Abstract
Synthetic oligodeoxynucleotides (ODN) expressing ‘suppressive’ TTAGGG motifs down-regulate a variety of proinflammatory and T helper type 1 (Th1)-mediated pathological immune responses. The ability of the archetypal suppressive ODN A151 to inhibit ocular inflammation was examined in two murine models: experimental autoimmune uveitis, induced by immunization with a retinal antigen, interphotoreceptor retinoid-binding protein (IRBP) and adoptively transferred ocular inflammation, induced by transferring Th1 cells specific to hen egg lysozyme (HEL) into recipient mice that express HEL in their eyes. A151 treatment suppressed the inflammation in both models. In addition, A151 inhibited IRBP-specific cytokine production and lymphocyte proliferation in mice immunized with IRBP. These findings suggest that suppressive ODN affects both afferent and efferent limbs of the immunopathogenic process and may be of use in the treatment of autoimmune ocular inflammation.
Keywords: EAU, immunopathogenic process, immunosuppression, ODN
Introduction
DNA has multiple and complex effects on the immune system. Unmethylated cytosine-guanine dinucleotide (CpG) motifs in bacterial DNA trigger an immune response characterized by the production of proinflammatory and T helper type 1 (Th1) cytokines [1–4]. In contrast, the telomeric regions of mammalian DNA contain ‘suppressive’ TTAGGG elements that inhibit immune activation [5–7]. Synthetic oligodeoxynucleotides (ODN) containing repetitive TTAGGG motifs, patterned after those present in mammalian telomeres, mimic this effect [8]. Studies examining suppressive TTAGGG ODNs established that these molecules could down-regulate the production of proinflammatory and Th1 cytokines [9] and inhibit immunopathogenic processes such as collagen-induced arthritis [10], lipopolysaccharide (LPS)-induced toxic shock [11] and lupus nephritis [12]. More recently, Ho et al.[13] reported that GpG ODNs enhance the efficiency of antigen-specific tolerizing DNA vaccine.
Intra-ocular inflammatory diseases, grouped under the term ‘uveitis’, are a major cause of vision loss in developed countries [14–16]. Included in this group are non-infectious conditions such as Behçet's disease, sarcoidosis, sympathetic ophthalmia and Vogt–Koyanagi–Harada syndrome. Available treatments for uveitic conditions are still inadequate, emphasizing the need for new and effective treatments [17,18].
The pathogenic mechanism underlying the development of uveitis, and approaches to modulate this disease, are investigated typically using animal models of experimental autoimmune uveitis (EAU) [19,20]. EAU is induced by immunization with eye-specific molecules such as S-antigen (‘arrestin’) [21,22] or interphotoreceptor retinoid-binding protein (IRBP) [23–25]. In another model, ocular inflammation is induced by adoptively transferring Th1 cells sensitized against hen egg lysozyme (HEL) into transgenic (Tg) mice that express HEL in their eyes [26,27]. Because the transferred cells are preactivated, the pathogenic mechanism in the latter model consists only of the efferent limb of the immune response.
In the present study we examined the capacity of the well-characterized suppressive ODN A151, which contains four tandem TTAGGG motifs, to inhibit the induction of ocular inflammation. A151 treatment was effective at reducing inflammation in both models, i.e. actively induced EAU by immunization with IRBP and adoptive transfer of ocular inflammation by activated Th1 cells.
Materials and methods
Mice
For the EAU model, female B10.A mice, 6–8 weeks old, were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). HEL-Tg mice, expressing membrane-bound HEL in the lens under the aA-crystallin promotor on the FVB/N background, were generated as detailed elsewhere [28]. HEL-specific T cell receptor (TCR) Tg mice, designated ‘3A9’, on the B10.BR background [29] were a gift from M. Davis (Stanford University, Stanford, CA, USA). Tg mice from each of the two lines were mated to produce (FVB/N × B10.BR) F1 hybrids, expressing either HEL in their eyes or the 3A9 TCR on their T cells. In this study, the mice expressing HEL in their eyes are designated ‘HEL-Tg’, whereas those expressing 3A9 TCR are named ‘3A9’. Only F1 hybrid mice of the two lines were used in the present study. In all adoptive transfer experiments the cells used were from 3A9 donors, whereas the recipients were HEL-Tg mice. The mice were housed in a pathogen-free facility, and all manipulations were performed in compliance with the National Institutes of Health Resolution on the Use of Animals in Research. All experimental procedures used in this study were approved by the National Eye Institute Animal Care and Use Committee, under NEI Animal Study Protocols 02-473 and 05-542.
