Abstract
Physiological properties involved in divergent cadmium (Cd) accumulation among rice genotypes were characterized using the indica cultivar ‘Habataki’ (high Cd in grains) and the japonica cultivar ‘Sasanishiki’ (low Cd in grains). Time-dependence and concentration-dependence of symplastic Cd absorption in roots were revealed not to be responsible for the different Cd accumulation between the two cultivars because root Cd uptake was not greater in the Cd-accumulating cultivar ‘Habataki’ compared with ‘Sasanishiki’. On the other hand, rapid and greater root-to-shoot Cd translocation was observed in ‘Habataki’, which could be mediated by higher abilities in xylem loading of Cd and transpiration rate as a driving force. To verify whether different abilities in xylem-mediated shoot-to-root translocation generally account for the genotypic variation in shoot Cd accumulation in rice, the world rice core collection, consisting of 69 accessions which covers the genetic diversity of almost 32 000 accessions of cultivated rice, was used. The results showed strong correlation between Cd levels in xylem sap and shoots and grains among the 69 rice accessions. Overall, the results presented in this study revealed that the root-to-shoot Cd translocation via the xylem is the major and common physiological process determining the Cd accumulation level in shoots and grains of rice plants.
Keywords: Cadmium, genotypic variation, Oryza sativa, uptake, xylem loading
Introduction
Many areas of arable soils in the world are moderately contaminated with cadmium (Cd) through the use of phosphate fertilizers, sludge, and irrigation water containing some levels of Cd (Sanità di Toppi and Gabbrielli, 1999; McGrath et al., 2001; Kikuchi et al., 2007). This moderate Cd contamination in soils leads to considerable Cd accumulation in edible parts of the crops, including rice (Wolnik et al., 1983; Arao et al., 2003; Arao and Ae, 2003; Ishikawa et al., 2005a). Rice and its derived products are the major source of Cd intake for Japanese people (Watanabe et al., 2004). To prevent negative effects on human health, it is necessary to reduce the rice Cd concentration below the maximum level indicated by the Codex Alimentarius Commission of FAO/WHO (CODEX, 2006). Several approaches have been proposed to reduce the Cd concentration in paddy soils, such as soil dressing, chemical remediation, and phytoextraction using crops (Ishikawa et al., 2006; Uraguchi et al., 2006; Murakami et al., 2007; Makino et al., 2008). Another promising approach for reducing the Cd concentration in rice grains is breeding or engineering low-Cd-accumulating cultivars. This requires a prior understanding of the Cd accumulation mechanisms in rice plants.
As suggested by many reports, there are three transport processes most likely to mediate Cd accumulation into the shoots and, subsequently, into the seeds: (i) uptake by roots, (ii) xylem-loading-mediated translocation to shoots, and (iii) further translocation to seeds via the phloem (Clemens et al., 2002). Uptake of Cd in roots has been considered a key process in overall plant Cd accumulation; the active uptake of Cd in roots has been demonstrated in various plants (Cataldo et al., 1983; Homma and Hirata, 1984; Costa and Morel, 1993; Hart et al., 1998; Zhao et al., 2002). Molecular approaches indicate that transporters for essential metals such as Zn2+ and Fe2+ can mediate Cd uptake in roots (Korshunova et al., 1999; Nakanishi et al., 2006). Xylem loading of Cd has been shown to be mediated by the P-type ATPase transporter AtHMA4 in Arabidopsis thaliana (Verret et al., 2004) and its homologues in the Cd-hyperaccumulator plants Thlaspi caerulescens and Arabidopsis halleri (Papoyan and Kochian, 2004; Hanikenne et al., 2008). Physiological experiments demonstrated the existence of phloem-mediated Cd transport into seeds/grains of various plants (Popelka et al., 1996; Herren and Feller, 1997; Harris and Taylor, 2001; Tanaka et al., 2003).
Among the major graminaceous crops, a series of studies on durum wheat (Triticum turgidum L. var. durum) have been conducted to characterize Cd uptake, root-to-shoot Cd translocation, and grain Cd accumulation (Hart et al., 1998, 2006; Harris and Taylor, 2001, 2004). However, despite the economical and agricultural importance of rice in monsoon Asia, including Japan, no such studies have been conducted in this crop. But some reports describe important observations related to Cd accumulation in japonica rice subspecies. Homma and Hirata (1984) revealed rapid and active uptake of Cd in rice roots. Rice iron transporters OsIRT1 and OsIRT2 (Nakanishi et al., 2006) and the zinc transporter OsZIP1 (Ramesh et al., 2003) showed Cd-transport activity when expressed in yeasts. Most of the Cd accumulated in rice grains is transported via the phloem (Tanaka et al., 2007). In addition to these observations, our prior studies revealed distinct phenotypes of indica and japonica rice cultivars related to Cd concentration in grains and vegetative tissues (Arao and Ae, 2003; Ishikawa et al., 2005a): Cd accumulation in shoot tissues is generally greater in indica rice cultivars than in japonica cultivars. Based on the grain Cd concentration, Ishikawa et al. (2005b) conducted QTL analysis and identified potential loci controlling Cd concentration in rice grains. More detailed physiological studies should provide insights into the genetic basis of Cd homeostasis. In particular, Cd uptake and root-to-shoot translocation has yet to be investigated in japonica and indica subspecies.
In the present study, Cd uptake and translocation in indica and japonica cultivars have been characterized. Two cultivars were chosen as being representative of each subspecies: ‘Habataki’ (indica subspecies; high Cd in grains) and ‘Sasanishiki’ (japonica subspecies; low Cd in grains; Arao and Ishikawa, 2006). Hydroponic experiments on ‘Sasanishiki’ and ‘Habataki’ suggested that Cd translocation via the xylem is the key process determining shoot Cd accumulation and subsequent grain Cd accumulation, rather than the root Cd uptake ability in the two cultivars. Furthermore, using a set of diverse rice germplasms from the world rice core collection (WRC; National Institute of Agrobiological Sciences Genebank URL: http://www.gene.affrc.go.jp/databases-core_collections_wr_en.php), covering the genetic diversity of 32 000 genotypes of cultivated rice (Kojima et al., 2005), it was examined whether the xylem-mediated root-to-shoot Cd translocation is the common key process in shoot and grain Cd accumulation in rice plants. Soil culture experiments using WRC and additional cultivars have shown that the root-to-shoot Cd translocation activity rather than that of root Cd uptake is strongly correlated with Cd accumulation in the shoot and grains. This is the first report demonstrating physiological differences of Cd accumulation between indica and japonica rice subspecies and the genetic diversity of Cd accumulation in graminaceous crops under lower Cd exposure as observed in the fields.
Materials and methods
Plant materials and growth conditions
Two rice cultivars (Oryza sativa L. cvs ‘Habataki’ and ‘Sasanishiki’) were used for all experiments in this study except for the field test. For soil culture experiments, in addition to ‘Habataki’ and ‘Sasanishiki’, 69 varieties from the WRC (National Institute of Agrobiological Sciences Genebank URL: http://www.gene.affrc.go.jp/databases-core_collections_wr_en.php), including three major japonica cultivars in Japan (‘Koshihikari’, ‘Akitakomachi’, and ‘Hitomebore’) and an indica cultivar ‘Cho-ko-koku’, which was reported as a unique cultivar showing extremely high Cd concentration in grains and shoots (Itoh et al., 2007), were tested. The WRC includes representative varieties of almost 32 000 cultivated rice and is convenient for surveying the genetic diversity of rice (Kojima et al., 2005).
