Abstract
Ani s 7 is currently the most important excretory/secretory (ES) Anisakis simplex allergen, as it is the only one recognized by 100% of infected patients. The allergenicity of this molecule is due mainly to the presence of a novel CX17–25CX9–22CX8CX6 tandem repeat motif not seen in any previously reported protein. In this study we used this allergen as a model to investigate how ES allergens are recognized during Anisakis infections, and the usefulness of a recombinant fragment of Ani s 7 allergen (t-Ani s 7) as a marker of true Anisakis infections. The possible antigenic relationship between native Ani s 7 (nAni s 7) from Anisakis and Pseudoterranova decipensantigens was also investigated. Our results demonstrate that nAni s 7 is secreted and recognized by the immune system of rats only when the larvae are alive (i.e. during the acute phase of infection), and that this molecule is not present in, or is antigenically different from, Pseudoterranova allergens. The t-Ani s 7 polypeptide is a useful target for differentiating immunoglobulin E antibodies induced by true Anisakis infections from those induced by other antigens that may cross-react with Anisakis allergens, including P. decipiens. The results also support the hypothesis that the Ani s 7 major allergen does not participate in maintaining the antigenic stimulus during chronic infections.
Keywords: allergen, Ani s 7, Anisakis, IgE, Pseudoterranova
Introduction
Anisakiasis (= Anisakiosis) is an important disease produced by ingestion of raw or undercooked fish infected with third-stage (L3) Anisakis spp. larvae [1]. Since the first case reported by van Thiel et al. in 1960 [2], thousands of cases of human anisakiosis have been reported worldwide, although infections occur mainly in Japan and some European countries [3–5]. Infection by Anisakis induces stimulation of both T helper type 1 (Th1) and Th2 responses, and provokes a strong specific immune response by all antibody isotypes [immunoglobulin (Ig)E, IgG, IgA and IgM][5]. The most frequent symptom reported in acute infections is abdominal pain of variable location and intensity, which follows a variable incubation period of 4–48 h after infection by the parasite [6]. However, more than 10% of gastrointestinal anisakiasis may be accompanied by allergic symptoms [7–9], ranging from intermediate urticaria to severe anaphylaxis [5]. In addition, several studies have detected the presence of anti-Anisakis IgE antibodies in more than 10% of healthy subjects [10], suggesting the existence of a large number of infected patients who do not develop clinical symptoms.
Unlike in marine mammals, Anisakis larvae do not usually reach the adult stage in humans and the larvae die during the course of 3 weeks after infection [11]. Consequently, it is expected that the immune response against Anisakis allergens from third- and/or fourth-stage larvae (L3/L4) occurs in response to two consecutive antigenic stimuli: (i) the excretory/secretory (ES) and cuticle antigens while the larvae is alive; and (ii) the cuticle and protease-resistant somatic and ES antigens, after the larvae die. Previous studies have shown that the Anisakis ES allergens are the most clinically important, as they are targeted by most of the anti-Anisakis IgE antibodies induced during infections by this parasite [12]. However, there are no data available to indicate whether these antigens stimulate the immune system only during the short period of time after infection in which the larvae remain alive, or also after the death of the larvae. Such data may be important in order to establish which Anisakis allergens cause the pathological changes observed during the acute and chronic stages of the infection.
Another characteristic of Anisakis-induced allergy is that either the ES allergens [12] or the allergens stored inside dead larvae [13–15] are not able to induce clinical symptoms when administered to previously sensitized patients by the oral route. These results suggest that: (i) immunization against clinically relevant Anisakis allergens is only possible during the course of an active infection; and (ii) that these allergens are destroyed during the digestion process. Accordingly, it would be expected that one or more Anisakis ES-specific allergens could be used as a marker of Anisakis infections, which is of clinical relevance to allow the differentiation of IgE antibodies induced by the parasite (true infection) from others induced by cross-reacting allergens frequently present in nature (false infection).
Several ES and somatic Anisakis allergens have been cloned and characterized in recent years (Allergen Nomenclature Sub-Committee; http://www.allergen.org). Among these, Ani s 1 (21 kDa) and Ani s 7 (139 kDa) are probably the most important major ES allergens described, as they were reported to be recognized by 85% and 100% of infected patients respectively [16,17]. Ani s 2 (paramyosin; 100 kDa) and Ani s 3 (tropomyosin; 41 kDa) are somatic A. simplex allergens that cross-react with other common allergens [18,19]. Finally, other cloned A. simplex allergens such as Ani s 4 (cystatin, 9 kDa), Ani s 6 (serine protease inhibitor, 7 kDa) and three SXP/RAL-2 family proteins, named Ani s 5 (15 kDa), Ani s 8 (15 kDa) and Ani s 9 (14 kDa), are all minor ES allergens recognized by fewer than 50% of infected patients [20–23].