Reagents
Whole IRBP protein was prepared from bovine retinas by concanavalin (ConA)-Sepharose affinity chromatography and fast protein liquid chromatography, as described in detail by Pepperberg et al.[30]. Phytohaemagglutinin (PHA) was purchased from Murex Diagnostics (Dartford, UK), pertussis toxin was provided by Sigma-Aldrich (St Louis, MO, USA) and purified protein derivative (PPD) was from Parke-Davis (Morris Plains, NJ, USA). Endotoxin-free suppressive ODNs and control ODNs were synthesized at the Center for Biologics Evaluation and Research (Food and Drug Administration core facility, Bethesda, MD, USA). The sequences of ODNs used in this study were 5′-TTAGGGTTAGGGTTAGGGTTAGGG-3′ (suppressive ODN: A151) and 5′-TCAAGCTTGA-3′ (control ODN: 1471).
Induction of EAU
The EAU was induced in mice by immunization with IRBP as described by Caspi et al.[31], with minor modifications. Briefly, B10.A mice were immunized with 40 µg IRBP emulsified in complete Freund's adjuvant (CFA) containing 2·5 mg/ml Mycobacterium tuberculosis H37RA. The emulsion was injected subcutaneously into the base of the tail and both thighs in a total volume of 0·2 ml. In addition, the mice were injected intraperitoneally (i.p.) with 0·25 µg pertussis toxin. Immunized mice were euthanized on day 14 post-immunization. Eyes were prepared for histological examination, while draining lymph nodes were collected for assessment of the cellular immune response.
Adoptive transfer of ocular inflammation
Adoptively transferred ocular inflammation was carried out as described in detail elsewhere [26,27]. Briefly, purified CD4 cells from the spleen and lymph nodes of 3A9 mice were stimulated in culture for 3 days with HEL in the presence of a Th1 polarizing cocktail, i.e. interleukin (IL)-12 and antibody against IL-4. Later, the cells were incubated with IL-2 for 4 days and then activated again with HEL and the Th1 polarizing cocktail. Reactivated Th1 cells were injected into HEL-Tg mice via the tail vein at the numbers indicated. Recipient mice were euthanized 7 days post-cell injection and their eyes were prepared for histological examination.
Treatment with the ODNs
Suppressive or control ODN, or phosphate-buffered saline (PBS), were administered i.p. at 300 µg in 0·2 ml per mouse on days 0, 3, 7 and 10, or on days 7, 10 and 12, as indicated, in mice developing EAU, or on days 0 and 3 in recipients of Th1 cells.
Assessment of disease
Enucleated eyes were fixed in 4% glutaraldehyde for 30 min and stored in 4% phosphate-buffered formaldehyde until processing. Eye tissues were embedded in methacrylate, and stained with haematoxylin and eosin. Stained sections were examined in a masked fashion by one of us, using score systems of severity of 0–4 for EAU or 0–9 for adoptive transfer, as detailed by Chan et al.[32] and Kim et al.[27] respectively.
Lymphocyte proliferation assay
Draining lymphocytes of mice immunized with IRBP were collected 14 days post-immunization, pooled within each group and cultured in triplicate in flat-bottomed 96-well plates. Cultures consisted of 4 × 105 cells in RPMI-1640 medium, supplemented with HL-1 (BioWhittaker, Walkersville, MD, USA), 2-mercaptoethanol (50 µM), methyl-α-D-mannoside (20 mg/ml) (Aldrich-Sigma, St Louis, MO, USA) and antibiotics, with or without stimulants, as indicated. As positive control, lymphocytes were cultured with PHA (at 1 µg/ml) to evaluate cellular viability and proliferative capacity. Following incubation for 72 h, the cultures were pulsed with [3H]-thymidine (0·5 µCi/10 µl/well) for an additional 16 h. The incorporated radioactivity was measured by a MicroBeta TriLux scintillation counter (PerkinElmer Life Science, Boston, MA, USA). Data are presented as mean delta counts per minute (Δcpm; cpm in stimulated cultures minus cpm in unstimulated cultures).
