Abstract
Human T lymphotropic virus-type 1 (HTLV-1) is the causal agent of the HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), adult T cell leukaemia/lymphoma and infective dermatitis associated with HTLV-1 (IDH). Over-production of proinflammatory cytokines and an increase in HTLV-1 proviral load are features of HAM/TSP, but the immunological basis of IDH has not been established. In addition to severe cutaneous manifestations, the importance of IDH relies on the observation that up to 30% of children with IDH develop HAM/TSP in childhood and adolescence. In this study we determined the immune response in patients with IDH measuring interleukin (IL)-4, IL-5, IL-10, interferon (IFN)-γ and tumour necrosis factor (TNF)-α levels as well as the HTLV-1 proviral load. Additionally, regulatory cytokines and anti-cytokines were added to cultures to evaluate the ability of these molecules to down-modulate TNF-α and IFN-γ synthesis. HTLV-1 carriers and patients with HAM/TSP served as controls. TNF-α and IFN-γ levels were higher in IDH than in HTLV-1 carriers. There was no difference in IFN-γ and TNF-α concentrations in IDH and HAM/TSP patients. There was a tendency for higher IL-4 mRNA expression and immunoglobulin E (IgE) levels in IDH than in HTLV-1 carriers, but the difference did not reach statistical significance. The HTLV-1 proviral load was significantly higher in IDH patients than in HTLV-1 carriers. IDH is characterized by an exaggerated Th1 immune response and high HTLV-1 proviral load. The similarities between the immunological response in patients with IDH and HAM/TSP and the high proviral load observed in IDH provide support that IDH is a risk factor for development of HAM/TSP.
Keywords: cytokines/interleukins, dermatological infections, HTLV-I/II
Introduction
Human T lymphotropic virus-type 1 (HTLV-1) is a retrovirus associated with a haematological disorder called adult T cell leukemia/lymphoma (ATLL), and with a neurological syndrome, HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). However, only a small percentage of infected individuals will develop disease. Peripheral blood mononuclear cells (PBMC) from HTLV-1 infected individuals proliferate spontaneously in vitro and secrete cytokines [1,2]. Patients with HAM/TSP present high HTLV-1 proviral load [3], an increased number of Tax-specific CD8+ T lymphocytes [4], a persistent and high titre of anti-HTLV-1 antibodies [5] and an increased expression of proinflammatory cytokines such as interferon (IFN)-γ, tumour necrosis factor (TNF)-α and chemokines in the peripheral blood and cerebral spinal fluid [6–8]. These immunological abnormalities are more pronounced in HAM/TSP patients, but evidence of enhanced T cell activation is also detected in HTLV-1 carriers [6,9].
Infective dermatitis associated with HTLV-1 (IDH) is a form of recurrent dermatitis that affects children infected with HTLV-1. Cases of IDH have been reported in Colombia, French Guyana and Senegal [10–12]. HTLV-1 is endemic in Salvador (Bahia, Brazil), an area with the highest prevalence of this infection among blood donors in Brazil [13] and where the majority of IDH cases have been reported [14]. The lesions in IDH are erythematous, scaly and crusted, and are located frequently on the scalp and on the retroauricular, cervical, peri-oral, inguinocrural and perinasal regions [15,16]. Patients present with mild to moderate pruritus, and with chronic nasal secretions and crusting on the nares. IDH is associated generally with Staphylococcus aureus and/or Streptococcus beta haemolyticus infection [16]. More recently, it has been shown that IDH may progress to ATLL and HAM/TSP [14,17–19]. IDH resembles some features of late lesions of severe atopic dermatitis, but the immunological basis of IDH has not been determined. In this work, we performed an analysis of in vitro cytokine patterns from PBMC of IDH patients, and the HTLV-1 proviral load was determined. Additionally, the ability of a regulatory cytokine [interleukin (IL)-10] and cytokine antagonists (anti-IL-2 and anti-IL-15) to down-regulate the spontaneous IFN-γ and TNF-α production in unstimulated cell culture was evaluated. These results were compared with those observed in HTLV-1 carriers and in patients with adult HAM/TSP.
