stain for CD19-PE-Cy5, CD27-PE, CD21-FITC and anti-CD20-PE-Cy5 (all from Immunotech) and IgD-FITC (BD Biosciences Pharmingen, San Diego, CA, USA), as detailed in Berglund et al.[15].
The CVID classification
Freiburg classification was applied based on published values [5]: Ia represented patients with a decrease in the isotype switched memory B cell fraction (namely CD19+/CD27+/IgD−/IgM− for the European cohorts, or CD19+/CD27+/IgD− for the Sydney cohort) below 0·4% of total lymphocytes, along with a proportion of CD21−/low B cells equal to or exceeding 20% of total B cells; Ib represented patients with low switched memory B cells as detailed above, but with a normal proportion of CD21−/low B cells; II represented patients with normal B cell subsets.
Cut-offs for the Paris classification varied between the two cohorts. In the Sydney and Freiburg cohorts, cut-offs were based on published values validated in local control populations [7,15,16], such that MB0 represented patients with a reduction in total memory B cells (CD19+/CD27+) below 11% of B cells; MB1 represented those with preserved total memory B cells but with an isotype switched fraction (see above) of fewer than 8% of B cells; and MB2, patients with normal memory B cell subsets. For the London cohort, cut-off values were defined based on a local control population, with the range based on two standard deviations below the mean, such that MB0 represented patients with a memory B cell proportion < 16·5% of B cells, MB1 represented patients with a memory B cell proportion ≥ 16·5% but with switched memory B cells < 7·1% of B cells, and MB2 patients had normal memory B cell subsets.
Statistical analysis
Statistical analysis was performed using spss version 15.0 (SPSS Inc., Chicago, IL, USA). The mean difference between continuous variables was analysed using the Mann–Whitney U-test, with P values < 0·05 considered significant. Subgroup analysis was performed with Fisher's exact test based on a two × two contingency table.
Results
Patients and analysis
A total of 99 CVID patients entered the study, 67 in the two European centres and 32 in Sydney, and were compared with 49 controls, 32 in the European centres and 17 in Sydney. Clinical characteristics and CVID classifications are summarized in Table 1. Analysis algorithms for the enumeration of Treg cells are depicted in Fig. 1. In the European cohorts, the frequency of Treg cells as a proportion of CD4+ cells was determined by intracellular FoxP3 and surface CD25 expression on fixed and permeabilized cells (Fig. 1a), while in Sydney values derived from expression of CD25 and CD127 on non-fixed non-permeabilized cells were used (Fig. 1b). In the London cohort, an additional analysis included CD127 expression on fixed and permeabilized cells, such that the Treg cell phenotype could be defined as either FoxP3+/CD25+ or FoxP3+/CD25+/CD127low CD4 cells. However, there was a strong correlation between the two estimates (r2 = 0·94, P < 0·0001), with FoxP3+/CD25+/CD127low values being consistently about 15% lower than FoxP3+/CD25+ ones (Supplementary Fig. S1). Given this correlation, and given that only the FoxP3+/CD25+ values were available for the entire European cohort, subsequent comparisons in the European patients were based on the latter phenotype.
A pilot study was carried out initially to ensure that Treg cell values were consistent in individual subjects over time. Treg cell proportions were determined at two time-points (mean 2·4 months apart) in a subset of 14 Freiburg patients (six CVID patients and eight controls). Values were found to be similar in both groups at either time-point (CVID: P = 0·992; controls: P = 0·315) (Supplementary Fig. S2a). Similarly, two clones of anti-FoxP3 (PCH101 and 236A/E7) were compared in three controls and three CVID patients; while PCH101 tended to over-estimate the frequency of Treg cells (Supplementary Fig. S2b), values were correlated closely and PCH101 was used in the remaining analyses.
The Treg cell proportions are reduced in CVID patients with specific clinical phenotypes
The Treg proportions of CD4 cells in peripheral blood, based on either the FoxP3+/CD25+ or the CD25+/CD127low phenotype, was not significantly different between CVID and controls (Fig. 2a and b). Similarly, no differences were noted when the Treg proportions of CD4 cells were based on FoxP3 expression alone, ignoring expression of CD25 and CD127 (data not shown). Conversely, there was a subset of CVID patients who had proportions of Treg cells well below that seen in healthy controls (Fig. 2), and given the heterogeneity of CVID this finding prompted the question as to whether Treg cell proportions varied in individual patients based on specific clinical manifestations. To test this, CVID patients were divided on the basis of the presence or absence of chronic infections, granulomatous disease, autoimmune manifestations or lymphoproliferation, and Treg cell proportions compared between the groups. In the European cohorts, there was no difference in Treg cell proportions among CVID patients with or without chronic infections (Fig. 3a) nor with lymphoproliferative disease (lymphadenopathy, splenomegaly) (Fig. 3b and c), but those with granulomatous disease (n = 17) had a significantly reduced FoxP3+/CD25+ CD4 Treg cell proportion, both in comparison with CVID patients without granulomata (n = 49) and also with healthy donors (Fig. 3d). Autoimmune disease was common in all cohorts; no differences in Treg cell proportions were detected after stratification based on this characteristic (Fig. 3e and f), but when analysis was directed specifically at those patients with autoimmune cytopenias (European cohorts: n = 6, Sydney cohort: n = 3), a strikingly lower Treg cell proportion was found in those patients with this complication (Fig. 3g and h, Supplementary Fig. S3).
