Abstract
Intense efforts are currently devoted to disentangling the relationships between plant carbon (C) allocation patterns and soil nitrogen (N) availability because of their consequences for growth and more generally for C sequestration. In cold ecosystems, only a few studies have addressed whole-plant C and/or N allocation along natural elevational or topographical gradients. 12C/13C and 14N/15N isotope techniques have been used to elucidate C and N partitioning in two alpine graminoids characterized by contrasted nutrient economies: a slow-growing species, Kobresia myosuroides (KM), and a fast-growing species, Carex foetida (CF), located in early and late snowmelt habitats, respectively, within the alpine tundra (French Alps). CF allocated higher labelling-related 13C content belowground and produced more root biomass. Furthermore, assimilates transferred to the roots were preferentially used for growth rather than respiration and tended to favour N reduction in this compartment. Accordingly, this species had higher 15N uptake efficiency than KM and a higher translocation of reduced 15N to aboveground organs. These results suggest that at the whole-plant level, there is a compromise between N acquisition/reduction and C allocation patterns for optimized growth.
Keywords: Alpine plants, 13C and 15N isotope labelling, Carex foetida, Kobresia myosuroides, photosynthesis, respiration, snow cover gradient
Introduction
Plant carbon assimilation, allocation, and yield are strongly affected by variations in soil fertility and nitrogen balance. Therefore, intense efforts are currently devoted to elucidating the relationships between plant carbon allocation patterns and nitrogen availability in order to improve our understanding of carbon cycles and sequestration pathways in terrestrial ecosystems (Waring, 1993; Farrar and Gunn, 1998; Trumbore, 2006). This is particularly important in the context of global climate change, in which carbon fixation and growth tend to be promoted by increased atmospheric CO2 but depend on the availability of N (or other nutrients) (Long et al., 2006).
Alpine ecosystems are particularly sensitive to such ecological effects because (i) alpine habitats are strongly N-limited in many instances (Bowman et al., 1993); (ii) snow cover gradients influence the nature of plant communities (Körner, 1999; Choler, 2005); and (iii) the composition of alpine vegetation varies in response to deposition of anthropogenic nitrogen (Bowman et al., 2006). However, the physiology of carbon allocation patterns of alpine plants in relation to N uptake is not well understood.
In fact, ecophysiological studies of alpine plants so far have mostly focused on carbon uptake (Körner, 1982; Körner and Diemer, 1987) or on the ecology of storage (Jaeger and Monson, 1992; Lipson et al., 1996; Monson et al., 2006). Only Körner (1999) presented a whole-plant C allocation of a dominant graminoid in alpine tundra, Carex curvula, after long-term 13C labelling. Other authors have examined the constraints of nutrient availability on leaf physiological properties and plant production of various alpine communities (Bowman et al., 1993, 1995; Theodose and Bowman, 1997), but they did not disentangle the C and N fluxes sustaining plant growth.
Furthermore, alpine plants are generally exposed to contrasting microenvironmental conditions that vary along the snow cover gradient (nutrients and water availability, temperature, length of the favourable period for carbon uptake), adding complexity to variations in the C and N balance. In late snowmelt locations, the growing season is shorter and accompanied by relatively high nutrient availability (particularly at the beginning of the growing season) (Bowman, 1992; Baptist and Choler, 2008). Plants growing under such conditions are thus energy limited rather than nutrient limited. In contrast, plants growing in early snowmelt habitats are much less energy limited but soil nutrients are less abundant. Contrasting growth rates (fast- and slow-growing species are observed in late and early snowmelt conditions, respectively) and leaf functional diversity related to nutrient economy (which is higher in fast-growing species, Wright et al., 2004) have been described along snow cover gradients (Kudo, 1996; Choler, 2005). However, to what extent the dynamic allocation of C and N is associated with these contrasting strategies remains unknown.
It is well known that carbon fixation and growth are promoted by higher N content at the leaf or the whole-plant level (Wright et al., 2004) and are accompanied by higher leaf and root respiration rates (Reich et al., 1997; Craine et al., 2002; Tjoelker et al., 2005; Atkinson et al., 2007). However, the extent to which carbon allocation patterns are related to N uptake efficiency and N allocation remains uncertain (Garnier, 1991; Osone et al., 2008). For example, fast-growing species have higher leaf N content and maximum carboxylation rates Vcmax compared with slow-growing species found at similar altitude (Baptist and Choler, 2008). These features are assumed to sustain rapid growth of the aboveground compartment at the expense of belowground organs, thereby supposedly causing lower root/shoot biomass ratios (Chapin, 1980). Nevertheless, there is no direct evidence of such a relationship, and thus that using shoot/root ratios as allocation estimates (as is commonly done: see, for example, Grulke and Bliss, 1985) may be questionable (Craine et al., 2002).
12C/13C and 14N/15N isotope techniques were used to investigate C fixation, N assimilation, and C and N allocation patterns of two alpine species. Carex foetida (CF) and Kobresia myosuroides (KM) were selected as case studies because they represent a fast-growing species inhabiting late snowmelt locations and a slow-growing species inhabiting early snowmelt locations, respectively. To our knowledge, the present work provides the first attempt to compare the coupled dynamics of C and N for alpine plants along a snow cover gradient. While the respiratory properties of the two species appear to be similar, the present labelling experiments show that, in contrast to KM, CF has different leaf/root allocation ratios for C and N. In other words, CF allocates more C to its roots whereas reduced N was more likely to be allocated to the aboveground compartment. It is therefore concluded that, in the present pair of species, the adaptation of fast-growing species for an optimal growth involves increased C and N assimilation efficiencies to feed belowground biomass production.
Materials and methods
Plant material
Twenty-eight plants each of KM and CF (both Cyperaceae) and their associated soil were collected in the vicinity of the Galibier pass [2646 m above sea level (a.s.l.)] and the Agnel pass (2744 m a.s.l.) in the French Alps in October 2005. They were transferred into pots (20×20×30 cm) in a common garden located in the Grand Galibier mountain range in the south-western Alps (45°7′N, 6°5′E, 2100 m a.s.l.). Snowmelt occurred on 15 May 2006. The pulse–chase labelling experiments (13C labelling and 15N labelling were independent) started on 5 July 2006 and ended on 26 July 2006. All the experiments were conducted at the Station Alpine Joseph Fourier, except the isotope measurements, which where carried out at the scientific facilities of the University of Barcelona. The δ13C of CO2 of the air at the Galibier Pass was approximately –11.5±0.1‰ (Nogués et al., 2006).
