Abstract
Cell cycle progression requires interaction between cyclin-dependent kinase B (CDKB) and cyclin B (CYCB). The seasonal expression patterns of the CDKB and CYCB homologues from Populus tomentosa Carr. were investigated, and effects of temperature and exogenous indole-3-acetic acid (IAA) on their expression were further studied in water culture experiments. Based on the differential responses of dormant cambium cells to exogenous IAA, four stages of cambium dormancy were confirmed for P. tomentosa: quiescence 1 (Q1), rest, quiescence 2-1 (Q2-1), and quiescence 2-2 (Q2-2). PtoCDKB and PtoCYCB transcripts were strongly expressed in the active phases, weakly in Q1, and almost undetectable from rest until late Q2-2. Climatic data analysis showed a correlation between daily air temperature and PtoCDKB and PtoCYCB expression patterns. Water culture experiments with temperature treatment further showed that a low temperature (4 °C) kept PtoCDKB and PtoCYCB transcripts at undetectable levels, while a warm temperature (25 °C) induced their expression in the cambium region. Meanwhile, water culture experiments with exogenous IAA treatment showed that induction of PtoCDKB and PtoCYCB transcription was independent of exogenous IAA. The results suggest that, in deciduous hardwood P. tomentosa growing in a temperate zone, the temperature in early spring is a vital environmental factor for cambium reactivation. The increasing temperature in early spring may induce CDKB and CYCB homologue transcription in the cambium region, which is necessary for cambium cell division.
Keywords: Cambium reactivation, CDKB, CYCB, Populus tomentosa Carr, temperature
Introduction
Environmental signals, especially photoperiod and temperature, are involved in cycles of activity–dormancy of trees growing in temperate zones. For example, short days (SDs) exclusively induce dormancy in poplar (Populus deltoides Bartr. ex Marsh.) (Jeknić and Chen, 1999; Park et al., 2008) and silver birch (Betula pendula Roth.) (Li et al., 2003); however, in apple (Malus pumila Mill.) and pear (Pyrus communis L.), low temperature controls growth cessation and dormancy induction independently of photoperiod (Heide and Prestrud, 2005). In addition, natural chilling is required for the transition from rest to quiescence in winter, and temperature in spring plays a main role during cambium reactivation (Little and Bonga, 1974; Antonova and Stasova, 1997; Druart et al., 2007; Venugopal and Liangkuwang 2007; Deslauriers et al., 2008). Interestingly, local heating of the cambium during the dormant period induces reactivation in the heated portions in some trees independently of the development of new buds (Oribe and Kubo, 1997; Oribe et al., 2001, 2003; Gričar et al., 2006; Begum et al., 2007), while heating (23–25 °C) or cooling (9–11 °C) the active cambium cells increases or slows down the rate of cell division in the stem portion of Norway spruce (Gričar et al., 2006, 2007). These data indicate that temperature has a great effect on cell division in the cambium.
Environmental signals are thought to regulate activity–dormancy cycling by modulating the cell division machinery, or by altering the sensitivity of cambium cells to hormones. For example, SD treatment decreases PttCDKB transcription and the histone H1 kinase activity of PttCDKA and PttCDKB complexes (Espinosa-Ruiz et al., 2004). In addition, SD and low temperature treatment decrease PcyclAt-gus and Pcdc2a-gus expression, respectively (Rohde et al., 1997). On the other side, cambium cells are rendered insensitive to auxin in the rest stage of dormancy (Little and Bonga, 1974; Mwange et al. 2003). In the resting vascular tissues of Eucommia ulmoides Oliv., auxin-binding protein 1 (ABP1) expression at both the protein and mRNA levels is undetectable, even after exogenous IAA application; but, after resting branches are incubated at 4 °C for 2 weeks, ABP1 is detected again after exogenous indole-3-acetic acid (IAA) application, indicating that low temperature can induce ABP1 transcription in the cambium and may change the sensitivity of cambium cells to IAA (Hou et al., 2006). Considering the effect of temperature on cambium dormancy, little is known about the link between this environmental signal and the cell cycle regulator in cambium cells.
The cell cycle function of a B2-type cyclin interacting with a B2-type cyclin-dependent kinase (CDK) has been demonstrated in rice (Lee et al., 2003). The CDKB2 family is expressed from the G2 to the M phase (Magyar et al., 1997; Umeda et al., 1999; Porceddu et al., 2001; Menges et al., 2005; Francis, 2007; Andersen et al., 2008) and is required both for normal cell cycle progression and for meristem organization (Andersen et al., 2008). Plant B2-type cyclins are also known to be expressed from the G2 to the M phase (Hirt et al., 1992; Francis, 2007). PttCDKB, a member of the CDKB2 family, is expressed only in the proliferation zone of the woody tissues of hybrid aspen stem, highly in the active stage of cambium, and weakly in the dormant stage (Espinosa-Ruiz et al., 2004; Schrader et al., 2004a, b). Here, the effect of environmental temperature on the expression of these core cell cycle genes during the distinct stages of the cambium activity–dormancy cycle, especially during the reactivation process, was investigated.