Materials and methods
Study subjects
This is a cross-sectional study with the participation of 20 IDH patients, 40 HTLV-1 carriers, 40 HAM/TSP patients and 15 HTLV-1 seronegative individuals used as controls. The IDH patients enrolled in the study comprise those who were followed at the dermatological clinic of the Hospital Universitário Professor Edgard Santos between September 2002 and August 2005. The skin lesions observed in IDH can be seen in Fig. 1. The diagnosis of IDH was made according to previously established criteria [16]. A differential diagnosis between IDH and atopic dermatitis was made on the basis of pre-existing criteria [20]. The HTLV-1 carriers were selected consecutively from blood bank donors, and patients with HAM/TSP have been followed in the HTLV-1 clinic of the Hospital Universitário Professor Edgard Santos, Federal University of Bahia, Brazil. Three IDH patients were excluded because at the time of the evaluation they already had HAM/TSP, or the diagnostic of myelopathy was performed close to the blood collection for the immunological studies. The diagnosis of HTLV-1 infection was performed by enzyme-linked immunosorbent assay (ELISA) (Murex HTLV-I + II; Abbot, Dartford, UK) and confirmed by Western blot analysis (HTLV 2·3–2·4; Genelabs, Singapore). The diagnosis of HAM/TSP was made according to World Health Organization guidelines. All HAM/TSP patients had HTLV-1 antibodies in their cerebral spinal fluid and had Osame's motor disability score =1 and expanded disability status scale = 3 [21,22]. Individuals who did not fulfill the criteria for HAM/TSP were classified as HTLV-1 carriers. Patients with positive serology for HIV-1 and -2 and hepatitis virus types B and C were excluded from the study. Participants of the study or their guardians gave informed consent prior to the drawing of blood samples, and the study was conducted with the approval of the Ethical Committee of the Hospital Universitário Professor Edgard Santos.
Fig. 1.

Infective dermatitis with severe involvement of scalp, forehead and external ear with exudative and crusted lesions.
Cell culture and IL-5, IL-10, IFN-γ and TNF-α levels
The PBMCs from 17 IDH patients, 40 HTLV-1 carriers and 40 HAM/TSP patients were isolated from heparinized blood by density gradient centrifugation with Ficcoll-Hypaque. The cell were cultured in RPMI-1640 (Life Technologies, Gibco brl, Grand Island, NY, USA), 10% human serum (Sigma, St Louis, MO, USA), glutamine, HEPES and antibiotics. Briefly, 3 × 106 cells/ml were plated in 24-well flat-bottomed microtitre plates (Falcon, Becton Dickinson, Lincoln Park, NJ, USA) and kept with media only (unstimulated) or were supplemented with 100 ng/ml IL-10 (DNAX Institute, Palo Alto, CA, USA), anti-IL-2 or anti-IL-15 (R&D Systems Inc, Minneapolis, MN, USA) at a concentration of 20 µg/ml. Cell cultures were incubated at 37°C with 5% CO2 for 72 h. Supernatants of the cell cultures were collected and stored at –20°C until use. IL-5, IL-10, IFN-γ and TNF-α levels were determined using the ELISA sandwich technique following the manufacturer's instructions (BD Bioscience Pharmingen, San Jose, CA, USA). The results were expressed in pg/ml based on a standard curve generated using recombinant cytokines.
Determination of total serum immunoglobulin E levels
Total immunoglobulin E (IgE) titres were obtained from the sera of 17 IDH patients and 16 HTLV-1 carriers by Immulite 2000 Total IgE kit, according to the manufacturer's instructions (EURO/DPC, Llanberis, UK).
Semi-quantitative reverse transcriptase–polymerase chain reaction to detect IL-4
Total RNA isolation from PBMCs after 72 h of culture was performed utilizing Trizol LS reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized using 3 µg of total RNA and reverse-transcribed by M-MLV reverse transcriptase. Polymerase chain reactions (PCRs) were then performed in a final volume of 50 µl containing 2·0 mM of MgCl2, 0·2 mM of deoxyribonucleoside triphosphate (dNTP) mix (dATP, dCTP, dTTP, dGTP), 10× PCR buffer, 2·5 U of the Taq DNA polymerase recombinant (Invitrogen) and specific primers at 25–50 pmol using the Veriti Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The human primer sequences were: HPRT forward: GCGTCGTGATTAGTGATGATGAAC and HPRT reverse: GGATTATACTGCCTGACCAAGG; IL-4 forward: GCGATATCACCTTACAGGAG and IL-4 reverse: TGTCCTGTGAAGGAAGCCAAC. Thermocycling conditions included 30 cycles of 1 min at 95°C for denaturation, 1 min 30 s at 60°C for annealing and extension for 1 min 30 s at 72°C, plus a final extension step of 10 min at 72°C. The amplification products of PCR were visualized in 1·3% agarose gel staining with 0·2% of ethidium bromide (Sigma). The band intensity was calculated using Quantity One Software 4.6.3 (Basic) (BioRad, Hercules, CA, USA). Results were expressed as relative units corrected for HPRT expression.