Cytokine production
Lymphocytes prepared as described above were cultured in 24-well plates at 5 × 106 cells per well in 1 ml of the above medium, with or without stimulants, as indicated. To examine cellular viability and response, lymphocytes were cultured with PHA in each group. Supernatants were collected after incubation for 48 h, and the level of cytokines was determined by Pierce Chemical Co. (Woburn, MA, USA) using the Multiplex SearchLight™ Arrays technology. In certain experiments, as indicated, the levels of IL-10, IL-17 and IL-22 were measured by enzyme-linked immunosorbent assay (ELISA) kits, purchased from R&D Systems (Minneapolis, MN, USA).
Statistical analysis
Statistical analysis of histological scores was performed by Mann–Whitney U-test, with each mouse (the average of both eyes) being treated as one statistical event. For EAU experiments, four or five mice were tested per group and the data, presented as mean ± standard error of the mean (s.e.m.), are compiled from independent experiments, as indicated. For adoptively transferred inflammation experiments, groups of three mice were used in each experiment and data are a composition of three separate experiments. Lymphocyte proliferation data were analysed by unpaired t-test.
Results
Suppressive ODN inhibits induction of EAU
To examine the capacity of suppressive ODN A151 to inhibit the development of EAU induced by active immunization, B10.A mice were immunized with IRBP on day 0 and treated by injecting A151 i.p. on days 0, 3, 7 and 10. Control mice were treated identically with control ODN (shown previously to have neither stimulatory nor suppressive activity) or with PBS. As seen in Fig. 1a, treatment with suppressive ODN inhibited EAU development significantly (P < 0·01). The typical reduction in histological changes is shown in Fig. 1b.
Fig. 1.
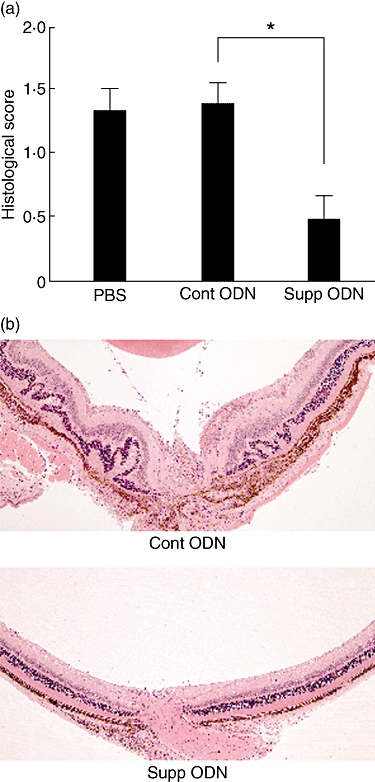
Inhibitory effects of suppressive oligodeoxynucleotide (ODN) on the development of experimental autoimmune uveitis (EAU). Groups of mice were immunized with 40 µg interphotoreceptor retinoid-binding protein (IRBP) and treated with phosphate-buffered saline, ODN A151 or control ODN on days 0, 3, 7 and 10 post-immunization. The two ODNs were administered at 300 µg/mouse. Eyes were collected on day 14 and examined for histological changes. (a) Data are the composition of five independent experiments, with four or five mice per group, each mouse being treated as one statistical event. Shown values are mean scores of pathological changes ± standard error of the mean (*P < 0·01; Mann–Whitney U-test). (b) Sections of posterior segments of eyes from representative mice treated with control (Cont) or suppressive (Supp) ODNs. Typical changes of EAU in the eye of mouse treated with control ODN include retinal folding, infiltration with inflammatory cells in the vitreous and retina. The infiltration in the retina is localized characteristically around blood vessels. On the other hand, only minimal changes are seen in the eye from the mouse treated with suppressive ODN. These changes include infiltration with inflammatory cells at the optic nerve head and vitreous (haematoxylin and eosin, original magnification ×100).