13C labelling procedure
In July 2006, at the peak of standing biomass, 12 out of 15 monoliths (hereafter referred to replicates) were labelled in a 13CO2-enriched atmosphere. The other three were used as controls (for the initial carbon isotope composition before labelling, the corresponding sampling time is denoted as Tinit). After labelling, a first set of three was immediately harvested (T0). For the other sets, the chase times lasted 24 h (1 d, T1), 82 h (3.5 d, T3), and 274 h (11.5 d, T11), respectively. The day before pulse labelling, the replicates were arranged in controlled conditions: 12 h photoperiod, and mean air temperature and PPFD (photosynthetic photon flux density) of 18 °C and 550 μmol m−2 s−1, respectively. Labelling was applied following a dark period of 12 h for all replicates. After labelling and until the end of the chase period, replicates were kept in the same controlled conditions. Every 2 d, the monoliths were watered with 0.5 l of distilled water.
The isotope label was applied over a period of 5 h by enclosing the monoliths, two by two, in a 36.0 l Perspex® labelling chamber. The atmospheric air was initially CO2 depleted (decarboxylated) by passing through a soda-lime column and then mixed with 13CO2 fluxes from a gas cylinder (enriched at 5%, Euriso-top, Saint-Aubin, France) in a mixing chamber. The mixing chamber was then connected to the sample air hose of the HCM 1000 Infra Red Gas Analyser (IRGA; Heinz Walz GmbH, Effeltrich, Germany) and the CO2 concentration was estimated. The CO2 concentration within the chamber was kept between 380 ppm and 420 ppm throughout the labelling procedure by mass flow controllers located before the mixing chamber (ROD-4, Aera, Fort Collins, CO, USA). The air flux passing through the labelling chamber was controlled by the HCM 1000 at a rate of 1.0 l min−1. A second pump was added at the output of the labelling chamber to avoid overpressurization. A fan was placed into the chamber to ensure air mixing. Aluminium tubes arranged at the bottom of the chamber and connected to a water bath maintained the chamber temperature at 22.5±0.4 °C during the pulses. Light intensity above the vegetation was kept at 550 μmol m−2 s−1.
At the end of each chase time (T0, T1, T3, T11) and also for Tinit, the three replicates were harvested. The living aboveground vegetation was harvested first by clipping the leaves at the soil surface. Half of the biomass was immediately lyophilized, whereas the rest was enclosed for 5 min in a 1.0 l Perspex® chamber to measure respiration (see below for procedure) and the isotopic signature of dark-respired 13CO2. To measure the latter, the 1.0 l chamber connected to an IRGA model Li-6200 (LI-COR, Inc., Lincoln, NE, USA) was flushed with CO2-free air to ensure that only the CO2 respired in the chamber was measured. CO2 was allowed to accumulate up to a concentration of ∼1000 ppm, and then air samples were collected using a special 50 ml syringe (SGE, Ringwood, Australia) and a needle (model microlance 3, BD, Plymouth, UK). The gas samples were passed through a magnesium perchlorate column (water vapour trap), then immediately injected into a 10 ml vacutainer (BD vacutainer, Plymouth, UK) as previously described (Noguès et al., 2008). To avoid contamination with the air present in the syringe and needle, both were flushed with N2 before taking each sample. The vacutainers were also overpressurized with N2, so the pressure inside the vacutainer was above the ambient pressure.
Non-woody (new) and woody (old) roots were subjected to a similar procedure after being harvested and washed. CO2 accumulation time was longer for old roots as respiration was much lower than that of new roots and leaves. Leaves and roots were subsequently lyophilized for isotopic analysis of organic matter (OM). OM analyses were done using an elemental analyser with a zero-blank autosampler (EA1108, Series 1, Carbo Erba Strumentazione, Milan, Italy) coupled to an isotope ratio mass spectrometer (Delta C, Finnigan Mat, Bremen, Germany) operating in continuous flow.
The δ13C of respired CO2 was measured using gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS) as previously described (Nogués et al., 2008). Briefly, water vapour and oxygen were removed from the gas samples, and carbon dioxide, argon, and nitrogen were separated by gas chromatography (6890N, Agilent Technologies, Palo Alto, CA, USA) coupled to a Deltaplus isotope ratio mass spectrometer through a GC-C Combustion III interphase (ThermoFinnigan, Bremen, Germany). The column used was a 30 m×0.32 mm i.d. GS-GASPRO (J&W Scientific Inc., Folsom, CA, USA). The carrier gas was helium at a flow rate of 1.2 ml min−1. Injection port temperature was 220 °C. The oven temperature was kept at 60 °C during the whole run. The injection was done in split mode (injected volume 0.3 ml, split flow 20 ml min−1).
CO2 gas exchange measurements
In order to evaluate 13C fixation during pulse labelling, CO2 fluxes were measured on each replicate with a Li-6200 IRGA (LI-COR) before and just after labelling. Light intensity, air temperature, and relative air humidity were recorded during all measurements. The net photosynthetic fixation rate (Anet) was calculated as the sum of the net CO2 fluxes in light (NEP) and in darkness (ER, which takes into account the CO2 evolution rate by both the belowground and aboveground compartments) as follows:
| (1) |
In darkness, respiration by the aboveground compartment was small compared with that of the belowground compartment. In other words, the contribution of photosynthetic organs to ER was small (typically <8%), so the overestimation of the net photosynthetic rate Anet was negligible. At the end of the chase period (see section below), leaf and new and old root respiration was estimated every minute for 5–10 min by enclosing them in a dark 1.0 l Perspex® chamber connected to the Li-6200 IRGA.
15N labelling procedure
The [15N]-NO3, [15N]-NH4, and [15N]-glycine uptake by KM and CF was assessed independently from the 13C labelling, i.e. on the remaining nine monoliths for each species (n=3 replicates for each compound and each species). Glycine was chosen as the amino acid as Lipson et al. (1999) indicated that it is the soil amino acid most available to plants. A 100 ml aliquot of a 1 mM solution of [15N]-NO3, [15N]-NH4, or [15N]-glycine was added with a 5 ml syringe; the concentration of N added was thus equivalent in the three treatments. The syringe was inserted to a depth of 5 cm in the soil following a 2×2 cm grid layout. Plants were then watered with 500 ml of demineralized water to ensure homogeneous labelling in the soil.