Fig. 2.
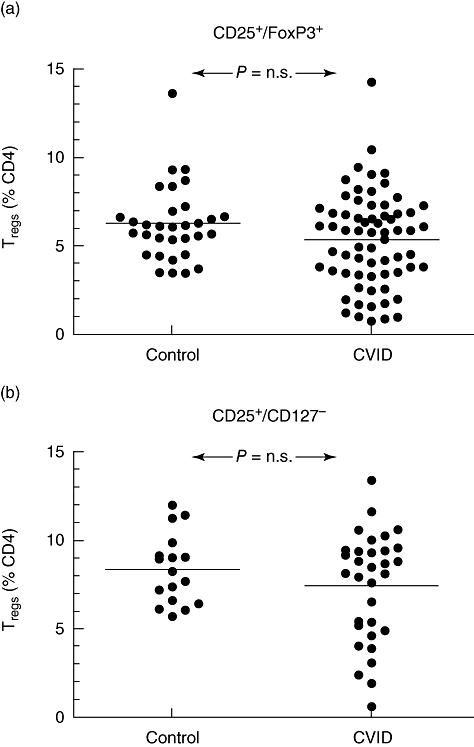
Regulatory T cell proportions (as a percentage of CD4 lymphocytes) in common variable immunodeficiency (CVID) patients and controls, analysed as either CD25+/forkhead box P3+ CD4 cells in the European patients (a) or as CD25+/CD127low CD4 cells in the Australian cohort (b).
Fig. 3.
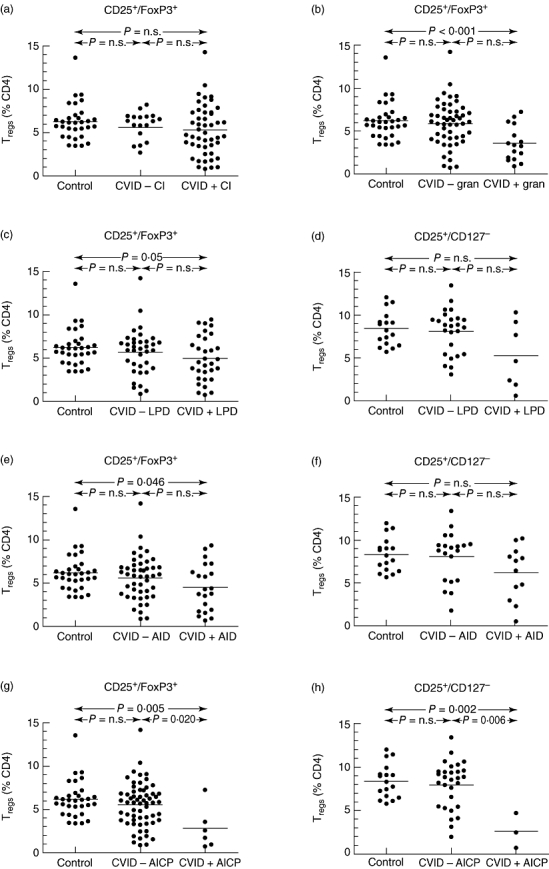
Regulatory T cell (Treg) proportions (as a percentage of CD4 lymphocytes) stratified by clinical characteristics. (a) Common variable immunodeficiency (CVID) patients with (CVID + CI) or without (CVID − CI) chronic infections (CI), as defined in the legend to Table 1; (b) CVID patients with (CVID + gran) or without (CVID − gran) granulomata (gran); (c,d) CVID patients with (CVID + LPD) or without (CVID − LPD) lymphoproliferative disease (LPD), including lymphadenopathy or splenomegaly. (e,f) CVID patients with (CVID + AID) or without (CVID − AID) autoimmune disease, Treg cell proportions in European patients defined by the phenotype CD25+/forkhead box P3 (FoxP3)+ (e), or CD25+/CD127low Treg cells from the Sydney cohort (f). (g,h) CVID patients with (CVID + AICP) or without (CVID − AICP) autoimmune cytopenias at any stage in the course of their disease, Treg cell proportions in European patients defined by the phenotype CD25+/FoxP3+ (g), or CD25+/CD127low Treg cells from the Sydney cohort (h). (a–c,e,g) Treg cell proportions in European patients defined by the phenotype CD25+/FoxP3+; (d,f,h) CD25+/CD127low Treg cells from the Sydney cohort.
