Abstract
Caterpillar feeding induces direct and indirect defences in brassicaceous plants. This study focused on the role of the octadecanoid pathway in induced indirect defence in Brassica oleracea. The effect of induction by exogenous application of jasmonic acid (JA) on the responses of Brussels sprouts plants and on host-location behaviour of associated parasitoid wasps was studied. Feeding by the biting–chewing herbivores Pieris rapae and Plutella xylostella resulted in significantly increased endogenous levels of JA, a central component in the octadecanoid signalling pathway that mediates induced plant defence. The levels of the intermediate 12-oxophyto-dienoic acid (OPDA) were significantly induced only after P. rapae feeding. Three species of parasitoid wasps, Cotesia glomerata, C. rubecula, and Diadegma semiclausum, differing in host range and host specificity, were tested for their behavioural responses to volatiles from herbivore-induced, JA-induced, and non-induced plants. All three species were attracted to volatiles from JA-induced plants compared with control plants; however, they preferred volatiles from herbivore-induced plants over volatiles from JA-induced plants. Attraction of C. glomerata depended on both timing and dose of JA application. JA-induced plants produced larger quantities of volatiles than herbivore-induced and control plants, indicating that not only quantity, but also quality of the volatile blend is important in the host-location behaviour of the wasps.
Keywords: Brussels sprouts, cabbage, induced indirect defence, jasmonate, parasitoid host-location behaviour, octadecanoid pathway, oxylipin, volatile emission
Introduction
In response to damage, the phenotype of an individual plant changes because of the induction of defences. This may affect the insect community associated with the plant, either directly by affecting the herbivores and their natural enemies or indirectly by modifying the interactions between the different trophic levels (Price et al., 1980; Van Zandt and Agrawal, 2004; Schoonhoven et al., 2005; Takabayashi et al., 2006; Poelman et al., 2008). This study addresses the effects of volatiles that plants release in response to herbivory, on the behaviour of members of the third trophic level. This was studied through a manipulative approach that exploits information on underlying mechanisms of the induction process.
Herbivore-induced plant volatiles (HIPVs) have been shown to attract natural enemies of the herbivores attacking the plant (Dicke et al. 1990; Turlings et al., 1990, 1993; Steinberg et al., 1992; Geervliet et al., 1994; Scascighini et al., 2005), and such HIPVs can also affect herbivore behaviour (Dicke and Vet, 1999; Sabelis et al., 1999). The types and amounts of volatiles emitted by the plants differ depending on plant species, attacking herbivore species, herbivore developmental stage, and abiotic factors (Vet and Dicke, 1992; Turlings et al., 1993; Takabayashi and Dicke, 1996; Dicke and Van Poecke, 2002; Gouinguené and Turlings, 2002; Hilker and Meiners, 2002; Heil, 2008). HIPVs play an important role in parasitoid host-location behaviour. For the model plant species in this study, Brussels sprouts, it is known that caterpillar-infested plants attract parasitoids such as Diadegma semiclausum and several Cotesia spp. (Blaakmeer et al., 1994; Geervliet et al., 1996; Ohara et al., 2003). Already within 1 h after herbivore infestation, females of Cotesia glomerata are attracted to Brussels sprouts plants (Scascighini et al., 2005).
The volatile blends emitted by plants can be manipulated by interfering with the signal transduction pathways leading to volatile emission. Manipulation of the volatile emission of a plant using a chemical elicitor allows for the investigation of the possible effects of plant volatiles on community ecology. The use of an elicitor has the advantage of being able to induce (part of) the volatile blend without removal of plant tissue, and offers the possibility to apply a controlled dose, whereas it is difficult to control the amount of damage inflicted by herbivore feeding. The major signal transduction pathway underlying insect-induced plant defences is the octadecanoid pathway (Dicke and Van Poecke, 2002; Kessler and Baldwin, 2002). The phytohormone jasmonic acid (JA) is the main octadecanoid of this pathway, while 12-oxophyto-dienoic acid (OPDA) is an intermediate that acts as a weaker elicitor of plant responses (Van Poecke and Dicke, 2002; Kessler and Baldwin, 2002). In this study, JA is used to manipulate the volatile emission of Brussels sprouts plants. Treatment of plants with JA or MeJA (methyl jasmonate) has been reported to induce volatile emission similar to herbivore induction, extrafloral nectar production, increased levels of endogenous secondary metabolites, reduced development and oviposition of herbivores, increased attraction of predators and parasitoids, and enhanced parasitism rates of herbivores for a wide variety of plant species (for example, Dicke et al., 1999; Thaler, 1999a; Meiners and Hilker, 2000; Kessler and Baldwin, 2001; Mumm et al., 2003; Heil, 2004). Jasmonates have also been used successfully for the induction of defence responses in several brassicaceous species such as Arabidopsis thaliana (Van Poecke and Dicke, 2002; Mewis et al., 2005), common cabbage (Ibrahim et al., 2005), and oilseed rape (Loivamäki et al., 2004). This wide range of species illustrates that this phytohormone is involved in many plant–insect interactions and is hypothesized to be involved in the defence of Brussels sprouts plants against herbivorous insects as well.
To gain insight into the effects of modified volatile production on interactions with parasitoid wasps, the effects of JA treatment on the volatile emission of Brussels sprouts, Brassica oleracea L. var gemmifera, and on the behaviour of several associated parasitoid wasps were investigated. Three species of parasitoids, Cotesia rubecula Marshall, Cotesia glomerata L. (Hymenoptera: Braconidae), and Diadegma semiclausum (Hellén) (Hymenoptera: Ichneumonidae) were selected, that all parasitize caterpillars on brassicaceous plants, but differ in their host range and specificity. While both Cotesia wasps use Pieris caterpillars as their hosts, D. semiclausum is a specialist parasitoid of Plutella xylostella. Cotesia glomerata accepts Pieris brassicae, P. rapae, and P. napi, whereas C. rubecula is more specialized in its choice of hosts for oviposition, and prefers P. rapae, only rarely accepting other hosts (Brodeur et al., 1996). Whether parasitoids that differ in host range and specificity also differ in their response to JA-induced plant volatiles was studied. Subsequently, the effect of JA induction on parasitoid behaviour was studied in more detail; C. glomerata attraction to plants was tested at several time points after induction and in response to treatment with different concentrations of JA. Oxylipin levels (JA and OPDA) in leaves with or without herbivory were analysed and, finally, the volatile blends emitted by non-induced, herbivore-damaged and JA-treated plants were analysed to compare the relative effects of herbivory and JA.
Materials and methods
Plant material
Brussels sprouts, B. oleracea L. var. gemmifera cultivar Cyrus (Brassicaceae), were grown from seed in a greenhouse in plastic pots (11×11 cm) at 20–28 °C, 40–80% relative humidity (RH), and a 16L:8D photoperiod. All experiments were conducted with 6- to 7-week-old plants with ∼8–9 fully expanded leaves.
Insects
Stock colonies of the large cabbage white butterfly P. brassicae L., the small cabbage white P. rapae L. (Lepidoptera: Pieridae), and the diamondback moth Plutella xylostella L. (Lepidoptera: Plutellidae) were maintained on Brussels sprouts plants in a climatized room at 20–22 °C, 50–70% RH, and a 16L:8D photoperiod. The parasitoid wasps C. rubecula and C. glomerata were reared in a greenhouse at 22–24 °C, 50–70% RH, and a 16L:8D photoperiod. Cotesia rubecula and C. glomerata were maintained on P. rapae and P. brassicae, respectively, both feeding on Brussels sprouts plants. Diadegma semiclausum was reared on P. xylostella feeding on Brussels sprouts plants in a climate room at 20–22 °C, 50–70% RH, under a 16L:8D photoperiod. All adult wasps emerged in cages without any plants or hosts, were fed with honey and water, and kept until use in the experiments under the same climate conditions as those used for rearing.
Parasitoid preference
Behavioural experiments with parasitoid wasps were conducted to compare the attractiveness of plants subjected to different induction treatments for the three wasp species. JA-treated plants were tested in dual-choice experiments against control plants and against caterpillar-infested plants.