The HTLV-1 proviral load
The PBMCs from 17 IDH patients, 32 HTLV-1 carriers and 31 HAM/TSP patients were isolated from ethylenediamine tetraacetic acid-treated blood samples by Ficoll density gradient centrifugation. DNA was extracted from 106 cells using the phenol/chloroform procedure. The HTLV-1 proviral load was quantified using a real-time TaqMan PCR method [23]. Albumin DNA was quantified in parallel to determine the input cell number and was used as an endogenous reference. Amplification and data acquisition were carried out using the ABI Prism 7700 Sequence detector system (Applied Biosystems). Standard curves were generated using a 10-fold serial dilution of a double-stranded plasmid (pcHTLV-ALB). The HTLV-1-infected human lymphocyte line MT2 was used as a control for quantification. The normalized value of the HTLV-1 proviral load was calculated as the ratio of (HTLV-1 DNA average copy number/albumin DNA average copy number) × 2 × 106 and expressed as the number of HTLV-1 copies per 106 PBMCs.
Statistical analysis
The Mann–Whitney U-test and Wilcoxon's matched-pairs signed-rank test were used to compare data. The software GraphPad Prism version 3·03 (San Diego, CA, USA) was applied for statistical analysis, and P values < 0·05 were considered to indicate a significant difference.
Results
Cytokines profile secreted by PBMC from IDH, HTLV-1 carriers and HAM/TSP patients
Spontaneous production of TNF-α, IFN-γ, IL-5 and IL-10 in HTLV-1 seronegative healthy controls, HTLV-1 carriers, IDH patients and in HAM/TSP is shown in Fig. 2. TNF-α and IFN-γ levels in patients with IDH were significantly higher than in controls. IFN-γ levels were slightly higher in HAM/TSP than in IDH patients, and TNF-α was slightly higher in IDH than in HAM/TSP, but these differences did not reach statistical significance. The median concentration of TNF-α in supernatants of IDH patients was 599 pg/ml compared with 122 pg/ml in HTLV-1 carriers (P = 0·005) and 442 pg/ml in HAM/TSP patients. IFN-γ levels were 1195 pg/ml, 424 pg/ml (P = 0·001) and 2588 pg/ml in IDH patients, HTLV-1 carriers and HAM/TSP patients respectively. There was no significant difference in IL-5 and IL-10 concentrations between the IDH patients and HTLV-1 carriers.
Fig. 2.
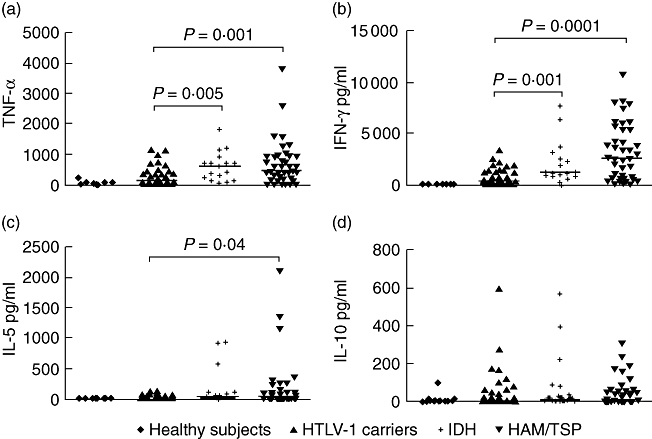
Comparison of interferon (IFN)-γ (b), tumour necrosis factor (TNF)-α (a), interleukin (IL)-5 (c) and IL-10 (d) concentrations in infective dermatitis associated with human T lymphotropic virus-type 1 (IDH) patients (n = 17), human T lymphotropic virus-type 1 (HTLV-1) carriers (40), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (40) patients and healthy controls (15). All cytokine data presented correspond to the value in unstimulated cultures. The levels of cytokines were measured by enzyme-linked immunosorbent assay and expressed as pg/ml. Statistical analysis was performed by Mann–Whitney U-test.
Correlation between TNF-α and IL-10 levels in IDH patients
The TNF-α has been associated with pathology in HTLV-1 infection, and IL-10 is one of the most important cytokines involved in down-modulating TNF-α levels [24]. As TNF-α and IL-10 levels were variable, we evaluated whether there was an inverse correlation between TNF-α and IL-10 levels in patients with IDH. The values of TNF-α and IL-10 plotted in Fig. 3 show that there was a direct correlation between TNF-α and IL-10 levels.
Fig. 3.