According to Little and Bonga (1974), cambium dormancy consists of rest and quiescence stages. The difference between these two stages is that, upon exposure to growth-promoting conditions, resting cambium does not restore activity but quiescent cambium does. In E. ulmoides, there exist one rest and two quiescence stages during the cambium dormancy period (Mwange et al., 2003). Two distinct dormant stages in hybrid aspen have been identified under controlled environmental conditions; the endodormant terminal bud does not break but the co-dormant terminal bud does upon exposure to growth-promoting conditions (Espinosa-Ruiz et al., 2004). Interestingly, PttCDKB transcript levels in stem tissues decline and then disappear with the establishment of endodormancy, and it was suggested to be a molecular marker for endodormancy (Espinosa-Ruiz et al., 2004).
In this study, four stages of cambium dormancy in P. tomentosa were identified according to the character of rest and quiescence described by Little and Bonga (1974) and the molecular marker suggested by Espinosa-Ruiz et al. (2004), the full-length cDNAs for CDKB and CYCB homologues from the tree were cloned, their seasonal expression patterns in the cambium region combined with climatic temperature data were analysed, and the induction of PtoCDKB and PtoCYCB transcription by temperature was further confirmed in water culture experiments. These results provide insights into the molecular basis of the cell division machinery of cambium cells regulated by environmental temperature during the cambium reactivation process.
Materials and methods
Plant material
Populus tomentosa trees with a stem diameter of 30–40 cm, located in Peking University campus (39°99′N, 116°30′E; Beijing, China), were sampled for all experiments. Sampling was performed from 8 March 2004 (before bud break) until 14 March 2006 (before bud break) at 28 time points, to cover all stages of the activity–dormancy cycle (Yin et al., 2002; Table 1).
Table 1.
Sampling dates
| Month | Sampling dates | ||
| 2004 | 2005 | 2006 | |
| January | – | 17 | 10, 26 |
| February | – | 22 | 10, 26 |
| March | 8 | 10 | 14 |
| May | 20 | 11 | – |
| June | – | 15 | – |
| July | – | 12 | – |
| August | – | 30 | – |
| September | 30 | 8, 22 | – |
| October | 26 | 13, 27 | – |
| November | 6, 14, 22 | 15, 30 | – |
| December | 19 | 9, 25 | – |
–, indicates no sampling.
At each sampling time, 10 small blocks of vascular tissue were excised from 10 twigs collected from different trees and fixed in formalin–alcohol–acetic acid (FAA) for anatomical observation.
Samples for total RNA isolation were carefully scraped with a scalpel from 1- or 2-year-old twigs. The scraped tissues were mainly cambium cells mixed with some phloem and xylem cells, as revealed by light microscopy. All the sampled materials were immediately frozen in liquid nitrogen and stored at –80 °C.
Meteorological data
The data for average, maximum, and minimum daily air temperatures near the experimental site in Beijing, China (39°48′N, 116°28′E), from 8 March 2004 to 10 March 2005 and from 15 June 2005 to 14 March 2006, were obtained from the Climatic Data Center, National Meteorological Information Center, CAM.
Water culture experiments
The stage of cambium dormancy in P. tomentosa grown in natural conditions was determined based upon the differential responses of cambium cells to exogenous IAA in water culture experiments according to the method of Little and Bonga (1974) and Cui et al. (1992). One- or 2-year-old dormant twigs were harvested at the indicated time points (Table 1); 10 twigs at each time point from normal growth trees were sampled as the intact control. Another 20 twigs were cut into 40 cm lengths, and divided into two groups of 10 cuttings each. Following removal of all buds, cuttings were treated with IAA and lanolin (1 mg IAA g−1 lanolin) or lanolin alone. IAA with lanolin or lanolin alone was spread over the excised tops of the cuttings. Cuttings were cultured in water. A water culture system was set up in a growth chamber with a 16 h photoperiod, light intensity of 150 μmol m−2 s−1, temperatures of 25/20 °C (day/night), and a relative humidity of 75–85%. The application of exogenous IAA was performed weekly; a fresh cut surface was prepared, and the part of the cutting in contact with the water was removed every 4 d. The water was changed daily to keep it fresh. After 3 weeks of water culture, a segment 2 cm thick was carefully removed and discarded from both ends of each cutting. Small blocks of vascular tissue were excised 5 cm below the top of the cutting after removal of both ends and fixed in FAA for anatomical observation, and the cambium tissues were collected from the remaining cutting for RNA extraction as above to test PtoCDKB and PtoCYCB expression.
To check the effect of temperature on PtoCDKB and PtoCYCB expression, 1-year-old dormant twigs were harvested on 30 December 2007, when PtoCDKB and PtoCYCB transcript levels were undetectable by reverse transcription-PCR (RT-PCR). Eighty cuttings of 20 cm in length were used for experimental treatment. All buds were removed from 40 cuttings and buds were left on the other 40. Cuttings were cultured in water. Water culture systems were set up in two growth chambers with a relative humidity of 75–85% and without light. Twenty cuttings with buds and 20 without were exposed to 25 °C in one chamber, and the other 40 cuttings were exposed to 4 °C in the other chamber. The water was changed daily to keep it fresh in the dark. After removal of a segment 2 cm from both ends of each cutting, the cambium tissues for RNA extraction were sampled from five cuttings with or without buds, separately, at 2, 4, 6, or 8 d after treatment as above.