15N isotope sampling and processing
After a 24 h chase, leaves and new and old roots were harvested, sorted, washed with demineralized water, and lyophilized for isotopic analysis. 15N/14N ratios were analysed at the scientific facilities of the University of Barcelona using an elemental analyser (Flash 1112 EA, ThermoFinningan, Germany) operating in continuous flow mode. The analysis for obtaining the natural abundance of 15N in leaves and new and old roots of KM and CF was performed on the 15 monoliths used for the 13C labelling experiment.
Isotope labelling calculations
To estimate 13C and 15N enrichment in each organ of the plants, %Atom (13C or 15N proportion) for 13C and 15N was calculated using the following equation:
| (2) |
where δ is the isotopic signature of CO2 respired or of OM. Rstandard is the international standard reference (i.e. 13C/12C, PeeDee Belemnite, and 15N/14N, atmospheric air).
%Atom excess was then calculated as the %Atom 15N or 13C differences between labelled and unlabelled organs (control, at Tinit):
The labelling-derived 13C content per DW (γ13C, in μg 13C g−1 DW) in each organ of the plant was calculated as follows:
| (3) |
where %C is the percentage of carbon in the organ. The dynamics of the labelling-derived 13C content in leaves were described by a double-exponential equation.
The labelling-derived 13C flux associated with root and leaf respiration (γ13CR, in μg 13C g−1 h−1) was calculated as follows:
| (4) |
where massorgan is the mass of the organ (g) considered, Rorgan is the respiration rate (μg C h−1), and %Atom excess is here the 13C atom excess in CO2.
As the plants experienced similar conditions during the chase period and the respiration measurements, the cumulative labelling-derived 13C content over time (in mg 13C g−1) was estimated by (i) fitting an exponential decay constant to the labelling-derived 13C flux over chase time (γ13CR):
| (5) |
(where t is the time in hours and a and b are constants); and (ii) integrating this exponential over time. For CO2 respired by new and old roots, the maximum concentration sampling point was used as the ‘time zero’ for the exponential curve fit.
Total labelling-derived 13C mass (γ13CM, μg or mg 13C) at chase time T was calculated by averaging the organ mass over the 15 replicates (see Table 1). For this purpose, the labelling-derived 13C mass for each organ and the loss through leaf and new/old root respiration from T0 to T were added as follows:
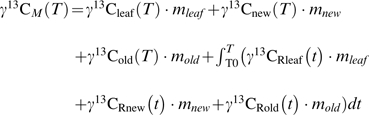 |
(6) |
By doing so, it was possible to see whether the labelling-derived 13C mass was balanced over time for each species, i.e. whether the total amount of 13C (remaining+respiratory losses) remained constant (Supplementary Table S1 available at JXB online). γ13CM was also compared with the amount of 13C fixed by each replicate during the 5 h labelling period based on Anet values integrated over the labelling period.
Table 1.
Morphological and physiological characteristics of C. foetida and K. myosuroides
| K. myosuroides | C. foetida | |
| Morphological characteristics | ||
| Mean dry biomass (g) | ||
| Aboveground | 2.6 (0.1) | 1.9 (0.1) |
| New roots | 0.51 (0.07) | 0.80 (0.09) |
| Old roots | 21.6 (1.8) | 28.00 (2.0) |
| Belowground productivity (g m−2 d−1) | 6.9 (1.8) a | 12.5 (0.6) b |
| Physiological characteristics | ||
| Assimilation rate during labelling (ng C g−1 leaf DW s−1) | 791.5 (20.3) a | 622.4 (13.3) b |
| Leaf respiration in darkness (ng C g−1 leaf DW s−1) | 63.5 (6.7) a | 100.3 (7.4) b |
| New root respiration (ng C g−1 new root DW s−1) | 233.6 (21.0) a | 206.3 (15.0) a |
| Old root respiration (ng C g−1 old root DW s−1) | 56.4 (4.3) a | 68.6 (13.0) a |
See text for further statistical details. Values are the mean ±SE (n=15, except for belowground productivity, where n=4, and assimilation rate, where n=12). Different letters indicate significant differences between the two species (P <0.05).
The labelling-derived 15N content per whole plant dry matter (γ15N, μg 15N g-1) was calculated as follows:
| (7) |
where %N is the percentage of nitrogen in the organ, massorgan, is the mass of the organ (g), and massplant is the mass of the whole plant (g).
Similar to the 13C data analysis, the labelling-derived 15N mass (μg15N) was calculated for each organ based on the average mass over the nine replicates of each species (see Table 1).
Belowground productivity
Belowground productivity was estimated using a root ingrowth core method. The ingrowth cores (5×15 cm) were installed on 30 May 2006 by removing one soil core in a set of four replicates for each species and by filling the holes with the same soil sieved through 2 mm mesh. Roots present within the cores were sampled on 15 July 2006, washed free of soil, dried at 60 °C for 48 h, and weighed. Root production was then calculated as the root biomass divided by time between 30 May 2006 and 15 July 2006, and expressed per unit core surface for each replicate.
Statistical procedure
For isotopic data, a non-parametric Kruskall–Wallis test was performed, except for 15N natural abundance for which a one-way analysis of variance (ANOVA) was used. Similarly, a non-parametric Kruskall–Wallis test was used to compare belowground productivity (n=4). The species effect on flux measurements and root/shoot ratios were analysed using a one-way ANOVA. Finally, an analysis of covariance (ANCOVA) was applied to test for the regression between (i) the labelling-derived quantity of 13C (γ13C) in new root OM against that of leaf OM and (ii) the γ13C of root-respired CO2 against that of leaf-respired CO2. Species was considered as a qualitative factor. All analyses were performed with Jump software (SAS Institute Inc., Cary, NC, USA).
Results
Biomass production
Mean biomass and belowground productivity data are indicated in Table 1 (upper part). Under the present experimental conditions, KM had a larger aboveground biomass and a lower belowground biomass than CF and hence a higher shoot/root ratio. In addition, the root productivity per unit ground area was higher in CF as compared with KM (χ2=5.01, P=0.02, Table 1).
