Abstract
In this study, it is shown that anti-sense suppression of Malus domestica 1-AMINO-CYCLOPROPANE-CARBOXYLASE OXIDASE (MdACO1) resulted in fruit with an ethylene production sufficiently low to be able to assess ripening in the absence of ethylene. Exposure of these fruit to different concentrations of exogenous ethylene showed that flesh softening, volatile biosynthesis, and starch degradation, had differing ethylene sensitivity and dependency. Early ripening events such as the conversion of starch to sugars showed a low dependency for ethylene, but a high sensitivity to low concentrations of ethylene (0.01 μl l−1). By contrast, later ripening events such as flesh softening and ester volatile production showed a high dependency for ethylene but were less sensitive to low concentrations (needing 0.1 μl l−1 for a response). A sustained exposure to ethylene was required to maintain ripening, indicating that the role of ethylene may go beyond that of ripening initiation. These results suggest a conceptual model for the control of individual ripening characters in apple, based on both ethylene dependency and sensitivity.
Keywords: ACC oxidase 1 (MdACO1), apple, ethylene, fruit ripening, Malus
Introduction
The importance of ethylene for fruit ripening has made the biosynthetic pathway for this hormone an attractive target for studies aiming to manipulate and understand the control of fruit ripening. Among the first transgenic fruit ever produced targeted ethylene synthesis in tomato. These included the reducing levels of the enzymes ACC SYNTHASE (ACS) (Oeller et al., 1991) and ACC OXIDASE (ACO) (Picton et al., 1993), and over-expressing ACC DEAMINASE to remove ACC from the ripening fruit (Klee et al., 1991). In other fruit species, researchers have targeted ACO in melon (Ayub et al., 1996), and ACO1 (Ross et al., 1992) in the apple cultivars, ‘Greensleeves’ and ‘Royal Gala’ (Dandekar et al., 2004; Schaffer et al., 2007). These studies demonstrated reducing ethylene in the climacteric fruit reduces fruit ripening, an effect reversed by adding exogenous ethylene.
Measuring the sensitivity of a tissue to a hormone gives insight into how the hormone acts. Due to the ambiguous nature of the word ‘sensitivity’, Firn (1986) proposed five measures of sensitivity. In this study, the term ‘sensitivity’ is used for category III sensitivity, namely ‘the minimum concentration needed to achieve a significant response’, ‘dependency’ is used for the category II sensitivity, namely ‘the ratio of the maximum response relative to the response to in the absence of the hormone’, and ‘responsivity’ is used to describe category I sensitivity ‘the magnitude of a maximum response which can be evoked’.
The significance of different sensitivities to the ripening hormone ethylene was not appreciated in early studies, as dependency was not considered alongside sensitivity. ACS tomatoes, demonstrating less than 0.5% of peak ethylene production, showed a delay in many ripening attributes, including chlorophyll loss and reddening (Oeller et al., 1991). It was also found that levels of the cell wall gene POLYGALACTURONASE (PG) were of a similar level to control fruit suggesting that this was regulated independently to ethylene (Oeller et al., 1991). This expression result was later found to be caused by the sensitivity of PG to ethylene and it has now been established that this PG is actually strongly regulated by ethylene in tomatoes, as suppression of ethylene production to 0.5% of the controls was still sufficient to induce 100% expression of this gene (Sitrit and Bennett, 1998). While the PG is obviously highly sensitive to low concentrations of ethylene, the ACS mutant tomatoes demonstrated a slower loss of chlorophyll (10–20 d) than the controls and, finally, they never turned bright red, suggesting that the colour response is less sensitive to ethylene. Work on the transgenic melon has demonstrated that many ripening attributes appear to be independent of ethylene (Ayub et al., 1996; Guis et al., 1997; Pech et al., 2008). In melon, it was found that the production of aroma volatiles and rind yellowing are considered to be ethylene-dependent ripening events (Guis et al., 1997), while starch breakdown, sugar accumulation, loss of acidity, and flesh colouration are considered ethylene-independent events. Fruit softening showed both dependent and independent effects with the knockout ACO fruit showing a significant softening albeit at a reduced rate. This ethylene-independent softening has been explained by the fact that some cell wall-modifying enzymes are strongly regulated by ethylene while others appeared to have an ethylene-independent expression (Nishiyama et al., 2007).
Currently, there is little known about the ripening sensitivity of apples to different ethylene concentrations. There is some evidence that the ripening sensitivity to ethylene increases during on-tree maturation, but these studies were limited to measurements of endogenous ethylene production and respiratory activity (Harkett et al., 1971; Knee et al., 1987). Ethylene concentrations inside the core cavity of apples increase from <0.1 μl l−1 to >500 μl l−1 during ripening (Johnston et al., 2001), but it is not known if this entire range of concentrations is physiologically active or if production is in excess of that required during the late stages of ripening. Apple ripening consists of the conversion of starch to sugars, reduced acidity, yellowing of the skin, reduced flesh firmness, and an increase in flavour volatiles. Inhibitors of ethylene action such as 1-MCP have been shown to reduce softening and volatile production, but have inconsistent or no effect on the conversion of starch to sugars (Fan et al., 1999). If similar processes were occurring in apple as in melon, this variation in efficacy could be explained by each ripening component having differing dependencies or sensitivities to ethylene.