Given the high potential of the Ani s 7 ES allergen as a target for Anisakis-induced allergy, and for immunodiagnosis of human anisakiasis [17], we used this allergen as a model to investigate how ES allergens are recognized during the Anisakis infections, and to evaluate the usefulness of a recombinant fragment of the Ani s 7 allergen (t-Ani s 7) as an indicator of true Anisakis infections.
Material and methods
Animals
Male Wistar rats weighing approximately 250 g at the start of experiments and Balb/c mice (4–6 months old) were obtained from the animal facilities of the University of Santiago de Compostela. All animals were provided with water and food ad libitum, and adequate measures were taken to minimize pain or discomfort in experimental animals. All experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC and RD 1201/2005) on the protection of experimental animals, and approved by the Ethics Committee of the University of Santiago de Compostela.
Obtainment of the parasites and parasite crude extracts
Third-stage larvae (L3) of A. simplex were extracted manually from the viscera and body cavity of blue whiting (Micromesistius poutassou) purchased from a local market. Pseudoterranova decipiens L3 were extracted manually from fillets of naturally infected monkfish (Lophius piscatorius) and washed thoroughly in water. The larvae were then washed with phosphate-buffered saline (PBS; pH 7·2) and their integrity was checked under a stereomicroscope. Crude soluble extract (CE) from third-stage larvae (L3) of P. decipiens and A. simplexwere prepared as described previously by Perteguer and Cuéllar [24] and Lorenzo et al.[25] respectively. The native Ani s 7 (nAni s 7) allergen was captured in enzyme-linked immunosorbent assay (ELISA) plates (see below) with monoclonal antibody (mAb) UA3.
Monoclonal antibodies
The mAb UA3 (IgG1/k) recognizing the Ani s 7 allergen [17,26] was purified from UA3 cell culture supernatants by affinity chromatography on protein-G Sepharose (Sigma-Aldrich, Madrid, Spain), according to the supplier's instructions.
Obtainment of Anisakis simplex Ani s 7 major allergen
An Anisakis cDNA library was screened with mAb UA3, and a cDNA clone (rAni s 7) encoding a 1096-amino acid fragment of Ani s 7 was identified. The encoded sequence (rAni s 7) has no known sequence homology, but as a distinctive characteristic it comprised 19 repeats of a novel CX17–25CX9–22CX8CX6 tandem repeat motif not seen in any previously reported protein sequence. An internal 435Met-713Arg fragment of the rAni s 7 (t-Ani s 7) was expressed in Escherichia coli[17] and used as target in an indirect ELISA (see below).
Sera from experimental infections and immunizations
The anti-Anisakis sera for kinetic studies (primary infection) were obtained from 16 Wistar rats inoculated intraperitoneally with either five live L3 larvae (eight rats; group 1) or five dead L3 larvae, killed by freezing at −30°C for 12 h (eight rats; group 2). Blood samples of the anaesthetized rats were collected by careful bleeding from the ophthalmic plexus vein with a capillary tube on day 5 before inoculation, and on days 8, 15, 22, 29, 36, 43, 63, 70, 75 and 90 post-infection (p.i.). On day 115 p.i., the Wistar rats from group 2 were reinoculated intraperitoneally with five L3 A. simplex dead larvae (four rats) or with five live larvae (four rats), and the animals were bled on days 3, 7, 37 and 65 post-reinfection, when all animals were exsanguinated by cardiac puncture. All the sera from the experimental animals were stored at −80°C until assayed.
For cross-reactivity experiments with P. decipiens, BALB/c mice were immunized by intramuscular injection of a single dose of 1 ml of A. simplexor P. decipiens CE antigens in Freund's complete adjuvant (1 mg/ml in final volume) in the quadriceps muscle at 0·5 cm above the knee. The mice were bled under general anaesthesia from the retro-orbital plexus 30 days after immunization and the sera obtained were stored at −80°C until assayed.
The ELISA methods to determine kinetics of anti-Anisakis antibodies
Coupling of ELISA plates with antigens and antibodies
Parasite-specific antibody isotypes were determined by indirect ELISA and by UA3-capture ELISA (UA3-ELISA) methods. For indirect ELISAs, 96-well microtitre plates were coupled overnight at 4°C with 100 µl of PBS containing 5 µg/ml of Anisakis CE antigen or 100 µl of 0·1 M Tris buffer pH 10·5 containing 0·6 µg/ml of recombinant t-Ani s 7 antigen, washed three times with PBS, and then blocked for 1 h at 37°C with 200 µl of Tris-buffered saline containing 0·2% Tween 20 (TBS-T) and 1% dry skimmed milk (TBS-T-SM), or with 200 µl of TBS-T containing 1% bovine serum albumin (BSA) (TBS-T-BSA) when biotinylated antibodies were used.
For the UA3-capture ELISA, the plates were coated overnight with UA3 purified Ig (1·4 µg/well) in PBS, and washed and blocked as above. The plates were then aspirated and 50 µg of A. simplexCE in 100 µl of TBS-T-SM or TBS-T-BSA was added to each well and incubated for 1 h at 37°C.