These data suggested the existence of a subgroup of CVID patients characterized by a decrease in Treg cell proportions and specific clinical manifestations. To test this, patients were divided into two subsets based on their Treg cell proportion, the ‘low Treg cell’ group having proportions below the lowest value in the control populations (cut-off of 3% of CD4 cells in the European group and 5% in the Australian cohort; see Fig. 2), and the ‘normal Treg cell’ group having values above these respective cut-offs. The low Treg cell group showed a significantly higher frequency of autoimmune cytopenias and granulomatous disease, as expected, but also autoimmune disease in general (Fig. 4).
Fig. 4.
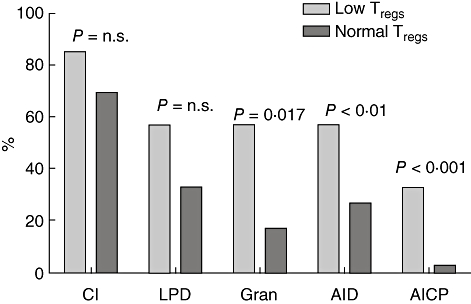
Clinical characteristics of the common variable immunodeficiency (CVID) cohort divided on the basis of having a low regulatory T cell proportion (cut-off of 3% of CD4 lymphocytes when defined by the phenotype CD25+/forkhead box P3+ in European patients, or 5% by the phenotype CD25+/CD127low from the Sydney cohort).
The Treg cell proportions are reduced in Freiburg group Ia
Classification of CVID patients based on variations in peripheral memory B cell subsets has been proposed as a possible approach to reducing the heterogeneity of this condition and to allow analysis of specific subgroups of patients. We examined whether Treg cell proportions varied according to these subgroups, and indeed there was a significant decrease in the proportion of Treg cells in Freiburg group Ia compared with either controls or either of the other two Freiburg groups (Fig. 5), although there were no significant differences in Treg cell proportions between the Paris groups MB0, MB1 and MB2 (data not shown).
Fig. 5.

Regulatory T cell (Treg) proportions (as a percentage of CD4 lymphocytes), stratified by the Freiburg group, in European patients defined by the phenotype CD25+/forkhead box P3+ (a), or as CD25+/CD127low Treg cells from the Sydney cohort (b).
Discussion
This study represents the largest cohort of CVID patients screened for Treg cells, and provides novel data linking changes in the proportion of this subset in the blood of patients with CVID with clinical manifestations of this condition. The frequency of Treg cells was analysed in 99 CVID patients from two European and one Australian centre. We found decreases in Treg cell proportions of CD4 cells in blood in CVID patients with granulomatous disease and immune cytopenias, and defined similarly a subset of CVID patients with abnormally low proportions of Treg cells who were predisposed to granulomatous disease and autoimmune disease, especially autoimmune cytopenias. Furthermore, Treg cells were particularly reduced in a subgroup of CVID patients characterized by loss of isotype-switched memory B cells in association with an increased CD21− B cell proportion (Freiburg Ia), a subset which itself has also been linked to autoimmune cytopenias. The decrease in proportion of Treg cells associated with Freiburg group Ia lends further weight to the existence of distinct but overlapping subcategories or clinicopathological ‘clusters’ within the broad umbrella of CVID.
Our results differ in many respects from the smaller study of 26 CVID patients reported by Fevang et al.[11]. In this Norwegian cohort there was an overall reduction in Treg cells in CVID patients compared with healthy controls, whereas our study showed this to be the case only in Freiburg Ia patients. As Fevang et al. did not differentiate these subgroups, alterations in cohort composition might explain this difference. We found no differences with respect to chronic infection, while the Norwegian study demonstrated an inverse correlation with serum neopterin levels, a marker of systemic chronic inflammation; however, these two are not necessarily linked, and Fevang et al. did not determine the presence of chronic infection clinically, in contrast to the present study. We were unable to confirm their observation of a relation to lymphoproliferation.