Treatment initiated on day 0 post-immunization inhibits the two phases of the immunopathogenic process, namely the ‘afferent’, or ‘prevention’ and ‘efferent’, or ‘treatment’ phases. To learn about the effect of A151 on the efferent phase only, we treated additional groups of mice on days 7, 10 and 12 post-immunization. At 7 days following immunization, T cells sensitized against IRBP are assumed to have acquired the features that characterize ‘effector’ cells, features that enable the cells to invade the eye tissues and initiate the inflammatory process. The data are summarized in Fig. 2 and show that histological changes in eyes of mice treated with A151 from day 7 were significantly lower than those in the control mice treated with the control ODN (P < 0·01).
Fig. 2.
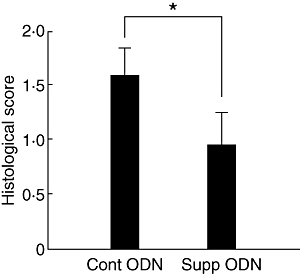
Suppressive oligodeoxynucleotide (ODN) inhibits the efferent phase of experimental autoimmune uveitis development. Groups of five mice were treated with A151 or control ODN on days 7, 10 and 12 post-immunization and their eyes were collected on day 14 for histological analysis of EAU. The data are the composition of two separate experiments. See legend for Fig. 1 for more technical detail. Values shown are mean scores of pathological changes ± standard error of the mean (*P < 0·01; Mann–Whitney U-test).
Suppressive ODN inhibits lymphocyte sensitization
Immune-mediated pathology in IRBP-induced EAU is initiated when lymphocytes, sensitized against that protein, recognize and respond to it in the retina. To determine the effect of A151 treatment on the induction of cellular immunity to IRBP, the in vitro proliferative response of lymphocytes from mice in each group treated from day 0 was examined. Lymphocytes from mice treated with PBS, control and suppressive ODNs showed high levels of proliferative response against PHA, and there was no significant difference in the responses among these three groups (Fig. 3a). In contrast, as seen in Fig. 3b, the proliferation of cells from the draining lymph nodes of mice treated with suppressive ODN was reduced significantly when tested with IRBP at 3 µg/ml (*P < 0·05) and at 30 µg/ml (**P < 0·01) when compared with those from control animals.
Fig. 3.
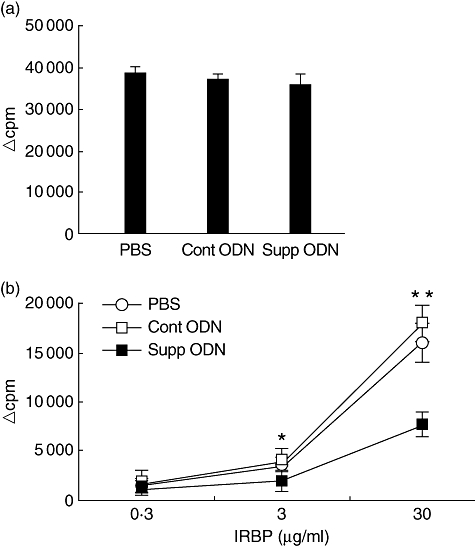
Treatment with suppressive oligodeoxynucleotide (ODN) inhibits interphotoreceptor retinoid-binding protein (IRBP)-dependent lymphocyte proliferation. Draining lymph node cells from mice treated as described in the legend for Fig. 1 were collected on day 14, pooled, and their proliferative response to phytohaemagglutinin (PHA) (a) or IRBP (b) was examined. Results are presented as delta counts per minute (Δcpm; mean cpm in stimulated cultures minus the background) of triplicate cultures. Lymphocytes collected from mice treated with suppressive ODN resembled controls in their response to PHA, but showed lower proliferative response against IRBP at 3 µg/ml (*P < 0·05) and at 30 µg/ml (**P < 0·01; unpaired t-test) compared with that of controls. Data are the representative of four independent experiments.