Photosynthetic rates and total 13C assimilation
During 13C pulse time, the net assimilation rate of the above compartment (Anet) was significantly larger in KM than in CF (F1,21=5.1, P=0.03). This does not reflect a general trend: in homogeneous light conditions, CF had higher photosynthetic rates (see Supplementary Fig. S1 at JXB online; Baptist and Choler, 2008). The lower value in CF observed here was caused by the non-saturating light conditions and is in contrast to values of maximal leaf CO2 assimilation (in saturated light, see Supplementary Fig. S1). Assimilation values were summed over the pulse time to calculate the total labelling-derived 13C mass (γ13CM) fixed by photosynthesis, giving 1.33±0.2 mg for KM and 0.93±0.1 mg for CF (Supplementary Table S1 at JXB online).
Respiratory properties
Leaf respiration in darkness was largely and significantly higher in CF (F1,28=13.2, P=0.001) (Table 1). Thus, in the present experimental conditions, the leaf respiration to net assimilation ratio was 8.0% in KM and 16.1% in CF. New and old root respiration did not differ significantly between the two species.
13C fixation and partitioning
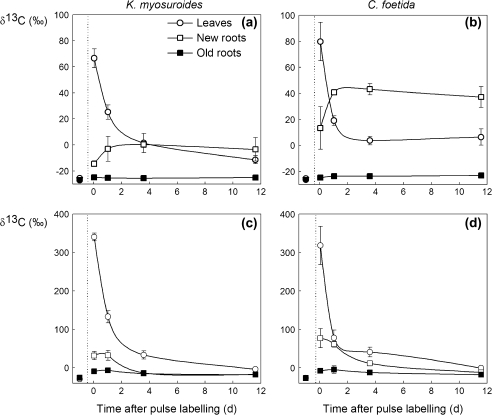
The 12C/13C isotope composition (δ13C) of OM and of respired CO2 after labelling is shown in Fig. 1. Clearly, at T0, leaves were the most labelled organs, followed by new roots (Fig. 1). Old roots showed a low degree of labelling throughout the experiment (i.e. until the end of the chase time). The kinetics were very different for leaves and roots: the δ13C value of leaves continuously declined during the chase time while that of new roots increased within 1.5 d and reached a plateau.
Fig. 1.
δ13C (‰) of leaves, and new and old roots of K. myosuroides (a) and C. foetida (b). δ13C (‰) of CO2 respired by the leaves, and new and old roots of K. myosuroides (c) and C. foetida (d) following pulse labelling. On the left of the dashed vertical line is shown the natural abundance of 13C in the OM of leaves, and new and old roots, and the CO2 respired by the leaves, and the new and the old roots. Leaves, open circles; new roots, open squares; old roots, filled squares. All x-axes show the time elapsed since pulse labelling (in days). Values are the mean ±SE (n=3).
The decline in the amount of 13C in leaves may be caused by: (i) isotopic dilution (natural 12CO2 fixed during the daytime of the chase period); (ii) a loss of 13C by respiration (dark-respired CO2 was strongly 13C enriched, Fig. 1c and d); and (iii) export (13C increases in roots within a couple of days). It is noteworthy that fixed 13C in CF leaf OM was exported more rapidly compared with KM [the calculated half-time of the exponential decay (t1/2 of the fastest turnover pool) is nearly 21 h for KM and only 13 h for CF].
A larger isotopic dilution is probably not responsible for such a difference: plants of both species experienced similar conditions, and KM had a slightly higher photosynthetic rate than CF under the conditions of the experiment (see paragraph above, Table 1). In contrast, respiration contributed to these leaf 13C kinetics simply because the respiration rate was larger in the case of CF (Table 1) while having a similar 13C enrichment (Fig. 1c, d).
The faster decline of leaf 13C in CF than KM also came from the export of larger amounts of assimilates to roots: the δ13C value of OM in new roots was higher in CF than in KM. Furthermore, in the steady state, the δ13C values of new root OM were 0‰ and 40‰ for KM and CF, respectively (Fig. 1a, b). That is, the labelling-derived carbon in new roots accounted for ∼0.6% and 1.8% of the total C in KM and CF, respectively.
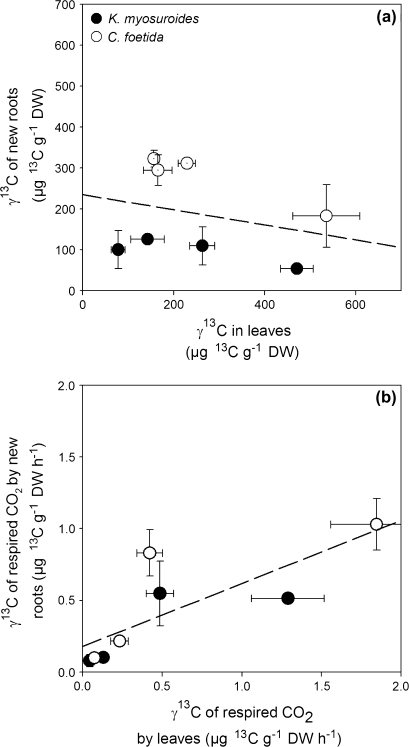
The labelling-derived quantity of 13C (γ13C) in new roots is plotted against that of leaves in Fig. 2. There was a significant correlation between the γ13C value of new roots and that of leaves when the species were considered together (F1,22=46.1, R2=0.71, P <0.0001, Fig. 2a). The slope of the regression was slightly (but not significantly) steeper in CF, again showing more rapid 13C kinetics in leaves. In addition, the more rapid transfer of carbon from leaves to roots was reflected by the shift of data points from the right-hand side (T0) to the left-hand side (the chase measurements, at T1–T3, clustered on the left, Fig. 2A, open symbols) in CF, while there were intermediate data points in KM. γ13CR values of leaf-respired CO2 and root-respired CO2 were correlated (F1,23=22.2, R2=0.56, P=0.0001, Fig. 2b) when species were considered together. However, neither the slope of the regression (F1,23=0.44, P=0.51) nor the mean value (F1,23=2.57, P=0.12) differed between the two species.