Plant treatments:
For the JA treatment in the first experiment, plants were sprayed with 20 ml of a 1 mM JA (± jasmonic acid Sigma-Aldrich, purity >97%) aqueous solution containing 0.1% Tween-20 (Sigma-Aldrich) as surfactant, which corresponds to ∼140 μg JA g−1 leaf fresh weight, and the control plants were treated with a 0.1% Tween-20 solution only (Van Poecke and Dicke, 2002). The caterpillar treatment consisted of plants infested with herbivores: 12 second-instar P. xylostella for D. semiclausum choice experiments (comparable with densities observed in cabbage plants in the field) and 30 first-to-second instar P. rapae for both Cotesia spp. (based on experiments with Brussels sprouts plants and JA by Z Szendrei, R Gols and M Dicke, unpublished results). The caterpillars were placed on the middle three leaves. Pieris rapae consumed on average 3–3.5 cm2 and P. xylostella 2–2.5 cm2 of leaf area. The plants were sprayed or infested 24±2 h before the experiments.
Preference behaviour of three parasitoid wasp species:
The behaviour of the three parasitoid wasp species was tested in a windtunnel (as described in detail by Geervliet et al., 1994). The windtunnel conditions were set at 26±2 °C, 60–70% RH, and a wind speed of 20 cm s−1 (Thermisches Anemometer, Lambrecht GmbH, Göttingen, Germany). The adult wasps were provided with water and honey, but had no experience with plants or caterpillars until the experiment. Female wasps were separated from male wasps on the day before the experiment. Wasps of 3–6 d old were used for all experiments and were assumed to have mated. The female parasitoid wasps were released at 60 cm distance downwind from the two plants. The parasitoids were individually released on a small piece of leaf damaged by their respective caterpillar host, from which caterpillars and faeces had been removed; this served to increase the responsiveness of the wasps but does not affect their choice. After release in the windtunnel, an individual parasitoid female was observed until it landed on one of the plants (choice) or for a maximum of 10 min without landing, after which it was recorded as not having made a choice (no choice). Wasps that did not make a choice were discarded from the analysis. Each wasp was used only once. The position of plants that had been exposed to different treatments was alternated after a maximum of five wasps to exclude possible directional bias. All experiments, control versus JA treatment and JA treatment versus herbivore-infested, for all three species were carried out on at least 5 d, with new sets of plants.
Dose–effect relationship:
After testing one JA concentration (1 mM) on different parasitoid species, the response of one parasitoid, C. glomerata, to plants treated with different concentrations of JA was tested. Plants treated with either 1 μM, 10 μM, 100 μM, or 1 mM JA were tested against control plants in the windtunnel; each plant was sprayed until run-off. Several concentrations were tested on the same experimental day and each concentration was tested on at least five different days. The effect on parasitoid behaviour was investigated in the windtunnel described above.
Effect of time since treatment:
To test when plants become attractive to the parasitoids after JA treatment and how long this attraction lasts, the time of testing after induction with 1 mM JA was varied from 1 h to 120 h. In the windtunnel, the response of C. glomerata to plants that were treated 1, 2, 3, 6, 24, 48, 72, or 120 h before with JA was investigated against a control plant (treated with a Tween-20 solution at the same time). New plants were used at each time point and parasitoid responses were tested on at least five different days per JA incubation time point. The set-up was the same as described above.
In order to compare induction with JA with induction by herbivores, herbivore-infested plants were prepared by infesting plants with five or 15 P. rapae first-instar larvae and allowing them to feed for 24 h. Subsequently, all caterpillars were removed and the plants were tested against intact plants 48 h and 120 h after removal.
Attraction to an inert substrate treated with JA:
To rule out that the observed effect of JA treatment is due to volatilized JA itself, rather than the result of induction of plant volatiles, the response of C. glomerata to an inert substrate treated with JA was tested. Just prior to the experiment, 1 ml of 5 mM JA was sprayed onto green paper (8×11.5 cm) with a Desaga chromatographic sprayer (Heidelberg, Germany), which amounts to a concentration of ∼10 μg JA cm−2 (roughly corresponding to the amount of JA that would be sprayed on the plant per cm2 at a dose of 2 mM). In the windtunnel, a control substrate (green paper sprayed with water) and JA-treated substrate were tested against each other; next to each substrate a leaf freshly excised from an intact Brussels sprouts plant was placed. Landings on both leaf and substrate were recorded.
Oxylipin analysis
For OPDA and JA analysis, leaf material was sampled from five plants infested with either 30 P. rapae caterpillars or 12 P. xylostella caterpillars, and from undamaged plants. Leaf samples were immediately frozen in liquid nitrogen after sampling and subsequently stored at –80 °C until analysis. For OPDA and JA analysis, frozen plant material (∼500 mg fresh weight) was transferred into a 2 ml vial. After addition of a ceramic bead (6 mm diameter), tissue was homogenized with a vibrating ball mill (20 s−1, 3 min). Methanol (1 ml), internal standards (100 ng of [D5]OPDA and 50 ng of dihydrojasmonic acid), and 50 μl of acetic acid were added and the mixture was homogenized again (30 s−1, 3 min). After centrifugation (10 min, 14 000 rpm, Centrifuge 5415C; Eppendorf, Hamburg, Germany), 800 μl of the supernatant was transferred to a 1.5 ml Eppendorf cup and dried in a vacuum centrifuge (Speed-Vac, Christ RVC 2-18). The residue was dissolved in 20 μl of acetonitrile and transferred to a microvial. Prior to HPLC-MS analysis, 80 μl of ammonium acetate (1 mM; pH 6.6) was added. Analysis was carried out on a Waters/Micromass (Milford, MA, USA) Quattro Premier Triple Quadrupole mass spectrometer coupled to an Agilent 1200 Series (Agilent, Waldbronn, Germany) HPLC system, equipped with a 1200 Binary Pump and 1200 Standard autosampler. A pre-column [Purospher Star 18e, 4×4 mm, 5 μm particle size (Merck, Darmstadt, Germany)] and Purospher Star RP 18e column (125×2 mm, 5 μm particle size; Merck, Darmstadt, Germany) were used. The injection volume was 15 μl, and the HPLC flow rate was 0.2 ml min−1 using the following gradient of ammonium acetate (1 mM, pH 6.6):acetonitrile mixtures: 10 min 95:5, 5 min 5:95, then at a flow rate of 0.3 ml min−1 15 min 95:5. Mass spectra were acquired using electrospray ionization in negative ion mode and multiple reaction monitoring (MRM). The capillary and cone voltage were set at 3.00 kV and 40.00 V, the flow rates of cone gas and desolvation gas were 50 l h−1 and 800 l h−1, and the source temperature and desolvation temperature were 120 °C and 400 °C, respectively. Data were acquired with MassLynx 4.1 software. Quantification of the compounds was performed by integration of the peak area in the MRM chromatograms. Oxylipin concentrations were calculated by reference to the integrated peak areas of the internal standards using response factors obtained from calibration curves. OPDA and JA levels determined in the samples were within the linear range of the analysis method and at least 5-fold over the limit of quantification.