All experiments, except for soil culture, were carried out in a growth chamber (14 h day with a light intensity of 400 μmol photons m−2 s−1 supplied using fluorescent lamps, and day/night temperatures of 25/20 °C). Seeds were soaked in deionized water overnight with aeration. Then seeds were transferred to a plastic mesh, which was in contact with a half-strength Kimura B solution (Ma et al., 2001) containing 2 mM MES [2-(N-morpholino) ethanesulphonic acid] buffer (pH 5.6) in a 10 l plastic container. The solution was aerated continuously and renewed once a week. The seedlings obtained were used for further experiments.
Soil culture experiments were carried out according to Ishikawa et al. (2005b). Cultivation was conducted in a greenhouse under natural light conditions. After germination, seedlings were grown in nursery boxes for a month and then used for further experiments. Soil used for pot experiments was collected from the upper layer (0–15 cm) of a paddy field in Japan that was contaminated with Cd after being irrigated using river water originating from an abandoned Cu mine area. The soil is classified as a Eutric Fluvisol; the Cd concentration was 1.8 mg Cd kg−1 dry weight of soil, as determined by 0.1 M HCl extraction according to the Agricultural Land–Soil Pollution Prevention Law in Japan. The concentrations of Fe, Mn, Cu, and Zn (mg kg−1 dry weight) were 248±4, 46.1±0.3, 10.9±0.1, and 16.4±0.1, respectively, when extracted by 0.1 M HCl (Ishikawa et al., 2006). The Cd concentration in the soil solution was 20 μg l−1 for soil moisture at 60% of field capacity.
Kinetic analyses of Cd uptake in roots
The Cd uptake in roots was determined using intact seedlings (10-d-old) according to Zhao et al. (2002) with some modifications. Hydroponically grown seedlings (9-d-old) were transferred to a solution containing 0.5 mM CaCl2 and 2 mM MES (pH 5.6) for 16 h with aeration before the uptake experiments. The uptake experiments were carried out after 2 h from the start of the light period and were carried out at both 25 °C and 2 °C. The experiment at 2 °C (ice-cold) was conducted to estimate the apoplastic binding of Cd by eliminating the metabolically dependent uptake at low temperature (Zhao et al., 2002). Before the uptake experiment at 2 °C started, plants were treated with an ice-cold solution (2 °C) containing 0.5 mM CaCl2 and 2 mM MES (pH 5.6) for 30 min.
For the dose-dependent Cd uptake experiment, four seedlings were transferred to a plastic vessel with 115 ml of the uptake solution containing 0.5 mM CaCl2 and 2 mM MES (pH 5.6) with different concentrations of CdSO4 (89 nM to 6.6 μM). Each Cd treatment was replicated in three vessels. The uptake solution was aerated continuously during the experiment. After 30 min uptake, plants were rinsed immediately three times in an ice-cold solution containing 0.5 mM CaCl2 and 2 mM MES (pH 5.6). Shoots and roots were subsequently weighed separately; the roots were digested in a microwave system with a mixture of HNO3 and H2O2 (4:1, v/v). The Cd concentration in the roots was determined using a Zeeman graphite furnace atomic absorption spectrometer (GF-AAS; SpectrAA 220Z, Varian Inc., Mulgrave, Victoria, Australia), as described previously (Ishikawa et al., 2005b). Values of Km and Vmax for each cultivar were calculated using software (GraphPad PRISM4; GraphPad Software Inc., CA, USA).
For the time-course Cd uptake experiment, four seedlings were transferred to a plastic vessel with 115 ml of the uptake solution containing 0.5 mM CaCl2 and 2 mM MES (pH 5.6) with 0.18 μM CdSO4. Plants were harvested after 0, 5, 10, 30, 60, 120, and 180 min Cd treatment, as described in the dose-dependent uptake experiment. Each sampling was in triplicate. The uptake solution was renewed every 30 min to maintain the Cd concentration in the solution. The concentration of Cd in roots was determined as described above.
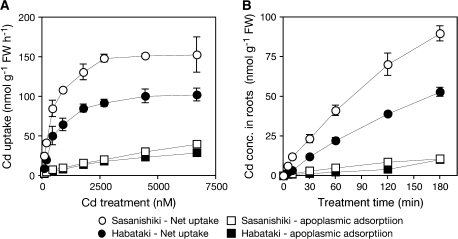
In the dose-dependent uptake experiment, the apparent uptake at 2 °C represented the linear concentration-dependency in both cultivars (Fig. 1A), indicating the passive adsorption of Cd in the apoplast. Symplasmic net uptake of Cd in roots was estimated by subtracting the apparent uptake at 2 °C from that of 25 °C in both dose-dependent and time-course uptake analyses.
Fig. 1.
Root Cd uptake in 10-d-old intact seedlings of a low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and a high-Cd-accumulating indica cultivar ‘Habataki’. (A) Dose-dependent Cd uptake by roots. Plants were exposed to 0.5 mM CaCl2 solution containing ranged concentrations of CdSO4 at 25 °C or 2 °C for 30 min. Net uptake of Cd (symplasmic fraction) in roots was estimated by subtracting the apparent uptake at 2 °C (apoplasmic fraction) from that of 25 °C. (B) Time-dependency of symplasmic Cd accumulation in roots. Plants were exposed to 0.5 mM CaCl2 solution containing 0.18 μM CdSO4 at 25 °C or 2 °C. Net uptake of Cd (symplasmic fraction) in roots was estimated by subtracting the apparent uptake at 2 °C (apoplasmic fraction) from that of 25 °C. Data are presented as means with SD (n=3). Error bars do not extend outside some data points.
Time-course analyses of xylem loading of Cd and shoot Cd accumulation
Seedlings (10-d-old) grown with a half-strength Kimura B solution were transferred to a 5.0 l plastic container containing a full-strength Kimura B solution buffered with 2 mM MES (pH 5.6). After 4 d culture, plants were treated with a solution containing a moderately low Cd concentration (0.18 μM CdSO4). The Cd concentration was based on Cd levels in soil solutions of moderately Cd-polluted paddy soils. Plants grown in Kimura B solution without added Cd were prepared as a control. Xylem sap, shoots, and roots were collected at 1 h, 6 h, 1 d, 2 d, 5 d, 7 d, and 14 d after Cd exposure started. Each sampling was replicated in three containers. Shoots and roots were separated and rinsed three times with deionized water. After drying and weighing, plant samples were ground to a fine powder and an aliquot was used for elemental analysis. Concentrations of Cd and chemically related heavy metals (Cu, Fe, Mn, and Zn) in digested samples were determined using inductively coupled plasma-optical-emission spectroscopy (ICP-OES, Vista-Pro; Varian Inc., Mulgrave, Australia). Xylem sap exuded from the cut surface was collected by means of trapping into a 1.5 ml plastic vial filled with a small piece of cotton for 2 h after cutting the shoots at 2 cm above the roots. The amount of collected sap was weighed; the Cd concentration in the sap was determined using GF-AAS.