Fig. 2.
(a) Labelling-derived 13C content of new roots in relation to labelling-derived 13C content of leaves and (b) labelling-derived 13C content of CO2 respired by the new roots in relation to the labelling-derived 13C content of CO2 respired by the leaves for K. myosuroides (filled symbols) and C. foetida (open symbols) at each chase time. Values are the mean ±SE (n=3). See text for further statistical details.
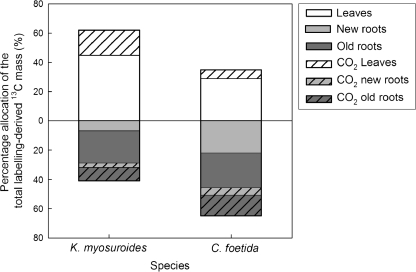
Whole-plant carbon partitioning
Eleven days after pulse labelling, carbon allocation patterns were calculated, taking into account integrated respiratory losses. The results are shown in Fig. 3 (raw data are shown in Supplementary Fig. S2 at JXB online). In KM, 45% of the labelling-derived 13C remained in the leaves, and nearly 5% of the labelling-derived 13C was allocated to new roots. For CF, 29% of the labelling-derived 13C remained in the leaves, while new roots accounted for ∼20%. Respiratory losses associated with leaves were more pronounced in KM than in CF, while those associated with new root respiration were larger in CF. However, in the case of new roots of CF, the respiration/OM ratio of 13C allocation was much smaller (Fig. 3, light grey), clearly suggesting that 13C was directed to new root growth to a greater extent than to respiration.
Fig. 3.
Percentage allocation of the total labelling-derived 13C mass (γ13CM) recovered in the leaves and the new and old roots and lost through leaf, and new and old root respiration 11^d after pulse labelling. Leaves, white; new roots, light grey; old roots, dark grey. Hatched patterns correspond to C lost through respiration.
Nitrogen allocation and partitioning
The natural 14N/15N isotope composition (δ15N) of unlabelled organs and soil is indicated in Table 2. Soil N was always 15N enriched (P <0.05) by 1.7–7.1‰, so a 14N/15N isotope fractionation during N reduction/assimilation is apparent. KM leaves were clearly and significantly 15N depleted compared with new and old roots, whereas CF leaves had nearly the same δ15N value as new and old roots and were slightly enriched compared with KM leaves.
Table 2.
15N natural abundance (δ15N, ‰) of unlabelled leaves, new roots, old roots, and soil in K. myosuroides and C. foetida monoliths
| δ15N (‰) | K. myosuroides | C. foetida |
| Leaf | –3.40 (0.37) a | 0.19 (0.23) a |
| New roots | 0.61 (0.62) b | –0.02 (0.37) a |
| Old roots | –0.76 (0.14) b | 2.73 (1.12) ab |
| Soil | 3.78 (0.12) c | 4.49 (0.95) b |
Values are the mean ±SE (n=15). Different letters indicate significant differences between the organs and the soil (P <0.05).
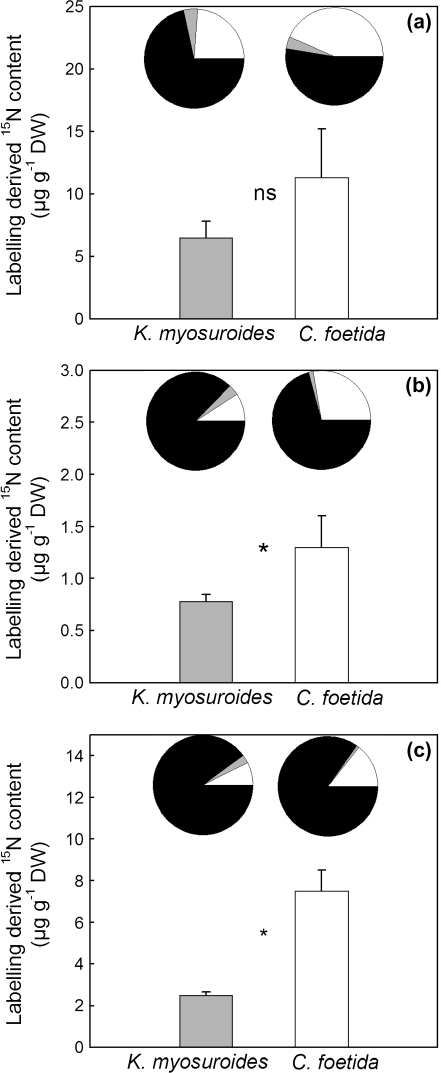
The nitrogen allocation after 24 h 15N labelling with [15N]-nitrate, [15N]-ammonium, or [15N]-glycine is indicated in Fig. 4. Clearly, CF was more 15N-labelled than KM at the whole-plant level, no matter which labelling molecule was applied. In other words, CF had a higher nitrogen uptake efficiency (NupE) than KM: the recovery of 15N from the labelling solution was 13.7±2.5% glycine, 19.8±3.1% NO3–, 2.9±1.2% NH4+ for CF; and 4.6±0.5% glycine, 10.4±3.8% NO3–, and 1.8±0.4% NH4+ for KM. For nitrate, the CF/KM ratio of NupE was then as high as 1.9.
Fig. 4.
Labelling-derived 15N content in whole-plant dry matter (μg 15N g-1 DW) after amendment with [15N]-NO3– (a), [15N]-NH4+ (b), or [15N]-glycine (c) in K. myosuroides (grey bar) and C. foetida (white bar). Circle graphs, percentage allocation of the total labelling-derived 15N mass recovered in the leaves (white), new roots (grey), and old roots (black) (μg 15N μg-1 15N in whole-plant dry matter). Values are the mean ±SE (n=3). ns, not significant, * indicates significant differences between both species (P <0.05). See text for further statistical details.
In addition, nearly twice as much N was allocated to leaves in CF as in KM when 15N was supplied as nitrate or ammonium (circle graphs, Fig. 4a, b). Such a difference was less pronounced with [15N]-glycine (Fig. 4c). However, old roots represent a large 15N sink in Fig. 4 simply because of their high biomass. In other words, the specific 15N abundance (μg 15N g−1 DW) was always low in old roots (data not shown). In the case of nitrate, the specific 15N abundances of leaves were 72.6±26.8 and 20.3±9.7 μg 15N g−1 DW in CF and KM, respectively, giving a CF/KM ratio of 3.5. Such a ratio is larger than the NupE ratio (1.9, see above), clearly demonstrating that preferential N allocation to leaves, rather than whole-plant uptake efficiency, was responsible for the larger 15N mass in CF leaves (Fig. 4a).