Volatile analysis
For the chemical analysis of the volatiles emitted by plants subjected to different treatments, plants were treated in the same way as for the parasitoid preference experiments. The headspace collection was performed in a climate room at 22–24 °C, 50–70% RH, and a light intensity of 95±5 μmol m−2 s−1 (Quantum meter QMSW-SS, Apogee instruments Inc., Logan, UT, USA). Pressurized air was filtered over silica gel, a molecular sieve (4 Å), and activated charcoal, and led through a 30.0 l clean glass jar. Overnight, clean air was led through the jar at a flow rate of 100 ml min−1 to remove any remaining volatile contaminants. Just before placing the plant in the jar, the pot of the plant was removed and the roots and soil were packed tightly in aluminium foil. The plant was placed in the jar, which was closed with a glass lid with a Viton® O-ring in between, and the lid was closed air-tight using a metal clamp. First, the jar with the plant was purged for 1 h with an air flow through the jar of 50 ml min−1. Subsequently, headspace volatiles were collected at the outlet of the jar on a glass tube filled with 90 mg of Tenax-TA 25/30 mesh (Grace-Alltech) for 4 h at a flow rate of 40 ml min−1. Two plants of different treatments were sampled at the same time, and five replicates per treatment were sampled and analysed. Headspace samples were analysed with a Varian 3400 GC (Varian, Palo Alto, USA) connected to a Finnigan MAT 95 MS (Thermo Scientific, Waltham, MA, USA). The collected volatiles were released from the Tenax by heating the trap in a Thermodesorption Cold Trap Unit (Chrompack, Middelburg, The Netherlands) at 250 °C for 10 min and flushing with helium at 14 ml min−1. The released compounds were cryofocused in a cold trap (0.52 mm ID deactivated fused silica) at a temperature of –85 °C. By ballistic heating of the cold trap to 220 °C the volatiles were transferred to the analytical column (DB-5ms, 60 m×0.25 mm ID, 0.25 μm film thickness; J&W, Folsom, CA, USA). The temperature program started at 40 °C (4 min hold) and rose 5 °C min−1 to 280 °C (4 min hold). The column effluent was ionized by electron impact (EI) ionization at 70 eV. Mass scanning was done from 24 to 300 m/z with a scan time of 0.7 s d−1 and an interscan delay of 0.2 s. Compounds were identified by comparison of the mass spectra with those in the Wiley library and in the Wageningen Mass Spectral Database of Natural Products, and by checking the retention index against those published in the literature.
Statistical analysis
The parasitoid choices between two odour sources were statistically analysed using the binomial test (MS Excel 2003) on the pooled data after testing for differences between experimental days using a n×2 contingency table test (SAS 9.1, n=number of experimental days). Differences among the percentages of wasps making a choice were tested using a contingency table test on the absolute numbers (SAS 9.1). Oxylipin levels were compared using an analysis of variance (ANOVA) with LSD (least significant difference) post hoc tests (SPSS 15.0). The volatile patterns of differently treated plants were analysed using principal component analysis (PCA) and projection to latent structures-discriminant analysis (PLS-DA) using the software program SIMCA-P 10.5 (Umetrics AB, Umeå, Sweden) (Wold et al., 1989; Eriksson et al., 2001). PCA obtains so-called scores by projecting data observations onto model planes, which are defined by the extracted principal components (PCs). The integrated peak areas, corrected for the fresh weight of the plants, were normalized, i.e. peak areas of all analysed compounds (X variables) were log-transformed [the constant 0.00001 was added to provide non-detectable components with a small non-zero value (Sjödin et al., 1989)] and mean-centred, scaled to unit variance, and represented as a matrix X (Eriksson et al., 2001). The objective of PLS-DA is to find an optimal model that discriminates the X data according to the plant treatments (Eriksson et al., 2001). PLS-DA is a supervised technique, so class memberships of the observations need to be pre-defined. Therefore, an additional Y matrix was made with G columns containing the values 1 and 0 as dummy variables for each of the plant treatments, respectively. The numbers of significant PCs and PLS components were determined by cross-validation (Wold et al., 1989; Eriksson et al., 2001). In addition, the variable importance in the projection (VIP) was calculated. Variables with VIP values >1 are most influential for the model (Eriksson et al., 2001; Paolucci et al., 2004).
Results
Parasitoid preference
Preference of three species of parasitoid wasps:
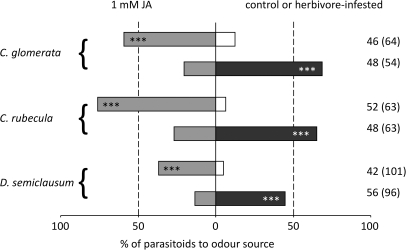
Testing the three parasitoid species for their preference for volatiles from herbivore-infested, JA-treated, or control plants yielded similar results for all species (3×2 contingency table test: control versus JA, χ2=2.16, df=2, P=0.340; JA versus herbivore, χ2=0.77, df=2, P=0.681). When control plants were tested against JA-treated plants in the windtunnel, C. rubecula, C. glomerata, and D. semiclausum all significantly preferred the volatiles from JA-treated plants (binomial test: all P<0.001; Fig. 1). When the wasps were presented with a choice between JA-treated plants and plants infested with herbivores (P. rapae for C. glomerata and C. rubecula, and P. xylostella for D. semiclausum), all three parasitoid species significantly preferred the herbivore-infested plants (binomial test: all P <0.001; Fig. 1).
Fig. 1.
Behavioural responses of three parasitoid wasp species, C. rubecula, C. glomerata, and D. semiclausum when offered two odour sources treated 24 h before testing in a windtunnel. For each parasitoid species the percentage (of the total number of parasitoids tested) of parasitoids that landed on control (white bars) versus 1 mM jasmonic acid (JA)-treated (grey bars) plants, and the distribution of choices for 1 mM JA-treated versus herbivore-infested (black bars) plants are shown. The numbers to the right of each bar represent the number of wasps that made a choice; between brackets are the total number of wasps tested (***P <0.001).
Dose–effect relationship:
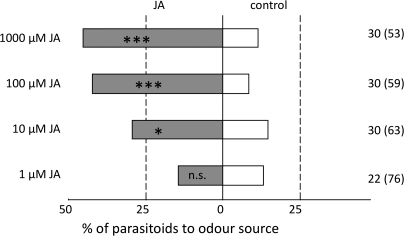
The preference of C. glomerata for the volatiles from JA-treated or control plants was tested in dual-choice tests in the windtunnel for four concentrations of JA, i.e. 1 μM, 10 μM, 100 μM, and 1 mM. The JA-treated plants were significantly more attractive than the control plants when treated with 10 μM (binomial test: n=30, P=0.049), 100 μM n=30, P <0.001) and 1 mM JA (n=30, P <0.001) (Fig. 2). Only for the lowest concentration tested did the parasitoids not show a preference for either of the plants (binomial test: n=21, P=0.50) (Fig. 2). With decreasing JA concentration, the response level of the parasitoids decreased from ∼60% to a level of <30% for the lowest concentration tested (Fig. 2). A negative correlation between the JA concentration and the percentage no choice was found (Spearman's ρ=–0.671, n=31, P <0.001).
Fig. 2.
Effect of the concentration of jasmonic acid (JA) applied to Brussels sprouts plants on the attraction of the parasitoid C. glomerata in a windtunnel. Plants treated with different concentrations of JA (24 h before the windtunnel test) are tested against control plants. The percentage of wasps that landed on the JA-treated and on control plants is shown (n.s. P >0.05, *P <0.05, ***P <0.001). The numbers to the right of each bar represent the number of wasps that made a choice; between brackets are the total number of wasps tested. The percentage of parasitoids that made no choice decreased with increasing JA concentration (Spearman rank correlation: P <0.001).
Effect of time since treatment:
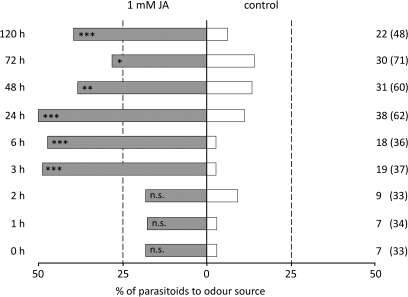
The response of C. glomerata to volatiles from JA-treated plants was tested after different time intervals. Already in the first hour after JA application more parasitoids landed on JA-treated plants than on control plants, although the response level in the first 3 h after application was very low and the difference, therefore, not significant (binomial test: P > 0.05; Fig. 3). After 3 h the preference for JA-treated plants was more pronounced and statistically significant (binomial test: P <0.001). The parasitoids still preferred the volatiles from JA-treated plants over the control plants after 120 h (binomial test: P <0.001). The percentage of wasps that responded, i.e. landed on a plant within 10 min, was highest after 24 h since JA application.
Fig. 3.