The controlled application of different concentrations of ethylene to determine ripening thresholds is not possible using standard apple cultivars, as most cultivars produce too much endogenous ethylene. This makes it difficult to determine if ripening responses are due to the added ethylene, or to changes in endogenous ethylene. The application of inhibitors of ethylene biosynthesis and action also do not sufficiently suppress endogenous production in apple to allow such studies. The present study circumvents this problem by using a recently developed ‘Royal Gala’ ACO1 antisense line that only produces background levels of ethylene (Schaffer et al., 2007). Using this system, the present study determines the sensitivity, responsivity, and dependency of early and late ripening events in apple. New knowledge is presented on the ethylene ripening thresholds for individual ripening characters of apples, and a new conceptual model is presented for the ethylene-mediated co-ordination of early and late ripening events in apples.
Materials and methods
Apple growth conditions
A single transgenic Malus×domestica ‘Royal Gala’ tree, containing the ACC OXIDASE 1 gene (Ross et al., 1992) in an antisense orientation (ACO1as), was selected as it produced only 0–0.13 ng l−1 levels of ethylene in its fruit at full climacteric (Schaffer et al., 2007). Seven scions were grafted onto ‘M9’ rootstocks and grown next to three ‘Royal Gala’ untransformed lines that act as a control in a greenhouse. At full bloom, flowers were pollinated by hand with M.×domestica ‘Granny Smith’ pollen.
Three hundred and forty three MdACO1as apples and 56 control apples were harvested at the same time based on the background colour of the control apples. The MdACO1as apples were randomly allocated into 14 groups of 14 apples, with 13 of these groups allocated to the ripening treatments described in Table 1, and one group assessed for maturity characteristics at harvest. The control apples were randomly allocated into four groups of 14 apples, with three of these groups used for ripening treatments (Table 1), and one group assessed for maturity at harvest. Fruit ripening assessments were performed 14 d after harvest for all ripening treatments.
Table 1.
Summary of ripening treatments for MdACO1as and control ‘Royal Gala’ apples
| Treatment no. | Genotype | Ethylene dosage (μl l−1) | Ethylene treatment duration | Ripening temperature (°C) | 600 nl l−1 MCP treatment |
| 1 | Control | – | – | 20 | No |
| 2 | – | – | 20 | Yes | |
| 3 | – | – | 4 | No | |
| 4 | ACO1as | – | – | 20 | No |
| 5 | – | – | 4 | No | |
| 6 | – | – | 20 | Yes | |
| 7 | 0.01 | 14 d | 20 | No | |
| 8 | 0.1 | 14 d | 20 | No | |
| 9 | 1 | 14 d | 20 | No | |
| 10 | 10 | 14 d | 20 | No | |
| 11 | 100 | 14 d | 20 | No | |
| 12 | 1000 | 14 d | 20 | No | |
| 13 | 100 | 30 min | 20 | Yes | |
| 14 | 100 | 4 h | 20 | Yes | |
| 15 | 100 | 1 d | 20 | Yes | |
| 16 | 100 | 7 d | 20 | Yes |
Ripening treatments included ethylene dosage, duration of ethylene treatment, 1-methylcyclopropene (MCP) treatment, and ripening temperature. Assessments for each treatment were performed 14 d after harvest.
Post-harvest treatments
Each group of apples was subjected to a different treatment (Table 1) each in a separate single-layered tray. Ethylene treatments were conducted at 20 oC in 340 l ripening bins with continuous air movement and lime to absorb CO2. Nil ethylene treatments were also carried out in sealed bins with lime and Purafil™ (Multimix MM-1000, Circul-Aire, Montreal, Canada) to remove any residual atmospheric ethylene. Ethylene was injected into the injection ports and assessed for correct concentration 1 h after injection. 1-Methylcyclopropane (1-MCP) (SmartFresh™, AgroFresh Ltd) treatments at a concentration of 600 nl l−1 were also performed for 24 h in 340 l sealed bins containing lime. In all treatments, the 340 l bin contained a maximum of 14 apples (a combined mass of 1100–1200 g).