Discussion
This study aimed to clarify C and N partitioning patterns at peak standing biomass of the alpine plant C. foetida (CF), a fast-growing species from late snowmelt locations, and K. myosuroides (KM), a slow-growing species from early snowmelt locations. Both natural isotopic abundances and isotopic labelling (13C, 15N) were used, and C and N assimilation patterns were followed.
Carbon fixation and partitioning
CF exhibits high relative growth rates (e.g. belowground productivity, Table 1) and photosynthetic capacity (Supplementary Fig. S1 at JXB online). This agrees with the larger maximal carboxylation rate Vcmax and higher leaf N elemental content in CF (Choler, 2005; Baptist and Choler, 2008), which is an indicator of the maximum velocity of ribulose bisphosphate (RuBP) carboxylation by Rubisco. Whole-plant carbon allocation favoured the root compartment in CF as indicated by the larger 13C transfer to new roots (Figs 1–3) as compared with KM. Such a pattern might correspond either to a larger flux of assimilates from aboveground to belowground organs or to a higher 13C-specific abundance of assimilates. Since the kinetics of the 13C decline in leaf OM are faster in CF (Fig. 1; t1/2 values of 13 h versus 21 h in KM; Fig. 2a) and the initial leaf 13C abundance (carbon source) was very similar in both species, the first hypothesis is favoured.
The mass balance after an 11 d chase period indicated that the larger carbon flow from leaves to roots was directed to new root growth rather than respiration in CF plants (Fig. 3). In fact, the 13C abundance in root-respired CO2 is dissimilar to the amount in total OM: while the 13C allocation (labelling-derived 13C content) to new root total OM is nearly three times higher in CF (Fig. 1, and Supplementary Fig. S2 at JXB online), the maximum 13C content in root-respired CO2 was only doubled. In other words, the turnover (consumption) of the root respiratory pool was lower in CF.
Nevertheless, a large difference was found between woody (old) and non-woody (new) roots. Old roots were weak 13C sinks (very low 13C abundance after labelling, Fig. 1) with low respiratory activity as compared with new roots (nearly 70% less, Table 1). This reflects differences in metabolic activities: new roots are responsible for root growth and nutrient absorption while old roots have a conduction and storage role (Comas et al., 2000; Lipp and Andersen, 2003; Volder et al., 2005). However, at the whole-plant level, old roots accounted for substantial 13C content because of their very large biomass (Table 1), while the C allocation pattern to old roots was somewhat similar in both species (Figs 1, 3).
Nitrogen uptake and assimilation
Variations in 15N uptake between both species (Fig. 4) are consistent with findings of previous studies that suggest that fast-growing species display higher specific nitrogen absorption rates than slow-growing species (Garnier, 1991; Poorter et al., 1991). In addition, CF and KM exhibited different N allocation patterns: CF experienced higher 15N allocation to leaves after a 24 h chase period as compared with KM (Fig. 4). Consequently, one may assume that N translocation toward the aboveground compartment was more efficient in CF than in KM plants.
However, there is a predominance of leaf N reduction over root N reduction in KM as compared with CF (Table 2). In fact, in normal conditions where nitrate is reduced by both leaves and roots, the natural 14N/15N isotope composition (δ15N) of leaves is higher (15N enriched) than that of roots, regardless of the δ15N and N content in the soil. This is because nitrate reduction fractionates against 15N, thereby enriching the remaining nitrate molecules exported to leaves (for a recent review, see Tcherkez and Hodges, 2007). This was typically the case in CF (Table 2). In contrast, KM leaves were not 15N enriched (and are in fact slightly 15N depleted) as compared with new roots, demonstrating that N reduction occurred mainly in leaves (Table 2). Accordingly, under non-limiting nutrient availability, shoots generally appear to be the predominant site of NO3– reduction because of the higher content of excess reductants produced by photosynthesis (Scheurwater et al., 2002).
The mechanisms underlying such a shoot versus root pattern of N reduction are uncertain. It is believed that it is influenced by the photosynthetic rate (which generates reductants and carbon skeletons) as well as biochemical parameters (maximum organ-specific velocity of nitrate reductase activity), biomass allocation, and environmental conditions (Gojon et al., 1994). Here, is it possible that the high C flux allocated to the belowground compartment in CF promotes significant levels of NO3– reduction in the roots (Pate, 1980). Although arctic and alpine slow-growing species such as KM are thought to have both a smaller NupE and a low nitrate reductase activity (Atkin, 1996), it remains plausible that a high rate of NO3– reduction would contribute to mitigating nitrate retroflux (back-diffusion to the soil), thereby increasing NupE in KM (Mata et al., 2000; Miller and Cramer, 2004).
Biomass and energetic root/shoot balance
A larger rate of root respiration was expected in CF as compared with KM plants as more respiratory energy was necessary to sustain higher root productivity and nitrogen uptake (almost 2-fold larger). Nevertheless, root respiration did not differ between the two species (Table 1), i.e. the fast-growing species respired at a lower rate than would be expected from their C and N metabolism. Previous studies have reported similar results concerning the absence of apparent relationships between the relative growth rate and the root respiration rate (Van der Werf et al., 1988; Poorter et al., 1991).
Root respiration can be decomposed into three components, i.e. the respiratory cost of root growth and of ion uptake, and maintenance respiration. A classical relationship has been proposed between total root respiration (denoted as R) and such components, as follows (modified version from Van der Werf et al., 1994):
where R is root respiration, Rm is maintenance respiration, NupE is nitrogen uptake efficiency, Pr is root productivity, and Cu and Cg are the specific respiratory costs of ion uptake and growth, respectively.