Dose-dependent analyses of xylem loading of Cd and shoot Cd accumulation
Seedlings (10-d-old) grown with a half-strength Kimura B solution were transferred to a 5.0 l plastic container containing a full-strength Kimura B solution buffered with 2 mM MES (pH 5.6). After 4 d culture, plants were treated with a solution containing different Cd concentrations (0.09–1.78 μM CdSO4). Xylem sap, shoots and roots were collected at 6 h after Cd exposure started and their Cd concentrations were determined using GF-AAS, as described elsewhere. Each treatment was performed in triplicate.
Measurement of the transpiration rate and the effect of ABA treatment on Cd accumulation
Seedlings (10-d-old) grown with a half-strength Kimura B solution were transferred to a 5.0 l plastic container containing a full-strength Kimura B solution buffered with 2 mM MES (pH 5.6). After 4 d culture, plants were used for measurement of the transpiration rate and ABA treatment. Ten seedlings were transferred to a vessel containing 500 ml of the nutrient solution 1 h after the light period started. The transpiration rate was measured according to the method used by Greger and Johansson (1992) before and during the ABA treatment and Cd treatment. For ABA treatment, plants were treated with a nutrient solution containing 100 μM ABA for 1 h. Plants treated with or without ABA were subsequently exposed to 0.18 μM CdSO4 for 6 h. Shoots, roots, and xylem sap were collected and their Cd concentrations were determined using GF-AAS. Plants treated with neither ABA nor Cd were prepared as controls. Each treatment was replicated in three vessels.
Soil culture experiments
Seedlings of ‘Sasanishiki’ and ‘Habataki’ grown in nursery boxes for 1 month were transplanted into a 1/2000-a Wagner pot containing 7.5 kg of the Cd-polluted soil under flooded conditions (two seedlings per pot). Fertilizer was applied to each pot at a rate of 0.75 g each of N, P2O5, and K2O as a base dressing. Half of those rates were also applied as a top dressing at the panicle formation stage. After 1 month of transplanting, water was drained from each pot; then water management was introduced by alternate flooding for 3 d and drainage for 2 d. At 10 d after heading, xylem sap was collected from one out of the two seedlings in a pot, as described elsewhere. After grain ripening, the shoot samples were harvested and separated into brown rice, upper leaf blades, lower leaf blades, and stems. Root samples were collected from the soil by carefully washing them with tap water and then with deionized water. The experiment was replicated in four pots.
Seedlings (1-month-old) of 69 genotypes from WRC and four additional cultivars (‘Koshihikari’, ‘Akitakomachi’, ‘Hitomebore’, and ‘Cho-ko-koku’) were transplanted from nursery boxes into a plastic pot filled with 0.3 kg of the Cd-polluted soil (one seedling per pot). After culture for 1 month under upland conditions, the xylem sap and shoots were collected from all genotypes. Then 0.1 ml of concentrated HNO3 was added to the collected xylem sap and their Cd concentrations were analysed immediately using GF-AAS. Based on xylem Cd concentrations, the roots were sampled from 11 genotypes of WRC which showed lower [‘Nipponbare’ (WRC01), ‘Asu’ (WRC13), ‘Kaluheenati’ (WRC41), ‘Tupa 729’ (WRC55), ‘Milyang 23’ (WRC57), ‘Hakphaynhay’ (WRC60), and Vandaran (WRC100)] or higher [‘Kasalath’ (WRC02), ‘Jarjan’ (WRC28), ‘Anjana Dhan’ (WRC30), and ‘Basilanon’ (WRC44)] Cd concentrations in xylem sap, ‘Cho-ko-koku’, which also showed higher Cd concentration in the xylem sap, and three genotypes of major japonica cultivars in Japan (‘Koshihikari’, ‘Akitakomachi’, and ‘Hitomebore’) on the day following xylem collection. The experiment was replicated in three pots. Metal concentrations (Cd, Cu, Fe, Mn, and Zn) of plant tissue samples were determined using ICP-OES, as described elsewhere.
The field experiment was conducted in a paddy field at the National Institute for Agro-Environmental Sciences (latitude 36° 01′ 32.31″ N, longitude 104° 06′ 23.88″ E, altitude 21 m), located in Tsukuba, Japan. The paddy soil was classified as a Fluvisol and contained naturally abundant level of Cd at 0.21 mg kg−1, when extracted with 0.1 M HCl. Seedlings of 69 genotypes from WRC were transplanted into the paddy field, at a rate of 20 seedlings/genotype in rows 0.15 m apart and seedlings within each row spaced 0.3 m apart. A commercial chemical fertilizer was applied at the rate of 10–10–10 kg N–P2O5–K2O as a base dressing before transplanting. Surface irrigation was applied for 1 month after transplanting and before the mid-season drainage was done for about 1week. Then, a full irrigation condition was applied again until grain harvesting. Three hills of each rice genotype were randomly collected from the field for analysis of grain Cd concentration. Of 69 accessions, nine varieties did not ripen during the rice growing season in Japan, so the concentrations of Cd in rice grains were compared among 60 varieties. Cadmium concentrations of grain were determined using ICP-MS (ELAN DRC-e, Perkin Elmer SCIEX, USA) after the acid digestion.
The significance of correlations among xylem sap, shoots, roots, and brown rice with regard to metal concentrations were tested by Spearman's rank correlation test using the SPSS computer program (Version 6.1 ; SPSS Inc., Tokyo, Japan).
Results
Cd uptake by roots
A dose-dependent Cd uptake assay using intact seedlings was first conducted to estimate the Cd uptake ability of two rice cultivars that differ in grain Cd accumulation. The apparent uptake at 2 °C represented the linear concentration-dependency in both cultivars (Fig. 1A), indicating the passive adsorption of Cd in the apoplast as described previously (Hart et al., 1998; Zhao et al., 2002). There is no significant difference of Cd adsorption at 2 °C between the two cultivars (Fig. 1A). However, the net uptake rate of Cd by root symplasts was higher in ‘Sasanishiki’ (lower Cd accumulation in grains) than in ‘Habataki’ (higher Cd accumulation in grains) under all Cd concentrations used in this assay (Fig. 1A). Cadmium uptake of both cultivars was saturated under higher Cd exposure (>2 μM), indicating the active transport of Cd into roots. Then, values of Km and Vmax were calculated (Table 1) based on these data: Vmax of ‘Sasanishiki’ (162±4.5 nmol g−1 FW h−1) was greater than that of ‘Habataki’ (115±3.7 nmol g−1 FW h−1). The value of Km was 0.44±0.04 μM in ‘Sasanishiki’ and 0.67±0.08 μM in ‘Habataki’. Supporting the results of the dose-dependent assay, the time-course of net uptake of Cd was 1.7–3.6-times higher in ‘Sasanishiki’ than in ‘Habataki’ through 180 min-Cd exposure (Fig. 1B). These results show that the Cd uptake ability of ‘Habataki’ is not superior to that of ‘Sasanishiki’, at least under the present experimental conditions.
Table 1.
Parameters of root Cd uptake in the low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and the high-Cd-accumulating indica cultivar ‘Habataki’
| Vmax (nmol g−1 FW h−1) | Km (μM) | r2 | |
| ‘Sasanishiki’ | 162.4±4.5 | 0.44±0.04 | 0.959 |
| ‘Habataki’ | 114.8±3.7 | 0.67±0.08 | 0.965 |
Data are presented as means ±SE (n=3).