Assessment of fruit maturity and ripening
For each fruit, flesh firmness was evaluated using a Texture Analyser TAXT plus (Stable Microsystems, United Kingdom) fitted with a 7.9 mm Effegi penetrometer probe. The probe was driven into the flesh at 4 mm s−1 to a depth of 9 mm, and the maximum force recorded as flesh firmness. Two readings were made on opposite sides of each fruit with the skin removed. Soluble solids concentration was used to estimate the sugar content, and was determined by placing an aliquot of the juice released during firmness measurement onto a digital refractometer (Atago, model PAL-1, Japan). Starch pattern index was determined by placing the cut surface of an apple into iodine solution (2.5 g l−1 iodine and 10 g l−1 potassium iodide in distilled water) for 60 s and then rating the staining pattern according to the ENZAFRUIT New Zealand International scale from 0 (cut surface completely stained, high starch) to 6 (no surface staining, low starch). Longitudinal quarter sections of fruit were held at –20 oC for the determination of titratable acidity. Titrations were performed according to Harker et al. (2002). Background skin colour was measured using a chromameter (Minolta, model CR-300, Japan), with two readings made per fruit on blush-free areas of skin. Internal ethylene concentration was determined by extracting a 1 ml core cavity gas sample and injecting it into a gas chromatograph (Hewlett Packard, 5890 series II) equipped with an injector at 160 oC, an activated alumina F1 column (Alltech, glass 1.5 m×6 mm×2 mm, mesh 80/100) set isothermally at 130 oC, N2 as the carrier gas (20 ml min−1), a flame ionization detector set at 200 oC (H2 at 20 ml min−1, air at 200 ml min−1), and an integrator (Hewlett Packard, model 3395) calibrated with certified gas standards. Volatiles were measured as described in Schaffer et al. (2007).
Data analysis
The significance of treatment effects were determined using analysis of variance, and mean separation using a protected least significant difference (5%). Ethylene response data were analysed by non-linear regression and a logistic function:
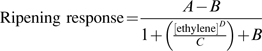 |
where A is the lower asymptote, B is the upper asymptote, C is the [ethylene] for 50% response, and D is the rate of change. Parameter estimates and standard errors for C were interpreted as sensitivity, and parameter D as responsivity. All statistical procedures were performed using Genstat, 9th edition.
Ethylene dependency was calculated as:
Results
Maturity characteristics at harvest
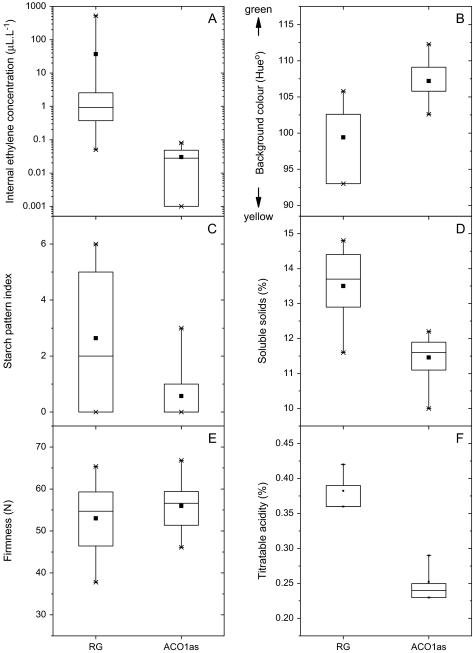
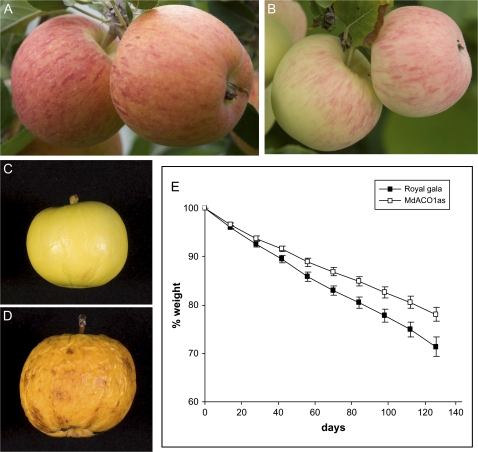
At harvest, the control fruit had internal ethylene concentrations ranging from 0.05 to 528 μl l−1, while the MdACO1as fruit had levels ranging from undetectable to 0.08 μl l−1 (Fig. 1A). Both the control fruit and MdACO1as fruit on exposed parts of the trees developed red blush at harvest (Fig. 2A, B), although the intensity and skin coverage of reddening was more pronounced on control fruit. Both fruit types had similar flesh firmness, but the MdACO1as fruit had lower concentrations of soluble solids and titratable acidity, less starch clearance, and a greener background skin colour (Fig. 1B–E). The starch pattern index for control fruit ranged from 0 to 6 and 0 to 3 in MdACO1as fruit.
Fig. 1.
Untransformed ‘Royal Gala’ (RG, wild type) and MdACC oxidase1 (ACO1as) fruit at harvest. Concentration of internal ethylene in the core cavity (A), background skin colour (B), starch pattern index (C), soluble solids concentration (D), flesh firmness (E), and titratable acidity (F).
Fig. 2.
