Abstract
Most modern wheat cultivars contain major dwarfing genes, but their effects on root growth are unclear. Near-isogenic lines (NILs) containing Rht-B1b, Rht-D1b, Rht-B1c, Rht8c, Rht-D1c, and Rht12 were used to characterize the effects of semi-dwarfing and dwarfing alleles on root growth of ‘Mercia’ and ‘Maris Widgeon’ wheat cultivars. Wheat seedlings were grown in gel chambers, soil-filled columns, and in the field. Roots were extracted and length and dry mass measured. No significant differences in root length were found between semi-dwarfing lines and the control lines in any experiment, nor was there a significant difference between the root lengths of the two cultivars grown in the field. Total root length of the dwarf lines (Rht-B1c, Rht-D1c, and Rht12) was significantly different from that of the control although the effect was dependent on the experimental methodology; in gel chambers root length of dwarfing lines was increased by ∼40% while in both soil media it was decreased (by 24–33%). Root dry mass was 22–30% of the total dry mass in the soil-filled column and field experiments. Root length increased proportionally with grain mass, which varied between NILs, so grain mass was a covariate for the analysis of variance. Although total root length was altered by dwarf lines, root architecture (average root diameter, lateral root:total root ratio) was not affected by reduced height alleles. A direct effect of dwarfing alleles on root growth during seedling establishment, rather than a secondary partitioning effect, was suggested by the present experiments.
Keywords: Rht1, Rht2, Rht3, Rht8, Rht10, Rht12, Triticum aestivum
Introduction
Today >70% of modern wheat cultivars contain major dwarfing genes (Evans, 1998). In northwest Europe, the semi-dwarfing alleles Rht-B1b (formerly Rht1) and Rht-D1d (formerly Rht2) are widely used; these have comparable mutation in two homeologous genes on chromosomes 4B and 4D in the hexaploid wheat genome (Boerner et al., 1996; McIntosh et al., 2003, 2006; McCartney et al., 2005). Cultivars containing these alleles show a reduced response to gibberellic acids (GAs) and a semi-dwarfing phenotype. Peng et al. (1997, 1999) identified Rht-B1b and Rht-D1b as homologues of the Arabidopsis thaliana GAI (gibberellic acid insensitive) and maize dwarf8 genes, which also cause a semi-dwarfing phenotype. These genes encode a transcription factor which belongs to the DELLA subgroup of the GRAS (GAI, RGA and SCARECROW-like) superfamily (Pysh et al., 1999) that act as negative regulators of the GA response. The Rht-B1b and Rht-D1b alleles used agronomically have dominant gain-of-function mutations in DELLA genes, which are predicted to be in the N-terminal DELLA motif, causing the protein to be resistant to GA-induced degradation. In addition to the wheat Rht-B1 and Rht-D1 genes, dominant mutations have been described in the highly conserved DELLA gene orthologues among other species, including maize, rice, barley, Brassica rapa, and grape (Peng et al., 1999; Ikeda et al., 2001; Boss and Thomas, 2002; Chandler et al., 2002; Gubler et al., 2002; Itoh et al., 2002; Hedden 2003; Muangprom and Osborn, 2004).
A third dwarfing gene used agronomically in wheat, Rht8, introduced into European wheats by the Italian breeder Strampelli in the 1930s, is linked to reduced plant height of the phenotype without reducing sensitivity to GA (Korzun et al., 1998; Worland et al., 1998). Only a few of the existing Rht genes are used agronomically, as typical features, such as reduced grain size and biomass, are not always compensated for by an increase in grain numbers and harvest index (Evans, 1998).
Many studies have investigated the effect of Rht alleles on the aerial parts of the plant, including, importantly, stem height (McCaig and Morgan, 1993) and coleoptile length (Ellis et al., 2004). McCaig and Morgan (1993) demonstrated that significant differences in stem height existed between tall near-isogenic lines (NILs), and semi-dwarfing and dwarf wheat lines within different genetic backgrounds, and that stem mass was reduced by dwarfing alleles, but not the leaf or head mass. Ellis et al. (2004) showed that different height-reducing alleles in NILs or induced mutants of wheat affected stem height, but not always coleoptile length. Botwright et al. (2001) demonstrated a difference in coleoptile length between tall wheat cultivars and GA-insensitive wheat lines, although there was no significant difference in coleoptile length between tall and Rht8 wheat cultivars.
The effects of the Rht alleles on root systems are, however, less clear, with studies in different growing conditions producing apparently contradictory results. Subbiah et al. (1968) reported that semi-dwarf wheat cultivars had shorter root systems than tall cultivars in field experiments. They argued that the consequence of a shorter root system might be decreased water uptake and yield in dry environments. Similarly Siddique et al. (1990) indicated that semi-dwarf wheat cultivars invested comparatively less dry matter into their root system than tall cultivars, resulting in a lower root:shoot mass ratio. McKey (1973) grew wheat in plexi-glass tubes filled with polytherm and perlite in a glasshouse environment and found that shoot mass was positively correlated with root mass in a study of progeny of wheat crosses and that cultivars containing Rht-B1b and Rht-D1b alleles had a smaller root mass compared with tall cultivars. However, Bush and Evans (1988) and Miralles et al. (1997) showed a negative correlation between shoot mass and root mass at anthesis in dwarfing lines of spring wheat grown in a 1:1 mixture of perlite and vermiculite and in a field experiment, respectively. In contrast, they observed a positive correlation between root and shoot mass before terminal spikelet formation in their isogenic dwarf wheat lines. Lupton et al. (1974) found no differences in root properties such as root length, root distribution, and phosphorus uptake between tall and semi-dwarfing wheat cultivars in field experiments, and other reports similarly indicate that there is little or no effect of Rht alleles on the wheat root system (Cholick et al., 1977—field experiment; Richards and Passioura, 1981—soil-filled tube experiment).