Anatomical observation and statistical analysis
Anatomical examination and statistical analysis were conducted to assess the response of cambium cells to exogenous IAA according to the methods of Cui et al. (1992) and Mwange et al. (2003). The small blocks fixed in FAA were dehydrated in an alcohol series and embedded in Spurr's resin (SPI, USA). Cross-sections 4 μm thick were cut on a microtome (Leitz 1512, Germany), stained with toluidine blue O, and observed under a Zeiss Axioskop 2 Plus microscope (Germany) equipped with a computer-assisted digital camera. Sections from twigs that were freshly harvested on the sampling day served as the intact control.
Six sections from different cuttings were used for anatomical observation and statistical analysis. Fifteen radial files per section were measured. The numbers of radial cell layers in the cambium region, and recently formed xylem and phloem, were counted to evaluate cambium activity. Data are shown as mean ±SD. Statistical analysis was performed with SPSS 11.0 using analysis of variance (ANOVA).
RNA extraction and cDNA synthesis
Scraped samples at the indicated time points were pulverized in liquid nitrogen. Total RNA was extracted from ∼0.1 g of tissue, with TRIzol reagent (Invitrogen) according to the manufacturer's protocol. A 5 μg aliquot of total RNA was reverse-transcribed into cDNA with a RevertAid™ H Minus First Strand cDNA Synthesis Kit (K1631, Fermentas, Life Sciences), and subsequently diluted for gene isolation and gene expression analysis.
Database search, cloning full-length cDNA, and sequence analysis
According to the sequences of PttCDKB (GenBank accession no. AY307372) and the uncharacterized gene estExt_fgenesh4_pm.C_LG_V0721 (identified from the genome sequence database of poplar using the sequence of PttCDKB; http://genome.jgi-psf.org/Poptr1_1/Poptr1_1.home.html; Protein ID: 831725, named PtCDKB), the primers 5′-GAGAGTAGCAACAGAAACGAACACG-3′ and 5′-CCCATACTAGTCGCAGCATTGTGATTC-3′ were used to identify the full-length cDNA sequence of the CDKB homologue from P. tomentosa. According to the sequence of the uncharacterized gene estExt_fgenesh4_pg.C_LG_IX0044 (identified from the genome sequence database of poplar using the sequence of CYCB2; 4 with the GenBank accession no. NP_177758.2; http://genome.jgi-psf.org/Poptr1_1/Poptr1_1.home.html; Protein ID: 820971, named PtCYCB), the primers 5′-ATGGGTCGATCAAATGAGAAC-3′ and 5′-TCAGAGCCCTAAGAGAAATAG-3′ were used to identify the full-length cDNA sequence of the CYCB homologue from P. tomentosa. The PCR products were purified using Qiagen PCR purification columns and subsequently cloned into the pGEM-T easy vector (Promega) and sequenced. The full-length cDNA sequences were submitted to GenBank with the accession no. EU822323 for PtoCDKB and FJ262735 for PtoCYCB. Multiple protein sequence alignments were made using ClustalX software (Thompson et al., 1997).
Semi-quantitative RT-PCR and quantitative RT-PCR (qRT-PCR)
Total RNA was extracted and reverse-transcribed into cDNA. cDNAs were diluted and used as templates for RT-PCR and qRT-PCR analysis. The concentration of cDNA templates was normalized according to the abundance of the ubiquitin-like (UBQ-L) PCR product. The PCR for UBQ-L was limited to 28 cycles in order to stay within the linear range of amplification and obtain a more accurate picture of the relative levels of gene expression. Amplification of PtoCDKB was carried out with 40 cycles and that of PtoCYCB with 35 cycles, to be sure of the relative levels of gene expression at specific time points and after water culture treatment. The primers were 5′-TGTCCCACCCACCACTCTTCGCGAAGTCTCC-3′ and 5′-GCCCATCCTTGTCCAAATTAGTAACAGCTGA-3′ for PtoCDKB (Espinosa-Ruiz et al., 2004), 5′-GTCCTCGATATGGAGAAAC-3′ and 5′-CTGTACTTCCTATGAACTCC-3′ for PtoCYCB, and 5′-TGAGGCTTAGGGGAGGAACT-3′ and 5′-TGTAGTCGCGAGCTGTCTTG-3′ for UBQ-L (Brunner et al., 2004). PCR products were analysed by electrophoresis on a 1.5% agarose gel stained with ethidium bromide. The RT-PCR data are representative of at least three experiments.
The qPCR analysis was performed on an MJ Research thermocycler, using a DyNAmo SYBR Green qPCR kit (Finnzymes, Finland) with UBQ-L as internal control. Each reaction was carried out on 2 μl of diluted cDNA sample, in a total reaction system of 10 μl. The reaction procedure was set up according to the manufacturer's protocol and the primers were: 5′-GTATCCCCAGTGGAAACCTCA-3′ and 5′-CGCTTTGAAGGGTCATACTGC-3′ for PtoCDKB, 5′-GACCGATTCTTGGAGCGTTGC-3′ and 5′-CACGAGTGGGACAGAGACCTC-3′ for PtoCYCB, and the same as above for UBQ-L. To check the specificity of amplification, the melting curve of the PCR products was detected. The expression levels of each gene were standardized to the constitutive expression level of UBQ-L. The ratio between the expression levels of PtoCDKB and UBQ-L or PtoCYCB and UBQ-L for each sample was calculated using the relative quantitative analysis method. Each sample was assayed in triplicate. Data are shown as mean ±SD.