Thymus-derived naturally occurring Treg cells were identified originally using the cell surface markers CD4 and CD25 [17]. In humans, however, CD25 expression is less reliable, as conventional CD4 T cells may also express CD25, even without activation, and there is controversy as to the level of CD25 expression that best defines a Treg cell [18,19]. The most specific marker that distinguishes regulatory from conventional T cells is expression of FoxP3, although one disadvantage is that its identification requires time-consuming intracellular staining of T cells, which also renders cells incompetent for functional studies. Thus the discovery of CD127 as a cell surface Treg cell marker has the potential to overcome these problems, and studies have shown a high rate of concordance between Treg cells defined by intracellular FoxP3 expression versus surface marker expression [10]. The strong correlation between values for FoxP3+/CD25+ and FoxP3+/CD25+/CD127low cells (Supplementary Fig. S2), however, suggests that the exclusion of CD127+ cells in the fixation-permeabilization assay does not add greatly to this analysis, whereas our ability to reproduce similar data using expression of surface markers alone adds validation to the use of the latter strategy as a surrogate for FoxP3 expression. One strength of our study was therefore that we were able to reproduce the data using either analysis method in three different cohorts in three different laboratories worldwide.
The CVID is associated with various autoimmune manifestations, chronic inflammation and granulomatous disease. Although immunodeficiency and autoimmunity seem to be at two ends of a spectrum of immunological activity, several hypotheses exist to explain this coincidence. Immunodeficiency might result in a failure to clear microbial antigens, and persistent antigenic exposure could then trigger both granulomatous disease as well as autoimmunity, the latter perhaps by molecular mimicry [20]. Alternatively, given that T cell abnormalities are often described in CVID patients, it is feasible that abnormalities in Treg cells themselves could be related to both complications. This was supported here by the finding of a higher rate of both granulomatous disease and autoimmunity in the subgroup with very low Treg cells (Fig. 4). The granulomas in CVID patients are non-caseating, ‘sarcoid-like’ lesions predominantly in the lungs, with frequent involvement of the spleen and liver, sometimes predating the diagnosis of antibody deficiency. The finding of diminished Treg cell frequency in these patients could relate to the sequestration of Treg cells at sites of chronic inflammation, although it is unknown whether Treg cells are predominant in granulomas from CVID patients. Studies of sarcoidosis in patients without CVID have given contradictory results, with two studies showing an increased frequency of CD4+/CD25+ Treg cells in peripheral blood and bronchoalveolar lavage fluid [21], and another which showed a reduced frequency of FoxP3+ Treg cells in the blood [22]. On the other hand, in immune thrombocytopenia in patients without CVID, a decrease in Treg cell proportion, number or function have all been well documented [23–27], a decrease which normalizes typically upon resolution of the thrombocytopenia, contrasting with the data presented here, where decreased Treg cell proportion was a stable phenotype and possibly an integral characteristic of this particular subset of CVID patients. One caveat to our conclusion that Treg cell proportions were decreased in CVID patients with immune cytopenias was the small number of CVID patients who demonstrated this complication; despite this, the study had at least 89% power to detect such differences in three of the four comparisons, and 74% power in the fourth study (European CVID patients, cytopenias compared with no cytopenias); however, more important than such power analyses is whether the values observed in our study are truly representative of the underlying (and unobserved) values. This cannot be determined until results from other independent studies are available.
In summary, we report decreases in the circulating frequency of Treg cells in Freiburg Ia CVID patients and in CVID patients complicated by granulomatous disease or autoimmune cytopenias, posing questions as to the underlying pathogenesis that might link these clinical manifestations. It will be important to determine whether numerical differences in the circulation translate to functional differences in terms of lack of suppression, and whether these changes reflect changes in immunoregulation within the microenvironment of the secondary lymphoid tissue.
Acknowledgments
The Sydney authors would like to acknowledge with thanks the help of Karen Byth-Wilson for the statistical analysis, Marjorie Bennetts for sample collection and Sue Wong for assistance with flow cytometry. The Freiburg authors would like to thank M. Schlesier and U. Salzer for valuable advice, and J. Birmelin for assistence with flow cytometry. The London investigators would like to thank Arthur McQuaid and Grahame Wright for their assistance and flow cytometry advice. We also appreciate the support of the patients and controls for agreeing to take part in the study. The study was funded in part by the DFG grant SFB620/C2 and EU grants MEXT-CT-2006-042316 and EURO-PADnet HEALTH-F2-2008-201549.