According to Lynch (2007), root system architecture may hold the key for a ‘second green revolution’, so how Rht alleles affect cereal roots is potentially of fundamental importance given the prevalence of these semi-dwarfing alleles in current wheat cultivars. The current literature is inconclusive about the effects of Rht alleles on root systems, possibly because comparisons have been carried out using wheat genotypes with different genetic backgrounds. This study aims to determine the effects of different Rht mutant alleles in a common genetic background on root growth. Seven NILs of Rht alleles in Trtitcum aestivum cv. Mercia and three NILs with a cv. Maris Widgeon background were used, and plants were grown using differing experimental methodologies.
Materials and methods
Seven NILs of wheat in a cv. Mercia background were grown in gel chambers, in soil-filled columns, and in the field to assess the effects of dwarfing alleles on root systems. All of the grains used in the experiments were obtained from plots that had been grown under identical conditions at Sonning Farm (51°29.0’ N, 0°53.9’ W), The University of Reading, UK in 2006. The NILs consisted of GA-insensitive, semi-dwarf (Rht-B1b, Rht-D1b from ‘Norin 10’) and dwarf (Rht-B1c from ‘Tom thumb’; Rht-D1c from ‘Ai-Bian’) lines, and GA-sensitive, semi-dwarf (Rht8c + Ppd-D1a from ‘Mara’), dwarf (Rht12 from ‘Karcagi 522’), and control (rht) lines (Addisu et al. 2009). Because the height of the control Mercia is already reduced, presumably due to the accumulation of several minor genes, three NILs (rht control, Rht-B1b, Rht-B1c) in a tall cultivar background (cv. Maris Widgeon) were also included in the field experiment.
Pre-weighed grains were surface sterilized and pre-germinated before being grown in gel chambers, and soil-filled columns. The grains were soaked in deionized water for 1 h, surface sterilized in saturated calcium hypochlorite solution for 15 min, washed twice for 5 min, and then left to imbibe in deionized water for 1 h. They were placed in rows on wet chromatography paper (Whatman 3MM Chr) within Petri dishes with the grain oriented vertically so that the radicles faced downwards. The Petri dishes were sealed with ‘Parafilm’ to avoid evaporation and ensure sufficient moisture during germination. The grains were kept at 12 °C (±1 °C) in the dark for 3 d, to allow germination.
At harvest, all root systems were recorded digitally using a flatbed scanner (Epson Expression 1600XL-Pro). Root properties (e.g. total root length) were estimated using ‘Winrhizo’ or ‘Winrhizotron Pro’ software (WinRhizo, Regent Instruments, Canada). Root and shoot dry mass were measured for material dried at 80 °C for at least 3 d. Analysis of variance (ANOVA) of all data was conducted using the statistical program ‘Genstat 11.0’ (VSN International Ltd, UK). Because NILs containing dwarfing alleles had a smaller mean grain mass (based on the mass of 1000 grains) compared with the control lines, the effect of grain mass on root growth was assessed using grains covering the range of variation of grain mass for each line in the gel chamber and field experiments. The correlation between grain mass (mg) and root system properties was assessed by ANOVA, and grain mass was used subsequently as a covariant in the gel chamber and field experiment.
Gel chambers
Eight grains representing the grain mass range of each NIL with a cv. Mercia background were used for each experiment. The grains were transferred to gel chambers comprising two closely spaced 2 mm deep layers of transparent gel (Agar Technical from Oxoid, Basingstoke, UK), on 215×300×3 mm plastic plates (one of which was transparent and the other black) allowing gas exchange through a 2 mm gap between the gel layers (described by Bengough et al., 2004). The seedlings were arranged with uniform orientation. The grains were placed 3.7 cm deep in the gel chamber and the chamber was placed in a thick, brown envelope to exclude light. The seedlings were grown at a constant temperature of 15 °C with 12 h day and night in a growth chamber. The total root length of each seedling was measured every second day by scanning the gel chamber using a flatbed scanner (Epson Expression 1600XL-PRO). The gel chambers were reshuffled after each measurement to avoid positional effects. At the end of the experiment (10 d), the plants had reached growth stage 12 on the Zadoks scale (second leaf unfolded), and root and shoot dry mass were measured. The experiment was repeated three times to give replicated results on the effect of grain size on early root growth.