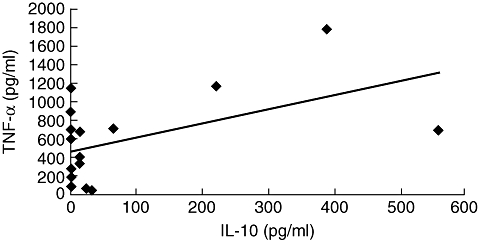
Correlation between tumour necrosis factor (TNF)-α and interleukin (IL)-10 levels in infective dermatitis associated with human T lymphotropic virus-type 1 (IDH) patients. A direct correlation between TNF-α and IL-10 levels was observed in peripheral blood mononuclear cell supernatants from IDH patients (P = 0·03). Analysis was performed by Pearson's signed-rank test.
The IL-4 mRNA expression in IDH patients and HTLV-1 carriers and total serum IgE levels
Expression of the IL-4 gene in unstimulated PBMC from healthy controls, HTLV-1 carriers and IDH patients was evaluated. We found an increased expression of IL-4 mRNA in IDH patients when compared with HTLV-1 carriers, but no significant difference was observed between these groups (Fig. 4). There was a tendency for higher total serum IgE in IDH patients when compared with HTLV-1 carriers (P > 0·05) (Fig. 4).
Fig. 4.
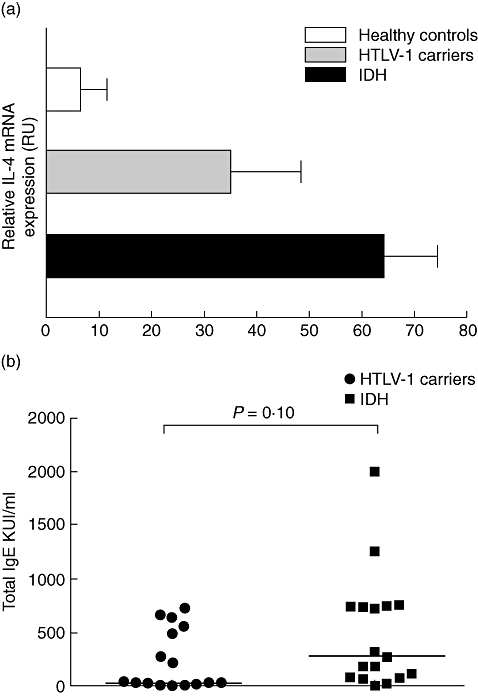
Relative interleukin (IL)-4 mRNA expression in the healthy controls, human T lymphotropic virus-type 1 (HTLV-1) carriers and infective dermatitis associated with HTLV-1 (IDH) patients (a). Total RNA from unstimulated peripheral blood mononuclear cells was extracted, reverse-transcribed and the cDNA amplified. The polymerase chain reaction products were visualized in 1·3% agarose gel staining with 0·2% of ethidium bromide. The band intensity was calculated using Quantity One Software version 4.6.3 (Basic). Results were expressed as relative units (RU) corrected for hypoxanthine phosphoribosyl transferase (HPRT). Total immunoglobulin E (IgE) antibody titres in sera from HTLV-1 carriers and infective dermatitis patients (b). The data are shown in KU/ml. Twelve IDH patients (57%) and seven (43%) HTLV-1 carriers had elevated IgE antibody titres. Statistical analysis was performed by Mann–Whitney U-test.
Quantification of HTLV-1 proviral load in HTLV-1 asymptomatic carrier, IDH patients and HAM/TSP patients
The proviral load in numbers of copies of HTLV-1 per 106 PBMCs in 32 HTLV-1 carriers, 17 IDH patients and 31 HAM/TSP patients is shown in Fig. 5. The median of proviral loads in IDH patients were much higher, with 244·369 copies per 106 PBMCs compared to 32·035 in HTLV-1 carriers (P = 0·0001) and 139·109 copies per 106 PBMCs in HAM/TSP patients.
Fig. 5.
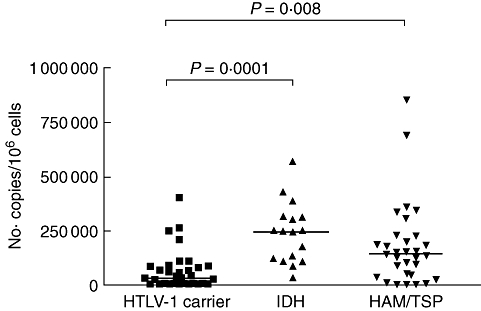
Human T lymphotropic virus-type 1 (HTLV-1) proviral load in peripheral blood mononuclear cells from 32 HTLV-1 carriers, 17 infective dermatitis associated with HTLV-1 (IDH) patients and 31 HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. HTLV-1 proviral load was significantly higher in IDH patients than HTLV-1 carriers. Statistical analysis was performed by Mann–Whitney U-test.