Time-course analysis of Cd accumulation into shoots
To estimate Cd translocation to the shoots in two rice cultivars, Cd accumulation in the shoots was analysed after the treatment of 0.18 μM Cd, which is equivalent to the Cd level (20 μg l−1) in the soil solution of the Cd-polluted soil used for this study. There was no significant effect of the Cd treatment on the dry weight of the shoots and roots. No visible symptom was observed in Cd-treated plants (data not shown). Moreover, the addition of Cd did not significantly alter the concentrations of essential metals in shoots, except for Cu and Fe in ‘Habataki’ and ‘Sasanishiki’, respectively (see Supplementary Fig. S1 at JXB online).
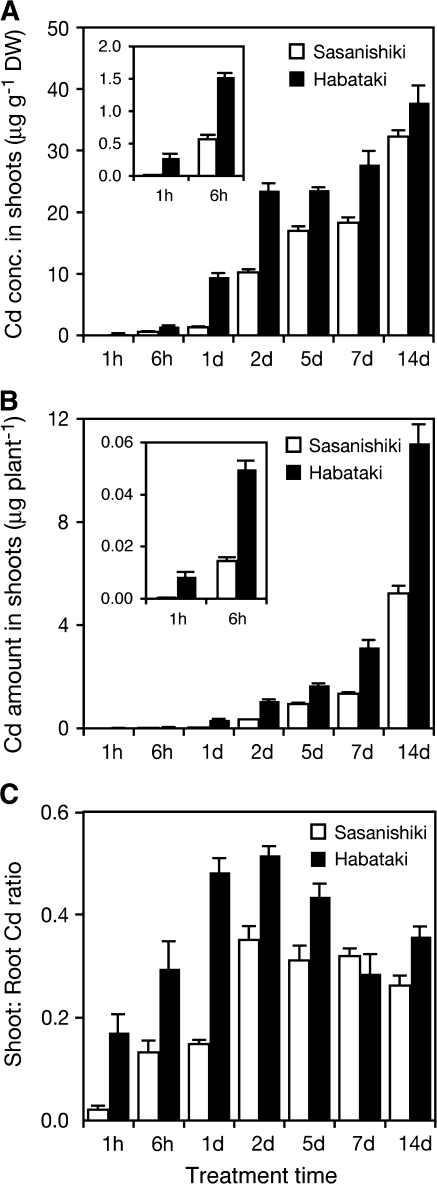
Cadmium concentration in the shoots increased according to the duration of the Cd treatment in both cultivars, and the concentration was 1.2–24-fold greater in ‘Habataki’ than in ‘Sasanishiki’ during the 14 d Cd treatment (Fig. 2A). The total amount of Cd accumulated in the shoots of ‘Habataki’ showed 1.8–34-times higher values than that of ‘Sasanishiki’ (Fig. 2B). It is noteworthy that a considerable accumulation of Cd was observed, even after only 1 h exposure in ‘Habataki’ (Fig. 2A, B). The shoot:root Cd ratio of ‘Habataki’ was 1.3–8.5-times higher than that of ‘Sasanishiki’ throughout the Cd treatment, except for 7 d after Cd exposure (Fig. 2C). These results demonstrate a more rapid and greater Cd translocation into the shoots of the Cd-accumulating cultivar ‘Habataki’ than into those of ‘Sasanishiki’.
Fig. 2.
Time-dependent Cd accumulation in shoots of a low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and a high-Cd-accumulating indica cultivar ‘Habataki’. 14-d-old seedlings were exposed to a nutrient solution containing 0.18 μM CdSO4 for 14 d. (A) Cd concentration in shoots. (B) Accumulated Cd amount in shoots. (C) Shoot:root ratio of accumulated Cd amount. Data are presented as means with SD (n=3).
Time-dependent and concentration-dependent analysis of xylem loading of Cd
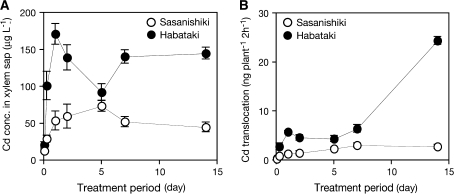
The Cd concentration in the xylem sap was determined to examine the translocation dynamics of Cd into the shoots. In both cultivars, the Cd concentration in the xylem sap always exceeded the concentration in the solution (20 μg l−1) and was maintained at certain levels 6 h from the start of the treatment (Fig. 3A). The Cd concentration in the xylem sap was 1.3–3.5 times higher in ‘Habataki’ than in ‘Sasanishiki’ throughout the treatment period (Fig. 3A). The concentration of Cd in the xylem sap was calculated and the amount of Cd transported for 2 h in the xylem sap was estimated (Fig. 3B). In ‘Habataki’, a greater amount of Cd was translocated through the xylem sap, which was 1.6–8.9 times higher than that of ‘Sasanishiki’ (Fig. 3B). The remarkable increase of Cd translocation via the xylem sap was observed in ‘Habataki’ after 14 d of the treatment, although ‘Sasanishiki’ exhibited a slight increase according to the duration of Cd exposure (Fig. 3B).
Fig. 3.
Time-dependent analysis of root-to-shoot Cd translocation in a low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and a high-Cd-accumulating indica cultivar ‘Habataki’. 14-d-old seedlings were exposed to a nutrient solution containing 0.18 μM CdSO4 for 14 d. (A) Cd concentration in xylem sap. (B) Cd amount in the xylem sap collected for 2 h. Data are presented as means with SD (n=3). Error bars do not extend outside some data points.
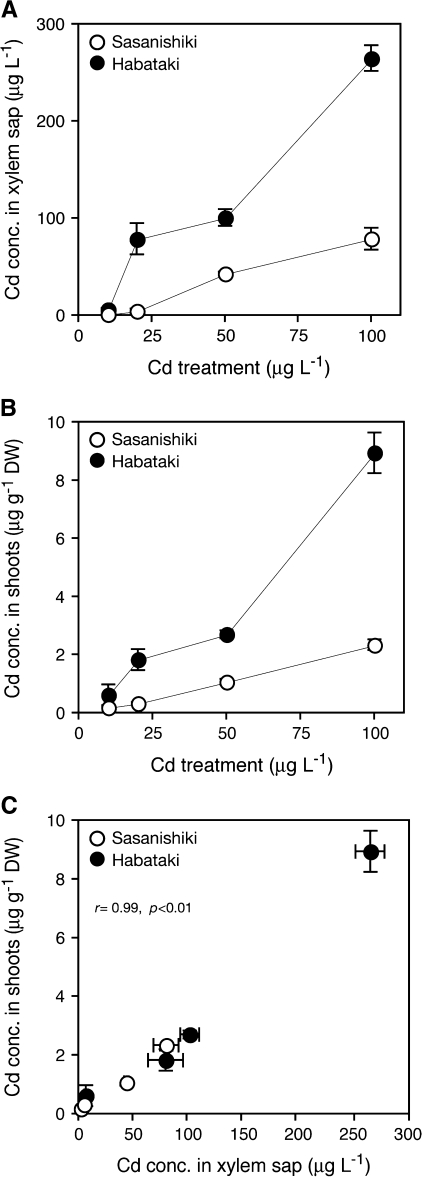
Dose-dependency of xylem loading of Cd was examined in two cultivars. After 6 h Cd exposure, the Cd concentration in the xylem sap showed a dose-dependent increase in both cultivars (Fig. 4A). The shoot Cd concentration was also increased in a dose-dependent manner in both cultivars, but was higher in ‘Habataki’ (Fig. 4B). Concentrations of Cd in xylem sap and shoots, as presented in Fig. 4C, showed a good correlation in both cultivars (r=0.98). These results suggest distinct differences in the xylem loading of Cd and subsequent shoot Cd accumulation between ‘Habataki’ and ‘Sasanishiki’.