Specific incubations
For determination of IgE and IgA isotypes, previously coupled ELISA plates were incubated for 2 h at 37°C with experimental rat sera (tested in duplicate) diluted 1:4 in TBS-T-SM (IgE) or 1:100 in TBS-T-SM (IgA). The plates were washed with TBS-T, then incubated for 1 h at 37°C with fluorescein isothiocyanate (FITC)-labelled mouse anti-rat IgE (AbD Serotec, Oxford, UK; 100 µl/well, dilution 1:500) or FITC-labelled mouse anti-rat IgA (Serotec Ltd, Oxford, UK; 100 µl/well, dilution 1:100). After further washing with TBS-T, the captured Ig-FITC was detected by incubation for 1 h at 37°C with peroxidase-labelled rabbit anti-FITC polyclonal antibodies (Abcam, Cambridge, UK; 100 µl/well, dilution 1:5000). Finally, the plates were washed again with TBS-T; buffered H2O2 and o-phenylenediamine (SigmaFast OPD; Sigma–Aldrich) were used as the substrate for peroxidase. The optical density (OD) was measured at 492 nm. A negative control serum (pooled sera obtained 5 days before inoculation with the Anisakis larvae) was included in each assay and the OD value subtracted from the corresponding values obtained for test sera.
For determination of IgM or IgG isotypes (IgG1, IgG2a, IgG2b or IgG2c), experimental rat sera diluted 1:100 in TBS-T-BSA (100 µl/well; tested in duplicate) were added to previously coupled ELISA plates and incubated for 2 h at 37°C. The plates were then washed with TBS-T and incubated for 1 h at 37°C with an appropriate dilution of the suitable biotinylated mouse anti-rat mAb in TBS-T-BSA (BD Bioscience Pharmingen, Madrid, Spain). The dilutions used were 1:100 for anti-rat IgM, 1:200 for anti-rat IgG2b, 1:400 for anti-rat IgG2a, 1:500 for anti-rat IgG2c and 1:1000 for anti-rat IgG1. At the end of the incubation period the plates were washed with TBS-T; 100 µl/well of Neutravidin™-horseradish peroxidase (Pierce Biotechnology, Rockford, USA) was applied at a dilution of 1:10 000, and the plates incubated again for 1 h at 37°C. Finally, the plates were washed with TBS-T and the reaction was revealed with buffered H2O2and o-phenylenediamine, as described above for IgE and IgA isotypes. As above, the OD value obtained for the preimmune serum was subtracted from the corresponding values obtained for test sera.
Cut-off calculations
The cut-off value for each Ig isotype was calculated as four times the standard deviation (+4) of the mean OD value obtained after individual testing of all rat sera collected 5 days before inoculation with the Anisakis larvae.
Indirect ELISA for the study of t-Ani s 7 genus specificity
The 96-well microtitre plates were reacted overnight at 4°C with 100 µl of PBS containing 5 µg/ml of Anisakis or Pseudoterranova CE antigens, or 0·6 µg/ml of recombinant t-Ani s 7 antigen in 0·1 M Tris buffer pH 10·5. Plates were then washed with TBS-T and non-specific binding sites were blocked by incubation (1 h at 37°C) with TBS-T-SM. After further washing, 100 µl of a 1:100 dilution of mouse immune sera (tested in quadruplicate) was added and the plates were incubated for 2 h at 37°C. After another washing step, specific IgG1 antibodies were detected with 100 µl/well of peroxidase-labelled goat anti-mouse IgG1 polyclonal antibodies (Caltag Laboratories, San Francisco, CA, USA; dilution 1:1000). The reaction was developed with buffered H2O2 and o-phenylenediamine and read at 492 nm, as described above. Sera from non-immunized BALB/c mice were used as negative controls.
Statistical analyses
Results from different groups were compared by a paired t-test. Differences were considered significant at P < 0·05. Data analysis was performed with the GraphPad InStat program (GraphPad Software Inc., San Diego, CA, USA).