Soil-filled columns
Ten grains (each 40±1 mg) of each NIL were selected, surface sterilized, and pre-germinated. A pre-germinated grain was transferred (sown at 4 cm) to each column on 12 July 2007. Each column consisted of 4.8 cm×4.8 cm×100 cm long PVC cable trunking with a removable side which facilitated irrigation of the soil along the entire length. Before packing the columns, the water content of the soil was determined using the average weight loss of three 100 g soil samples, which were dried at 105 °C for 24 h. The columns were filled with a sieved sandy silt loam (sand 71%, silt 19%, clay 10%, and pH 6.2) arable topsoil (4 mm mesh, Carpow Series; Walker et al., 1982) from the SCRI (Scottish Crop Research Institute, Dundee, UK) farm to a dry bulk density of 1.1 Mg m−3. After packing, known volumes of water were sprayed evenly along the entire column over a period of several hours until the required volumetric water contents of 27% (‘wet’) and 18.5% (‘moist’) were achieved. Any water lost from the ‘wet’ columns during the experiment was subsequently replaced by spraying water (according to the weight loss) along the entire length of the root column every third day. No water was replaced in the ‘moist’ columns, which led to a water loss of 3% during the experiment. The plants were placed in a glasshouse in five randomized blocks and surrounded by guard plants to avoid edge effects. The experiment lasted for 26 d (July 2007) until the main shoot started tillering (Zadoks scale: growth stage 20). Root systems were washed from the soil, and root length and diameter, and root and shoot dry mass were measured.
Field experiment
The experiment was designed with five blocks containing three randomized subplots containing each NIL. The mass of each grain was recorded and the individual grains (including the three NILs of T. aestivum cv. Maris Widgeon) planted directly (sowing depth: 5 cm) on 26 October 2007, without pre-germination, in PVC tubes (20 cm long×7.5 cm diameter) that had previously been pushed into a power-harrowed and rolled sandy loam soil [Sonning series (Jarvis, 1968) at Sonning Farm, Reading University, UK]. The soil had the following properties: pH 6.9, and available phosphate, potassium, magnesium, and sulphate concentrations (mg l−1): 27.2, 95, 45, and 16.5, respectively. The field was fertilized according to standard recommendations with 117 kg of muriate of potash ha−1, equivalent to 70 kg K2O ha−1, on 17 September 2007. The plants were grown for 283 °Cd (30 d, November 2007), reaching the same growth stage (second leaf unfolded, Zadoks scale: 12) as plants in the gel chamber experiment. The tubes facilitated sampling of intact root systems which were gently washed from the tubes before measurement of the total root length.
Results
Effects of grain mass on root growth
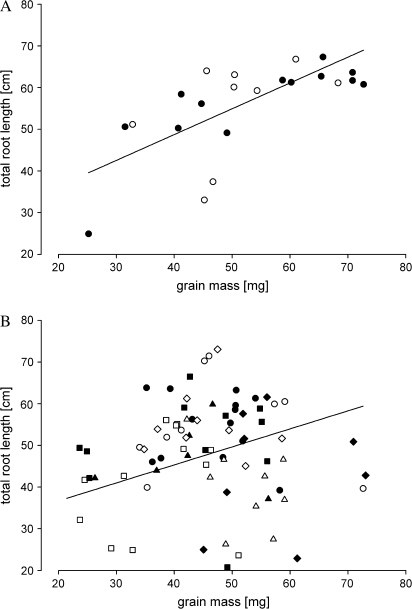
There were differences between wheat NILs in mean grain mass in comparison with both Mercia and Maris Widgeon. For Mercia, the mean grain mass was control (43 mg), Rht-B1b (36 mg), Rht-D1b (34 mg), Rht-B1c (28 mg), Rht8c (36 mg), Rht-D1c (24 mg), and Rht12 (27 mg), and in Maris Widgeon control (52.6 mg), Rht-B1b (43.2 mg), and Rht-B1c (43.6 mg). The grains of NILs showing a dwarfing phenotype (Rht-B1c, Rht-D1c, and Rht12) had a shrunken grain phenotype. Linear regression showed that grain mass and total root length of seedlings at 30 d were correlated (Fig. 1); greater grain mass was significantly (controls: P=0.001 and semi-dwarfs and dwarfs: P=0.020) associated with increased total root length in the field experiment. Grain mass was therefore used as a covariant for the ANOVA of roots in gel chamber and field experiments. Linear regression of the gel chamber results demonstrated an increase of total seminal root length of 36 mm mg−1 grain mass. A similar result was obtained in the field-grown plants in which total seminal root length increased by 41 mm mg−1 grain mass.
Fig. 1.
Relationship between total root lengths measured at 30 d and grain mass for (A) control (rht) cv. Mercia (open circles) and cv. Maris Widgeon control (rht) (filled circles). The line is the linear regression (y=0.619x+23.959; r2=0.48).(B) Semi-dwarf and dwarf NILs in cv. Mercia Rht-B1b (open circles), Rht-D1b (filled circles), Rht-B1c (open squares), Rht8c (filled squares), Rht-D1c (open triangles), Rht12 (filled triangles) and in cv. Maris Widgeon Rht-B1b (open diamonds), Rht-B1c (filled diamonds). The line is the linear regression (y=0.4320x+28.0470; r2 = 0.12).
Shoot
Shoot dry mass of the Rht-D1c isoline was less than that of the control in the gel chamber experiment, while the other lines were similar to the control plants. Furthermore, there were no significant differences in shoot dry mass between the isogenic lines and their controls and different cultivars in the soil-filled column and field experiments (Table 1).
Table 1.