Apples with MdACC oxidase1 (MdACO1as) knocked out and untransformed ‘Royal Gala’. ‘Royal Gala’ apples on tree at harvest (A). MdACO1as fruit at harvest (B). MdACO1as apple after storage at room temperature for 6 months (C). ‘Royal Gala’ apples after storage at room temperature for 6 months (D). Weight loss from ‘Royal Gala’ (filled boxes) and MdACO1as apples (open boxes) over a 14 week period (E).
MdACO1as fruit have improved water retention and reduced incidence of ripening-related rots
Fourteen MdACO1as-suppressed apples and 14 ‘Royal Gala’ untransformed fruit (control fruit) were kept at 20 °C for 30 weeks. These were weighed and visually inspected every 2 weeks for shrivel and ripening-related rots. After 6–8 weeks the control fruit began to show signs of shrivel much earlier than the MdACO1as fruit that began to shrivel 20–26 weeks during this treatment (Fig. 2C, D). By 20 weeks five of the control fruit had completely rotted while there were no visual rots on any of the MdACO1as fruit for the whole of this period. In both the MdACO1as lines and control lines the water loss from the fruit was linear (Fig. 2E), with a slower rate of water loss from the MdACO1as lines (average 1.6 mg g−1 FW d−1) compared to the controls (2.2 mg g−1 FW d−1).
Comparison of ripening characters between MdACO1as and untransformed fruit
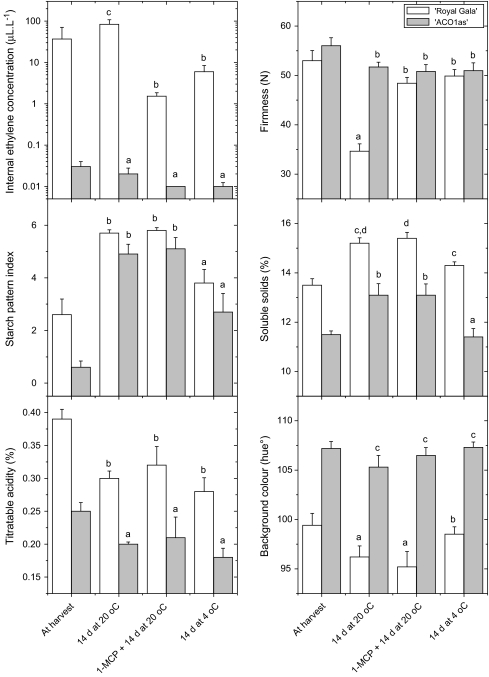
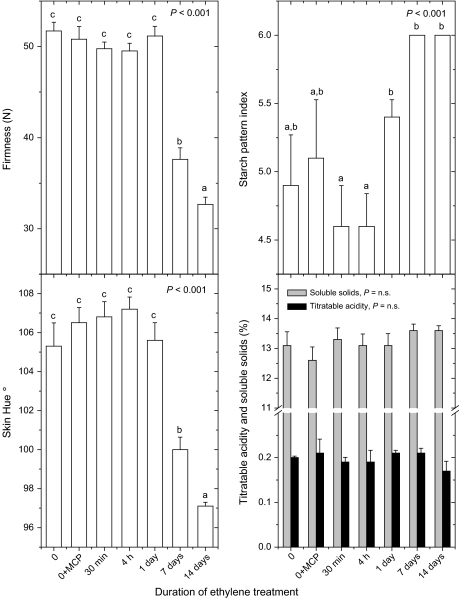
Three groups of 14 control fruit and three groups of 14 MdACO1as fruit were assessed following a 14-d period of ripening. The first group of control and MdACO1as apples were stored with no treatment at 20 °C. After this time the ‘Royal Gala’ control fruit had a higher internal ethylene concentration, had softened by approximately 20 N, had near-complete starch clearance, a more yellow background colour, and decreased titratable acidity (Fig. 3). The MdACO1as fruit had no detectable increase in internal ethylene concentration after 14 d at 20 °C, with ethylene levels ranging from undetectable to 0.094 μl l−1, and only changed slightly in skin background colour and firmness. Both the untransformed fruit and the MdACO1as fruit had a large degree of starch clearance, an increase in soluble solids concentration, and a loss of titratable acidity during the 14 d ripening period.
Fig. 3.
Untransformed ‘Royal Gala’ (wild type, unfilled bars) and MdACO1as fruit (filled bars) at harvest and after ripening following three different storage treatments. Analysis of variance P values for all ripening characters were <0.001. Treatments labelled with the same letter are similar according to a 5% least significant difference.
To assess whether there were any ripening effects caused by the very low ethylene concentrations detected in some of the MdACO1as fruit, the second group of MdACO1as fruit were treated with the inhibitor of ethylene action, 1-methylcyclopropene (1-MCP) before being left to ripen for 14 d at 20 °C. A group of control fruit were also treated. Treatment of the control fruit with 1-MCP reduced the internal ethylene concentration to between 0.023 and 4.2 μl l−1. There was also reduced loss of firmness compared with the untreated fruit but 1-MCP had little effect on the clearance of starch and the associated increase in soluble solids concentration. MdACO1as fruit treated with 1-MCP showed no significant differences from the untreated fruit suggesting that the MdACO1as lines have a complete ethylene biosynthesis knockout with respect to ripening (Fig. 3).