Results
The stage of cambium dormancy in P. tomentosa
The stage of cambium dormancy in P. tomentosa grown in natural conditions was determined based upon the differential responses of cambium cells to exogenous IAA according to the character of rest and quiescence described by Little and Bonga (1974). Anatomical examination and statistical analysis were conducted to assess the response of cambium cells to exogenous IAA according to the methods of Cui et al. (1992) and Mwange et al. (2003). After 3 weeks of water culture with exogenous IAA treatment, in the cambium of lanolin-treated bud-free cuttings sampled from late September to late January of the following year, no change in cell layers was found (Figs 1C, F, I, and 2; P >0.05), compared with their intact control. Interestingly, in the cuttings receiving the same treatment harvested from early February to the middle of March, cell layers increased compared with their intact control (Figs 1L and 2; P <0.05).
Fig. 1.
Photomicrographs of cambium cells in cross-sections of dormant cuttings of P. tomentosa in response to exogenous IAA in water culture. (A) Intact control cutting (13 October 2005). (B) IAA-treated cutting (13 October 2005). (C) Lanolin-treated cutting (13 October 2005). (D) Intact control cutting (9 December 2005). (E) IAA-treated cutting (9 December 2005). (F) Lanolin-treated cutting (9 December 2005). (G) Intact control cutting (26 January 2006). (H) IAA-treated cutting (26 January 2006). (I) Lanolin-treated cutting (26 January 2006). (J) Intact control cutting (10 February 2006). (K) IAA-treated cutting (10 February 2006). (L) Lanolin-treated cutting (10 February 2006). Arrowheads indicate thin newly formed cell walls. Double-headed arrows indicate cell layers to be counted. C, cambium region; P, phloem; X, xylem. Scale bar = 50 μm.
Fig. 2.
Statistical analysis of the effect of exogenous IAA on cambium reactivation of 1- or 2-year-old dormant cuttings of P. tomentosa in water culture. Cell layers of IAA-treated cuttings from late September to the end of October and from late December to the middle of March increased (P <0.05), while from early November to late December they showed no change (P >0.05. Cell layers of lanolin-treated cuttings from late September to late January showed no change (P >0.05), but they increased from early February to the middle of March (P <0.05). Cell layers = cambium cell layers+recently formed xylem cell layers+recently formed phloem cell layers. The P-value was generated between an IAA-treated cutting and an intact control cutting or beteeeen a lanolin-treated cutting and an intact control cutting at each time point.
However, the cambium of bud-free cuttings sampled from late September to the middle of March of the following year showed differential responses to exogenous IAA. From late September to the end of October, and from late December to the middle of March, in IAA-treated cuttings, cell layers increased, compared with their intact control (Figs. 1B, H, K, and 2; P <0.05), while in IAA-treated cuttings from early November to late December, no change of cell layers was detected (Figs 1E and 2; P >0.05).
Based upon the differential responses of dormant cambium cells to exogenous IAA, four stages of cambium dormancy were identified in P. tomentosa grown under natural conditions: quiescence 1 (Q1) from late September to the end of October, rest from early November to late December, quiescence 2-1 (Q2-1) from late December to early February, and quiescence 2-2 (Q2-2) from early February to the middle of March (Fig. 2). Compared with Q2-1, exogenous IAA was dispensable for cambium reactivation in Q2-2 under the same water culture conditions.
Cloning and analysing the sequences of PtoCDKB and PtoCYCB in P. tomentosa
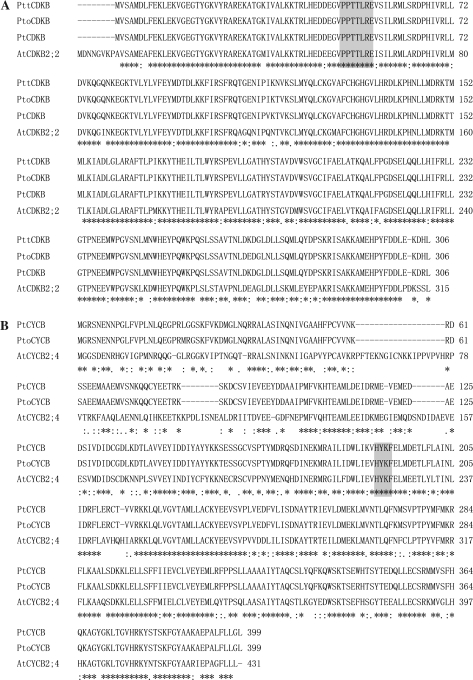
The full-length cDNAs for the CDKB and CYCB homologues from P. tomentosa, PtoCDKB and PtoCYCB, were cloned. The deduced amino acid sequence for PtoCDKB displayed high homology to PttCDKB from P. tremula×P. tremuloides and PtCDKB from P. trichocarpa, with sequence identity of up to 99% (303/306 amino acids) (Fig. 3A). The sequence was also 306 amino acids long and contained a PPTTLRE motif characteristic of the CDKB2; 2 protein type (Vandepoele et al., 2002) (Fig. 3A).