Root dry mass (DM), shoot DM, and root:total plant dry mass ratio of cv. Mercia measured in a gel chamber, a soil-filled column, and field experiments
| Experiment | Control (rht) | Rht-B1b | Rht-D1b | Rht-B1c | Rht8c | Rht-D1c | Rht12 | SED | lsd 5% | |
| Root DM (mg) | Gel chamber | 5.57 | 6.41 | 6.24 | 6.87 | 6.31 | 6.84 | 7.49 | 0.78 | 1.54 |
| Soil-filled columns | 144 | 169 | 183 | 105 | 205 | 97 | 135 | 30 | 61 | |
| Field | 2.96 | 4.66 | 6.49 | 4.42 | 3.42 | 2.87 | 3.45 | 1.07 | 2.13 | |
| Shoot DM (mg) | Gel chamber | 6.79 | 6.01 | 6.58 | 5.82 | 6.84 | 5.47 | 7.45 | 0.61 | 1.21 |
| Soil-filled columns | 471 | 507 | 545 | 387 | 460 | 315 | 422 | 80 | 160 | |
| Field | 8.97 | 8.45 | 8.15 | 10.67 | 9 | 7.76 | 8.59 | 2.01 | 3.98 | |
| Root:total plant ratio | Gel chamber | 0.45 | 0.51 | 0.48 | 0.54 | 0.47 | 0.55 | 0.50 | 0.02 | 0.05 |
| Soil-filled columns | 0.23 | 0.24 | 0.25 | 0.22 | 0.29 | 0.25 | 0.24 | 0.03 | 0.06 | |
| Field | 0.25 | 0.31 | 0.34 | 0.23 | 0.31 | 0.23 | 0.23 | 0.05 | 0.09 |
Shoot length of the field-grown plants was measured at the two-leaf stage. No significant differences were found between NILs in the Maris Widgeon background, but the shoot length of Rht12 (8.24 cm) was significantly less (P <0.05) than that of the control (11.22 cm) in the Mercia background. A comparison of shoot length between the NILs for the same mutant allele in different cultivar backgrounds showed that Rht-B1c was significantly shorter in the Mercia (9.75 cm) background than in that of the Maris Widgeon (12.13 cm) background. The least significant difference (P <0.05) was 1.6 cm for both measurements.
Roots
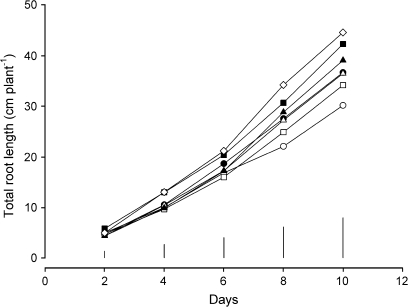
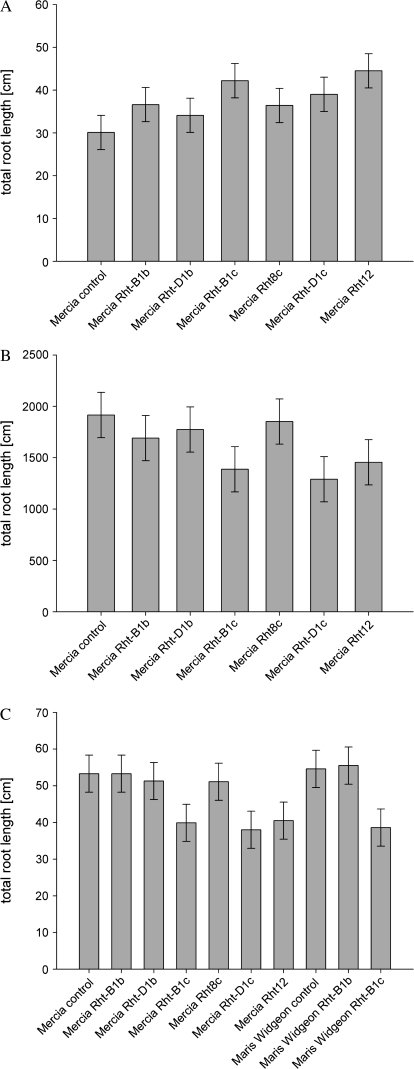
In the gel chamber experiment, total root length (Fig. 2) of the dwarf lines (Rht-B1c, Rht-D1c, and Rht12) was significantly increased compared with the control and semi-dwarf lines over time. The NILs with a dwarf genotype had a significantly increased total root length compared with the control at day 4 (Rht12, P=0.04) and day 8 (Rht-B1c and Rht-D1c, P=0.02). Mean total root length adjusted for the covariate of the control was 30.1 cm (Fig. 3a), while that of the dwarf genotypes was significantly increased (P=0.02) by 40% (Rht-B1c), 29% (Rht-D1c), and 48% (Rht12) at day 10 of the experiment. In contrast to the gel chamber experiment, total root length was significantly reduced in the dwarf lines compared with the control in both the soil column and field experiments. In the soil-filled column experiment, total root length of the dwarf lines was significantly [P= 0.035, least significant difference (lsd) 5%=446.3 cm per plant] decreased by 29% (Rht-B1c), 33% (Rht-D1c), and 24% (Rht12) compared with the control (mean control: 1932 cm) and semi-dwarfing lines (Rht-B1b, 1691 cm; Rht-D1b, 1775 cm; and Rht8c, 1852 cm) (Fig. 3b). Similarly in the field experiment (Fig. 3c), the dwarf lines of both backgrounds had significantly (P <0.001, lsd 5%=10.04 cm per plant) shorter total root length compared with the controls (Mercia, 53.3 cm and Maris Widgeon, 54.6 cm): [Mercia Rht-B1c (–25%), Mercia Rht-D1c (–29%), and Mercia Rht12 (–24%); and Maris Widgeon Rht-B1c (–29%)]. However, there were no significant differences between the semi-dwarfing lines (Mercia Rht-B1b, 53.3 cm; Mercia Rht-D1b, 51.3 cm; Mercia Rht8c, 51.1 cm; and Maris Widgeon Rht-B1b, 55.5 cm) and the control line in any of the experiments, nor was there a significant difference between the total root lengths of the two cultivars grown in the field.