The third group of MdACO1as and control apples were cold-stored at 4 °C for 14 d to assess the effect of cold storage on ripening characters. MdACO1as fruit showed no significant differences in ripening compared with the fruit stored at 20 °C for internal ethylene concentration, firmness, and skin colour. Cold-stored MdACO1as fruit had slightly less starch clearance and an increase in soluble solids concentration (Fig. 3). Cold storage of the control fruit suppressed internal ethylene concentrations to 0.091–27.2 μl l−1, and reduced softening, skin yellowing, and starch clearance compared with fruit stored at 20 °C.
Ethylene sensitivity for individual ripening characteristics
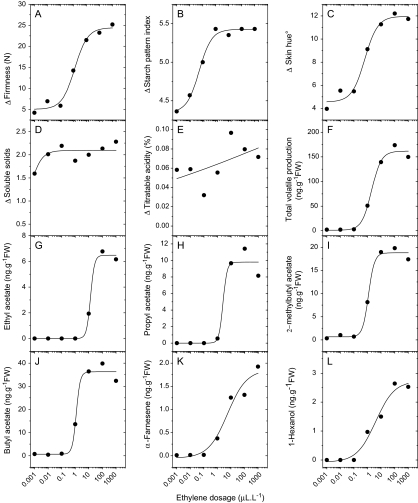
To establish the level of ethylene needed to induce a ripening response, MdACO1as lines were exposed continuously to a range of ethylene concentrations from 0.01 to 1000 μl l−1 for 14 d at 20 °C. Ethylene response curves were then produced for the six main ripening traits and for the six dominant volatiles (Fig. 4). While there was no clear response to ethylene for titratable acidity and soluble solids concentration, distinct response curves were established for volatile production, firmness, skin background colour, and starch clearance. The general pattern for these curves was sigmoidal, with little or no change in ripening at low concentrations, followed by a rapid increase in response from 0.1 to 10 μl l−1, and little or no change thereafter at which point the process was saturated. However, there were subtle differences in the responses of individual ripening traits, where the majority of the response occurred between 0.1 and 10 μl l−1 for softening, background colour and total volatiles, while in contrast a lower range of 0.01 to 1 μl l−1 was required for starch clearance. Results from the 0.01 and 0.1 μl l−1 ethylene concentrations were indistinguishable from the no-ethylene treatment controls for volatiles, firmness, and background colour suggesting that these concentrations are below the response threshold for these processes. A small increase in starch degradation was observed for the 0.01 μl l−1 ethylene-treated fruit, suggesting a lower response threshold for this trait.
Fig. 4.
Response curves of different ripening characters for MdACO1as fruit treated with different concentrations of ethylene. Flesh firmness (A) starch pattern index (B), skin background colour (C), soluble solids concentration (D), titratable acidity (E), total volatiles (F), and individual volatiles (G–L). Each point depicts the mean of 14 fruit.
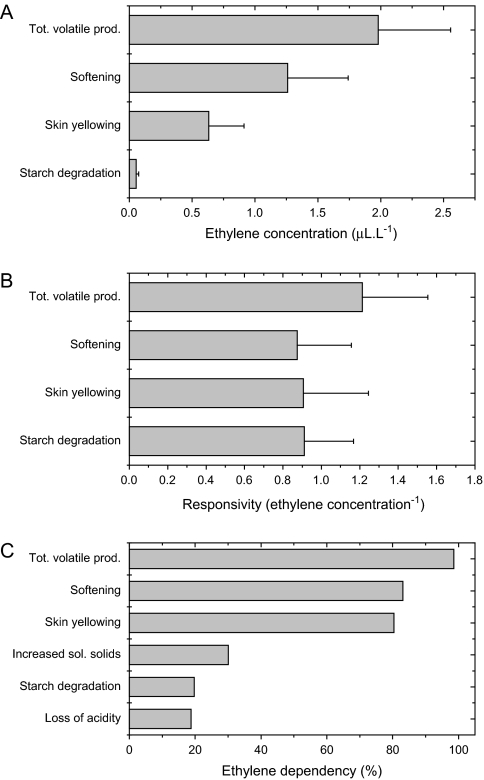
The differential sensitivity of the different ripening traits was further quantified using non-linear regression to estimate the ethylene concentration required to induce a half-maximum response. This approach showed that the half-maximal response for starch clearance occurred at low ethylene concentrations, with skin yellowing, softening, and volatile production requiring progressively higher ethylene concentrations to induce a similar 50% response (Fig. 5A). The rate of change parameter estimated from this analysis was similar for all ripening characters (Fig. 5B), suggesting the responsivity to ethylene was similar despite differences in sensitivity.
Fig. 5.