Response of the parasitoid C. glomerata to B. oleracea plants at different time intervals since the treatment with 1 mM jasmonic acid (JA) in a windtunnel. The percentage (of the total number of wasps tested) of wasps choosing JA-treated or control plants is shown. The numbers to the right of each bar represent the number of wasps that made a choice; between brackets are the total number of wasps tested (n.s. P >0.05; *P <0.05; **P <0.01; ***P <0.001).
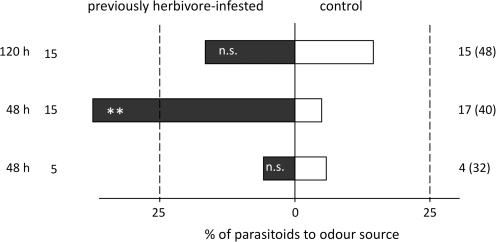
After removing the caterpillars that had been feeding for 24 h, the attractiveness of these plants waned more rapidly than after JA treatment (Fig. 4). Forty-eight hours after removal of the caterpillars, the plants that had been fed on by 15 caterpillars were still attractive (binomial test: P=0.001), but attractiveness was absent after 120 h (binomial test: P=0.5). The plants that had been fed on by five caterpillars had already lost their attractiveness 48 h after removal (binomial test: P=0.69).
Fig. 4.
Response of C. glomerata wasps in a windtunnel to previously infested plants (black bars) or undamaged plants (white bars) (n.s. P >0.05; **P <0.01). Pieris rapae caterpillars were removed after 24 h of feeding; attraction of the parasitoids was tested 48 h or 120 h after removing the caterpillars. Infestation levels (five or 15 caterpillars per plant) are indicated on the left. The numbers to the right of each bar represent the number of wasps that made a choice; between brackets are the total number of wasps tested.
Attraction to an inert substrate treated with JA:
When JA was offered on an inert substrate, the response of the parasitoids was very low. Only one out of 34 parasitoids landed on a leaf next to the JA-treated substrate, and none on the substrates. Eight parasitoids flew within a distance of 5 cm of a substrate; six flew near the water-treated substrate, and two flew near the JA-treated substrate.
Oxylipin analysis
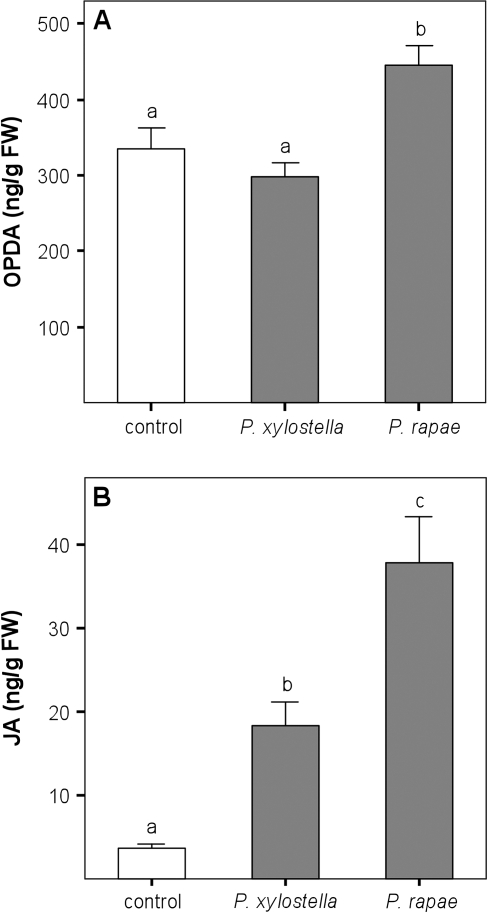
Caterpillar feeding induced oxylipin accumulation (ANOVA: OPDA, F=5.779, df=2, P=0.012; JA, F=42.2, df=2, P <0.001; Fig. 5). JA levels rose from an average of 3.6 ng g FW−1 in control leaves to 18.2 ng g FW−1 for P. xylostella-infested and 37.9 ng g FW−1 for P. rapae-infested leaves. After 24 h of feeding by 12 P. xylostella caterpillars, OPDA levels were not significantly higher than in control leaves, but JA levels had increased significantly (post hoc test comparing JA levels in control, P. xylostella-infested, and P. rapae-infested leaves with Bonferroni correction P=0.003). Feeding by 30 P. rapae caterpillars showed a significant increase for both OPDA and JA levels (post hoc tests comparing OPDA and JA levels in control, P. xylostella-infested, and P. rapae-infested leaves with Bonferroni correction: OPDA, P=0.038; JA, P <0.001).
Fig. 5.
Levels of (A) OPDA and (B) JA in B. oleracea plants with and without herbivory. Twelve P. xylostella or 30 P. rapae caterpillars were allowed to feed on the plants for 24 h. Different letters indicate significant differences between treatments (one-way ANOVA with post hoc tests with Bonferroni correction); error bars indicate the SE.
Volatile analysis
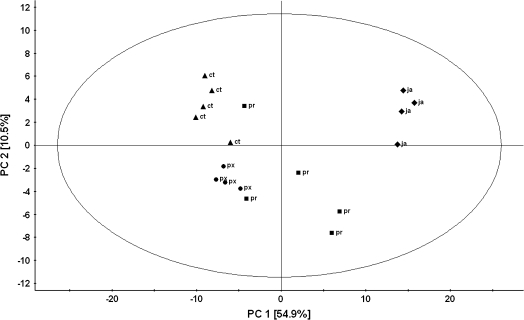
Volatile blends of control, 1 mM JA-treated, and herbivore-infested plants were analysed. Fifty-three compounds were identified (Table 1). Among the identified compounds were terpenoids, ketones, alcohols, an aldehyde, nitriles, sulphides, and esters. The compounds that were produced in highest amounts were (Z)-3-hexen-1-yl acetate, sabinene, limonene, 1,8-cineole, β-myrcene, and α-thujene (Table 1). JA-treated plants emitted the highest amounts of volatiles, followed by P. rapae-infested plants, whereas control plants emitted the lowest amounts and number of volatile compounds. PCA resulted in a model with three significant PCs explaining a total variation (R2X cum) of 73%. The score plot of PC1 versus PC 2 shows that the volatile blend composition of P. rapae- and P. xylostella-infested samples slightly overlapped, with P. xylostella-infested plants showing the lowest degree of within-treatment variation, whereas P. rapae-infested plants varied most (Fig. 6). The first PC, explaining 54.9% of the variation of the data, separated control and JA-treated plants, while the second PC (explaining 10.5%) separated the control and JA treatment from the herbivore treatments (Fig. 6).
Table 1.