It has been shown that both nitrate uptake efficiency and root growth are 2-fold higher in CF, while both species exhibit similar root respiration rates (Table 1). Consequently, it may be assumed that the respiratory costs of ion uptake and growth (Cg and Cu coefficients) are much lower in CF as compared with KM unless there is a large difference in the value of maintenance respiration. Such an assumption is not likely. Scheurwater et al. (1998) and Van der Werf et al. (1988) demonstrated that within a plant life form (e.g. Graminoids), maintenance respiration differs only very slightly between fast- and slow-growing species.
Although several studies have suggested that the construction costs of roots and leaves may not differ between fast- and slow-growing species (Van der Werf et al., 1988; Navas et al., 2003; Roumet et al., 2006), it was not possible to obtain experimentally the cost coefficients (Cg and Cu) in the present study, and thus it remains plausible that both coefficients differ between the two species.
Conclusions
CF and KM represent contrasting examples of C and N dynamic allocation during the growth period. Relative to KM, CF exhibits an improved photosynthetic capacity and a lower N assimilation capacity in its leaves, which are compensated for by (i) a preferential carbon allocation to roots favouring root growth (storage) and NO3– reduction and (ii) a larger translocation of reduced N to aboveground organs. Unexpectedly, then, CF root respiration was much lower than expected, possibly due to a lower respiratory cost for ion uptake. The large C allocation to the belowground compartment during the growing season might also ensure sufficient root storage for sustaining the extremely rapid greening of snowbed plants just after snowmelt in the following year.
The results obtained here suggest that an allocation-based balance between root N reduction and leaf CO2 assimilation is involved in growth strategies of alpine species growing under short, energy-limited vegetation periods. This coupling between C and N fluxes was less apparent in the case of KM, which benefited from a longer growing season but was nutrient limited. Nevertheless, it is recognized that other environmental parameters may be involved in C and N allocation patterns (e.g. temperature, CO2 mole fraction), impacting on growth, respiration, and N metabolism. The importance of temperature-mediated changes in whole-plant C allocation as a result of growing season progress also needs to be established (Atkin et al., 2007). These aspects will be addressed in a future work.
Supplementary data
Supplementary data, comprising Table S1, and Figures S1 and S2, are available at JXB online.
Supplementary Material
Acknowledgments
We gratefully acknowledge Laurent Vanbostal, Céline Flahaut, Peter Streb, Richard Bligny, and Marie-Pascale Colace for their help in the laboratory. We also thank Fabien Quétier and Jaleh Ghashghaie for their helpful discussions, and Sophie Rickebusch for English-language editing of the manuscript. Logistical support was provided by the ‘Laboratoire d'Ecologie Alpine’ (UMR 5553 CNRS/UJF, University Joseph Fourier) and the ‘Station Alpine Joseph Fourier’, the alpine field station of the University Joseph Fourier. The financial support of CNRS and Ministerio de Ciencia e Innovacion (PR2008-0247) and BIODIVERSA-VITAL is gratefully acknowledged.
References
- Atkin OK. Reassessing the nitrogen relations of arctic plants: a mini-review. Plant, Cell and Environment. 1996;19:695–704. [Google Scholar]
- Atkin OK, Scheurwater I, Pons TL. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytologist. 2007;174:367–380. doi: 10.1111/j.1469-8137.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- Atkinson LJ, Hellicar MA, Fitter AH, Atkin OK. Impact of temperature on the relationship between respiration and nitrogen concentration in roots: an analysis of scaling relationships, Q10 values and thermal acclimation ratios. New Phytologist. 2007;173:110–120. doi: 10.1111/j.1469-8137.2006.01891.x. [DOI] [PubMed] [Google Scholar]
- Baptist F, Choler P. A simulation on the importance of growing season length and canopy functional properties on the seasonal gross primary production of temperate alpine meadows. Annals of Botany. 2008;101:549–559. doi: 10.1093/aob/mcm318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WD. Inputs and storage of nitrogen in winter snowpack in an alpine ecosystem. Arctic and Alpine Research. 1992;24:211–215. [Google Scholar]
- Bowman WD, Gartner JR, Holland K, Wiedermann M. Nitrogen critical loads for alpine vegetation and terrestrial ecosystem response: are we there yet? Ecological Applications. 2006;16:1183–1193. doi: 10.1890/1051-0761(2006)016[1183:nclfav]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bowman WD, Theodose TA, Fisk MC. Physiological and production responses of plant growth forms to increases in limiting resources in alpine tundra: implications for differential community response to environmental change. Oecologia. 1995;101:217–227. doi: 10.1007/BF00317287. [DOI] [PubMed] [Google Scholar]
- Bowman WD, Theodose TA, Schardt JC, Conant RT. Constraints of nutrient availability on primary production in two alpine communities. Ecology. 1993;74:2085–2097. [Google Scholar]
- Chapin FS., III. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics. 1980;11:37–52. [Google Scholar]
- Choler P. Consistent shifts in alpine plant traits along a mesotopographical gradient. Arctic, Antarctic and Alpine Research. 2005;37:444–453. [Google Scholar]
- Comas LH, Eissenstat DM, Lakso AN. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytologist. 2000;147:171–178. [Google Scholar]
- Craine JM, Tilman D, Wedin D, Reich P, Tjoelker M, Knops J. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Functional Ecology. 2002;16:563–574. [Google Scholar]
- Farrar J, Gunn S. Allocation: allometry, acclimation—and alchemy? In: Lambers H, Poorter H, Van vuren MMI, editors. Inherent variation in plant growth. Leiden: Backhuys Publishers; 1998. pp. 183–197. [Google Scholar]
- Garnier E. Resource capture, biomass allocation and growth in herbaceous plants. Trends in Ecology and Evolution. 1991;6:126–131. doi: 10.1016/0169-5347(91)90091-B. [DOI] [PubMed] [Google Scholar]