Fig. 2.
Effects of different Rht NILs on total root length over time. Pre-germinated seedlings were grown in gel chambers and the root system was measured every second day. Control (open circles), Rht-B1b (filled circles), Rht-D1b (open squares), Rht-B1c (filled squares), Rht8c (open triangles), Rht-D1c (filled triangles), and Rht12 (open diamonds) in cv. Mercia (the bars=lsd 5%).
Fig. 3.
Effects of Rht NILs on total root length. (A) Total root length of NILs grown in gel chambers after 10 d (the bar is ±1 SED: lsd 5%=7.90 cm per plant). (B) Total root length of plants grown in soil-filled columns after 26 d (the bar is ±1 SED: lsd 5% = 441.4 cm per plant). (C) Effects of Rht NILs on total root length of field-grown plants. NILs of two wheat cultivars (tall cultivar cv. Maris Widgeon and cv. Mercia) were measured after 30 d (the bar is ±1 SED: lsd 5%=9.02 cm per plant).
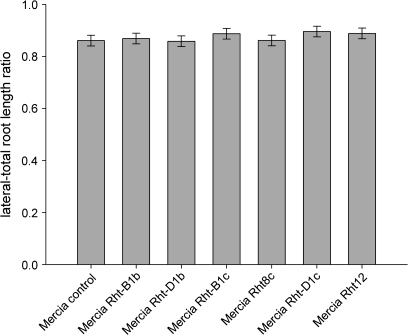
Seminal root and lateral root growth and average root diameter were not significantly altered by dwarfing alleles in the soil-filled column experiment, and the ratio between lateral root and seminal root lengths, and the lateral root:total root length ratio were also unaffected (Fig. 4). The average root diameter was not significantly (P=0.502, lsd 5%=0.09 mm per plant) affected by the different reduced height alleles: control (0.61 mm per plant), Rht-B1b (0.592 mm per plant), Rht-D1b (0.610 mm per plant), Rht-B1c (0.564 mm per plant), Rht8c (0.621 mm per plant), Rht-D1c (0.543 mm per plant), and Rht12 (0.557 mm per plant). There was no effect of volumetric water content of the soil, whether as a main effect or as an interactive with genotype using the ANOVA model: total root length or average root diameter=mean+block+genotype+volumetric water content+genotype×volumetric water content interaction.
Fig. 4.
Lateral root:total root length ratio of plants grown in a soil-filled column (cv. Mercia) measured after 26 d (the bar is ±1 SD: lsd 5%=0.04).
Root dry mass of Rht12 (7.49 mg per plant) was significantly (lsd 5% 1.54 cm per plant) greater than that of the control (5.57 mg per plant) in the gel chamber experiment, but the other lines showed no significant difference. No significant differences between the root:plant dry mass ratios of NILs were observed in the soil-filled column experiment. There was a significant difference in root dry mass between some lines in the field experiment as Mercia Rht-D1b had a root dry mass (adjusted for the covariant) of 6.49 mg per plant compared with that of the control of 2.96 mg per plant (lsd 5% 2.13 mg per plant). Root dry mass was 22–30% of the total dry mass in the soil-filled column and field experiments. However, the root:total plant dry mass ratios of Rht-B1b (0.51 per plant), Rht-B1c (0.54 per plant), Rht-D1c (0.55 per plant), and Rht12 (0.50 per plant) were significantly greater (lsd 5% 0.05 per plant) than the control plants (0.45 per plant) in the gel chamber experiment (Table 1).
Discussion
Neither total root length, nor any of the components constituting the total length, of NILs with a semi-dwarfing shoot phenotype (Rht-B1b, Rht-D1b, and Rht8c) were significantly different compared with the control plants in any experiment. This result supports a previous study comparing control (rht) with isogenic semi-dwarfing lines (Miralles et al., 1997) and a range of studies comparing tall and semi-dwarfing cultivars (Lupton et al., 1974; Cholick et al., 1977; Holbrook and Welsh 1980; Miralles et al., 1997). The findings do not support Siddique et al. (1990) who postulated that semi-dwarfing wheat cultivars reduced investment of dry mass in their root systems compared with tall cultivars. Similarly, our findings are not in agreement with those of Hurd (1974) who found that semi-dwarfing lines had a larger root system compared with a tall control in the same genetic background. These different findings may be due to the effect of either different genetic backgrounds or different growing environments, or both. Bush and Evans (1988) demonstrated that root dry mass did not vary between lines with semi-dwarfing and dwarfing alleles [a comparsion of a tall control (rht) with Rht-B1b+Rht-D1b (Rht1+2) in an isogenic line in a cv. Ciano-67 genetic background], but was influenced by different temperature regimes. In the present study, root elongation rate (data not shown) was affected by the different temperatures in the column and field experiments, but the relative effects of the dwarfing alleles on total root length compared with the controls were unchanged. Interactions with the environment are evident in Hargreaves et al. (2009), and the present study also showed contrasting results for root systems grown in gel chambers and soil-grown plants; such environmental effects evidently complicate comparisons between experiments with different methodologies. For example, Laperche et al. (2006) found significant effects of Rht-B1b alleles on root architecture in a root trait quantitative trait locus (QTL) study, when plants were grown in gel chambers. The study showed a significant effect of Rht-B1b on lateral root length. However, in the present study there was no significant effect of Rht-alleles (Rht-B1b and Rht-D1b) on lateral root:total root length ratios. Although total root length was significantly decreased in NILs with a dwarf as opposed to a semi-dwarf phenotype, no significant difference of average root diameter and lateral root:total root ratio was found (Fig. 4).