Volatile compounds detected in the headspace of Brussels sprouts, sprayed with a Tween-20 solution (control, n=5), infested with either 30 Pieris rapae (n=5) or 12 Plutella xylostella larvae (n=4), or sprayed with 1 mM jasmonic acid solution with Tween-20 (n=4) 24 h before headspace collection
| Compound | Control | Plutella xylostella | Pieris rapae | Jasmonic acid | VIP-values* | |
| Alcohols | ||||||
| 1 | 2-Methyl-1-propanol | 44.8±16.5 | n.d. | 74.6±25.2 | 163.8±58.7 | 1.58 |
| 2 | 1-Penten-3-ol | 2.2±2.2 | n.d. | 194.5±82.0 | 482.2±101.8 | 1.48 |
| 3 | 3-Pentanol | 3.8±3.8 | n.d. | 60.9±20.7 | 220.1±42.3 | 1.42 |
| 4 | 3-Methyl-1-butanol | n.d. | n.d. | 3.1±2.4 | 23.5±3.7 | 0.78 |
| 5 | 1-Pentanol | n.d. | n.d. | 10.4±4.4 | 24.9±3.9 | 0.89 |
| 6 | (Z)-2-Pentanol-1-ol | n.d. | n.d. | 8.7±4.3 | 30.7±5.2 | 0.87 |
| 7 | 3-Methyl-2-pentanol | n.d. | n.d. | 4.6±2.9 | 80.5±13.8 | 0.77 |
| 8 | (Z)-3-Hexen-1-ol | 157.5±95.4 | 46.7±8.3 | 364.8±104.4 | 902.1±103.3 | 0.82 |
| 9 | 1-Hexanol | 5.4±5.4 | 3.0±3.0 | 17.5±8.2 | 61.4±10.4 | 0.74 |
| 10 | 2-Methyl-3-hexen-1-ol | n.d. | n.d. | 3.1±2.0 | 20.4±4.6 | 0.78 |
| 11 | 3-Methyl-3-hexen-1-ol | n.d. | n.d. | 3.1±2.0 | 33.8±16.6 | 0.78 |
| Aldehydes | ||||||
| 12 | Hexanal | 5.3±3.3 | 2.3±2.3 | 6.5±3.4 | 27.5±18.9 | |
| Esters | ||||||
| 13 | 3-Methyl-1-butanol acetate | n.d. | n.d. | 4.1±3.0 | 19.5±4.7 | 0.78 |
| 14 | (Z)-2-Penten-1-yl acetate | n.d. | 20.1±8.4 | 296.8±154.4 | 607.8±180.4 | 1.54 |
| 15 | Pentyl acetate | n.d. | n.d. | 29.6±15.0 | 63.6±20.2 | 1.14 |
| 16 | (Z)-3-Hexen-1-yl acetate | n.d. | 364.3±80.9 | 4249.1±2010.7 | 9873.6±2934.9 | 1.97 |
| 17 | Hexyl acetate | n.d. | 12.0±2.8 | 135.9±68.8 | 605.7±172.7 | 1.59 |
| 18 | Methyl salicylate | 0.9±0.6 | 2.3±2.3 | 2.8±1.6 | 2.0±1.1 | 0.78 |
| Isothiocyanate | ||||||
| 19 | Methyl (iso)thiocyanate | n.d. | n.d. | 1.3±1.3 | 25.9±3.6 | 0.86 |
| Ketones | ||||||
| 20 | 3-Pentanone | n.d. | n.d. | 20.6±16.3 | 456.2±170.3 | 0.84 |
| 21 | 3-Methyl-2-pentanone | n.d. | n.d. | n.d. | 169.0±29.0 | 1.21 |
| 22 | Cyclopropyl-2-propen-1-one | 2.7±2.7 | 1.0±1.0 | 17.9±8.2 | 22.2±4.6 | 0.70 |
| 23 | 3-Heptanone | 21.4±13.7 | 13.1±7.1 | 8.7±2.5 | 16.0±6.5 | 0.28 |
| 24 | 2-Heptanone | 5.9±3.3 | 5.1±2.7 | 3.4±1.5 | 10.1±4.4 | 0.42 |
| 25 | 2-Methyl-2-cyclopenten-1-one | 2.7±2.7 | n.d. | 15.9±6.8 | 4.0±4.0 | 1.45 |
| 26 | 2-Methyl-6-methylene-1,7-ocatadiene-3-one | n.d. | 2.3±2.3 | 8.3±4.6 | 55.5±7.0 | 0.78 |
| Nitriles/N-containing | ||||||
| 27 | 2-Methylbutanenitrile | n.d. | n.d. | n.d. | 194.0±49.9 | 1.21 |
| 28 | 3-Methylbutanenitrile | n.d. | n.d. | 5.0±3.2 | 329.9±46.4 | 0.84 |
| 29 | Benzonitrile | 28.1±5.2 | 14.4±6.3 | 7.5±6.3 | 25.3±15.6 | 0.90 |
| 30 | Benzyl cyanide | 4.4±2.7 | 2.3±2.3 | 15.9±5.3 | 40.2±12.3 | 0.76 |
| Terpenoids | ||||||
| 31 | α-Thujene | 94.6±29.4 | 112.3±49.5 | 417.9±65.7 | 2892.1±261.1 | 0.37 |
| 32 | α-Pinene | 44.0±14.8 | 51.5±24.2 | 224.9±20.8 | 1098.3±40.7 | 0.39 |
| 33 | Thuja-2,4(10)-diene | n.d. | n.d. | n.d. | 111.8±13.3 | 1.20 |
| 34 | Sabinene | 318.4±102.5 | 733.0±227.8 | 1851.7±373.8 | 10996.5±959.3 | 0.39 |
| 35 | β-Pinene | 30.9±8.5 | 39.4±11.7 | 148.1±25.0 | 825.5±43.6 | 0.37 |
| 36 | β-Myrcene | 108.6±32.6 | 146.0±41.3 | 396.4±65.3 | 3448.3±608.7 | 0.35 |
| 37 | α-Phellandrene | n.d. | n.d. | 3.0±3.0 | 21.0±8.5 | 0.94 |
| 38 | α-Terpinene | 21.5±13.5 | 2.2±2.2 | 46.7±27.1 | 156.1±26.7 | 0.81 |
| 39 | p-Cymene | 10.0±4.0 | 1.1±1.1 | 17.3±6.0 | 92.8±54.4 | 1.16 |
| 40 | Limonene | 294.8±94.4 | 397.3±96.8 | 1135.9±200.7 | 9658.5±1160.4 | 0.36 |
| 41 | β-Phellandrene | 13.3±7.3 | 8.6±3.2 | 32.5±10.2 | 25.3±24.9 | 0.64 |
| 42 | 1,8-Cineole | 131.8±39.4 | 237.5±73.1 | 704.2±127.0 | 6110.3±773.4 | 0.38 |
| 43 | (E)-β-Ocimene | n.d. | n.d. | n.d. | 49.0±18.4 | 1.17 |
| 44 | γ-Terpinene | 28.6±15.1 | 2.2±2.2 | 56.9±30.8 | 239.5±42.7 | 1.22 |
| 45 | (E)-4-Thujanol | 1.6±1.6 | 13.2±8.1 | 41.5±13.6 | 413.0±113.9 | 1.04 |
| 46 | Terpinolene | 8.9±5.9 | n.d. | 5.5±4.2 | 81.6±14.3 | 1.11 |
| 47 | (Z)-4-Thujanol | n.d. | n.d. | 5.6±3.8 | 90.0±56.1 | 0.78 |
| 48 | (E)-DMNT | n.d. | 25.1±10.1 | 84.7±29.3 | 68.3±5.8 | 1.59 |
| 49 | Pinocarvone | n.d. | n.d. | n.d. | 64.9±13.6 | 1.18 |
| 50 | Terpinen-4-ol | n.d. | n.d. | n.d. | 17.6±5.9 | 0.91 |
| 51 | Carvone | n.d. | n.d. | n.d. | 24.9±9.4 | 0.93 |
| 52 | Longifolene | 12.3±2.2 | 9.6±4.1 | 11.5±4.0 | 20.3±3.7 | 0.57 |
| Unknown | ||||||
| 53 | C10H14O, 107,108B† | n.d. | n.d. | n.d. | 68.5±17.6 | 1.18 |
| Total amount | 1399±433 | 2225±635 | 10752±2531 | 51138±5813 |
Mean (±SE) of GC peak area (units g FW−1).
VIP, variable importance in the projection for PLS-DA. VIP-values >1 are most influential for separation of the treatments.
Numbers indicate ion masses of unknown compounds.
DMNT, (E)-4,8-dimethyl-1,3,7-nonatriene; n.d., not detected.
Fig. 6.
Principal component analysis (PCA) of the volatile pattern of plants infested with P. xylostella (px), P. rapae (pr), jasmonic acid-treated plants (ja), and control plants (ct). First (PC1) and second (PC2) principal component plotted against each other. Percentage variation explained between brackets. The ellipse defines the Hotelling's T2 confidence region (95%).