Sensitivity (A), responsivity (B), and dependency (C) of the MdACO1as apples to exogenous ethylene. Sensitivity and responsivity was determined as the ethylene concentration required for a 50% response and the rate of change, respectively, using non-linear regression and a logistic function. Ethylene dependency (%) was calculated as the difference between the 0 μl l−1 and 1000 μl l−1 ethylene treatments divided by the 1000 μl l−1 ethylene treatment, and multiplying by 100 to give a percentage: 0%=ethylene independent, 100%=ethylene dependent.
The ethylene response curves also differed between volatiles. The production of α-farnesene, butyl acetate, and 2-methylbutyl acetate occurred once the ethylene concentration exceeded 0.1 μl l−1, while the production of ethyl acetate and propyl acetate only occurred once the ethylene concentration exceeded a higher concentration of 1 μl l−1 (Fig. 4). The esters tended to have an abrupt increase in production in response to increased ethylene dosage, whereas volatiles such as α-farnesene and hexanol had a more gradual increase and seemed to respond to a wider range of ethylene concentrations. Alpha-farnesene and 1-hexanol also showed a higher saturation threshold concentration of >100 μl l−1, whereas most other volatiles were saturated at concentrations >10 μl l−1.
Ethylene dependency for individual ripening characteristics
To establish the degree of ethylene dependence for each ripening trait, MdACO1as fruit treated with 1000 μl l−1 ethylene, and untreated fruit stored at 20 °C, were compared with the harvest samples. Taking the value for each trait exposed to 1000 μl l−1 as 100% the percentage ripening for each trait in the absence of ethylene (ethylene-independent traits) could be determined (Fig. 5C). Two groups of ripening traits emerged, those that were strongly ethylene-dependent (increased total volatiles, loss of firmness and skin yellowing), and those that were only weakly ethylene-dependent (starch clearance, loss of titratable acidity, and increased soluble solids concentration).
A continuous exposure to ethylene is required for ripening
To assess if ethylene acts as a trigger for ripening or whether a continuous exposure to ethylene is required, MdACO1as fruit were exposed to 100 μl l−1 ethylene for different periods of time followed by treatment with 600 nl l−1 1-MCP to stop further ethylene action. Fruit were exposed to ethylene for 30 min, 4 h, 1 d, and 7 d, and the same ripening characters as assessed above were measured after 14 d at 20 oC. The results from these fruit were also compared with those from fruit that had been treated with 100 μl l−1 for 14 d in the previous experiment.
For the ripening traits showing a moderate-to-strong dependency for ethylene (background skin colour, firmness, and volatiles), the responses of fruit receiving a short period of ethylene (30 min, 4 h, and 1 d) exposure followed by treatment of 1-MCP were indistinguishable from the responses of no-ethylene treatment MdACO1as fruit. However, for starch clearance (a ripening processes with a weak dependence for ethylene), one day was sufficient to significantly accelerate starch loss. Fruit treated with ethylene for 7 d had an intermediate level of ripening characters compared with fruit receiving the 14 d treatment (Fig. 6), suggesting a continued exposure of ethylene is needed for ripening to occur.
Fig. 6.
Fruit ripening in MdACO1as fruit treated with 100 μl l−1 ethylene for different periods of time followed by treatment with 1-MCP. Fruit was measured after 14 d. Analysis of variance P values are displayed, treatments labelled with the same letter are similar according to a 5% least significant difference.
Discussion
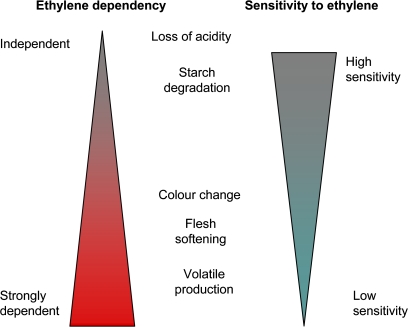
Ripening in apples is a complex progression of developmental steps. These steps are not completely controlled by ethylene, as there is always a degree of ethylene-independent progression. This suggests a model of ethylene action where ethylene is acting as a modulator of ripening rather than a ripening trigger. While the morphological changes that occur in apples during ripening are well documented, it is shown that these traits seem to follow an overlapping sequence of events, starting with the conversion of starch to sugars and loss of acidity, yellowing of the skin, softening of the flesh, and finally an increase in aroma volatiles. As this sequence progresses, the dependency for ethylene becomes stronger and the sensitivity to ethylene decreases (Fig. 7). For most apple cultivars, rather than having a constant level, there is an increase in ethylene during the ripening process (Johnston et al., 2001). This increase in ethylene concentration may allow the different ripening characters to be co-ordinated so that starch conversion always occurs before excessive fruit softening or the production of attractant volatiles. The low ethylene dependency for starch clearance suggests that this process is mainly driven by other developmental factors, but the high sensitivity of this process to low ethylene concentrations may provide a backup stimulus should the non-ethylene developmental cues fail to develop.