Suppressive ODN inhibits production of inflammation-related cytokines
Inflammatory processes such as EAU are mediated by cytokines produced by lymphoid cells. To assess the effect of suppressive ODN on this immunological parameter, the cytokines produced by lymph node cells from mice in the different groups, treated from day 0, were measured, following incubation in vitro with IRBP for 2 days. Levels of cytokines of IL-2, IL-4, IL5, IL-6, IL-10, IL-12p70, interferon (IFN)-γ and tumour necrosis factor (TNF)-α were measured using the SearchLight technology. When cultures were activated non-specifically by PHA, there was no significant difference among the groups in the level of any of the cytokines tested (data not shown). On the other hand, when stimulated with the immunizing antigen, IRBP, the levels of IFN-γ, TNF-α and IL-6 were decreased significantly in cultures of cells from mice treated with suppressive ODN, compared with the two other control groups (*P < 0·05) (Fig. 4a). Levels of IL-2, IL-4, IL-5 and IL-12p70 were lower in the suppressive ODN-treated group, but did not reach significant difference. The level of one cytokine, IL-10, was the same among the three groups (Fig. 4a).
Fig. 4.
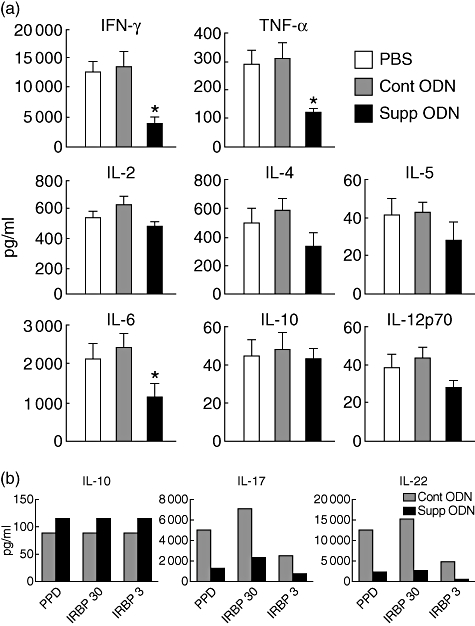
Treatment with suppressive oligodeoxynucleotide (ODN) inhibits production of cytokines. Draining lymph node cells of immunized mice as described in the legend for Fig. 1 were collected on day 14, pooled, and cultured in the presence of immunizing antigens. After 48 h, culture supernatants were harvested and examined for the levels of cytokines. (a) Supernatants were collected from cultures stimulated with interphotoreceptor retinoid-binding protein (IRBP) at 30 µg/ml and cytokine levels were measured using the SearchLight technology. The levels of interferon-γ, tumour necrosis factor-α and interleukin-6 were significantly lower in the supernatants of mice treated with suppressive ODN than in those of controls (P < 0·05). Data are the composition of five independent experiments. (b) Supernatants were collected from cultures stimulated with purified protein derivative at 5 µg/ml or IRBP at 30 or 3 µg/ml, and cytokine levels were measured by enzyme-linked immunosorbent assay kits. Data are from a representative experiment; the same pattern of data was obtained in another experiment.
Recently, a new population of effector cells, designated Th17, has been discovered and found to play a major role in the pathogenesis of autoimmune diseases [33,34], including EAU [35,36]. The signature cytokines of Th17 cells are IL-17 and IL-22 [33,34]; we determined the effect of treatment with A151 on the production of these two cytokines by comparing their level in lymphocyte cultures from mice treated with A151 or the control ODN. The cell cultures were stimulated with IRBP or PPD, a component of the CFA, and the cytokine levels were measured by ELISA kits. In addition to IL-17 and IL-22 we also determined the levels of another cytokine, IL-10. Data of a representative experiment are depicted in Fig. 4b; similar results were obtained in another experiment. The production of both IL-17 and IL-22 was remarkably lower in cultures of cells from A151 treated mice compared with the controls. In contrast, only marginal differences were noted in the levels of IL-10 in cultures from the two groups of mice (Fig. 4b), in line with data collected on this cytokine using the SearchLight technology (Fig. 4a).
Suppressive ODN inhibits ocular inflammation induced by adoptive transfer of activated Th1 cells
To examine further the effect of suppressive ODN on the immune response, we tested its capacity to inhibit the efferent limb of the immunopathogenic process. Ocular inflammation was induced in Tg mice that express HEL in their eyes by adoptively transferring Th1 cells sensitized against HEL [26,27]. Groups of recipient mice were treated with suppressive or control ODN or PBS on days 0 and 3 post-cell transfer. As shown in Fig. 5, treating the recipient mice with suppressive ODN reduced significantly the severity of ocular inflammation, compared with the two groups of controls (P < 0·01).
Fig. 5.