Fig. 3.
CLUSTALX (1.81) multiple sequence alignment of CDKB2; 2 and CYCB2; 4 amino acid sequences. Family-specific protein signatures are shaded grey. Identical residues are indicated by asterisks. (A) Multiple sequence alignment of CDKB2; 2. The GenBank accession no. for AtCDKB2; 2 is NP_173517.1. (B) Multiple sequence alignment of CYCB2; 4.
The deduced amino acid sequence for PtoCYCB displayed high homology to PtCYCB from P. trichocarpa, with sequence identity of up to 98% (391/399 amino acids) (Fig. 3B). The sequence was 399 amino acids long with the B-type-specific HxKF signature (Vandepoele et al., 2002) (Fig. 3B).
Seasonal expression patterns of PtoCDKB and PtoCYCB in the cambium region and their relationships with climatic air temperature
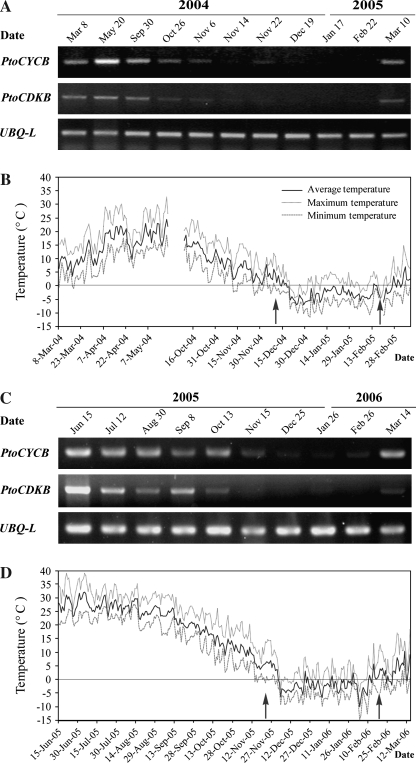
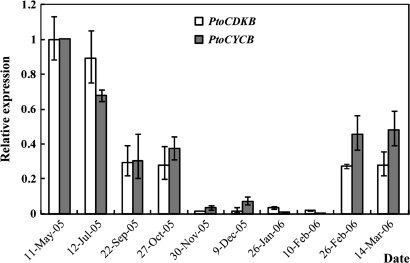
The seasonal expression patterns of PtoCDKB and PtoCYCB in the cambium region were assayed by RT-PCR from March 2004 to March 2006. The PtoCDKB and PtoCYCB transcripts were strongly expressed in the active phases from May to August (Fig. 4A, C). The PtoCYCB transcripts were weakly expressed from September and undetectable after late November until early March of 2005 (Fig. 4A). Likewise, the PtoCDKB transcripts were weakly expressed from September and undetectable after the middle of November until early March of 2005 (Fig. 4A). Although a small difference existed in PtoCYCB expression, almost the same expression patterns of PtoCDKB and PtoCYCB were observed from 2005 to 2006 (Fig. 4C). The qRT-PCR analysis of PtoCDKB and PtoCYCB transcripts from May 2005 to March 2006 further confirmed these expression patterns (Fig. 5).
Fig. 4.
Seasonal expression patterns of PtoCDKB and PtoCYCB in the P. tomentosa cambium region and the climatic daily temperatures during the sampling period. (A) Periodic expression patterns of PtoCDKB and PtoCYCB investigated by RT-PCR using UBO-L as the internal control in 2004 and 2005. (B) Climatic daily temperatures in 2004 and 2005. (C) Periodic expression patterns of PtoCDKB and PtoCYCB investigated by RT-PCR in 2005 and 2006. (D) Climatic daily temperatures in 2005 and 2006. During the time between the left and right arrows, the PtoCDKB and PtoCYCB transcript levels were almost undetectable by RT-PCR.
Fig. 5.
Seasonal expression patterns of PtoCDKB and PtoCYCB in the P. tomentosa cambium region assayed by qRT-PCR in 2005 and 2006 using UBO-L as the internal control.
From 1 December 2004 to 28 February 2005, of these 90 d there were 85 d when the average daily air temperatures were <4 °C, and the PtoCDKB and PtoCYCB transcript levels were almost undetectable by RT-PCR; in contrast, of the 31 d of March 2005, there were 21 d when the average daily air temperatures were >4 °C, and PtoCDKB and PtoCYCB transcripts were detectable (Fig. 4A, B). Quite similarly, from 1 December 2005 to 28 February 2006, there were 87 d out of these 90 d when the average daily air temperatures were <4°C, and the PtoCDKB and PtoCYCB transcript levels were almost undetectable, while there were 25 d in the 31 d of March 2005 when the average daily air temperatures were >4 °C and PtoCDKB and PtoCYCB transcripts were detectable (Fig. 4C, D). Thus, the relationship between average daily air temperature and the PtoCDKB and PtoCYCB expression patterns from December 2005 to March 2006 was similar to that from December 2004 to March 2005. It therefore appears that when the average daily air temperature remains below 4 °C from December to February of the next year, the PtoCDKB and PtoCYCB transcript levels are undetectable, and, after exposure to several days of average daily air temperature above 4 °C, the PtoCDKB and PtoCYCB transcripts become detectable in early spring.