Fig. 7.
Conceptual model for control of individual ripening characters in apples. Ethylene sensitivity calculated from the concentration of ethylene required for a 50% ripening response. Ethylene dependency measured by the amount of ripening measured in the absence of ethylene. (This figure is available in colour at JXB online.)
When the MdACO1as apples are left for a considerable period of time, the greatest change that occurs is weight loss, albeit at a lower rate than the control apples. This suggests that only a certain progression of ripening can be achieved in the absence of ethylene. The ethylene dependency for starch clearance appears less, as it would be likely that there would be a complete clearing of starch if the 2-week ripening period were extended.
Because of apple number constraints, a limitation to this study was that ripening was measured at a single time point in apple fruit development. It has been well documented that, as apples mature, they become more sensitive to ethylene (Harkett et al., 1971). This means it is possible that the ethylene response curves may shift to the left or the right for fruit harvested at different maturities. It is also likely that these response curves may shift for different cultivars and for fruit exposed to different conditions during growth; factors which warrant further investigation. Nevertheless, despite these potential shifts in the response curve, it is likely that the relative sensitivities and dependencies of the different ripening characters for ethylene would remain in the same order, as most apple cultivars tend to follow the same sequence of ripening events (e.g. starch clearance is always an early ripening event). The present research also demonstrates that ethylene was most physiologically active for ripening between 0.01 and 10 μl l−1, which is similar to those concentrations measured in most apple cultivars during the harvest period. These concentrations also fit with storage recommendations for ethylene concentrations to be less than 0.1 μl l−1 to reduce ripening (Stow et al., 2000). While research has been initiated to unravel the ethylene dependency of ripening pathways (Pech et al., 2008), there is a lack of research on the molecular basis for ethylene sensitivity. The differing ethylene sensitivities of individual ripening characters raises questions as to whether this is mediated through different signalling systems for each ripening pathway, or through differences in the numbers of ethylene-binding domains for key genes associated with each ripening process.
There is considerable variation in the ripening process in fleshy fruits, but when the individual traits are examined separately, there are remarkable consistencies. The starch to sugar conversion is common to many fruit. In some cultivars of apple it has been reported as ethylene independent, while in others it is more ethylene dependent (Thammawong and Arakawa, 2007). In melons, starch has also been suggested to be independent to ethylene (Ayub et al., 1996), it would be interesting if this starch clearance could be accelerated in this fruit with exogenous ethylene. The dependency on ethylene for changes in skin colouration has been recorded in melon (Ayub et al., 1996) and tomato (Oeller et al., 1991). The ethylene dependency of fruit softening and volatile production has been extensively studied, with responses to ethylene by many candidate cell wall genes in apples responding to ethylene (Atkinson et al., 1998; Wakasa et al., 2003; Goulao et al., 2008) and in other fruit, (Brummell, 2006), and by aroma biosynthesis genes (Defilippi et al., 2005; Schaffer et al., 2007). Interestingly, work with melon has shown that many of the cell-wall related genes are regulated independently of ethylene, in addition to those that are regulated by ethylene (Nishiyama et al., 2007). While much progress has been made there is still much to learn about the molecular control of these genes.
Finally, it appears from this and previous work that the ethylene signal is constantly controlling the ripening process. As well as this study, it was shown in tomato (Oeller et al., 1991) that ripening could be stopped if ethylene fruit were exposed to only a short period of ethylene. As ripening occurs through enzymic action, it is surprising that residual ripening does not occur even after a short period of ethylene exposure. This suggests that enzyme turnover is high during ripening or that there are additional layers of regulation for enzyme action to progress in the absence of ethylene.
Conclusion
This study has characterized for the first time the ethylene response curves for individual ripening characters in apple. It has revealed that ethylene-mediated ripening is controlled at two levels, firstly, through differing dependencies for ethylene, and, secondly, through differing sensitivities to ethylene. This dual control mechanism means that the sequence of individual ripening events is tightly co-ordinated so that early ripening events are less dependent on ethylene but are also highly sensitive to ethylene should the ethylene-independent factors fail to develop. This model warrants further investigation in relation to applicability to other fruits, particularly in relation to the ripening physiology of climacteric and non-climacteric fruit.
Acknowledgments
The authors would like to acknowledge Nihal De Silva and Mark Wohlers for statistical advice, Andrew Allan and Ian Ferguson for critically reading the manuscript, and the New Zealand Foundation for Research, Science and Technology (C06X0705; Pipfruit a juicy future) for funding this research.