Down-regulation of IFN-γ production by cytokine and anti-cytokines (IL-10, anti-IL-2 and anti-IL-15) in HTLV-1 carriers and IDH patients
We have previously shown that while PBMCs from HTLV-1 carriers can be modulated when IL-10 and anti-IL-2 are added in vitro, cells from HAM/TSP patients exhibit poor modulation of the immune response [25]. To evaluate the ability of IL-10 to decrease the spontaneous IFN-γ production observed in HTLV-1-infected patients, PBMCs of IDH patients and HTLV-1 carriers were cultured in the presence of IL-10. The addition of 100 ng/ml of IL-10 significantly reduced IFN-γ production from 796 pg/ml to 180 pg/ml in HTLV-1 carriers and from 844 pg/ml to 558 pg/ml in IDH patients, showing a decrease in spontaneous IFN-γ synthesis of 71% and 39% respectively (P < 0·05) (Fig. 6a). IL-2 and IL-15 are cytokines participating in T cell activation and proliferation, and both are involved in the spontaneous lymphoproliferation observed in HTLV-1-infected patients [26,27]. The effects of neutralizing antibodies against IL-2 and IL-15 were assayed in PBMC cultures of IDH patients and HTLV-1 carriers. Anti-IL-2 addition reduced IFN-γ production in unstimulated cell cultures by 11·5% in IDH patients (844–778 pg/ml, P > 0·05) and by 52% in HTLV-1 carriers (796–258 pg/ml, P = 0·01) (Fig. 6b). Anti-IL-15 reduced IFN-γ production by 33% in IDH patients (844–534 pg/ml, P < 0·05) and by 36% in HTLV-1 carriers (796–347 pg/ml, P < 0·05) (Fig. 6c).
Fig. 6.
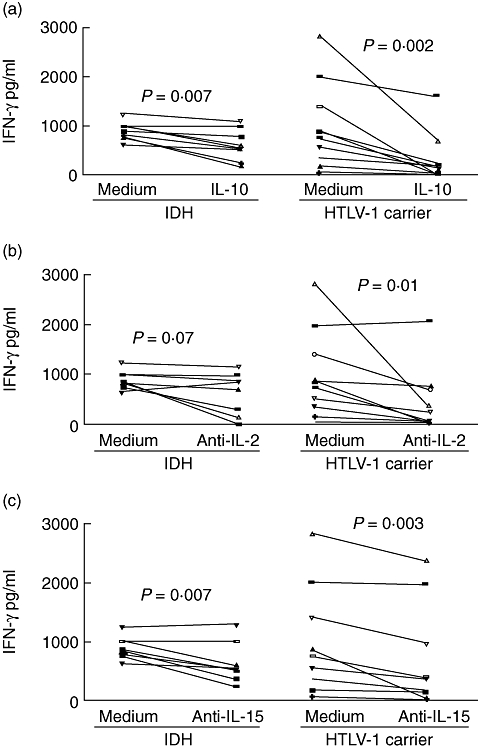
Effect of interleukin (IL)-10 (a), anti-IL-2 (b) and anti-IL-15 (c) on spontaneous interferon (IFN)-γ production in peripheral blood mononuclear cells cultures of infective dermatitis associated with human T lymphotropic virus-type 1 (IDH) patients (eight) and asymptomatic human T lymphotropic virus-type 1 (HTLV-1) carriers (10). The data represent the variation in spontaneous IFN-γ levels after the addition of cytokine and cytokine antagonists (P < 0·05, Wilcoxon matched-pairs signed-rank test).
Discussion
The IDH is a very severe cutaneous manifestation of HTLV-1 infection observed predominantly in childhood [16]. Despite the eventual improvement of cutaneous lesions in the majority of patients, recent studies have indicated that a large percentage of IDH patients may experience a rapid progression to HAM/TSP [19]. In the present study, we have documented that patients with IDH have higher production of TNF-α and IFN-γ than HTLV-1 carriers, pointing out the possible importance of these immunological abnormalities in the pathogenesis of the disease. As observed in HTLV-1 carriers, IDH patients are able to modulate IFN-γ production when anti-IL-15 was added. However, anti-IL-2 was not able to reduce significantly the immunological response in these patients. Moreover, our data show that proviral load was significantly higher in IDH patients than in HTLV-1 carriers. These data indicate that IDH is characterized by an exaggerated type 1 immune response and high proviral load.