To gain more insight into which compounds differ between control, herbivore-damaged, and JA-treated plants, further analyses of the data were made using PLS-DA. This analysis showed that the volatile blends of the four treatments were significantly different, as it extracted four PLS components by cross-validation [PLS-DA: four PLS components, R2X (cum)=0.77, R2Y (cum)=0.916, Q2 (cum)=0.67], although the fourth component did not add any predictive value to the model. In addition, the VIP was calculated, which is a numerical value describing the importance of the X variables, for both the X and the Y parts (Wold et al., 1993, 2001). Compounds with a VIP-value >1 are considered to influence the separation between the groups (Eriksson et al., 2001; Paolucci et al., 2004). In this model, 19 compounds had a VIP-value >1 (Table 1). The most important compounds for separation were (in order of decreasing VIP-values): (Z)-3-hexen-1-yl acetate (1.97), hexyl acetate (1.59), (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) (1.59), 2-methyl-1-propanol (1.58), and (Z)-2-penten-1-yl acetate (1.54). Compounds that had little influence on the separation of the groups were (in order of increasing VIP): 3-heptanone (0.28), β-myrcene (0.35), limonene (0.36), β-pinene (0.37), α-thujene (0.37), 1,8-cineole (0.38), sabinene (0.39), α-pinene (0.39), and 2-heptanone (0.42).
Discussion
Previous studies documented that caterpillar-infested brassicaceous plants are more attractive than control plants for both Cotesia species as well as D. semiclausum (Blaakmeer et al., 1994; Geervliet et al., 1994; Van Poecke and Dicke, 2002; Bukovinszky et al., 2005). In the present study, JA-induced plants were tested against control plants, and all three parasitoid species preferred the volatiles from the JA-induced plants. However, the results also show that HIPV blends were more attractive to the parasitoids than the JA-induced volatile blends. These results are analogous to the results shown for predatory mite attraction to spider mite-induced Lima bean volatiles (Dicke et al., 1999), and parasitoid attraction to P. rapae-induced A. thaliana volatiles (Van Poecke and Dicke, 2002) and common armyworm-induced corn volatiles (Ozawa et al., 2004). This indicates that also in Brussels sprouts plants JA induces part of the plant defence response that is induced by herbivore damage, which renders the plant attractive for parasitoids, but that exogenous application of this phytohormone cannot fully mimic the plant response, indicating the involvement of other factors in the plant defence response.
In Brussels sprouts plants, the JA-induced volatile blend differed from that of the herbivore-infested plants and was less attractive to all three species of parasitoid wasps. The JA-treated as well as the herbivore-infested B. oleracea plants emitted larger amounts of DMNT, (E)-4-thujanol, hexyl acetate, (Z)-3-hexen-1-yl acetate, and (Z)-2-penten-1-yl acetate than control plants. These compounds also contributed most to the separation of the volatile blends of different treatments. JA-induced plants differed from herbivore- or non-induced plants mostly in terms of higher emission of several terpenes, such as β-ocimene, thuja-2,4(10)-diene, and terpinene. Previous studies on jasmonate-induced brassicaceous plants also showed larger amounts of several terpenoids and green leaf volatiles, such as DMNT and (Z)-3-hexen-1-yl acetate, compared with non-induced plants (Loivamäki et al., 2004; Ibrahim et al., 2005). JA-induced plants emitted the largest amount of volatiles of all treatments. This suggests that spraying the whole plant with 1 mM JA induces a stronger response than infestation with 30 P. rapae or 12 P. xylostella caterpillars for 24 h. Possibly, JA induced the whole plant, while herbivores were only feeding on small parts of the plant, removing <1% of total leaf area, and elicited a more local response. In A. thaliana, however, the volatile emission after 1 mM JA application was ∼10-fold lower than herbivore-induced volatile emission, but these plants were infested with ∼15-fold more caterpillars per gram fresh weight compared with infestation rates in this study (Van Poecke et al., 2002). While non-induced Brussels sprouts plants emitted the lowest amounts of volatiles and JA-treated plants emitted the largest amounts of volatiles, the parasitoids preferred the herbivore-induced plants, which emitted intermediate total amounts of volatiles, over JA-induced plants. This implies that not only the quantity (Geervliet et al., 1998), but also the qualitative composition of the volatile blend is important for parasitoid attraction. This corresponds to findings on the attractiveness of infested Brussels sprouts and mustard plants to D. semiclausum wasps, which also preferred the plants that emitted lower overall quantities of volatiles (Bukovinszky et al., 2005). Moreover, when volatiles emitted by JA-treated Lima bean plants were supplemented with MeSA (methyl salicylate), which is induced by spider-mite feeding but not by JA application (Dicke et al., 1999), the competitiveness of the JA-induced blend compared with the prey-induced blend was restored (De Boer and Dicke, 2004). Another explanation could be that JA induces not only attractive compounds, but also repellent volatiles that could mask the attractiveness (D'Alessandro et al., 2006), or perhaps some compounds become repellent at higher concentrations, as was shown, for example, for attraction of predatory mites to higher concentrations of MeSA (De Boer and Dicke, 2004).
Herbivore-infested cabbage plants already attract C. glomerata parasitoids within 1 h after infestation (Scascighini et al., 2005). The response increased after the first hour and this preference remained for at least 16 h. In the present study, similar results were obtained for the treatment with JA, although the response of the parasitoids was very weak in the first hours after treatment, i.e. 20–25% of the wasps responded. At 3 h after JA treatment the response increased and the parasitoids significantly preferred the JA-induced plants to control plants. The JA-induced volatile blends attracted parasitoids at least 5 d after the treatment. After 5 d the parasitoids still preferred the JA-treated plants to control plants, although the response level of the parasitoids declined slightly with time after 24 h. The effect of herbivore induction did not last as long: herbivore-damaged plants had lost their attractiveness 5 d after removal of the caterpillars. Mattiacci et al. (2001) obtained similar results when they tested the attractiveness of leaves excised from herbivore-induced cabbage plants. They found these were attractive to C. glomerata 1 d after removal of the P. brassicae caterpillars, but 2 d after removal the parasitoids no longer discriminated between previously infested and control leaves. Although removal of all herbivores after such a short time interval and JA application are both artificial treatments, these results indicate a difference between the induction by herbivory and JA treatment. Possibly, JA residues remained on the JA-treated plants, thus sustaining induction, while the local induction by caterpillar feeding stopped soon after removal of the caterpillars. In tomato plants in the field, increases in polyphenol oxidase and proteinase inhibitors can be measured 3 weeks after initial application of JA (Thaler, 1999b) and the numbers of parasitoid pupae were twice as high on JA-sprayed plants than on control plants at that time (Thaler, 1999a).
Endogenous JA levels increase soon after damage; for example, in Nicotiana sylvestris, the JA level in damaged roots was 100 ng g FW−1 after 90 min, a 10-fold increase compared with undamaged roots (Baldwin et al., 1997). In A. thaliana the JA level also increased ∼10-fold after 24 h of P. rapae feeding to 315 ng g FW−1 (Reymond et al., 2004). Brussels sprouts plants have a slightly lower endogenous JA level of 3.5 ng g FW−1, which increases to 38 ng g FW−1 after 24 h of P. rapae feeding. If we compare these levels with the dosages used in the present experiments, these are lower than the lowest JA dosage applied to the plant (the 1 μM JA application amounts to 140 ng g FW−1), and lower than all JA concentrations tested that resulted in significant changes in parasitoid behaviour (1.4, 14, and 140 μg g FW−1). However, it is difficult to compare exogenous amounts applied to the plants with endogenous concentrations since feeding by biting–chewing insects will induce plants continuously for 24 h, cause mechanical damage, and induce high levels locally and lower levels in the surrounding tissue. In contrast, for the JA treatment, the phytohormone was applied at one time point 24 h before testing the plants to intact plants and JA was sprayed evenly over the leaf surface. Furthermore, it is difficult to estimate losses due to spraying, and the percentage of JA effectively taken up by the leaves. Previous experiments indicated that a residue of JA remains on the leaves, that can be transferred by touching from one plant to another (M Bruinsma and TAL Snoeren, unpublished results), but it remains to be elucidated how much exactly is taken up by the plants after exogenous application. The differences between P. rapae and P. xylostella treatments may be due to the differences in leaf area eaten in the two treatments, which was larger for P. rapae.