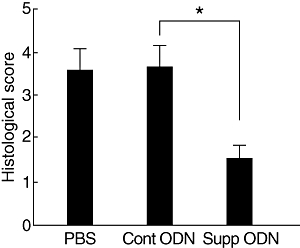
Treatment with suppressive oligodeoxynucleotide (ODN) inhibits the development of adoptively transferred ocular inflammation. Groups of hen egg lysozyme-transgenic (HEL-Tg) mice (three mice per group) were injected with 2 × 105 HEL-specific T helper type 1 cells that had been activated in vitro. Recipients were treated with phosphate-buffered saline or suppressive or control ODN (300 µg/mouse) on days 0 and 3. Eyes were collected on day 7, and the severity of histological changes was scored on a scale of 0–9. See Materials and methods for more details. Mean scores of pathological changes ± standard error of the mean are shown. Each mouse (the average of both eyes) is treated as one statistical event. Data are the composition of three independent experiments (*P < 0·01; Mann–Whitney U-test).
Discussion
Findings reported here show that treatment with suppressive ODN A151 inhibits the development of ocular inflammation in two murine models of ocular inflammation, i.e. induction of EAU by immunization with IRBP and adoptively transferred disease in recipients of sensitized lymphocytes. In the EAU system, the suppressive activity of ODN was tested on the two phases of the immunopathogenic process, i.e. the ‘afferent’ or ‘prevention’ phase, in which lymphocytes become ‘sensitized’ against IRBP, and the ‘efferent’ or ‘treatment’ phase, during which the sensitized lymphocytes migrate to the eye where they trigger the complex process of inflammation [37]. Treatment from day 0 of mice developing EAU examined the suppressive effect on both the afferent and efferent phases, whereas treating from day 7 provided information on the A151 effect on the efferent phase only. Both treatment schedules were found to be effective (Figs 1 and 2) and suppression of the efferent phase by A151 was demonstrated further in the model of adoptively transferred inflammation, in which in vitro activated lymphocytes [26,27] were exposed to suppressive ODN only in vivo (Fig. 5).
The ability of A151 to suppress the afferent phase in mice immunized with IRBP was indicated by the observation that antigen-specific lymphocyte proliferation and cytokine production were reduced significantly in mice treated with this ODN (Figs 3 and 4 respectively). The inhibition of EAU in mice treated from day 0 could be attributed, at least in part, to the lowered level of lymphocyte sensitization in these mice. It is of note that treatment with A151 inhibited in particular the production of cytokines specific to the immunopathogenic Th1 (IFN-γ) and Th17 cells (IL-17 and IL-22), whereas the production of cytokines specific to the non-pathogenic Th2 cells (IL-4 and IL-5) was affected to a lesser extent (Fig. 4).
Unlike its effect on the specific response of lymphocytes against IRBP (Fig. 3b), treatment with A151 did not inhibit the lymphocyte response to PHA (Fig. 3a). This difference is attributed to the selective effect of suppressive ODN on the process of lymphocyte sensitization against the immunizing antigen, IRBP. As a result of this suppressive effect, the small population of IRBP-specific lymphocytes in the A151-treated mice is assumed to expand less than the IRBP-specific population in the control mice and, consequently, the response to the antigen is lower in the treated mice. On the other hand, PHA stimulates the whole population of T cells, most of which were not involved in the specific response to IRBP, and therefore responsiveness to PHA remains intact in the treated mice and similar to that in control mice.
Previous data indicate that one of the mechanisms by which suppressive ODNs can inhibit immune reactivity is by blocking the stimulatory effect of CpG motifs [8]. Mycobacterial CpG sequences represent pivotal components of CFA [2], the adjuvant that is used for induction of EAU by immunization [31], and it is possible that in the IRBP-induced EAU model the inhibitory activity of A151 was related to blocking this effect. However, the inhibition of CpG activity could not account for the suppression of adoptively transferred inflammation by A151, a system in which adjuvant is not used. In addition to blocking CpG-induced immune stimulation, suppressive ODN can also inhibit the signal transduction cascade associated with the production of Th1-related cytokines such as IFN-γ or IL-12 [9]. We hypothesize, therefore, that the inhibitory effect of A151 in the adoptively transferred inflammation model could be attributed to this mode of action. Although the adoptively transferred ocular inflammation was elicited by in vitro activated Th1 cells, we have shown previously that the injected Th1 cells undergo additional stimulation in the eye upon exposure to their target antigen (HEL) [38]. The molecular processes that take place during this lymphocyte restimulation in the eye has not yet been dissected, but it is conceivable that cytokine production plays an important role in the process, and could be affected by suppressive ODN. Moreover, it is possible that A151 inhibits the efferent phase of EAU by this same mode of action.