Results
In vivo recognition of ES Anisakis allergens
To investigate how the Anisakis ES antigens are recognized in natural infections, Wistar rats were inoculated intraperitoneally with dead or live Anisakis L3 larvae and the kinetics of specific antibodies produced against three target antigens: t-Ani s 7 (internal 435Met-713Arg recombinant fragment of the nAni s 7) [17], nAni s 7 and Anisakis CE were tested. The antibody levels were measured by indirect ELISA with immobilized t-Ani s 7, by capture ELISA with immobilized mAb UA3 capturing nAni s 7 from Anisakis CE and by an indirect ELISA containing immobilized Anisakis CE. As shown in Fig. 1, the t-Ani s 7 antigen was recognized only by IgE antibodies induced by live larvae, and the IgE response peaked on day 30 p.i. When nAni s 7 was used as target, the IgE response induced by live larvae also peaked on day 30 p.i., but compared with t-Ani s 7 the IgE response induced by dead larvae was low and peaked on day 42 post-immunization. As far as the IgE response against Anisakis CE is concerned, a discrete response was induced by either live or dead larvae covering the entire observation period from the second week of infection/immunization. The pattern of the anti-Anisakis IgA antibody response was very similar to that obtained for IgE, except that: (i) the maximum response peaked later (at about 2 months after infection); (ii) there was very little IgA response with t-Ani s 7 from the animals immunized with dead larvae; and (iii) the anti-t-Ani s 7 IgA antibodies decreased more slowly than IgE antibodies (Fig. 1). Considering IgM antibodies, good responses against t-Ani s 7 and nAni s 7 were observed only in sera from infected animals. This response peaked by day 21 after infection and then decreased quickly. Finally, the levels of anti-CE IgM antibodies were low throughout the entire observation period with rats inoculated either with live or dead L3 Anisakis larvae (Fig. 1).
Fig. 1.
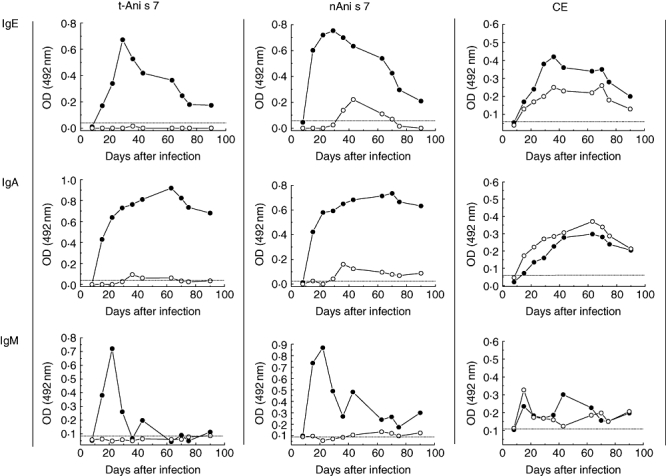
Kinetics of immunoglobulin (Ig)E, IgA and IgM antibodies induced in rats after intraperitoneal inoculation with live (closed circles) or dead (open circles) L3 Anisakis larvae. Enzyme-linked immunosorbent assays were carried out with recombinant Ani s 7 allergen (t-Ani s 7), native Ani s 7 (nAni s 7) or crude extract (CE) as target antigens. Each serum sample was tested in duplicate. Cut-off values are represented by a horizontal dashed line.
The data in Fig. 2 show the kinetics of IgG antibody responses. Considering the four IgG rat isotypes and the three antigens assayed, we observed three main characteristics of these antibody responses: (i) high IgG1, IgG2a and IgG2b antibody responses against t-Ani s 7 antigen in infected rats, with minimal or no IgG antibodies induced against this antigen in animals immunized with dead larvae; (ii) high IgG1 levels against nAni s 7 antigen induced with either live or dead larvae; and (iii) low IgG2c responses induced by either live or dead larvae against t-Ani s 7 and nAni s 7, which contrasted with the relatively good biphasic responses obtained against the antigens contained in the Anisakis CE. In addition, after reaching maximal values, anti-t-Ani s 7 and anti-nAni s 7 IgG1 antibodies remained stable throughout the entire observation period (Fig. 2: t-Ani s 7 and nAni s 7).
Fig. 2.
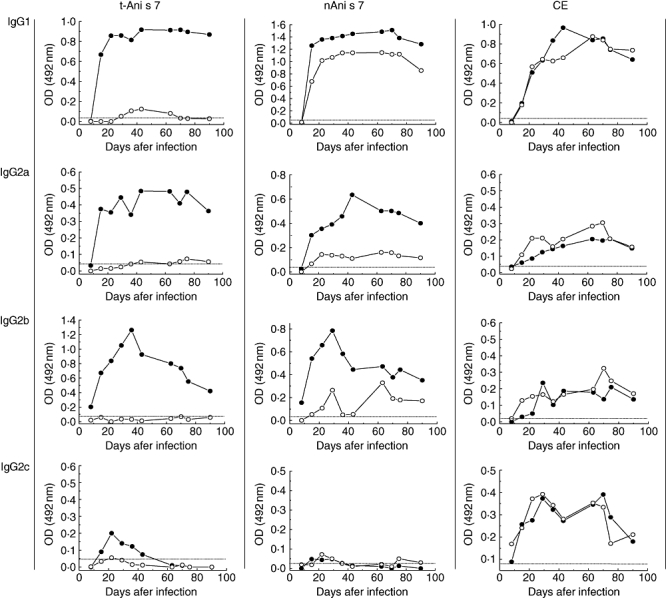
Kinetics of immunoglobulin (Ig)G (IgG1, IgG2a, IgG2b and IgG2c) antibodies induced in rats after intraperitoneal inoculation with live (closed circles) or dead (open circles) L3 Anisakis larvae. Enzyme-linked immunosorbent assays were obtained with recombinant Ani s 7 allergen (t-Ani s 7), native Ani s 7 (nAni s 7) or crude extract (CE) as target antigens. Each serum sample was tested in duplicate. Cut-off values are represented by a horizontal dashed line.