Fig. 4.
Dose-dependent analysis of root-to-shoot Cd translocation in a low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and a high-Cd-accumulating indica cultivar ‘Habataki’. 14-d-old seedlings were exposed to a nutrient solution containing ranged concentration of CdSO4 for 6 h. (A) Cd concentration in xylem sap. (B) Cd concentration in shoots. (C) Relationship between Cd concentration in xylem sap and shoots. Data are presented as means with SD (n=3). Error bars do not extend outside some data points.
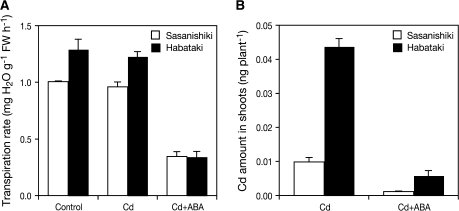
Transpiration activity and effect of ABA treatment on root-to-shoot Cd translocation
The transpiration rate was measured using 14-d-old seedlings. Under normal and Cd-treated conditions, ‘Habataki’ exhibited significantly higher activity of transpiration (P <0.05) compared to ‘Sasanishiki’ (Fig. 5A). No significant effect of Cd treatment was found on the transpiration activity in either cultivar (Fig. 5A).
Fig. 5.
Transpiration rate (A) and the effect of ABA treatment on shoot Cd accumulation (B) in a low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and a high-Cd-accumulating indica cultivar ‘Habataki’. 14-d-old seedlings were exposed to a nutrient solution with or without 100 μM ABA for 1 h. Then plants were exposed to a nutrient solution containing 0.18 μM CdSO4 for 6 h. Data are presented as means with SD (n=3).
The ABA treatment reduced the transpiration rate by 72% in ‘Habataki’ and 64% in ‘Sasanishiki’, which resulted in the same level of transpiration in the two cultivars (Fig. 5A). Throughout the Cd treatment experiment, transpiration rates were maintained equal between the two cultivars (data not shown). The ABA treatment decreased Cd accumulation in both cultivars (Fig. 5B). However, Cd accumulation in shoots was still 5.1-times higher in ‘Habataki’ than in ‘Sasanishiki’.
Cd concentration in grains and xylem sap under the soil culture polluted with Cd
Cadmium accumulation in grains, leaves, stem, and roots as well as Cd in the xylem sap of ‘Habataki’ and ‘Sasanishiki’ grown under the soil culture were analysed at the heading or grain-filling stage (Table 2). No significant difference was found in the root Cd concentration between the two cultivars. However, except for the lower leaf blades, all the analysed shoot tissues, including those of brown rice, represented 2.4–5.9 times higher Cd concentrations in ‘Habataki’ than in ‘Sasanishiki’. The cadmium concentration in the xylem sap was also 4.3 times higher in ‘Habataki’ than in ‘Sasanishiki’ under paddy soils. Concentrations of Fe, Mn, Cu, and Zn in shoots and grains of ‘Habataki’ and ‘Sasanishiki’ were also determined (see Supplementary Fig. S2 at JXB online). The shoots of ‘Habataki’ contained significantly higher concentrations of Mn, Cu, and Zn than those of ‘Sasanishiki’ (P <0.05), although there was no significant difference in Fe concentrations of two cultivars (see Supplementary Fig. S2A at JXB online). In grains, only the Cu concentration of ‘Habataki’ was significantly higher than that of ‘Sasanishiki’ (see Supplementary Fig. S2B at JXB online).
Table 2.
Cadmium concentrations in shoot and root tissues (μg g−1 DW) and in xylem sap (μg l−1) of the low-Cd-accumulating japonia cultivar ‘Sasanishiki’ and the high-Cd-accumulating indica cultivar ‘Habataki’ cultivated with the moderately Cd-polluted paddy soil under intermittent flooded conditions
| Cultivar |
Ratioa | ||
| ‘Sasanishiki’ | ‘Habataki’ | ‘Habataki’/‘Sasanishiki’ | |
| Brown rice | 0.30±0.03 | 1.57±0.14 | 5.2* |
| Spikelets | 0.37±0.05 | 2.20±0.31 | 5.9* |
| Upper leaf blades | 0.22±0.01 | 0.53±0.05 | 2.4* |
| Lower leaf blades | 0.15±0.01 | 0.11±0.02 | 0.7 |
| Stems | 0.99±0.09 | 3.11±0.37 | 3.1* |
| Xylem sap | 5.1±0.4 | 21.9±4.7 | 4.3* |
| Roots | 18.2±1.20 | 17.0±3.07 | 0.9 |
Data are presented as means with SD (n=4). Xylem sap was collected at the heading stage.
Asterisks represent significant difference between each value of two cultivars tested by Student's t test (P <0.05).
Correlation between Cd in the xylem sap and shoots including grains in diverse rice genotypes
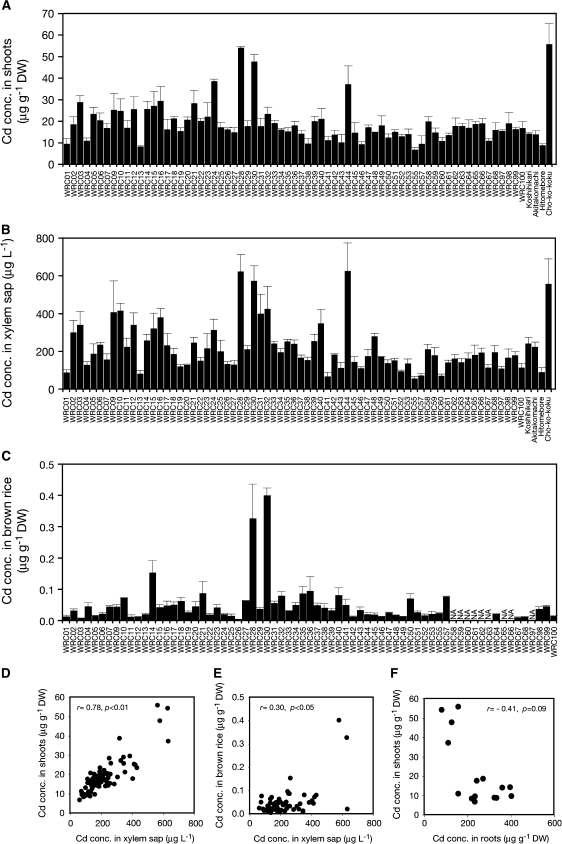
To examine whether the correlation between Cd in the xylem sap and in the shoots exists in various rice genotypes, 69 varieties from WRC and four additional cultivars were grown with the Cd-polluted soil under aerobic conditions; the Cd concentrations in shoots and their xylem sap were analysed. Cadmium concentrations in shoots varied among genotypes; those of most varieties of representative Japanese cultivars ‘Nipponbare’ (WRC01), ‘Koshihikari’, ‘Akitakomachi’, and ‘Hitomebore’ were in the range of 10–20 μg g−1 DW (Fig. 6A). However, the varieties ‘Pinulupot 1’ (WRC24), ‘Jarjan’ (WRC28), ‘Anjana Dhan’ (WRC30), ‘Basilanon’ (WRC44), and ‘Cho-ko-koku’ showed more than 30 μg g−1 DW Cd concentration in their shoots (Fig. 6A). The results of Cd concentration in the xylem sap showed similar trends among the varieties: ‘Jarjan’ (WRC28), ‘Anjana Dhan’ (WRC30), ‘Basilanon’ (WRC44), and ‘Cho-ko-koku’ represented excessive Cd concentration in the xylem sap (Fig. 6B). Grains of 60 varieties of the WRC were harvested from the paddy field which contained naturally abundant levels of Cd and the Cd concentrations in brown rice were determined (Fig. 6C). As the results of xylem sap and shoots in the Cd-polluted soil, brown rice of ‘Jarjan’ (WRC28) and ‘Anjana Dhan’ (WRC30) contained more than 20-times more Cd than that of ‘Nipponbare’ (WRC01) (Fig. 6C). The Cd concentrations in the xylem sap and shoots (r=0.78, P <0.01; Fig. 6D) and in the xylem sap and brown rice (r=0.30, P <0.05; Fig. 6E) were significantly correlated, whereas those of roots and shoots of 15 selected genotypes were negatively correlated (r= –0.41, P=0.09; Fig. 6F).