Variations of PtoCDKB and PtoCYCB expression in water culture conditions with exogenous IAA and temperature treatment
On 9 December 2005 and 26 January 2006, the PtoCDKB and PtoCYCB transcripts were not detectable in dormant cambium regions by RT-PCR, but after 3 weeks of water culture at 25/20 °C (day/night), the transcription of PtoCDKB and PtoCYCB was reactivated, whether or not exogenous IAA was present (Fig. 6A, C). Similar results were achieved by qRT-PCR analysis (Fig. 6B, D).
Fig. 6.
Variations of PtoCDKB and PtoCYCB expression after water culture with exogenous IAA treatment. Cuttings were sampled on 9 December 2005 (A, B) and 26 January 2006 (C, D); after bud removal, they were treated with lanolin or IAA with lanolin (1 mg IAA g−1 lanolin) and cultured in water for 3 weeks. Variations of PtoCDKB and PtoCYCB expression in the cambium region were analysed by RT-PCR (A, C) or qRT-PCR (B, D) using UBO-L as the internal control. 1, intact control cutting; 2, IAA-treated cutting; 3, lanolin-treated cutting.
Based on the relationships between reactivation of PtoCDKB and PtoCYCB transcription and climate temperatures in early spring and the influence of water culture on their expression, the effects of temperature on PtoCDKB and PtoCYCB expression were explored further. Dormant cuttings at the Q2-1 stage were cultured at 25 °C or 4 °C in the dark, and variations of PtoCDKB and PtoCYCB expression were analysed separately in cuttings with or without buds by RT-PCR and qRT-PCR (Fig. 7). RT-PCR and qRT-PCR analysis showed that PtoCDKB transcription in cambium regions was induced after 4 d in cuttings without buds and after 6 d in cuttings with buds in response to 25 °C (Fig. 7A, B), whereas PtoCYCB transcription was induced after 4 d in both kinds of cuttings in response to 25 °C (Fig. 7C, D). In contrast, the PtoCDKB and PtoCYCB transcripts were almost undetectable in both kinds of cuttings after 8 d of exposure to 4 °C (Fig. 7).
Fig. 7.
Variations of PtoCDKB and PtoCYCB expression during water culture with temperature treatment. When PtoCDKB and PtoCYCB transcript levels were undetectable (30 December 2007), 1-year-old dormant cuttings were harvested, with one half debudded, and were cultured in water at 4 °C or 25 °C for 2, 4, 6, or 8 d in the dark. The induction of PtoCDKB (A, B) and PtoCYCB (C, D) transcription was analysed by RT-PCR (A, C) or qRT-PCR (B, D) using UBO-L as the internal control.
Discussion
PtoCDKB and PtoCYCB expression patterns during the cambium periodicity of P. tomentosa
Expression of several cell cycle genes is related to the radial growth of woody plants (Yamaguchi et al., 2000; Goué et al., 2003; Espinosa-Ruiz et al., 2004). Transition from the G2 to the M phase in the cell cycle requires interaction of CDKB with CYCB (Francis, 2007). In the present work, high levels of CDKB and CYCB homologue transcripts in the active stage also reflect a positive correlation between cambium cell division activity and key cell cycle gene expression.
PttCDKB transcript levels persist during the ecodormant stage and stop with entry into the endodormant stage of cambium dormancy, and are suggested to be a molecular marker of endodormancy (Espinosa-Ruiz et al., 2004). The seasonal expression pattern of PtoCDKB in the cambium region from trees growing under natural conditions was checked. Based on the PtoCDKB expression pattern during cambium periodicity, it was found that, partly consistent with the results of Espinosa-Ruiz et al. (2004), the PtoCDKB transcript levels were undetectable during rest (endodormancy), Q2-1, and the early stage of Q2-2, especially from early January to early March, when the dormant cambium cells regained sensitivity to exogenous IAA and reactivated when treated with exogenous IAA. These results show that PtoCDKB transcript levels cannot be used as a molecular marker of endodormancy (rest), because they were not detected at rest, in Q2-1, and in the early stage of Q2-2, while they can mark the establishment of the rest stage (endodormancy). These results undoubtedly enlarge our understanding about cambium dormancy of poplar under complex environmental conditions.
After 3 weeks of water culture, the transcription of PtoCDKB and PtoCYCB was triggered in cuttings in the rest and Q2 stages treated with IAA and lanolin or lanolin alone, suggesting that the reactivation of PtoCDKB and PtoCYCB transcription is independent of exogenous IAA and the stage of cambium dormancy. The resting cambium cells did not divide, but the increase in PtoCDKB and PtoCYCB transcript levels was triggered in water culture, and the presence of PtoCDKB and PtoCYCB mRNA after cessation of cambium cell division in natural conditions suggests that the transcripts of CDKB and CYCB homologues may be insufficient for cambium cell division in poplar species. It is known that post-transcriptional modification of CDKs is required for cell cycle progression (Lew and Kornbluth, 1996; Mészáros et al., 2000; Porceddu et al., 2001; Espinosa-Ruiz et al., 2004).