T regulatory (Treg) cells play major roles in certain immunosuppressive processes [39]. Treg cells are identified by the surface markers CD4 and CD25 and by expression of the intracellular forkhead box P3 transcription factor (FoxP3). We examined the possibility that Treg cells participate in the suppressive effect of A151 by comparing the proportions of CD4+CD25+FoxP3+ cells in the spleen of mice treated with A151 and their controls. Using the method described by Chen et al.[40], we found essentially no difference between the two groups of mice in the percentage of Treg cells: 3·54% and 3·64% respectively. In addition, incubation in vitro of murine T lymphocytes with A151 had no effect on the expression of the Treg markers or on their regulatory activity (data not shown).
Our finding that both afferent and efferent limbs of the immune response are affected by ODN A151 suggests that this suppressive molecule inhibits a process basic to both limbs, i.e. the T cell activation by the antigen presented on antigen-presenting cells. Additional studies are needed to investigate this hypothesis.
Previous studies demonstrated that ODN A151 could inhibit the development of glomerulonephritis in lupus-prone (NZB × NZW) F1 mice [12] and improve survival of mice challenged with lethal doses of LPS [11]. Thus, our results recorded here document further that suppressive ODN can block immunopathogenic processes in which CpG plays no role. It is noteworthy that the capacity of suppressive ODNs to inhibit the efferent limb of immune responses makes these agents good candidates for use in treatment of immune-mediated disease in humans, where presensitized lymphocytes should be targeted by treatment.
Acknowledgments
This research was supported by the Intramural Research Program of the National Eye Institute, NIH. We thank Robert S. Lee for tail DNA analysis, Ricardo Dreyfuss for digital microphotography and the National Eye Institute Histology Core Facility for tissue section preparations.
Disclosure
C. F., D. M. K. and I. G. are co-inventors on several patents associated with the use of suppressive ODN. All rights to these patents are assigned to the US government. Other authors have no financial conflict of interest.
References
- 1.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–16. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 3.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 4.Krieg AM. The role of CpG motifs in innate immunity. Curr Opin Immunol. 2000;12:35–43. doi: 10.1016/s0952-7915(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 5.Zakian VA. Telomeres: beginning to understand the end. Science. 1995;270:1601–7. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph KL, Chang S, Lee HW, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1996;96:701–12. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 7.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 8.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 9.Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol. 2004;173:5002–7. doi: 10.4049/jimmunol.173.8.5002. [DOI] [PubMed] [Google Scholar]
- 10.Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligonucleotides protect against collagen-induced arthritis in mice. Arthritis Rheum. 2004;50:1686–9. doi: 10.1002/art.20263. [DOI] [PubMed] [Google Scholar]
- 11.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–83. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 12.Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB × NZW mice. Arthritis Rheum. 2005;52:651–8. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- 13.Ho PG, Fontoura P, Platten M, et al. A suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J Immunol. 2005;175:6226–34. doi: 10.4049/jimmunol.175.9.6226. [DOI] [PubMed] [Google Scholar]
- 14.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussenblatt RB. Treating intraocular inflammatory disease in the 21st century. Arch Ophthalmol. 2005;123:1000–1. doi: 10.1001/archopht.123.7.1000. [DOI] [PubMed] [Google Scholar]
- 16.Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–62. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol. 2002;21:273–89. doi: 10.1080/08830180212067. [DOI] [PubMed] [Google Scholar]