Fig. 6.
Genotypic variation of root-to-shoot Cd translocation ability in various rice cultivars. Seedlings (1-month-old) of 69 genotypes from the world rice core collection (WRC), three major japonica cultivars in Japan (‘Koshihikari’, ‘Akitakomachi’, and ‘Hitomebore’), and a Cd-accumulating indica cultivar ‘Cho-ko-koku’ were cultured under upland condition for 1 month in a pot filled with paddy soil that had been moderately contaminated with Cd. The Cd concentrations are shown for shoots (A) and xylem sap (B). Data are presented as means with SD (n=3). (C) Seedlings of rice genotypes from WRC were grown in the paddy fields with a naturally abundant level of Cd under flooded condition until the grain ripened and grains were harvested. The Cd concentration in brown rice is shown (C). Data are presented as means with SD (n=3). NA: not analysed on account of grain immaturity. (D) Relationships between Cd concentration in xylem sap (shown in B) and shoots (shown in A) of 73 genotypes. Data are presented as means (n=3). (E) Relationships between Cd concentration in xylem sap (shown in B) and brown rice (shown in C) of varieties from WRC. Data are presented as means (n=3). (F) Relationships between Cd concentrations in roots and shoots of 15 selected genotypes differing in xylem sap Cd concentration shown in (A) and (B). Data are presented as means (n=3).
Concentrations of Fe, Mn, Cu, and Zn in shoots of 69 varieties from WRC and four additional cultivars grown with the Cd-polluted soil under aerobic conditions were also determined (see Supplementary Fig. S3 at JXB online). There was some genotypic variation in these metal concentrations, however, which was smaller than that of Cd. Relationships between Cd and other metals concentrations in the shoots are shown in Supplementary Fig. S4 at JXB online. Positive and strong correlations in shoot metal concentrations were observed between Cd and Cu, Cd and Zn, and Cd and Mn (P <0.01), but not between Cd and Fe (P=0.59). However, these observed correlations seemed inapplicable to several high Cd-accumulating cultivars such as ‘Jarjan’ (WRC28), ‘Anjana Dhan’ (WRC30), and ‘Cho-ko-koku’ (see Supplementary Fig. S4 at JXB online).
Discussion
Root Cd uptake ability of two rice cultivars
In this study, the uptake and root-to-shoot translocation of Cd in two representative rice cultivars have been characterized so as to understand the basis for their different accumulation of Cd in grains. Accumulation of heavy metals such as Cd in shoot tissues has been proposed to be the result of several transport processes (Clemens et al., 2002). As Cd is initially taken up from the roots, many studies evaluated the potential of Cd uptake in an attempt to explain the differences in shoot Cd accumulation between ecotypes, cultivars, and relatives (Hart et al., 1998, 2006; Zhao et al., 2002; Chan and Hale, 2004). The Cd uptake ability in rice cultivars ‘Habataki’ and ‘Sasanishiki’ was examined first. Interestingly, ‘Sasanishiki’, which has a low Cd content in grains, had a higher Cd uptake rate than ‘Habataki’ which shows higher Cd levels in grains (Fig. 1A, B; Table 1). No significant difference in root Cd concentration was observed between these two cultivars when grown in soils (Table 2). Higher root Cd concentrations were observed in the various rice cultivars showing lower Cd levels in shoots (Fig. 6F). Similarly, in durum wheat, the potential of Cd uptake in roots was comparable between near isogenic lines differing in grain Cd levels (Harris and Taylor, 2004; Hart et al., 2006). Overall, the results presented here on rice demonstrate that root Cd uptake is not a major process leading to the divergent difference in shoot and grain Cd accumulation between ‘Habataki’ and ‘Sasanishiki’.
Xylem loading and translocation of Cd into shoots
Following the uptake of metals by roots, xylem loading of metals is suggested as the next important transport process for metal-accumulation in plant shoots (Clemens et al., 2002). Although root Cd uptake ability was not superb in ‘Habataki’ compared to that in ‘Sasanishiki’, more rapid and greater root-to-shoot Cd translocation was observed in ‘Habataki’ (Figs 2A, C, 4A, C). Moreover, under equal transpiration conditions mediated by ABA treatment, Cd accumulation in the shoots remained higher in ‘Habataki’ (Fig. 5A, B). The Cd concentration in xylem sap was up to four times higher in ‘Habataki’ than in ‘Sasanishiki’ (Figs 3A, 4A). These results suggest the higher loading ability of Cd to the xylem in ‘Habataki’, which leads to higher shoot Cd accumulation (Fig. 4C). No gene encoding transporter involved in metal xylem loading has been identified in rice so far. However, it has been demonstrated that a P-type ATPase, AtHMA4, of A. thaliana and its homologue in the Cd/Zn hyperaccumulator A. halleri are involved in root-to-shoot translocation of Cd (Verret et al., 2004; Hanikenne et al., 2008). Given that one homologue of AtHMA4 in rice (Williams and Mills, 2005) mediates xylem loading of Cd, differential shoot accumulation of essential metals as Zn is expected between the cultivars, because AtHMA4 and its homologue in A. halleri function in the xylem loading of Zn as well as Cd (Verret et al., 2004; Hanikenne et al., 2008). In fact, 1.3–1.6 times higher accumulation of Zn, Cu, and Mn was observed in shoots of ‘Habataki’ compared to those of ‘Sasanishiki’ (see Supplementary Fig. S2A at JXB online). Transporters for essential metals including Zn, Mn, and Cu have been also demonstrated to transport Cd in plants (Korshunova et al., 1999; Ramesh et al., 2003; Nakanishi et al., 2006). These results suggest the possibility that the transport system mediating xylem loading of Zn and/or other essential metals is involved in Cd xylem loading in rice. Moreover, the difference in metal accumulation between the cultivars was much greater for Cd than for Zn, Cu, and Mn (Table 2; see Supplementary Fig. S2A at JXB online). This suggests that differences in expression level as well as in the affinity for Cd of xylem loading transporter(s) are responsible for the different Cd accumulation between the cultivars.