The IgE and IgG1 secondary antibody response induced against t-Ani s 7 in rats inoculated previously with dead larvae and then reinoculated with either dead or live larvae was also investigated (Fig. 3). A similar pattern to that obtained in the primary response was observed, with good levels of IgE and IgG1 induced by live L3 Anisakis larvae and poor responses induced in rats reinoculated with dead larvae.
Fig. 3.
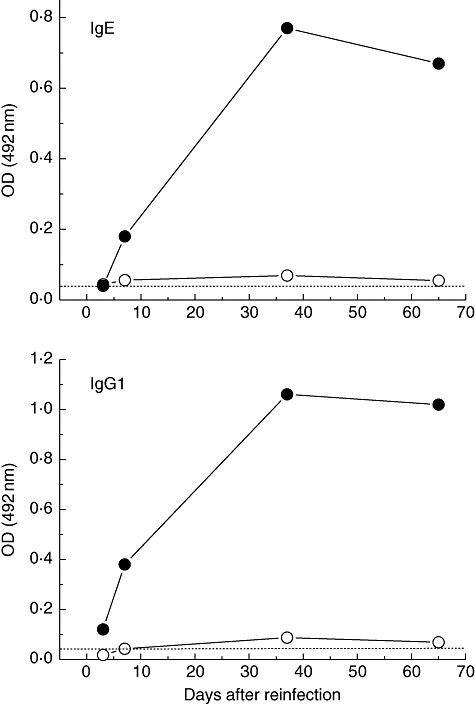
Kinetics of immunoglobulin (Ig)G1, and IgE antibodies induced in rats after intraperitoneal challenge (secondary response) with live (closed circles) or dead (open circles) L3 Anisakis larvae. All the enzyme-linked immunosorbent assays were carried out with recombinant Ani s 7 allergen (t-Ani s 7) as target antigen. Each serum sample was tested in duplicate. Cut-off values are represented by a horizontal dashed line.
Testing of the genus specificity of the t-Ani s 7 polypeptide
The above results, showing that the antibodies induced by the antigens contained in dead Anisakis larvae did not recognize the t-Ani s 7 polypeptide, suggest strongly that: (i) the antigenic stimulation by ES antigens ceases after the infecting larvae die; and (ii) the t-Ani s 7 polypeptide does not have any epitopes in common with other Anisakis antigens, and thus may be a candidate target for use as a specific marker of Anisakis infections. However, a doubt arises as to whether this polypeptide is sufficiently specific to enable differentiation between infections provoked by Anisakis or by other members of the Anisakidae family, notably P. decipiens. To address this question, recognition of the t-Ani s 7 polypeptide by serum IgG1 antibodies from mice immunized with CEs from A. simplex and P. decipiens was compared by indirect ELISA. The CE antigens from A. simplex and P. decipiens were also used as positive controls for cross-reactivity in ELISA. The results showed that the antibody response induced by Pseudoterranova and Anisakis CEs were cross-reactive, whereas only IgG1 antibodies from mice immunized with A. simplex CE were recognized in indirect ELISA with the t-Ani s 7 antigen as target (P < 0·0001) (Fig. 4).
Fig. 4.
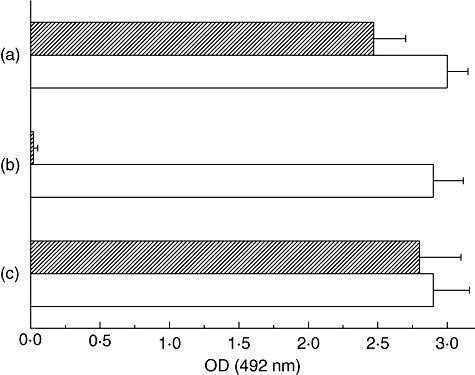
Analysis of cross-reactivity between crude extract (CE) antigens of Anisakis simplex and Pseudoterranova decipiens. Mice were injected intramuscularly with CE antigens from P. decipiens (crossed bars) or A. simplex (white bars) and their IgG1 serum antibodies were tested (in duplicate) by enzyme-linked immunosorbent assay against P. decipiens CE antigen (a), recombinant Ani s 7 allergen (t-Ani s 7) (b) and A. simplex CE antigen (c). Values are represented as mean optical density plus standard deviation.