- Gojon A, Passard C, Bussi C. Root/shoot distribution of NO3– assimilation in herbaceous and woody species. In: Roy R, Garnier E, editors. A whole plant perspective on carbon–nitrogen interactions. The Hague: SPB Academic Publishing; 1994. pp. 131–147. [Google Scholar]
- Grulke NE, Bliss LC. Growth forms, carbon allocation, and reproductive strategies of high arctic saxifrages. Arctic Alpine Research. 1985;17:241–250. [Google Scholar]
- Jaeger CH, Monson RK. Adaptative significance of nitrogen storage in Bistorta bistortoides, an alpine herb. Oecologia. 1992;92:121–131. doi: 10.1007/BF00317852. [DOI] [PubMed] [Google Scholar]
- Körner C. CO2 exchange in the alpine sedge Carex curvula as influenced by canopy structure, light and temperature. Oecologia. 1982;53:165–175. doi: 10.1007/BF00377142. [DOI] [PubMed] [Google Scholar]
- Körner C. Alpine plant life. Berlin: Springer Verlag; 1999. [Google Scholar]
- Körner C, Diemer M. In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Functional Ecology. 1987;1:313–343. [Google Scholar]
- Kudo G. Intraspecific variation of leaf traits in several deciduous species in relation to length of growing season. Ecoscience. 1996;3:483–489. [Google Scholar]
- Lipp CC, Andersen CP. Role of carbohydrate supply in white and brown root respiration of Ponderosa pine. New Phytologist. 2003;160:523–531. doi: 10.1046/j.1469-8137.2003.00914.x. [DOI] [PubMed] [Google Scholar]
- Lipson DA, Monson RK, Bowman WD. Luxury uptake and storage of nitrogen in the rhizomatous alpine herb, Bistorta bistortoides. Ecology. 1996;77:569–576. [Google Scholar]
- Lipson DA, Raab TK, Schmidt SK, Monson RK. Variation in competitive abilities of plants and microbes for specific amino acids. Biology and Fertility of Soils. 1999;29:257–261. [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nosberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- Mata C, van Vemde N, Clarkson DT, Martins-Louçao MA, Lambers H. Influx, efflux and net uptake of nitrate in Quercus suber seedlings. Plant and Soil. 2000;221:25–32. [Google Scholar]
- Miller AJ, Cramer MD. Root nitrogen acquisition and assimilation. Plant and Soil. 2004;274:1–36. [Google Scholar]
- Monson RK, Rosenstiel TN, Forbis TA, Lipson DA, Jaeger CH. Nitrogen and carbon storage in alpine plants. Integrative and Comparative Biology. 2006;46:35–48. doi: 10.1093/icb/icj006. [DOI] [PubMed] [Google Scholar]
- Navas ML, Ducout B, Roumet C, Richarte J, Garnier J, Garnier E. Leaf life span, dynamics and construction cost of species from Mediterranean old-fields differing in successional status. New Phytologist. 2003;159:213–228. doi: 10.1046/j.1469-8137.2003.00790.x. [DOI] [PubMed] [Google Scholar]
- Nogués S, Aranjuelo I, Pardo T, Azcon-Bieto J. Assessing the stable-carbon isotopic composition of intercellular CO2 in a CAM plant at two CO2 levels. Rapid Communication in Mass Spectrometry. 2008;22:1017–1022. doi: 10.1002/rcm.3460. [DOI] [PubMed] [Google Scholar]
- Nogués S, Tcherkez G, Streb P, Pardo A, Baptist F, Bligny R, Ghashghaie J, Cornic G. Respiratory carbon metabolism in the high mountain plant species Ranunculus glacialis. Journal of Experimental Botany. 2006;57:3837–3845. doi: 10.1093/jxb/erl149. [DOI] [PubMed] [Google Scholar]
- Osone Y, Ishida A, Tateno M. Correlation between relative growth rate and specific leaf area requires associations of specific leaf area with nitrogen absorption rate of roots. New Phytologist. 2008;179:417–427. doi: 10.1111/j.1469-8137.2008.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate JS. Transport and partitioning of nitrogenous solutes. Annual Review of Plant Physiology. 1980;31:313–340. [Google Scholar]
- Poorter H, Van Der Werf A, Atkin OK, Lambers H. Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiologia Plantarum. 1991;83:469–475. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences, USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumet C, Urcelay C, Diaz S. Suites of root traits differ between annual and perennial species growing in the field. New Phytologist. 2006;170:357–368. doi: 10.1111/j.1469-8137.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- Scheurwater I, Cornelissen JHC, Dictus F, Welschen R, Lambers H. Why do fast- and slow-growing grass species differ so little in their rate of root respiration, considering the large differences in rate of growth and ion uptake? Plant, Cell and Environment. 1998;21:995–1005. [Google Scholar]
- Scheurwater I, Koren M, Lambers H, Atkin OK. The contribution of roots and shoots to whole plant nitrate reduction in fast- and slow-growing grass species. Journal of Experimental Botany. 2002;53:1635–1642. doi: 10.1093/jxb/erf008. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Hodges M. How stable isotopes may help to elucidate primary nitrogen metabolism and its interaction with (photo)respiration in C3 leaves. Journal of Experimental Botany. 2007;59:1685–1693. doi: 10.1093/jxb/erm115. [DOI] [PubMed] [Google Scholar]
- Theodose TA, Bowman WD. Nutrient avaibility, plant abundance, and species diversity in two alpine tundra communities. Ecology. 1997;78:418–429. [Google Scholar]
- Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytologist. 2005;167:493–508. doi: 10.1111/j.1469-8137.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- Trumbore S. Carbon respired by terrestrial ecosystems—recent progress and challenges. Global Change Biology. 2006;12:141–153. [Google Scholar]
- Van der Werf A, Kooijman A, Welschen R, Lambers H. Respiratory energy costs for the maintenance of biomass, for growth and for ion uptake in roots of Carex diandra and Carex acutiformis. Physiologia Plantarum. 1988;72:483–491. [Google Scholar]
- Van der Werf A, Poorter H, Lambers H. Respiration as dependent on a species’ inherent growth rate and on the nitrogen supply to the plants. In: Roy J, Garnier E, editors. A whole plant perspective on carbon–nitrogen interactions. The Hague: SPB Academic Publishing; 1994. pp. 91–110. [Google Scholar]
- Volder A, Smart DR, Bloom AJ, Eissenstat DM. Rapid decline in nitrate uptake and respiration with age in fine lateral roots of grape: implications for root efficiency and competitive effectiveness. New Phytologist. 2005;165:493–502. doi: 10.1111/j.1469-8137.2004.01222.x. [DOI] [PubMed] [Google Scholar]
- Waring RH. How ecophysiologists can help scale from leaves to landscapes. In: Ehleringer JR, Field CB, editors. Scaling physiological processes: leaf to globe. New York: Academic Press; 1993. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.