Induction of PtoCDKB and PtoCYCB transcription in the cambium region by temperature in spring
Temperature plays the main role during cambium reactivation (Antonova and Stasova, 1997; Druart et al., 2007; Venugopal and Liangkuwang, 2007; Deslauriers et al., 2008). However, little is known about the molecular mechanism of this regulation. A strong correlation was noted between the restart of PtoCDKB and PtoCYCB expression in the cambium region and increasing air temperatures in spring. Water culture experiments with temperature treatment further showed that PtoCDKB and PtoCYCB transcription can be induced in response to 25 °C, suggesting that this regulatory mechanism might be involved in cambium reactivation in spring. The present experiments provide primary molecular clues about regulation of the cell division machinery of cambium cells by environmental temperature during cambium reactivation.
Temperature sensing by cambium
Localized heating in a deciduous hardwood hybrid poplar induces cambium reactivation independently of bud burst (Begum et al., 2007), indicating that the cambium cells themselves sense the temperature signal and cambium reactivation can be independent of bud swelling and newly developing leaves, regardless of endogenous IAA produced in the swelling bud and newly developing leaves (Sundberg and Uggla, 1998). In the present study, cell layers in bud-free lanolin-treated cuttings sampled in the Q2-2 stage increased after water culture, in support of the occurrence of cambium growth in the debudded, lanolin control cuttings (Little and Bonga, 1974), indicating that cambium reactivation and the function of cell cycle regulators such as CDKB and CYCB homologues can be initiated independently of bud burst. The increases of PtoCDKB and PtoCYCB transcript levels before bud burst in spring and the temperature treatment experiment confirmed that the cambium cells themselves sense the temperature signal (Little and Bonga, 1974). Therefore, it is concluded that, in deciduous hardwood P. tomentosa growing in a temperate zone, the temperature in early spring is a vital environmental factor for cambium reactivation. The increasing temperature in early spring sensed by cambium cells may induce CDKB and CYCB homologue transcription in the cambium region, which is necessary for cambium cell division.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30530620; 30670120; 30872001). The authors thank the National Meteorological Information Center, CAM, for providing meteorological data, Dr Iain C Bruce (Zhejiang University) for critical reading of the manuscript, Dr Dahai Yu, Yong Guo, and Ms Aiqing Song (Peking University) for statistical and image analysis, and the anonymous reviewers for their constructive comments on an earlier version of this manuscript.
References
- Andersen SU, Buechel S, Zhao Z, Ljung K, Novák O, Busch W, Schuster C, Lohmann JU. Requirement of B2-type Cyclin-Dependent Kinases for meristem integrity in Arabidopsis thaliana. The Plant Cell. 2008;20:88–100. doi: 10.1105/tpc.107.054676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova GF, Stasova VV. Effects of environmental factors on wood formation in larch (Larix sibirica Ldb.) stems. Trees. 1997;11:462–468. [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii×P. grandidentata) Annals of Botany. 2007;100:439–447. doi: 10.1093/aob/mcm130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biology. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui KM, Little CHA, Sundberg B. The cambial activity and on which the effects of exogenous IAA in the stem of Pinus sylvestris L. Acta Botanica Sinica. 1992;34:515–522. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T, Saracino A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiology. 2008;28:863–871. doi: 10.1093/treephys/28.6.863. [DOI] [PubMed] [Google Scholar]
- Druart N, Johansson A, Baba K, Schrader J, Sjödin A, Bhalerao RR, Resman L, Trygg J, Moritz T, Bhalerao RP. Environmental and hormonal regulation of the activity–dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. The Plant Journal. 2007;50:557–573. doi: 10.1111/j.1365-313X.2007.03077.x. [DOI] [PubMed] [Google Scholar]
- Espinosa-Ruiz A, Saxena S, Schmidt J, Mellerowicz E, Miskolczi P, Bakó L, Bhalerao RP. Differential stage-specific regulation of cyclin-dependent kinases during cambial dormancy in hybrid aspen. The Plant Journal. 2004;38:603–615. doi: 10.1111/j.1365-313X.2004.02070.x. [DOI] [PubMed] [Google Scholar]
- Francis D. The plant cell cycle—15 years on. New Phytologist. 2007;174:261–278. doi: 10.1111/j.1469-8137.2007.02038.x. [DOI] [PubMed] [Google Scholar]
- Goué N, Montiel G, Levert I, Gaudet M, Jay-Allemand C, Label P. CDKA orthologue isolation and its expression during cambial activity in hybrid walnut (Juglans nigra×Juglans regia) Trees. 2003;17:316–324. [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Koch G, Schmitt U, Oven P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies) Annals of Botany. 2006;97:943–951. doi: 10.1093/aob/mcl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Oven P. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Science and Technology. 2007;41:463–475. [Google Scholar]
- Heide OM, Prestrud AK. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiology. 2005;25:109–114. doi: 10.1093/treephys/25.1.109. [DOI] [PubMed] [Google Scholar]