The IDH has distinct clinical features from atopic dermatitis; however, sometimes skin lesions not only resemble atopic dermatitis but are also frequently colonized by S. aureus, as observed in atopic patients [28]. The majority of children suffering from atopic dermatitis have a raised serum level of IgE, which correlates in turn with severity of the disease [29,30]. HTLV-1 infection is characterized by a strong type 1 immune response, but compared with seronegative individuals HTLV-1 carriers have higher levels of IL-4, IL-5 and IL-10 [6]. Herein we show that IDH patients had higher levels of IgE than HTLV-1 carriers, but this difference did not reach statistical significance. Moreover, the levels of specific IgE in IDH patients, healthy controls and HTLV-1 carriers for a panel of inhalant and food allergens did not show a statistical difference when they were compared (data not shown). IL-4 plays a pivotal role in differentiation of T helper type 0 (Th0) to Th2 cells which, in turn, produce predominantly IL-4, IL-5, IL-13 and IL-10. As IL-4 levels in supernatants of PBMC cultures may not be elevated even in situations associated clearly with a type 2 immune response [31], mRNA expression for IL-4 was determined. The observation that no difference was found between mRNA in IDH and controls gives support that the immunological response in IDH differs from atopic dermatitis and that a type 2 immune response is not responsible for the tissue damage observed in IDH. In fact, immunohistochemical studies performed in IDH patients showed a predominance of CD8+ T cells expressing T cell intracellular antigen 1 and granzyme B, data that differ from those observed in atopic dermatitis [32].
The HTLV-1 infection is characterized by an exaggerated type 1 immune response [6]. TNF-α has been associated with pathology of chronic inflammatory and autoimmune diseases [33,34], and IL-10 is one of the most important cytokines in down-modulating TNF-α synthesis [24]. Here we show that there is a direct correlation between TNF-α and IL-10, probably suggesting that the increase in IL-10 concentration is an attempt to down-modulate TNF-α production. Alternatively, it has been shown that human CD4 T cells may secrete simultaneously IL-10 and IFN-γ[35], and CD4 T lymphocytes are the cells infected predominantly by HTLV-1. Therefore, it cannot be ruled out that the source of IL-10 is mainly activated T cells during HTLV-1 infection.
Proviral load has been associated with pathology associated with HTLV-1. PBMC from adult HAM/TSP have a higher proviral load than HTLV-1 carriers [9,36], and more recently it was shown that this difference is more significant in the central nervous system (CNS) fluid than in PBMC [37]. This increase in HTLV-1 proviral load in the CNS could be associated with the passage of infected and activated CD4 T cells through the blood–brain barrier, followed by infiltration of the cytotoxic cells producing cytokines, chemokines and other molecules that will mediate CNS tissue damage [37]. In this study we showed that HTLV-1 proviral load was higher in IDH than in HTLV-1 carriers. T cell infiltration is observed in skin lesions from IDH patients [32], and it is possible that both HTLV-1-infected cells and activated T cells migrate to the skin of these patients [38].
We have previously shown that IL-10, anti-IL-2 and anti-IL-15 down-modulate IFN-γ production in HTLV-1 carriers but do not suppress IFN-γ synthesis in HAM/TSP [25]. In this study we evaluated whether the exogenous addition of IL-10 and neutralization of IL-2 and IL-15 was able to down-modulate IFN-γ synthesis in cell cultures. While IL-10, anti-IL-2 and anti-IL-15 significantly decreased IFN-γ production in HTLV-1 carriers, only IL-10 and anti-IL-15 significantly down-modulated IFN-γ production in patients with IDH. Additionally, in all cases cytokines and antibodies anti-cytokines were more effective in decreasing IFN-γ production in HTLV-1 carriers than in patients with IDH.
This study characterizes the immunological changes observed in IDH patients, allowing a better understanding of the immunopathogenesis of this severe dermatological manifestation observed in children infected with HTLV-1. Our data indicate that pathogenesis of IDH is related to an exaggerated type 1 immune response with increased concentration of TNF-α and IFN-γ in supernates of PBMC and high proviral load. These abnormalities are similar to those which have been observed in adult HAM/TSP [39,40], giving support to the clinical observation that a large proportion of IDH patients develop HAM/TSP [19]. The only difference between these two groups of patients in the immunological evaluation performed was the documentation that IL-10 and anti-IL-15 are able to decrease IFN-γ production in IDH, while patients with HAM/TSP had poor modulation of the type 1 immune response. It is possible that IDH patients who do not modulate the immune response appropriately are more likely to progress to HAM/TSP. This emphasizes the need for an effective anti-retroviral therapy associated with immunotherapy to treat patients at high risk of developing HAM/TSP. While no anti-retroviral drug is effective against HTLV-1, the use of immunomodulators such as pentoxifylline, a TNF-α inhibitor, should be considered, as TNF-α is highly produced in these patients. Patients with IDH are therefore natural candidates to receive therapy in an attempt to prevent the development of irreversible neurological damage.