References
- Atkinson RG, Bolitho KM, Wright MA, Iturriagagoitia-Bueno T, Reid SJ, Ross GS. Apple ACC-oxidase and polygalacturonase: ripening-specific gene expression and promoter analysis in transgenic tomato. Plant Molecular Biology. 1998;38:449–460. doi: 10.1023/a:1006065926397. [DOI] [PubMed] [Google Scholar]
- Ayub R, Guis M, BenAmor M, Gillot L, Roustan JP, Latché A, Bouzayen M, Pech JC. Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nature Biotechnology. 1996;14:862–866. doi: 10.1038/nbt0796-862. [DOI] [PubMed] [Google Scholar]
- Brummell DA. Cell wall disassembly in ripening fruit. Functional Plant Biology. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- Dandekar AM, Teo G, Defilippi BG, Uratsu SL, Passey AJ, Kader AA, Stow JR, Colgan RJ, James DJ. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Research. 2004;13:373–384. doi: 10.1023/b:trag.0000040037.90435.45. [DOI] [PubMed] [Google Scholar]
- Defilippi BG, Dandekar AM, Kader AA. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. Journal of Agricultural and Food Chemistry. 2005;53:3133–3141. doi: 10.1021/jf047892x. [DOI] [PubMed] [Google Scholar]
- Fan XT, Blankenship SM, Mattheis JP. 1-Methylcyclopropene inhibits apple ripening. Journal of the American Society for Horticultural Science. 1999;124:690–695. [Google Scholar]
- Firn RD. Growth substance sensitivity: the need for clearer ideas, precise terms and purposeful experiments. Physiologia Plantarum. 1986;67:267–272. [Google Scholar]
- Goulao LF, Cosgrove DJ, Oliveira CM. Cloning, characterisation and expression analyses of cDNA clones encoding cell wall-modifying enzymes isolated from ripe apples. Postharvest Biology and Technology. 2008;48:37–51. [Google Scholar]
- Guis M, Botondi R, BenAmor M, Ayub R, Bouzayen M, Pech JC, Latché A. Ripening-associated biochemical traits of Cantaloupe Charentais melons expressing an antisense ACC oxidase transgene. Journal of the American Society for Horticultural Science. 1997;122:748–751. [Google Scholar]
- Harker FR, Marsh KB, Young H, Murray SH, Gunson FA, Walker SB. Sensory interpretation of instrumental measurements. 2. Sweet and acid taste of apple fruit. Postharvest Biology and Technology. 2002;24:241–250. [Google Scholar]
- Harkett PJ, Hulme AC, Rhodes MJC, Wooltorton LSC. The threshold value for physiological action of ethylene on apple fruits. Journal of Food Technology. 1971;6:39–45. [Google Scholar]
- Johnston JW, Hewett EW, Hertog MLATM, Harker FR. Temperature induces differential softening responses in apple cultivars. Postharvest Biology and Technology. 2001;23:185–196. [Google Scholar]
- Klee HJ, Hayford MB, Kretzmer KA, Barry GF, Kishore GM. Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. The Plant Cell. 1991;3:1187–1193. doi: 10.1105/tpc.3.11.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee M, Hatfield GS, Bramlage WJ. Response of developing apple fruits to ethylene treatment. Journal of Experimental Botany. 1987;38:972–979. [Google Scholar]
- Nishiyama K, Guis M, Rose JKC, et al. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. Journal of Experimental Botany. 2007;58:1281–1290. doi: 10.1093/jxb/erl283. [DOI] [PubMed] [Google Scholar]
- Oeller PW, Wong LM, Taylor LP, Pike DA, Theologis A. Reversible inhibition of tomato fruit senescence by antisense RNA. Science. 1991;254:437–439. doi: 10.1126/science.1925603. [DOI] [PubMed] [Google Scholar]
- Pech JC, Bouzayen M, Latché A. Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Science. 2008;175:114–120. [Google Scholar]
- Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D. Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. The Plant Journal. 1993;3:469–481. [Google Scholar]
- Ross GS, Knighton ML, Layyee M. An ethylene-related cDNA from ripening apples. Plant Molecular Biology. 1992;19:231–238. doi: 10.1007/BF00027344. [DOI] [PubMed] [Google Scholar]
- Schaffer RJ, Friel EN, Souleyre EJF, et al. A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiology. 2007;144:1899–1912. doi: 10.1104/pp.106.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitrit Y, Bennett AB. Regulation of tomato fruit polygalacturonase mRNA accumulation by ethylene: a re-examination. Plant Physiology. 1998;116:1145–1150. doi: 10.1104/pp.116.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J, Dover CJ, Genge PM. Control of ethylene biosynthesis and softening in ‘Cox's Orange Pippin’ apples during low-ethylene, low-oxygen storage. Postharvest Biology and Technology. 2000;18:215–225. [Google Scholar]
- Thammawong M, Arakawa O. Starch degradation of detached apple fruit in relation to ripening and ethylene. Journal of the Japanese Society for Horticultural Science. 2007;76:345–350. [Google Scholar]
- Wakasa Y, Hatsuyama Y, Takahashi A, Sato T, Niizeki M, Harada T. Divergent expression of six expansin genes during apple fruit ontogeny. European Journal of Horticultural Science. 2003;68:253–259. [Google Scholar]