Concentrations of JA similar to those used here have been employed in other behavioural, chemical, and molecular studies (for example, Dicke et al., 1999; Koch et al., 1999; Thaler, 1999a; Van Poecke and Dicke, 2002; Ozawa et al., 2004, 2008; Zheng et al., 2007). For example, in Brussels sprouts plants, Pieris butterflies preferred to oviposit on control leaves rather than on JA-treated leaves, when treated with 100 μM or 1 mM (Bruinsma et al., 2007). However, at a concentration of 10 μM the butterflies did not distinguish between treated and control plants, whereas the wasps still did. In this tritrophic system, the parasitoid was more sensitive to induction than its host, possibly because of the use of different cues for host location by herbivores and parasitoids. This indicates that several trophic levels of the insect community can be affected by JA-induced changes in plants. Bruinsma et al. (2008) describe a study in which JA is applied to flowering brassicaceous plants to investigate the response of another important group of associated arthropods, i.e. pollinators. While nectar secretion was affected by JA treatment, pollinator visitation did not change after JA treatment.
To eliminate the possibility that the observed attractiveness of JA-treated plants in this study was due to the olfactory perception of JA itself rather than to the induction of plant-produced infochemicals, JA was tested on an inert substrate. Similar to what was demonstrated for the herbivores (Bruinsma et al., 2007), JA itself did not influence the behaviour of C. glomerata. Therefore, the results are to be ascribed to induction processes in the plant.
JA biosynthesis is suggested to be regulated by positive feedback (Wasternack, 2007). This fits well with the observation that JA application to Brussels sprouts plants induces expression of BoLOX, a lipoxygenase gene from Brassica oleracea of the octadecanoid pathway upstream of JA, that is also induced by insect-herbivore feeding (Zheng et al., 2007). JA application will therefore not only induce compounds downstream, but also oxylipins and gene expression upstream of JA. OPDA, an intermediate of the octadecanoid pathway, has been demonstrated to mediate resistance in A. thaliana in the absence of JA (Stintzi et al., 2001) and accumulates in Brussels sprouts plants in response to herbivore infestation (Fig. 5). This study shows that also in Brussels sprouts JA-induced processes play an important role in the attraction of parasitoid wasps to the plants and that both timing and dose of JA induction affect attraction of parasitoids. JA-induced plants were attractive compared with control plants, but even though they emitted more volatiles, the parasitoids were still more attracted to herbivore-induced plants. This shows that parasitoids can discriminate between herbivore induction and artificial induction of plants, and that for actual herbivore induction more factors than JA alone are involved, such as the salicylic acid (SA) and ethylene pathway, and visual cues (reviewed in Dicke and Van Poecke, 2002; Kessler and Baldwin, 2002; Van Poecke, 2007). Many studies demonstrated a negative effect of SA on JA-inducible defences (Doares et al., 1995; Thaler et al., 2002; Cipollini et al., 2004). Ethylene was also shown to interact with JA-inducible defences (Kahl et al., 2000; Stotz et al., 2000). This suggests that while JA and other oxylipins play a central role in defence against herbivorous insects, as shown in this study, cross-talk between different phytohormones can fine-tune attacker-specific defence responses (Pieterse and Dicke, 2007), which offers interesting possibilities for future research.
Acknowledgments
We thank Rieta Gols for advice and help with volatile trapping and analysis, Monique Buurman for helping with behavioural experiments, Tjeerd Snoeren and Gabriele Gresser for their contribution to oxylipin analysis, and Leo Koopman, Frans van Aggelen, André Gidding, and Unifarm for rearing insects and plants. This work was supported by a VICI grant from the Netherlands Organisation for Scientific Research, NWO (865.03.002).
References
- Baldwin IT, Zhang ZP, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta. 1997;201:397–404. [Google Scholar]
- Blaakmeer A, Geervliet JBF, Van Loon JJA, Posthumus MA, Van Beek TA, De Groot A. Comparative headspace analysis of cabbage plants damaged by two species of Pieris caterpillars: consequences for in-flight host location by Cotesia parasitoids. Entomologia Experimentalis et Applicata. 1994;73:175–182. [Google Scholar]
- Brodeur J, Geervliet JBF, Vet LEM. The role of host species, age and defensive behaviour on ovipositional decisions in a solitary specialist and gregarious generalist parasitoid (Cotesia species) Entomologia Experimentalis et Applicata. 1996;81:125–132. [Google Scholar]
- Bruinsma M, IJdema H, Van Loon JJA, Dicke M. Differential effects of jasmonic acid treatment of Brassica nigra on the attraction of pollinators, parasitoids and butterflies. Entomologia Experimentalis et Applicata. 2008;128:109–116. [Google Scholar]
- Bruinsma M, Van Dam NM, Van Loon JJA, Dicke M. Jasmonic acid-induced changes in Brassica oleracea affect oviposition preference of two specialist herbivores. Journal of Chemical Ecology. 2007;33:655–668. doi: 10.1007/s10886-006-9245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovinszky T, Gols R, Posthumus MA, Vet LEM, van Lenteren JC. Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellen) Journal of Chemical Ecology. 2005;31:461–480. doi: 10.1007/s10886-005-2019-4. [DOI] [PubMed] [Google Scholar]
- Cipollini D, Enright S, Traw MB, Bergelson J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Molecular Ecology. 2004;13:1643–1653. doi: 10.1111/j.1365-294X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- D'Alessandro M, Held M, Triponez Y, Turlings TCJ. The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. Journal of Chemical Ecology. 2006;32:2733–2748. doi: 10.1007/s10886-006-9196-7. [DOI] [PubMed] [Google Scholar]
- De Boer JG, Dicke M. The role of methyl salicylate in prey searching behaviour of the predatory mite Phytoseiulus persimilis. Journal of Chemical Ecology. 2004;30:255–271. doi: 10.1023/b:joec.0000017976.60630.8c. [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in Lima bean plants. Journal of Chemical Ecology. 1999;25:1907–1922. [Google Scholar]
- Dicke M, Van Beek TA, Posthumus MA, Ben Dom N, Van Bokhoven H, De Groot AE. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions: involvement of host plant in its production. Journal of Chemical Ecology. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- Dicke M, Van Poecke RMP. Signaling in plant–insect interactions: signal transduction in direct and indirect plant defence. In: Scheel D, Wasternack C, editors. Plant signal transduction. Vol. 38. Oxford: Oxford University Press; 2002. pp. 289–316. [Google Scholar]
- Dicke M, Vet LEM. Plant–carnivore interactions: evolutionary and ecological consequences for plant, herbivore and carnivore. In: Olff H, Brown VK, Drent RH, editors. Herbivores: between plants and predators. Oxford: Blackwell Science; 1999. pp. 483–520. [Google Scholar]
- Doares SH, Narváez-Vásquez J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiology. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. Multi- and megavariate data analysis: principles and applications. Umeå, Sweden: Umetrics Academy; 2001. [Google Scholar]
- Geervliet JBF, Ariens S, Dicke M, Vet LEM. Long-distance assessment of patch profitability through volatile infochemicals by the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae) Biological Control. 1998;11:113–121. [Google Scholar]
- Geervliet JBF, Vet LEM, Dicke M. Volatiles from damaged plants as major cues in long-range host-searching by the specialist parasitoid Cotesia rubecula. Entomologia Experimentalis et Applicata. 1994;73:289–297. [Google Scholar]
- Geervliet JBF, Vet LEM, Dicke M. Innate responses of the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae) to volatiles from different plant–herbivore complexes. Journal of Insect Behavior. 1996;9:525–538. [Google Scholar]
- Gouinguené SP, Turlings TCJ. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiology. 2002;129:1296–1307. doi: 10.1104/pp.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M. Induction of two indirect defences benefits Lima bean (Phaseolus lunatus, Fabaceae) in nature. Journal of Ecology. 2004;92:527–536. [Google Scholar]
- Heil M. Indirect defence via tritrophic interactions. New Phytologist. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- Hilker M, Meiners T. Induction of plant responses to oviposition and feeding by herbivorous arthropods: a comparison. Entomologia Experimentalis et Applicata. 2002;104:181–192. [Google Scholar]