Discussion
In this study we have shown that rats inoculated intraperitoneally with dead Anisakis larvae failed to produce significant amounts of IgE or other antibody isotypes against the highly specific Anisakis t-Ani s 7 recombinant polypeptide, comprising the internal 435Met-713Arg of the nAni s 7 major allergen [17]. Indeed, these rats were also unable to produce significant amounts of anti-t-Ani s 7 antibodies after reinoculation with more dead L3 Anisakis larvae. Together these findings suggest strongly that: (i) the antigenicity of the nAni s 7 molecule, or at least that corresponding to the t-Ani s 7 fragment, is destroyed inside the dead larvae; and (ii) after the larvae die, there are no remaining Anisakis antigens that share the same epitopes with the t-Ani s 7 polypeptide. These results are consistent with a scenario in which Ani s 7, and probably other relevant allergens produced and accumulated in the excretory cell of the parasite while it remains alive, are cleaved inside the L3 dead larvae by endogenous proteases until the outer cuticle becomes damaged. Accordingly, we hypothesize that nAni s 7, and probably other Anisakis ES allergens, stimulate the immune system only during the short time during which the infecting larvae remain alive (i.e. during the acute phase of infection), whereas other Anisakis antigens (e.g. protease-resistant somatic and cuticle antigens) probably maintain the antigenic stimulus during the chronic stage of anisakiasis. This hypothesis is consistent with previous findings that have shown that reactivity against Anisakis larvae is maintained during the chronic phase of anisakiasis when the larvae have already been destroyed and only cuticle debris remains [27].
The present finding that no antigen present in dead Anisakis larvae was capable of inducing antibodies against the t-Ani s 7 recombinant sequence is of clinical relevance, as it confirms that all epitopes contained in this sequence are specific to the nAni s 7 ES allergen. This suggests the possibility of using this polypeptide to differentiate patients with true acute or chronic Anisakis infections (i.e. with a positive IgE response against t-Ani s 7) from those with false anti-Anisakis IgE antibodies induced by other antigens that may cross-react with some Anisakis allergens. Furthermore, as the anti-Anisakis antibody response against the t-Ani s 7 polypeptide was genus-specific (see Fig. 4), differentiation between Anisakis and Pseudoterranova infection may also be possible. Although human Anisakis infections are much more prevalent, pseudoterranovosis is currently arising as an emerging infection in some countries where consumption of raw fish in different dishes such as sushi, sashimi and ceviche is becoming popular [28,29].
In contrast to the failure of the Anisakis dead larvae to induce a good antibody response against the polypeptide t-Ani s 7 in rats, dead larvae were able to induce a strong IgG1 response and slight IgE, IgA, IgG2b and IgG2c responses when nAni s 7 was used as target antigen in capture ELISA. These antibodies were probably produced by other Anisakis protease-resistant antigens (e.g. somatic and/or cuticle antigens) with some common epitopes, e.g. O-glycans, which we have demonstrated previously to be involved in the formation of cross-reacting anti-nAni s 7 antibodies [30]. Alternatively, this antibody response may be due to the presence of one or more protease-resistent epitopes in the nAni s 7 allergen, located in a different part of that corresponding to the t-Ani s 7. Using CE Anisakis antigens as target (Fig. 1: CE and Fig. 2: CE), we observed that dead Anisakis larvae were able to induce a very similar antibody response to that induced by the live larvae, which indicates that, unlike Ani s 7, some Anisakis antigens are resistant to enzymatic cleavage inside the larvae, and are consequently able to stimulate the host immune system after the larvae are destroyed.
Previous studies on Anisakis-induced allergy have suggested that the adverse reactions observed in some patients after ingestion of well-cooked or even canned fish could be related to Anisakis allergens that are resistant to heat and/or pepsin treatments [22,31,32]. However, these studies did not establish whether the IgE antibodies reacting with heat/enzyme resistant Anisakis allergens were induced by direct contact with these allergens at a gastrointestinal level (i.e. without previous infection), or whether they were produced as a consequence of previous infection by the parasite. The use of the t-Ani s 7 polypeptide to detect true Anisakis infections may help allergologists to solve this question, which is important in order to establish preventive measures for Anisakis-induced allergy.
It was reported recently that Anisakis infections in rodents are similar to those in humans in terms of antibody and cytokine production [33,34]. As in human cases of gastroallergic anisakiasis [35], in the present study we observed that anti-t-Ani s 7 and anti-nAni s 7 IgE antibodies induced by live Anisakis larvae peaked at about day 30 p.i. and then decreased slowly over the course of 2 months (Fig. 1: t-Ani s 7 and nAni s 7). However, IgG1 antibodies were maintained at maximal values from day 20 p.i. throughout the observation period (Fig. 2: t-Ani s 7 and nAni s 7). This contrasted with our previous observations that anti-t-Ani s 7 antibodies are mainly of the IgE class in humans, and that IgE levels often remain high for several years (unpublished results). This may reflect a different antibody response to t-Ani s 7 in humans, but more probably indicates that most positive human cases of anisakiasis are in fact reinfections.