Acknowledgments
This study was supported by NIH/Fogarty International Center grant D43 TW007127, Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and Brazilian National Research Council (CNPq). EMC and ALB are senior investigators of the CNPq. We acknowledge Dr Aurélia Porto and Dr André Muniz in providing medical assistance, Dr Carlos Brites for the performance of serological testing and the secretarial assistance of Elbe Silva in the preparation of this manuscript. We also thank Roberto Novoa for reviewing the manuscript and editing the English.
References
- 1.Prince H, Kleinman S, Doyle M, Lee H, Swanson P. Spontaneous lymphocyte proliferation in vitro characterizes both HTLV-I and HTLV-II infection. J Acquir Immune Defic Syndr. 1990;3:1199–200. [PubMed] [Google Scholar]
- 2.Jacobson S, Gupta A, Mattson D, Mingioli E, McFarlin DE. Immunological studies in tropical spastic paraparesis. Ann Neurol. 1990;27:149–56. doi: 10.1002/ana.410270209. [DOI] [PubMed] [Google Scholar]
- 3.Kira J, Koyanagi Y, Yamada T, et al. Increased HTLV-I proviral DNA in HTLV-I-associated myelopathy: a quantitative polymerase chain reaction study. Ann Neurol. 1991;29:194–201. doi: 10.1002/ana.410290214. [DOI] [PubMed] [Google Scholar]
- 4.Nagai M, Kubota R, Greten TF, Schneck JP, Leist TP, Jacobson S. Increased activated human T cell lymphotropic virus type I (HTLV-I) Tax11-19-specific memory and effector CD8+ cells in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with HTLV-I provirus load. J Infect Dis. 2001;183:197–205. doi: 10.1086/317932. [DOI] [PubMed] [Google Scholar]
- 5.Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–10. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho EM, Bacellar O, Porto AF, Braga S, Galvao-Castro B, Neva F. Cytokine profile and immunomodulation in asymptomatic human T-lymphotropic virus type 1-infected blood donors. J Acquir Immune Defic Syndr. 2001;27:1–6. doi: 10.1097/00126334-200105010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Umehara F, Izumo S, Ronquillo AT, Matsumuro K, Sato E, Osame M. Cytokine expression in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1994;53:72–7. doi: 10.1097/00005072-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Guerreiro JB, Santos SB, Morgan DJ, et al. Levels of serum chemokines discriminate clinical myelopathy associated with human T lymphotropic virus type 1 (HTLV-1)/tropical spastic paraparesis (HAM/TSP) disease from HTLV-1 carrier state. Clin Exp Immunol. 2006;145:296–301. doi: 10.1111/j.1365-2249.2006.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–93. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 10.Mahe A, Meertens L, Ly F, et al. Human T-cell leukaemia/lymphoma virus type 1-associated infective dermatitis in Africa: a report of five cases from Senegal. Br J Dermatol. 2004;150:958–65. doi: 10.1111/j.1365-2133.2004.05834.x. [DOI] [PubMed] [Google Scholar]
- 11.Clyti E, Reynier C, Couppie P, et al. [Infective dermatitis and recurrent strongyloidiasis in a child] Ann Dermatol Venereol. 2004;131:191–3. doi: 10.1016/s0151-9638(04)93569-7. [DOI] [PubMed] [Google Scholar]
- 12.Blank A, Herrera M, Lourido MA, Rueda R, Blank M. Infective dermatitis in Colombia. Lancet. 1995;346:710. doi: 10.1016/s0140-6736(95)92326-8. [DOI] [PubMed] [Google Scholar]
- 13.Galvao-Castro B, Loures L, Rodriques LG, et al. Distribution of human T-lymphotropic virus type I among blood donors: a nationwide Brazilian study. Transfusion. 1997;37:242–3. doi: 10.1046/j.1537-2995.1997.37297203532.x. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira Mde F, Brites C, Ferraz N, Magalhaes P, Almeida F, Bittencourt AL. Infective dermatitis associated with the human T cell lymphotropic virus type I in Salvador, Bahia, Brazil. Clin Infect Dis. 2005;40:e90–6. doi: 10.1086/430064. [DOI] [PubMed] [Google Scholar]
- 15.Walshe MM. Infective dermatitis in Jamaican children. Br J Dermatol. 1967;79:229–36. doi: 10.1111/j.1365-2133.1967.tb11479.x. [DOI] [PubMed] [Google Scholar]
- 16.La Grenade L, Manns A, Fletcher V, et al. Clinical, pathologic, and immunologic features of human T-lymphotrophic virus type I-associated infective dermatitis in children. Arch Dermatol. 1998;134:439–44. doi: 10.1001/archderm.134.4.439. [DOI] [PubMed] [Google Scholar]
- 17.Hanchard B, LaGrenade L, Carberry C, et al. Childhood infective dermatitis evolving into adult T-cell leukaemia after 17 years. Lancet. 1991;338:1593–4. doi: 10.1016/0140-6736(91)92413-v. [DOI] [PubMed] [Google Scholar]