- Hirt H, Mink M, Pfosser M, Bögre L, Györgyey J, Jonak C, Gartner A, Dudits D, Heberle-Bors E. Alfalfa cyclins: differential expression during the cell cycle and in plant organs. The Plant Cell. 1992;4:1531–1538. doi: 10.1105/tpc.4.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou HW, Zhou YT, Mwange KN, Li WF, He XQ, Cui KM. ABP1 expression regulated by IAA and ABA is associated with the cambium periodicity in Eucommia ulmoides Oliv. Journal of Experimental Botany. 2006;57:3857–3867. doi: 10.1093/jxb/erl150. [DOI] [PubMed] [Google Scholar]
- Jeknić Z, Chen THH. Changes in protein profiles of poplar tissues during the induction of bud dormancy by short-day photoperiods. Plant and Cell Physiology. 1999;40:25–35. [Google Scholar]
- Lee J, Das A, Yamaguchi M, Hashimoto J, Tsutsumi N, Uchimiya H, Umeda M. Cell cycle function of a rice B2-type cyclin interacting with a B-type cyclin-dependent kinase. The Plant Journal. 2003;34:417–425. doi: 10.1046/j.1365-313x.2003.01736.x. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Current Opinion in Cell Biology. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- Li C, Junttila O, Ernstsen A, Heino P, Palva ET. Photoperiodic control of growth, cold acclimation and dormancy development in silver birch (Betula pendula) ecotypes. Physiologia Plantarum. 2003;117:206–212. [Google Scholar]
- Little CHA, Bonga JM. Rest in the cambium of Abies balsamea. Canadian Journal of Botany. 1974;52:1723–1730. [Google Scholar]
- Magyar Z, Mészáros T, Miskolczi P, et al. Cell cycle phase specificity of putative cyclin-dependent kinase variants in synchronized alfalfa cells. The Plant Cell. 1997;9:223–235. doi: 10.1105/tpc.9.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M, de Jager SM, Gruissem W, Murray JAH. Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. The Plant Journal. 2005;41:546–566. doi: 10.1111/j.1365-313X.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- Mészáros T, Miskolczi P, Ayaydin F, Pettkó-Szandtner A, Peres A, Magyar Z, Horváth GV, Bakó L, Fehér A, Dudits D. Multiple cyclin-dependent kinase complexes and phosphatases control G2/M progression in alfalfa cells. Plant Molecular Biology. 2000;43:595–605. doi: 10.1023/a:1006412413671. [DOI] [PubMed] [Google Scholar]
- Mwange KN, Wang XW, Cui KM. Mechanism of dormancy in the buds and cambium of Eucommia ulmoides. Acta Botanica Sinica. 2003;45:698–704. [Google Scholar]
- Oribe Y, Funada R, Kubo T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees. 2003;17:185–192. [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Kubo T. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiology. 1997;17:81–87. doi: 10.1093/treephys/17.2.81. [DOI] [PubMed] [Google Scholar]
- Park S, Keathley DE, Han KH. Transcriptional profiles of the annual growth cycle in Populus deltoides. Tree Physiology. 2008;28:321–329. doi: 10.1093/treephys/28.3.321. [DOI] [PubMed] [Google Scholar]
- Porceddu A, Stals H, Reichheld JP, Segers G, De Veylder L, Barrôco RP, Casteels P, Van Montagu M, Inzé D, Mironov V. A plant-specific cyclin-dependent kinase is involved in the control of G2/M progression in plants. Journal of Biological Chemistry. 2001;276:36354–36360. doi: 10.1074/jbc.M011060200. [DOI] [PubMed] [Google Scholar]
- Rohde A, Van Montagu M, Inzé D, Boerjan W. Factors regulating the expression of cell cycle genes in individual buds of Populus. Planta. 1997;201:43–52. [Google Scholar]
- Schrader J, Moyle R, Bhalerao R, Hertzberg M, Lundeberg J, Nilsson P, Bhalerao RP. Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. The Plant Jouranl. 2004a;40:173–187. doi: 10.1111/j.1365-313X.2004.02199.x. [DOI] [PubMed] [Google Scholar]
- Schrader J, Nilsson J, Mellerowicz E, Berglund A, Nilsson P, Hertzberg M, Sandberg G. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. The Plant Cell. 2004b;16:2278–2292. doi: 10.1105/tpc.104.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg B, Uggla C. Origin and dynamics of indoleacetic acid under polar transport in Pinus sylvestris. Physiologia Plantarum. 1998;104:22–29. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The clustalx windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda M, Umeda-Hara C, Yamaguchi M, Hashimoto J, Uchimiya H. Differential expression of genes for cyclin-dependent protein kinases in rice plants. Plant Physiology. 1999;119:31–40. doi: 10.1104/pp.119.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D. Genome-wide analysis of core cell cycle genes in Arabidopsis. The Plant Cell. 2002;14:903–916. doi: 10.1105/tpc.010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal N, Liangkuwang MG. Cambial activity and annual rhythm of xylem production of elephant apple tree (Dillenia indica Linn.) in relation to phenology and climatic factor growing in sub-tropical wet forest of northeast India. Trees. 2007;21:101–110. [Google Scholar]
- Yamaguchi M, Fabian T, Sauter M, Bhalerao RP, Schrader J, Sandberg G, Umeda M, Uchimiya H. Activation of CDK-activating kinase is dependent on interaction with H-type cyclins in plants. The Plant Journal. 2000;24:11–20. doi: 10.1046/j.1365-313x.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- Yin YF, Jiang XM, Cui KM. Seasonal changes in the ultrastructure of the vascular cambium in shoots of Populus tomentosa. Acta Botanica Sinica. 2002;44:1268–1277. [Google Scholar]