Disclosure
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Correlation between regulatory T cell (Treg) proportions (as a percentage of CD4 lymphocytes) in the London cohort as determined by either the phenotype CD25+/forkhead box P3 (FoxP3)+ (x-axis) or CD25+/CD127low/FoxP3+ (y-axis) on the same samples. The dotted line represents line of best fit, with correlation coefficient as shown,while the solid line represents theoretical equivalence between the two assays.
Fig. S2. (a) Regulatory T cell (Treg) cell proportions, defined by the phenotype CD25+/forkhead box P3 (FoxP3)+, in a subsample of 14 patients [six common variable immunodeficiency (CVID) and eight controls] in the Freiburg cohort at two different time-points, separated by a mean of 2·4 months, showing stability of these values over this timeperiod. (b) Comparison of two clones of anti-FoxP3 (PCH101 and 236A/E7) in six subjects (three controls and three CVID patients). Values plotted represent percentages of CD4 cells staining positive for FoxP3.
Fig. S3. Representative dot-plots depicting regulatory T cell (Treg) proportions in two common variable immunodefi- ciency patients, one with autoimmune cytopenias (right panel) and one without (left panel). Percentages represent proportions of CD4 cells in the specified gate.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 2.Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C, Cooper DL, Duffy TP, Strauchen J. Lymphomas of mucosal-associated lymphoid tissue in common variable immunodeficiency. Am J Hematol. 2002;69:171–8. doi: 10.1002/ajh.10050. [DOI] [PubMed] [Google Scholar]
- 4.Knight AK, Cunningham-Rundles C. Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmun Rev. 2006;5:156–9. doi: 10.1016/j.autrev.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 6.Agematsu K, Futatani T, Hokibara S, et al. Absence of memory B cells in patients with common variable immunodeficiency. Clin Immunol. 2002;103:34–42. doi: 10.1006/clim.2001.5197. [DOI] [PubMed] [Google Scholar]
- 7.Piqueras B, Lavenu-Bombled C, Galicier L, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- 8.Giovannetti A, Pierdominici M, Mazzetta F, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–43. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fevang B, Yndestad A, Sandberg WJ, et al. Low numbers of regulatory T cells in common variable immunodeficiency: association with chronic inflammation in vivo. Clin Exp Immunol. 2007;147:521–5. doi: 10.1111/j.1365-2249.2006.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geha RS, Notarangelo LD, Casanova J-L, et al. International Union of Immunological Societies Primary Immunodeficiency Diseases Classification C, Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120:776–94. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 14.Ferry BL, Jones J, Bateman EA, et al. Measurement of peripheral B cell subpopulations in common variable immunodeficiency (CVID) using a whole blood method. Clin Exp Immunol. 2005;140:532–9. doi: 10.1111/j.1365-2249.2005.02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berglund LJ, Wong SWJ, Fulcher DA. B-cell maturation defects in common variable immunodeficiency and association with clinical features. Pathology. 2008;40:288–94. doi: 10.1080/00313020801911470. [DOI] [PubMed] [Google Scholar]
- 16.Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry Part B. 2008;74B:261–71. doi: 10.1002/cyto.b.20432. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 18.Banham AH, Powrie FM, Suri-Payer E. FOX P3+ regulatory T cells: current controversies and future perspectives. Eur J Immunol. 2006;36:2832–6. doi: 10.1002/eji.200636459. [DOI] [PubMed] [Google Scholar]
- 19.Rouse BT. Regulatory T cells in health and disease. J Intern Med. 2007;262:78–95. doi: 10.1111/j.1365-2796.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 20.Brandt D, Gershwin ME. Common variable immune deficiency and autoimmunity. Autoimmun Rev. 2006;5:465–70. doi: 10.1016/j.autrev.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–70. doi: 10.1084/jem.20050648. erratum appears in J Exp Med 2006 Feb 20;203(2):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idali F, Wahlström J, Müller-Suur C, Eklund A, Grunewald J. Analysis of regulatory T cell-associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin Exp Immunol. 2008;152:127–37. doi: 10.1111/j.1365-2249.2008.03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling Y, Cao X, Yu Z, Ruan C. Circulating dendritic cells subsets and CD4+Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol. 2007;79:310–6. doi: 10.1111/j.1600-0609.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Zhao H, Poon M-C, et al. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol. 2007;78:139–43. doi: 10.1111/j.1600-0609.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 25.Sakakura M, Wada H, Tawara I, et al. Reduced CD4+CD25+ T cells in patients with idiopathic thrombocytopenic purpura. Thromb Res. 2007;120:187–93. doi: 10.1016/j.thromres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112:1147–50. doi: 10.1182/blood-2007-12-129262. see comment. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Heck S, Patel V, et al. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112:1325–8. doi: 10.1182/blood-2008-01-135335. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