In summary, we conclude that the t-Ani s 7 polypeptide fragment of the Ani s 7 major Anisakis allergen is genus-specific and that IgE antibodies against this polypeptide can be induced only when the living larva inoculates it into host tissues. Consequently, the specific detection of IgE antibodies against t-Ani s 7 appears to be an excellent way to differentiate IgE antibodies induced in true Anisakis infections from IgE antibodies induced by other common allergens such as those present in cockroaches, chironomids and dustmites, which may display cross-reactivity with Anisakis antigens such as, for example, paramyosin (Ani s 2) and tropomyosin (Ani s 3) [18,19,36]. These aspects may be of interest not only for the specific serodiagnostic of human anisakiosis but also to enable investigation of which Anisakis antigens are clinically relevant, and whether or not there exist other mechanisms of sensitization, apart from infection, in Anisakis-induced allergy.
Acknowledgments
The present study was supported by grants SAF2002-04057 (Ministerio de Sanidad y Consumo, Spain) and MPY 1337/01 (Instituto de Salud Carlos III, Spain). The nucleotide and deduced amino acid sequences of nAni s 7 are available at GenBank (Accession no.: EF158010). Ana María Anadón is supported by a fellowship from Xunta de Galicia (Program María Barbeito).
Disclosure
All authors declare that there are no conflicts of interest.
References
- 1.Sakanari JA, Mckerrow JH. Anisakiasis. Clin Microbiol Rev. 1989;2:278–84. doi: 10.1128/cmr.2.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Thiel PH, Kuipers FC, Roskam TH. A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop Geogr Med. 1960;12:97–113. [PubMed] [Google Scholar]
- 3.Chai JY, Darwin Murrell K, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35:1233–54. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Petithory JC. New data on anisakiasis. Bull Acad Natl Med. 2007;191:53–65. [PubMed] [Google Scholar]
- 5.Audícana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008;21:360–79. doi: 10.1128/CMR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daschner A, Pascual CY. Anisakis simplex: sensitization and clinical allergy. Curr Opin Allergy Clin Immunol. 2005;5:281–5. doi: 10.1097/01.all.0000168795.12701.fd. [DOI] [PubMed] [Google Scholar]
- 7.Repiso Ortega A, Alcántara Torres M, González de Frutos C, et al. Gastrointestinal anisakiasis. Study of a series of 25 patients. Gastroenterol Hepatol. 2003;26:341–6. doi: 10.1016/s0210-5705(03)70370-7. [DOI] [PubMed] [Google Scholar]
- 8.Ohtaki H, Ohtaki R. Clinical manifestation of gastric anisakiasis. In: Ishikura H, Namiki M, editors. Gastric anisakiasis in Japan. Epidemiology, diagnosis and treatment. Tokyo: Springer-Verlag; 1989. pp. 37–46. [Google Scholar]
- 9.Kakizoe S, Kakizoe H, Kakizoe K, et al. Endoscopic findings and clinical manifestation of gastric anisakiasis. Am J Gastroenterol. 1995;90:761–3. [PubMed] [Google Scholar]
- 10.Puente P, Anadón AM, Rodero M, Romarís F, Ubeira FM, Cuéllar C. Anisakis simplex: the high prevalence in Madrid (Spain) and its relation with fish consumption. Exp Parasitol. 2008;118:271–4. doi: 10.1016/j.exppara.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Jones RE, Deardorff TL, Kayes SG. Anisakis simplex: histopathological changes in experimentally infected CBA/J mice. Exp Parasitol. 1990;70:305–13. doi: 10.1016/0014-4894(90)90112-p. [DOI] [PubMed] [Google Scholar]
- 12.Baeza ML, Rodríguez A, Matheu V, et al. Characterization of allergens secreted by Anisakis simplex parasite: clinical relevance in comparison with somatic allergens. Clin Exp Allergy. 2004;34:296–302. doi: 10.1111/j.1365-2222.2004.01883.x. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Gómez A, Moreno-Ancillo A, López-Serrano MC, et al. Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol Res. 2004;93:378–84. doi: 10.1007/s00436-004-1085-9. [DOI] [PubMed] [Google Scholar]
- 14.García F, Blanco JG, Garcés M, Juste S, Fuentes M, Herrero D. Freezing protects against allergy to Anisakis simplex. J Investig Allergol Clin Immunol. 2001;11:49–52. [PubMed] [Google Scholar]