The effects of dwarfing alleles in addition to semi-dwarfing alleles in the same genetic background (Rht-B1c, Rht-D1c, and Rht12) on root growth were also measured in this study. The effect of these Rht alleles, causing a dwarf phenotype, were not affected by the genetic background [comparison between a tall cultivar (Maris Widgeon) and a height-reduced cultivar (Mercia), probably due to the accumulation of several minor genes], but were heavily influenced by the experimental methodology and environment. The NILs with these dwarfing alleles showed an increase in total root length compared with the control plants in the gel chamber experiment, but a decrease in the soil-filled column and field experiment. An explanation for this difference in behaviour might lie in the different availability of nutrients in the two experiments. The results of the current study contrast with those of Landjeva et al. (2007) who found no significant difference in root length between Rht-B1c (130 mm per plant) and the control (123.3 mm per plant−1, SED 11.11 mm) in Maris Widgeon, but found a significant difference (P=0.05) comparing Rht-B1c (123.3 mm per plant) with the control (144.5 mm per plant, SED 10.81 mm) in Mercia, when the plants were grown on filter paper moistened with distilled water.
Miralles et al. (1997) and Richards (1992) suggested that decreased shoot biomass is positively correlated with root growth as roots act as a sink for photosynthates, with the surplus of photoassimilates being allocated to roots. Two studies have shown an increase of root mass ratio with height reduction in isogenic wheat lines for Rht alleles (Bush and Evans, 1988; McCaig and Morgan, 1993). Miralles et al. (1997) demonstrated that root dry mass of dwarfs was 15% of total plant biomass, compared with 10% in the control and semi-dwarfing plants at anthesis, and that root dry mass of plants with a dwarf phenotype was increased by 5% at anthesis and 7% at maturity in a comparison of the double dwarfs Rht-B1b+Rht-D1b (Rht1+2) and Rht-D1b+Rht-B1c (Rht2+3) with a semi-dwarf Rht-D1b. Interestingly, there were no significant differences in shoot lengths or leaf dry masses among the NILs with reduced height alleles in the present experiments, but Rht-B1c, Rht-D1c, and Rht12 still affected total root lengths. This implies that there is a more direct effect of these alleles on root growth during seedling establishment and not simply an influence on the partitioning of dry matter between root and shoot at least in these early stages of growth. However, it is possible that root growth may be influenced by indirect effects of the reduced shoot biomass in later developmental stages. For example, the increased root dry mass of spring wheat lines at anthesis and maturity found by Miralles et al. (1997) could be a result of partitioning of surplus C to roots in those lines with severely limited shoot growth. Partitioning in wheat is influenced by ontogeny and nutrient availability as well as genotype (Siddique et al. 1990; Rengel and Graham 1995; Gregory and Eastham 1996), so more work is required to distinguish whether the observed partitioning effects are determined only by the dwarfing alleles.
In the present study, results were corrected for grain mass in the gel chamber and field experiments, as increased grain mass was correlated with increasing total root length. Richards and Passioura (1981) described a correlation between grain mass and seminal root numbers and xylem vessel numbers and diameter within seminal roots of wheat plants. An increase in grain mass also resulted in an increase of root numbers, xylem vessel diameters, and xylem vessels per seminal root. This finding supports the conclusions of Lopez-Castaneda et al. (1996) who found a positive correlation between seed mass and embryo size and concluded that these properties were the principal factor increasing shoot and root mass and therefore early vigour. De Marco (1990) also demonstrated that root length increased as grain mass increased in wheat. In the present experiment, the measured differences in total root lengths between the control and Rht-D1c were not significantly different when data were not corrected for grain mass as an ANOVA covariant in the gel chamber experiment though Rht-B1c and Rht12 still showed an increased total root length. When the field experiment data were not corrected for differences of average grain mass, the significance of the difference between the control plants and the Rht-B1c, Rht-D1c, and Rht12 NILs increased.
This study has shown that the dwarfing alleles Rht-B1c, Rht-D1c, and Rht12 have significant effects on root length of young seedlings compared with control and semi-dwarf lines, but no effect on early shoot growth (except Rht12 in the field experiment). This would seem to indicate a direct effect of dwarfing alleles on roots during seedling establishment rather than the secondary partition effect suggested by Richards (1992) and Miralles et al. (1997). The mechanisms underlying such a direct effect on roots are uncertain, but Ubeda-Tomas et al. (2008) demonstrated that root cell expansion was regulated by the DELLA gene (orthologues of Rht-B1b, RhtB1c, Rht-D1b, and Rht-D1c) in the endodermis in A. thaliana. However, while the reduced root growth of the isogenic line with a GA-sensitive Rht12 allele was significant it presumably occurred without a direct effect of the DELLA genes.