- Ibrahim MA, Nissinen A, Holopainen JK. Response of Plutella xylostella and its parasitoid Cotesia plutellae to volatile compounds. Journal of Chemical Ecology. 2005;31:1969–1984. doi: 10.1007/s10886-005-6071-x. [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gabler R, Kuhnemann F, Preston CA, Baldwin IT. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta. 2000;210:336–342. doi: 10.1007/PL00008142. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Annual Review of Plant Biology. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung V, Engelberth J, Boland W. Differential induction of plant volatile biosynthesis in the Lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiology. 1999;121:153–162. doi: 10.1104/pp.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loivamäki M, Holopainen JK, Nerg AM. Chemical changes induced by methyl jasmonate in oilseed rape grown in the laboratory and in the field. Journal of Agricultural and Food Chemistry. 2004;52:7607–7613. doi: 10.1021/jf049027i. [DOI] [PubMed] [Google Scholar]
- Mattiacci L, Rocca BA, Scascighini N, D'Alessandro M, Hern A, Dorn S. Systemically induced plant volatiles emitted at the time of ‘danger’. Journal of Chemical Ecology. 2001;27:2233–2252. doi: 10.1023/a:1012278804105. [DOI] [PubMed] [Google Scholar]
- Meiners T, Hilker M. Induction of plant synomones by oviposition of a phytophagous insect. Journal of Chemical Ecology. 2000;26:221–232. [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiology. 2005;138:1149–1162. doi: 10.1104/pp.104.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm R, Schrank K, Wegener R, Schulz S, Hilker M. Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. Journal of Chemical Ecology. 2003;29:1235–1252. doi: 10.1023/a:1023841909199. [DOI] [PubMed] [Google Scholar]
- Ohara Y, Takafuji A, Takabayashi J. Response to host-infested plants in females of Diadegma semiclausum Hellen (Hymenoptera: Ichneumonidae) Applied Entomology and Zoology. 2003;38:157–162. [Google Scholar]
- Ozawa R, Shiojiri K, Sabelis MW, Arimura GI, Nishioka T, Takabayashi J. Corn plants treated with jasmonic acid attract more specialist parasitoids, thereby increasing parasitization of the common armyworm. Journal of Chemical Ecology. 2004;30:1797–1808. doi: 10.1023/b:joec.0000042402.04012.c7. [DOI] [PubMed] [Google Scholar]
- Ozawa R, Shiojiri K, Sabelis MW, Takabayashi J. Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomologia Experimentalis et Applicata. 2008;129:189–199. [Google Scholar]
- Paolucci U, Vigneau-Callahan KE, Shi HL, Matson WR, Kristal BS. Development of biomarkers based on diet-dependent metabolic serotypes: characteristics of component-based models of metabolic serotypes. Omics—A Journal of Integrative Biology. 2004;8:221–238. doi: 10.1089/omi.2004.8.221. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Dicke M. Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends in Plant Science. 2007;12:564–569. doi: 10.1016/j.tplants.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Poelman EH, van Loon JJA, Dicke M. Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends in Plant Science. 2008;13:534–541. doi: 10.1016/j.tplants.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annual Review of Ecology and Systematics. 1980;11:41–65. [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. A conserved transcript pattern in response to a specialist and a generalist herbivore. The Plant Cell. 2004;16:3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelis MW, Janssen A, Pallini A, Venzon M, Bruin J, Drukker B, Scutareanu P. Behavioral responses of predatory and herbivorous arthropods to induced plant volatiles: from evolutionary ecology to agricultural applications. In: Agrawal AA, Tuzun S, Bent E, editors. Induced plant defenses against pathogens and herbivores. Biochemistry, ecology and agriculture. St. Paul, MN: APS Press; 1999. pp. 269–296. [Google Scholar]
- Scascighini N, Mattiacci L, D'Alessandro M, Hern A, Rott AS, Dorn S. New insights in analysing parasitoid attracting synomones: early volatile emission and use of stir bar sorptive extraction. Chemoecology. 2005;15:97–104. [Google Scholar]
- Schoonhoven LM, Van Loon JJA, Dicke M. Insect–plant biology. Oxford: Oxford University Press; 2005. [Google Scholar]
- Sjödin K, Schroeder LM, Eidmann HH, Norin T, Wold S. Attack rates of scolytids and composition of volatile wood constituents in healthly and mechanically weakend pine trees. Scandinavian Journal of Forest Research. 1989;4:379–391. [Google Scholar]
- Steinberg S, Dicke M, Vet LEM, Wanningen R. Response of the braconid parasitoid Cotesia (= Apanteles) glomerata to volatile infochemicals. Effects of bioassay set-up, parasitoid age and experience and barometric flux. Entomologia Experimentalis et Applicata. 1992;63:163–175. [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proceedings of the National Academy of Sciences, USA. 2001;98:12837–12842. doi: 10.1073/pnas.211311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz HU, Pittendrigh BR, Kroymann J, Weniger K, Fritsche J, Bauke A, Mitchell-Olds T. Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of Arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiology. 2000;124:1007–1017. doi: 10.1104/pp.124.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi J, Dicke M. Plant–carnivore mutualism through herbivore-induced carnivore attractants. Trends in Plant Science. 1996;1:109–113. [Google Scholar]
- Takabayashi J, Sabelis MW, Janssen A, Shiojiri K, van Wijk M. Can plants betray the presence of multiple herbivore species to predators and parasitoids? The role of learning in phytochemical information networks. Ecological Research. 2006;21:3–8. [Google Scholar]
- Thaler JS. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature. 1999a;399:686–688. [Google Scholar]
- Thaler JS. Jasmonic acid mediated interactions between plants, herbivores, parasitoids, and pathogens: a review of field experiments in tomato. In: Agrawal AA, Tuzun S, Bent E, editors. Induced plant defenses against pathogens and herbivores. Biochemistry, ecology and agriculture. St. Paul, MN: APS Press; 1999b. pp. 319–334. [Google Scholar]
- Thaler JS, Karban R, Ullman DE, Boege K, Bostock RM. Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia. 2002;131:227–235. doi: 10.1007/s00442-002-0885-9. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH. An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. Journal of Chemical Ecology. 1993;19:411–425. doi: 10.1007/BF00994314. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP. Arabidopsis–insect interactions. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis book. Rockville, MD: American Society of Plant Biologists; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poecke RMP, Dicke M. Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. Journal of Experimental Botany. 2002;53:1793–1799. doi: 10.1093/jxb/erf022. [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Posthumus MA, Dicke M. Signal transduction in Arabidopsis: molecular genetic and chemical analysis of different genotypes. In: Van Poecke RMP, editor. Indirect defence of Arabidopsis against herbivorous insects: combining parasitoid behaviour and chemical analysis with a molecular genetic approach. Wageningen: Thesis; 2002. pp. 81–101. [Google Scholar]
- Van Zandt PA, Agrawal AA. Community-wide impacts of herbivore-induced plant responses in milkweed (Asclepias syriaca) Ecology. 2004;85:2616–2629. [Google Scholar]
- Vet LEM, Dicke M. Ecology of infochemical use by natural enemies in a tritrophic context. Annual Review of Entomology. 1992;37:141–172. [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold S, Albano C, Dunn WJ, III, et al. Multivariate data analysis: converting chemical data tables to plots. Intelligent Instruments and Computers. 1989:197–216. [Google Scholar]
- Wold S, Johansson E, Cocchi M. PLS—partial least squares projections to latent structures. In: Kubinyi H, editor. 3D QSAR in drug design: theory, methods, and applications. Leiden, The Netherlands: ESCOM Science Publishers; 1993. pp. 523–550. [Google Scholar]
- Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometrics and Intelligent Laboratory Systems. 2001;58:109–130. [Google Scholar]
- Zheng S-J, Van Dijk J, Bruinsma M, Dicke M. Sensitivity and speed of induced defense of cabbage (Brassica oleracea L.): dynamics of BoLOX expression patterns during insect and pathogen attack. Molecular Plant-Microbe Interactions. 2007;20:1332–1345. doi: 10.1094/MPMI-20-11-1332. [DOI] [PubMed] [Google Scholar]