The final Cd content in the grain depends on the Cd concentration in the xylem sap and the xylem flow rate. Cd concentration in the xylem sap was higher in ‘Habataki’ (Fig. 3A). A 1.3-times higher rate of transpiration (P <0.05) was also observed for ‘Habataki’ than for ‘Sasanishiki’ (Fig. 5A). In the experiment using ABA, Cd accumulation in shoots decreased under the lowered transpiration rate (Fig. 5A, B). In line with our results, ABA treatment decreased the root-to-shoot Cd translocation in Brassica juncea (Salt et al., 1995) and A. halleri (Zhao et al., 2006). These observations also indicate the role of the transpiration stream as a driving force of Cd-translocation into shoot tissues. Taken together, it is suggested that the differences in the activity of loading Cd into xylem and the transpiration rate lead to different Cd accumulation in shoots of ‘Habataki’ and ‘Sasanishiki’.
Characteristics of Cd accumulation in rice
The distinct difference in the Cd accumulation pattern between ‘Habataki’ and ‘Sasanishiki’ was confirmed under soil culture conditions, which is in agreement with results of hydroponic cultures (Table 2). It is noteworthy that the grain Cd concentration was strongly associated with those of the xylem sap and vegetative tissues rather than that of the root (Table 2). As with essential heavy metals, Cd is thought to be transported into seeds or grains via the phloem in many plants (Popelka et al., 1996; Herren and Feller, 1997; Tanaka et al., 2003). In rice, 91–100% of Cd in grains are transported via the phloem (Tanaka et al., 2007). Thus it is hypothesized that in ‘Habataki’, showing higher Cd content in grains, exceeding root-to-shoot Cd translocation via the xylem first leads to higher Cd accumulation in vegetative tissues (‘source’ organ). Subsequently, the greater Cd accumulated in source organs is likely to be transported to grains (‘sink’ organ) via the phloem, leading to higher Cd concentrations in grains of ‘Habataki’ compared to ‘Sasanishiki’. It is also possible that greater Cd in the xylem is transported directly into grains after the xylem–phloem transfer, which can also cause higher Cd accumulation in grains. In fact, the tracer analysis on the grain-filling-stage of rice indicated that Cd loaded to xylem was transferred to the phloem at nodes and was transported directly into grains via the phloem (personal communication with Dr Shu Fujimaki, Japan Atomic Energy Agency). In either case, the root-to-shoot Cd transport via the xylem is proposed to be the major factor determining grain Cd concentration in ‘Habataki’ and ‘Sasanishiki’.
It was verified whether different abilities in xylem-mediated shoot-to-root translocation generally account for genotypic variation in shoot Cd accumulation in rice. The pot experiments using WRC clearly demonstrated that Cd levels in xylem sap and shoots were strongly correlated among various cultivars (Fig. 6D). Cd concentrations in xylem sap and brown rice were also significantly and positively correlated in 60 varieties of WRC (Fig. 6E). These results on diverse rice cultivars support the hypothesis formulated from the results on ‘Habataki’ and ‘Sasanishiki’. With regard to the relationships between Cd and chemically related heavy metals (Cu, Fe, Mn, and Zn), positive and significant correlations were found between Cd and essential metals (Cu, Zn, and Mn) accumulation in the shoots of various genotypes (see Supplementary Fig. S4 at JXB online). These results are also in agreement with those of ‘Habataki’ and ‘Sasanishiki’ (see Supplementary Fig. S2A at JXB online), indicating that the transport process common to heavy metals is involved in root–shoot translocation of metals in rice. However, some varieties accumulating extremely high amounts of Cd such as ‘Jarjan’ (WRC28), ‘Anjana Dhan’ (WRC30), and ‘Cho-ko-koku’ unambiguously deviated from the obtained correlations (see Supplementary Fig. S2A at JXB online). In these specific varieties, the affinity for Cd in the process of loading metals into xylem might be dramatically higher compared with those of other varieties tested.
In conclusion, it has been demonstrated that, in rice, the ability of root-to-shoot Cd translocation via the xylem is the main process in shoot and grain Cd accumulation. It should be noted that mapping populations of ‘Sasanishiki’ crossed with ‘Habataki’ are available for the initial genetic analysis (Ando et al., 2008; Rice Genome Resource Center URL: http://www.rgrc.dna.affrc.go.jp/index.html.en). Because rice is a model plant of graminaceous crops and has a high degree of synteny with other cereal crops, such as wheat and maize, further investigations based on the present results in rice would provide useful information for the regulation of Cd accumulation in graminaceous crops.
Supplementary data
Supplementary data can be found at JXB online.
Fig. S1. Metal concentrations in shoots of a low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and a high-Cd-accumulating indica cultivar ‘Habataki’ exposed to a nutrient solution with or without 0.18 μM CdSO4 for 14 d.
Fig. S2. Metal concentrations in shoots (A) and grains (B) of a low-Cd-accumulating japonica cultivar ‘Sasanishiki’ and a high-Cd-accumulating indica cultivar ‘Habataki’ cultivated with the moderately Cd-polluted paddy soil under intermittent flooded conditions.
Fig. S3. Genotypic variation of metal concentrations in shoots of diverse rice cultivars.
Fig. S4. Relationships between Cd and essential metals concentrations in shoots of various genotypes.
Acknowledgments
This work was supported by a Grant-in-aid (Hazardous Chemicals) for the Ministry of Agriculture, Forestry, and Fisheries of Japan (HC-1150) and by the Program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN). The core collection of rice consisting of 69 accessions was kindly supplied by Genebank at the National Institute of Agro-biological Sciences (NIAS) in Japan. We gratefully acknowledge Mr Masashi Itoh, Akita Prefectural Agriculture, Forestry, and Fisheries Research Centre, Japan, for supplying seeds of Cho-ko-koku, Ms Hiroko Abe and Mr Daishi Misawa for their excellent technical assistance, and Dr Fabien Lombardo for helpful advice on the manuscript.