- 18.Whitcup SM, Nussenblatt RB. Treatment of autoimmune uveitis. Ann NY Acad Sci. 1993;696:307–18. doi: 10.1111/j.1749-6632.1993.tb17166.x. [DOI] [PubMed] [Google Scholar]
- 19.Forrester JV, Liversidge J, Dua HS, Dick A, Harper F, McMenamin PG. Experimental autoimmune uveoretinitis: a model system for immunointervention: a review. Curr Eye Res. 1992;11(Suppl.):33–40. doi: 10.3109/02713689208999509. [DOI] [PubMed] [Google Scholar]
- 20.Caspi RR. Immune mechanisms in uveitis. Springer Semin Immunopathol. 1999;21:113–24. doi: 10.1007/BF00810244. [DOI] [PubMed] [Google Scholar]
- 21.Wacker WB, Donoso LA, Kalsow CM, Yankeelov JA, Jr, Organisciak DT. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977;119:1949–58. [PubMed] [Google Scholar]
- 22.Nussenblatt RB, Kuwabara T, de Monasterio FM, Wacker WB. S-antigen uveitis in primates. A new model for human disease. Arch Ophthalmol. 1981;99:1090–2. doi: 10.1001/archopht.1981.03930011090021. [DOI] [PubMed] [Google Scholar]
- 23.Gery I, Wiggert B, Redmond TM, et al. Uveoretinitis and pinealitis induced by immunization with interphotoreceptor retinoid-binding protein. Invest Ophthalmol Vis Sci. 1986;27:1296–300. [PubMed] [Google Scholar]
- 24.Hirose S, Kuwabara T, Nussenblatt RB, Wiggert B, Redmond TM, Gery I. Uveitis induced in primates by interphotoreceptor retinoid-binding protein. Arch Ophthalmol. 1986;104:1698–702. doi: 10.1001/archopht.1986.01050230136049. [DOI] [PubMed] [Google Scholar]
- 25.Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–5. [PubMed] [Google Scholar]
- 26.Foxman EF, Zhang M, Hurst SD, et al. Inflammatory mediators in uveitis: differential induction of cytokines and chemokines in Th1- versus Th2-mediated ocular inflammation. J Immunol. 2002;168:2483–92. doi: 10.4049/jimmunol.168.5.2483. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Zhang M, Vistica BP, et al. Induction of ocular inflammation by T-helper lymphocytes type 2. Invest Ophthalmol Vis Sci. 2002;43:758–65. [PubMed] [Google Scholar]
- 28.Lai JC, Fukushima A, Wawrousek EF, et al. Immunotolerance against a foreign antigen transgenically expressed in the lens. Invest Ophthalmol Vis Sci. 1998;39:2049–57. [PubMed] [Google Scholar]
- 29.Ho WY, Cooke MP, Goodnow CC, Davis MM. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994;179:1539–49. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepperberg DR, Okajima TL, Ripps H, Chader GJ, Wiggert B. Functional properties of interphotoreceptor retinoid-binding protein. Photochem Photobiol. 1991;54:1057–60. doi: 10.1111/j.1751-1097.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 31.Caspi RR, Chan CC, Leake WC, Higuchi M, Wiggert B, Chader GJ. Experimental autoimmune uveoretinitis in mice. Induction by a single eliciting event and dependence on quantitative parameters of immunization. J Autoimmun. 1990;3:237–46. doi: 10.1016/0896-8411(90)90143-g. [DOI] [PubMed] [Google Scholar]
- 32.Chan CC, Caspi RR, Ni M, et al. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun. 1990;3:247–55. doi: 10.1016/0896-8411(90)90144-h. [DOI] [PubMed] [Google Scholar]
- 33.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.McGeachy MJ, Dua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–53. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Amadi-Obi A, Yu C-R, Liu X, et al. Th17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–18. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 36.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caspi RR. Regulation, counter-regulation, and immunotherapy of autoimmune responses to immunologically privileged retinal antigens. Immunol Res. 2003;27:149–60. doi: 10.1385/IR:27:2-3:149. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Vistica BP, Takase H, et al. A unique pattern of up- and down-regulation of chemokine receptor CXCR3 on inflammation-inducing Th1 cells. Eur J Immunol. 2004;34:2885–94. doi: 10.1002/eji.200425318. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T-cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM. Pertussis toxin as an adjuvant suppresses the number and function of CD+CD25+ T regulatory cells. Eur J Immunol. 2006;36:671–80. doi: 10.1002/eji.200535353. [DOI] [PMC free article] [PubMed] [Google Scholar]