- 18.Tschachler E, Franchini G. Infective dermatitis: a pabulum for human T-lymphotrophic virus type I leukemogenesis? Arch Dermatol. 1998;134:487–8. doi: 10.1001/archderm.134.4.487. [DOI] [PubMed] [Google Scholar]
- 19.Primo JR, Brites C, Oliveira Mde F, Moreno-Carvalho O, Machado M, Bittencourt AL. Infective dermatitis and human T cell lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis in childhood and adolescence. Clin Infect Dis. 2005;41:535–41. doi: 10.1086/432058. [DOI] [PubMed] [Google Scholar]
- 20.Hanifin JM. An overview of atopic dermatitis. Dermatol Nurs. 2003;15(Suppl):6–9. [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 22.Izumo S, Goto I, Itoyama Y, et al. Interferon-alpha is effective in HTLV-I-associated myelopathy: a multicenter, randomized, double-blind, controlled trial. Neurology. 1996;46:1016–21. doi: 10.1212/wnl.46.4.1016. [DOI] [PubMed] [Google Scholar]
- 23.Dehee A, Cesaire R, Desire N, et al. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J Virol Methods. 2002;102:37–51. doi: 10.1016/s0166-0934(01)00445-1. [DOI] [PubMed] [Google Scholar]
- 24.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–47. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos SB, Porto AF, Muniz AL, et al. Modulation of T cell responses in HTLV-1 carriers and in patients with myelopathy associated with HTLV-1. Neuroimmunomodulation. 2006;13:145–51. doi: 10.1159/000097259. [DOI] [PubMed] [Google Scholar]
- 26.Tendler CL, Greenberg SJ, Blattner WA, et al. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc Natl Acad Sci USA. 1990;87:5218–22. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariner JM, Lantz V, Waldmann TA, Azimi N. Human T cell lymphotropic virus type I Tax activates IL-15R alpha gene expression through an NF-kappa B site. J Immunol. 2001;166:2602–9. doi: 10.4049/jimmunol.166.4.2602. [DOI] [PubMed] [Google Scholar]
- 28.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–30. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 29.Akdis M, Simon HU, Weigl L, Kreyden O, Blaser K, Akdis CA. Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol. 1999;163:466–75. [PubMed] [Google Scholar]
- 30.Hofer MF, Harbeck RJ, Schlievert PM, Leung DY. Staphylococcal toxins augment specific IgE responses by atopic patients exposed to allergen. J Invest Dermatol. 1999;112:171–6. doi: 10.1046/j.1523-1747.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 31.Araujo MI, Hoppe B, Medeiros M, et al. Impaired T helper 2 response to aeroallergen in helminth-infected patients with asthma. J Infect Dis. 2004;190:1797–803. doi: 10.1086/425017. [DOI] [PubMed] [Google Scholar]
- 32.Bittencourt AL, Oliveira Mde F, Brites C, Van Weyenbergh J, da Silva Vieira MG, Araujo I. Histopathological and immunohistochemical studies of infective dermatitis associated with HTLV-I. Eur J Dermatol. 2005;15:26–30. [PubMed] [Google Scholar]
- 33.Lessa HA, Machado P, Lima F, et al. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg. 2001;65:87–9. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 34.Brennan FM, Maini RN, Feldmann M. TNF alpha – a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992;31:293–8. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- 35.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25 (-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–97. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuzaki T, Nakagawa M, Nagai M, et al. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol. 2001;7:228–34. doi: 10.1080/13550280152403272. [DOI] [PubMed] [Google Scholar]
- 37.Nagai M, Yamano Y, Brennan MB, Mora CA, Jacobson S. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann Neurol. 2001;50:807–12. doi: 10.1002/ana.10065. [DOI] [PubMed] [Google Scholar]
- 38.Nobre V, Guedes AC, Martins ML, et al. Dermatological findings in 3 generations of a family with a high prevalence of human T cell lymphotropic virus type 1 infection in Brazil. Clin Infect Dis. 2006;43:1257–63. doi: 10.1086/508177. [DOI] [PubMed] [Google Scholar]
- 39.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004:4·7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biddison WE, Kubota R, Kawanishi T, et al. Human T cell leukemia virus type I (HTLV-I)-specific CD8+ CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. J Immunol. 1997;159:2018–25. [PubMed] [Google Scholar]