Further work is needed to determine how root growth is affected by the GA pathway, and the sites of Rht gene expression in roots. The experiments reported here also raise methodological issues in the characterization of wheat roots. The differences between the results obtained in the gel and soil experiments cast some doubt on the relationship of results found in any gel-based experiment to field performance in soil. The agronomic use of the dwarf shoot phenotype (e.g. Rht-B1c) has not been successful because these phenotypes with reduced above-ground biomass also have reduced light interception and use, lower resistance to leaf diseases, and exhibit poorer crop establishments when sown deep in dry environments. However, future work should explore the possibility of enhancing root growth via the GA pathway to enable crops to grow in drier and more infertile soils.
Acknowledgments
We thank Professor John Snape and the John Innes Centre for providing the NILs, and Richard Casebow and Rebecca Kiff for the field preparation at Sonning farm. Drs Tim George and Joanne Russell and Professor Robbie Waugh provided valuable improvements to an earlier draft.
References
- Addisu M, Snape JW, Simmonds JR, Gooding MJ. Reduced height (Rht) and photoperiod insensitivity (Ppd) allele associations with establishment and early growth of wheat in contrasting production systems. Euphytica. 2009;166:249–267. [Google Scholar]
- Bengough AG, Gordon DC, Al-Menaie H, Ellis RP, Allan D, Keith R, Thomas WTB, Forster BP. Gel observation chamber for rapid screening of root traits in cereal seedlings. Plant and Soil. 2004;262:63–70. [Google Scholar]
- Boerner A, Plaschke J, Korzun V, Worland AJ. The relationships between the dwarfing genes of wheat and rye. Euphytica. 1996;89:69–75. [Google Scholar]
- Boss P, Thomas M. Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature. 2002;416:847–850. doi: 10.1038/416847a. [DOI] [PubMed] [Google Scholar]
- Botwright T, Rebetzke G, Condon T, Richard R. The effect of Rht genotype and temperature on coleoptile growth and dry matter partitioning in young wheat seedlings. Australian Journal of Plant Physiology. 2001;28:417–423. [Google Scholar]
- Bush MG, Evans LT. Growth and development in tall and dwarf isogenic lines of spring wheat. Field Crops Research. 1988;18:243–270. [Google Scholar]
- Chandler PM, Marion-Poll A, Ellis M, Gubler F. Mutants at the slender1 locus of barley cv Himalaya. Molecular and physiological characterization. Plant Physiology. 2002;129:181–190. doi: 10.1104/pp.010917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholick FA, Welsh JR, Cole CV. Rooting patterns of semi-dwarf and tall winter wheat cultivars under dryland field conditions. Crop Science. 1977;17:637–640. [Google Scholar]
- De Marco DG. Effect of seed weight and seed phosphorus and nitrogen concentrations on the early growth of wheat seedlings. Australian Journal of Experimental Agriculture. 1990;30:545–550. [Google Scholar]
- Ellis MH, Rebetzke G, Chandler P, Bonnett D, Spielmeyer W, Richards RA. The effect of different height reducing genes on early growth of wheat. Functional Plant Biology. 2004;31:583–589. doi: 10.1071/FP03207. [DOI] [PubMed] [Google Scholar]
- Evans LT. Feeding the ten billion: plants and population growth. Cambridge: Cambridge University Press; 1998. pp. 133–150. [Google Scholar]
- Gregory PJ, Eastham J. Growth of shoots and roots, and interception of radiation by wheat and lupin crops on a shallow, duplex soil in response to time of sowing. Australian Journal of Agricultural Research. 1996;47:427–447. [Google Scholar]
- Gubler F, Chandler PM, White RG, Llewellyn DJ, Jacobsen JV. Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression. Plant Physiology. 2002;129:191–200. doi: 10.1104/pp.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves CE, Gregory PJ, Bengough AG. Measuring root traits in barley (Hordeum vulgare ssp. vulgare and ssp. spontaneum) seedlings using gel chambers, soil sacs and x-ray microtomography. Plant and Soil. 2009;316:285–297. [Google Scholar]
- Hedden P. The genes of the green revolution. Trends in Genetics. 2003;19:5–9. doi: 10.1016/s0168-9525(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Holbrook FS, Welsh JR. Soil-water use by semidwarf and tall winter wheat cultivars under dryland field conditions. Crop Science. 1980;20:244–246. [Google Scholar]
- Hurd EA. Phenotype and drought tolerance in wheat. Agricultural Meteorology. 1974;14:39–55. [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J. Slender Rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. The Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. The Plant Cell. 2002;14:57–70. doi: 10.1105/tpc.010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis RA. Soils of the Reading District. Memoirs of the Soil Survey of Great Britain, England and Wales. Harpenden, UK: Agricultural Research Council; 1968. pp. 77–78. [Google Scholar]
- Korzun V, Roder MS, Ganal MW, Worland AJ, Law CN. Genetic analysis of the dwarfing gene (Rht8) in wheat. Part I. Molecular mapping of Rht8 on the short arm of chromosome 2D of bread wheat (Triticum aestivum L.) Theoretical and Applied Genetics. 1998;96:1104–1109. [Google Scholar]