Glossary
Abbreviations
- ABA
abscisic acid
- GF-AAS
graphite furnace atomic absorption spectrometer
- ICP-OES
inductively coupled plasma-optical-emission spectroscopy
- MES
2-(N-morpholino) ethanesulphonic acid
- WRC
world rice core collection
References
- Ando T, Yamamoto T, Shimizu T, Ma X, Shomura A, Takeuchi Y, Lin S, Yano M. Genetic dissection and pyramiding of quantitative traits for panicle architecture using chromosomal segment substitution lines in rice. Theoretical and Applied Genetics. 2008;116:881–890. doi: 10.1007/s00122-008-0722-6. [DOI] [PubMed] [Google Scholar]
- Arao T, Ae N. Genotypic variations in cadmium levels of rice grain. Soil Science and Plant Nutrition. 2003;49:473–479. [Google Scholar]
- Arao T, Ae N, Sugiyama M, Takahashi M. Genotypic differences in cadmium uptake and distribution in soybeans. Plant and Soil. 2003;251:247–253. [Google Scholar]
- Arao T, Ishikawa S. Genotypic differences in cadmium concentration and distribution of soybean and rice. Japan Agricultural Research Quarterly. 2006;40:21–30. [Google Scholar]
- Cataldo DA, Garland TR, Wildung RE. Cadmium uptake kinetics in intact soybean plants. Plant Physiology. 1983;73:844–848. doi: 10.1104/pp.73.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DY, Hale BA. Differential accumulation of Cd in durum wheat cultivars: uptake and retranslocation as sources of variation. Journal of Experimental Botany. 2004;55:2571–2579. doi: 10.1093/jxb/erh255. [DOI] [PubMed] [Google Scholar]
- Clemens S, Palmgren MG, Kramer U. A long way ahead: understanding and engineering plant metal accumulation. Trends in Plant Science. 2002;7:309–315. doi: 10.1016/s1360-1385(02)02295-1. [DOI] [PubMed] [Google Scholar]
- CODEX. Report of the 38th session of the CODEX Committee on Food Additives and Contaminants. Codex Alimentarius Commission. 2006 ALINORM 06/29/12:1–12. [Google Scholar]
- Costa G, Morel JL. Cadmium uptake by Lupinus albus (L.): cadmium excretion, a possible mechanism of cadmium tolerance. Journal of Plant Nutrition. 1993;16:1921–1929. [Google Scholar]
- Greger M, Johansson M. Cadmium effects on leaf transpiration of sugar beet (Beta vulgaris) Physiologia Plantarum. 1992;86:465–473. [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Kramer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- Harris NS, Taylor GJ. Remobilization of cadmium in maturing shoots of near isogenic lines of durum wheat that differ in grain cadmium accumulation. Journal of Experimental Botany. 2001;52:1473–1481. doi: 10.1093/jexbot/52.360.1473. [DOI] [PubMed] [Google Scholar]
- Harris NS, Taylor GJ. Cadmium uptake and translocation in seedlings of near isogenic lines of durum wheat that differ in grain cadmium accumulation. BMC Plant Biology. 2004;4:4. doi: 10.1186/1471-2229-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV. Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiology. 1998;116:1413–1420. doi: 10.1104/pp.116.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JJ, Welch RM, Norvell WA, Kochian LV. Characterization of cadmium uptake, translocation and storage in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytologist. 2006;172:261–271. doi: 10.1111/j.1469-8137.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Herren T, Feller U. Transport of cadmium via xylem and phloem in maturing wheat shoots: comparison with the translocation of zinc, strontium and rubidium. Annals of Botany. 1997;80:623–628. [Google Scholar]
- Homma Y, Hirata H. Kinetics of cadmium and zinc absorption by rice seedling roots. Soil Science and Plant Nutrition. 1984;30:527–532. [Google Scholar]
- Ishikawa S, Ae N, Sugiyama M, Murakami M, Arao T. Genotypic variation in shoot cadmium concentration in rice and soybean in soils with different levels of cadmium contamination. Soil Science and Plant Nutrition. 2005a;51:101–108. [Google Scholar]
- Ishikawa S, Ae N, Yano M. Chromosomal regions with quantitative trait loci controlling cadmium concentration in brown rice (Oryza sativa) New Phytologist. 2005b;168:345–350. doi: 10.1111/j.1469-8137.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Ae N, Murakami M, Wagatsuma T. Is Brassica juncea a suitable plant for phytoremediation of cadmium in soils with moderately low cadmium contamination? Possibility of using other plant species for Cd-phytoextraction. Soil Science and Plant Nutrition. 2006;52:32–42. [Google Scholar]
- Itoh M, Nakagawa S, Itoh C, Matsumoto S, Masaki S, Itani T. Practicability of using high Cd-accumulating rice varieties for phytoremediation of the paddy fields contaminated with Cd. Abstract of the Annual Meeting. Japanese Society of Soil Science and Plant Nutrition. 2007;53:180. (in Japanese) [Google Scholar]
- Kikuchi T, Okazaki M, Toyota K, Motobayashi T, Kato M. The input–output balance of cadmium in a paddy field of Tokyo. Chemosphere. 2007;67:920–927. doi: 10.1016/j.chemosphere.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Ebana K, Fukuoka S, Nagamine T, Kawase M. Development of an RFLP-based rice diversity research set of germplasm. Breeding Science. 2005;55:431–440. [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Molecular Biology. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Ma JF, Goto S, Tamai K, Ichii M. Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiology. 2001;127:1773–1780. [PMC free article] [PubMed] [Google Scholar]
- Makino T, Takano H, Kamiya T, Itou T, Sekiya N, Inahara M, Sakurai Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere. 2008;70:1035–1043. doi: 10.1016/j.chemosphere.2007.07.080. [DOI] [PubMed] [Google Scholar]
- McGrath SP, Zhao FJ, Lombi E. Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant and Soil. 2001;232:207–214. [Google Scholar]
- Murakami M, Ae N, Ishikawa S. Phytoextraction of cadmium by rice (Oryza sativa L.), soybean (Glycine max (L.) Merr.), and maize (Zea mays L.) Environmental Pollution. 2007;145:96–103. doi: 10.1016/j.envpol.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Science and Plant Nutrition. 2006;52:464–469. [Google Scholar]
- Papoyan A, Kochian LV. Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase. Plant Physiology. 2004;136:3814–3823. doi: 10.1104/pp.104.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popelka JC, Schubert S, Schulz R, Hansen AP. Cadmium uptake and translocation during reproductive development of peanut (Arachis hypogaea L.) Angewandte Botanik. 1996;70:140–143. [Google Scholar]
- Ramesh SA, Shin R, Eide DJ, Schachtman DP. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiology. 2003;133:126–134. doi: 10.1104/pp.103.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology. 1995;109:1427–1433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanità di Toppi LS, Gabbrielli R. Response to cadmium in higher plants. Environmental and Experimental Botany. 1999;41:105–130. [Google Scholar]
- Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H. Cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.) treated with a nutrient solution containing cadmium. Soil Science and Plant Nutrition. 2003;49:311–313. [Google Scholar]
- Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H. Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.) Soil Science and Plant Nutrition. 2007;53:72–77. [Google Scholar]
- Uraguchi S, Watanabe I, Yoshitomi A, Kiyono M, Kuno K. Characteristics of cadmium accumulation and tolerance in novel Cd-accumulating crops, Avena strigosa and Crotalaria juncea. Journal of Experimental Botany. 2006;57:2955–2965. doi: 10.1093/jxb/erl056. [DOI] [PubMed] [Google Scholar]
- Verret F, Gravot A, Auroy P, Leonhardt N, David P, Nussaume L, Vavasseur A, Richaud P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Letters. 2004;576:306–312. doi: 10.1016/j.febslet.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Shimbo S, Nakatsuka H, Koizumi A, Higashikawa K, Matsuda-Inoguchi N, Ikeda M. Gender-related difference, geographical variation and time trend in dietary cadmium intake in Japan. Science of the Total Environment. 2004;329:17–27. doi: 10.1016/j.scitotenv.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Williams LE, Mills RF. P1B-ATPases: an ancient family of transition metal pumps with diverse functions in plants. Trends in Plant Science. 2005;10:491–502. doi: 10.1016/j.tplants.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Wolnik KA, Fricke FL, Capar SG, Braude GL, Meyer MW, Satzger RD, Bonnin E. Elements in major raw agricultural crops in the United States. 1. Cadmium and lead in lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. Journal of Agricultural and Food Chemistry. 1983;31:1240–1244. doi: 10.1021/jf00120a024. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP. Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2002;53:535–543. doi: 10.1093/jexbot/53.368.535. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Jiang RF, Dunham SJ, McGrath SP. Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytologist. 2006;172:646–654. doi: 10.1111/j.1469-8137.2006.01867.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.