- 15.Sastre J, Lluch-Bernal M, Quirce S, et al. A double-blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy. 2000;55:560–4. doi: 10.1034/j.1398-9995.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 16.Moneo I, Caballero ML, Gómez F, Ortega E, Alonso MJ. Isolation and characterization of a major allergen from the fish parasite Anisakis simplex. J Allergy Clin Immunol. 2000;106:177–82. doi: 10.1067/mai.2000.106732. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez E, Anadón AM, García-Bodas E, et al. Novel sequences and epitopes of diagnostic value derived from the Anisakis simplex Ani s 7 major allergen. Allergy. 2008;63:219–25. doi: 10.1111/j.1398-9995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 18.Johansson E, Aponno M, Lundberg M, van Hage-Hamsten M. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy. 2001;56:660–6. doi: 10.1034/j.1398-9995.2001.00798.x. [DOI] [PubMed] [Google Scholar]
- 19.Guarneri F, Guarneri C, Benvenga S. Cross-reactivity of Anisakis simplex: possible role of Ani s 2 and Ani s 3. Int J Dermatol. 2007;46:146–50. doi: 10.1111/j.1365-4632.2006.03091.x. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Mahillo AI, González-Muñoz M, Gómez-Aguado F, et al. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int J Parasitol. 2007;37:907–17. doi: 10.1016/j.ijpara.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Pérez R, Moneo I, Rodríguez-Mahillo A, Caballero ML. Cloning and expression of Ani s 9, a new Anisakis simplex allergen. Mol Biochem Parasitol. 2008;159:92–7. doi: 10.1016/j.molbiopara.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Caballero ML, Moneo I, Gómez-Aguado F, Corcuera MT, Casado I, Rodríguez-Pérez R. Isolation of Ani s 5, an excretory-secretory and highly heat-resistant allergen useful for the diagnosis of Anisakis larvae sensitization. Parasitol Res. 2008;103:1231–3. doi: 10.1007/s00436-008-1105-2. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi Y, Ishizaki S, Shimakura K, Nagashima Y, Shiomi K. Molecular cloning and expression of two new allergens from Anisakis simplex. Parasitol Res. 2007;100:1233–41. doi: 10.1007/s00436-006-0396-4. [DOI] [PubMed] [Google Scholar]
- 24.Perteguer MJ, Cuéllar C. Isotype-specific immune responses in murine experimental anisakiasis. Zentralbl Veterinarmed B. 1998;45:603–10. doi: 10.1111/j.1439-0450.1998.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo S, Iglesias R, Leiro J, et al. Usefulness of currently available methods for the diagnosis of Anisakis simplex allergy. Allergy. 2000;55:627–33. doi: 10.1034/j.1398-9995.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias R, Leiro J, Santamarina MT, Sanmartín ML, Ubeira FM. Monoclonal antibodies against diagnostic Anisakis simplex antigens. Parasitol Res. 1997;83:755–61. doi: 10.1007/s004360050335. [DOI] [PubMed] [Google Scholar]
- 27.Oshima T. Anisakis and anisakiasis in Japan and adjacent area. In: Morishita K, Komiya Y, Matsubayashi H, editors. Progress of medical parasitology in Japan. Tokyo: Meguro Parasitological Museum; 1972. pp. 301–93. [Google Scholar]
- 28.Torres P, Jercic MI, Weitz JC, Dobrew EK, Mercado RA. Human pseudoterranovosis, an emerging infection in Chile. J Parasitol. 2007;93:440–3. doi: 10.1645/GE-946R.1. [DOI] [PubMed] [Google Scholar]
- 29.Jofré ML, Neira OP, Noemí HI, Cerva CJL. Pseudoterranovosis and sushi. Rev Chilena Infectol. 2008;25:200–5. [PubMed] [Google Scholar]
- 30.Lorenzo S, Romarís F, Iglesias R, et al. O-glycans as a source of cross-reactivity in determinations of human serum antibodies to Anisakis simplex antigens. Clin Exp Allergy. 2000;30:551–9. doi: 10.1046/j.1365-2222.2000.00758.x. [DOI] [PubMed] [Google Scholar]
- 31.Caballero ML, Moneo I. Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol Res. 2004;93:248–51. doi: 10.1007/s00436-004-1099-3. [DOI] [PubMed] [Google Scholar]
- 32.Audícana MT, Ansotegui IJ, de Corres LF, Kennedy MW. Anisakis simplex: dangerous – dead and alive? Trends Parasitol. 2002;18:20–5. doi: 10.1016/s1471-4922(01)02152-3. [DOI] [PubMed] [Google Scholar]
- 33.Cho SW, Lee HN. Immune reactions and allergy in experimental anisakiasis. Korean J Parasitol. 2006;44:271–83. doi: 10.3347/kjp.2006.44.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baeza ML, Conejero L, Higaki Y, et al. Anisakis simplex allergy: a murine model of anaphylaxis induced by parasitic proteins displays a mixed Th1/Th2 pattern. Clin Exp Immunol. 2005;142:433–40. doi: 10.1111/j.1365-2249.2005.02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daschner A, Cuéllar C, Sánchez-Pastor S, Pascual CY, Martín-Esteban M. Gastro-allergic anisakiasis as a consequence of simultaneous primary and secondary immune response. Parasite Immunol. 2002;24:243–51. doi: 10.1046/j.1365-3024.2002.00458.x. [DOI] [PubMed] [Google Scholar]
- 36.Weiler CR. Anisakis simplex and cross-reacting antigens. Int J Dermatol. 2007;46:224–5. doi: 10.1111/j.1365-4632.2006.03090.x. [DOI] [PubMed] [Google Scholar]