- Landjeva S, Korzun V, Stoimenova E, Truberg B, Ganeva G, Rner A. The contribution of the gibberellin-insensitive semi-dwarfing Rht genes to genetic variation in wheat seedling growth in response to osmotic stress. Journal of Agricultural Science. 2007;146:275–286. [Google Scholar]
- Laperche A, Vienne-Barret F, Maury O, Gouis JL, Ney B. A simplified conceptual model of carbon/nitrogen functioning for QTL analysis of winter wheat adaptation to nitrogen deficiency. Theoretical and Applied Genetics. 2006;113:1131–1146. doi: 10.1007/s00122-006-0373-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Castaneda C, Richards RA, Farquhar GD, Williamson RE. Seed and seedling characteristics contributing to variation in early vigor among temperate cereals. Crop Science. 1996;36:1257–1266. [Google Scholar]
- Lupton FGH, Oliver RH, Ellis FB, Barnes BT, Howse KR, Welbank PJ, Taylor PJ. Root and shoot growth of semi-dwarf and taller winter wheats. Annals of Applied Biology. 1974;77:129–144. [Google Scholar]
- Lynch JP. Roots of the second green revolution. Australian Journal of Botany. 2007;55:493–512. [Google Scholar]
- McCaig TN, Morgan JA. Root and shoot dry matter partitioning in near-isogenic wheat lines differing in height. Canadian Journal of Plant Science. 1993;73:679–689. [Google Scholar]
- McCartney CA, Somers DJ, Humphreys DG, Lukow O, Ames N, Noll J, Cloutier S, McCallum BD. Mapping quantitative trait loci controlling agronomic traits in the spring wheat cross RL4452בAC Domain’. Genome. 2005;48:870–883. doi: 10.1139/g05-055. [DOI] [PubMed] [Google Scholar]
- McIntosh RA, Devos KM, Dubcovsky J, Rogers WJ, Morris CF, Appels R, Anderson OA. Catalogue of gene symbols for wheat: 2006 supplement. 2006 In: Integrated Database of Wheat http://www.shigen.nig.ac.jp/wheat/komugi/genes/macgene//supplement2006.pdf. [Google Scholar]
- McIntosh R, Yamazaki Y, Devos K, Dubcovsky J, Rogers W, Appels R. Catalogue of gene symbols for wheat (MacGene 2003) [CD-ROM]. In: Pogna NE. Proceedings of the 10th International Wheat Genetics Sympsium. 2003;Vol. 4 Pasetum, Italy. 1–6 September 2003. Istituto Sperimentale per la Cerealicoltura, Rome, Italy. [Google Scholar]
- McKey J. The wheat root. Proceedings 4th International Wheat Genetics Symposium. 1973 Missouri Agricultural Experiment Station, College of Agriculture, University of Missouri, Columbia, Missouri, USA, 827–842. [Google Scholar]
- Miralles DJ, Slafer GA, Lynch V. Rooting patterns in near-isogenic lines of spring wheat for dwarfism. Plant and Soil. 1997;197:79–86. [Google Scholar]
- Muangprom A, Osborn TC. Characterization of a dwarf gene in Brassica rapa, including the identification of a candidate gene. Theoretical and Applied Genetics. 2004;108:1378–1384. doi: 10.1007/s00122-003-1551-2. [DOI] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards D, King K, Cowling R, Murphy G, Harberd N. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes and Development. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature. 1999;400:256–261. doi: 10.1038/22307. [DOI] [PubMed] [Google Scholar]
- Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN. The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. The Plant Journal. 1999;18:111–119. doi: 10.1046/j.1365-313x.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- Rengel Z, Graham RD. Importance of seed Zn content for wheat growth on Zn-deficient soil 1. Vegetative growth. Plant and Soil. 1995;173:259–266. [Google Scholar]
- Richards RA. The effect of dwarfing genes in spring wheat in dry environments. 2. Growth, water use and water-use efficiency. Australian Journal of Agricultural Research. 1992;43:529–539. [Google Scholar]
- Richards RA, Passioura JB. Seminal root morphology and water use of wheat. I. environmental effects. Crop Science. 1981;21:249–252. [Google Scholar]
- Siddique HM, Belford RK, Tennant D. Root:shoot ratios of old and modern, tall and semi-dwarf wheats in a mediterranean environment. Plant and Soil. 1990;121:89–98. [Google Scholar]
- Subbiah BV, Katyal JC, Narasimh RL, Dakshina C. Preliminary investigations on root distribution of high yielding wheat varieties. International Journal of Applied Radiation and Isotopes. 1968;19:385–390. [Google Scholar]
- Ubeda-Tomas S, Swarup R, Coates J, Swarup K, Laplaze L, Beemster GTS, Hedden P, Bhalerao R, Bennett MJ. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nature Cell Biology. 2008;10:625–628. doi: 10.1038/ncb1726. [DOI] [PubMed] [Google Scholar]
- Walker AD, Campbell CGB, Heslop REF, Gauld JH, Laing D, Chem C, Shipley BM, Wright GG. Soil survey of Scotland. The Macaulay Institute for Soil Research Aberdeen. 1982:78–79. [Google Scholar]
- Worland AJ, Korzun V, Roder MS, Ganal MW, Law CN. Genetic analysis of the dwarfing gene Rht8 in wheat. Part II. The distribution and adaptive significance of allelic variants at the Rht8 locus of wheat as revealed by microsatellite screening. Theoretical and Applied Genetics. 1998;96:1110–1120. [Google Scholar]