Abstract
BRCA2 is a breast tumour susceptibility factor with functions in maintaining genome stability through ensuring efficient double-strand DNA break (DSB) repair via homologous recombination. Although best known in vertebrates, fungi, and higher plants also possess BRCA2-like genes. To investigate the role of Arabidopsis BRCA2 genes in DNA repair in somatic cells, transposon insertion mutants of the AtBRCA2a and AtBRCA2b genes were identified and characterized. atbrca2a-1 and atbrca2b-1 mutant plants showed hypersensitivity to genotoxic stresses compared to wild-type plants. An atbrca2a-1/atbrca2b-1 double mutant showed an additive increase in sensitivity to genotoxic stresses compared to each single mutant. In addition, it was found that atbrca2 mutant plants displayed fasciation and abnormal phyllotaxy phenotypes with low incidence, and that the ratio of plants exhibiting these phenotypes is increased by γ-irradiation. Interestingly, these phenotypes were also induced by γ-irradiation in wild-type plants. Moreover, it was found that shoot apical meristems of the atbrca2a-1/atbrca2b-1 double mutant show altered cell cycle progression. These data suggest that inefficient DSB repair in the atbrca2a-1/atbrca2b-1 mutant leads to disorganization of the programmed cell cycle of apical meristems.
Keywords: Arabidopsis, BRCA2, cell cycle, double-strand DNA breaks, fasciation, homologous recombination, γ-ray
Introduction
Double-strand breaks (DSBs) are the most threatening type of DNA damage in living cells. There are two major DSB repair pathways: non-homologous end-joining (NHEJ) and homologous recombination (HR). NHEJ repair is error-prone, and represents the predominant repair pathway during G1 to early S-phase of the cell cycle (although this pathway is able to operate throughout the cell cycle). HR is important as an error-free repair pathway during late S to G2 phase of the cell cycle, when sister chromatids are available (Essers et al., 2000; Couedel et al., 2004; Bleuyard et al., 2006).
HR processes are accomplished via the co-operation of a set of proteins known as RAD52 epistasis group proteins, of which MRE11/RAD50/XRS2 (NBS1) processes DSBs to generate 3′ single-strand DNA (ssDNA) tails, RAD52 and RAD51 paralogues assemble the eukaryotic recombinase, RAD51 forms RAD51-single-strand DNA (ssDNA) nucleoprotein filaments with replication protein A, and RAD54 assists in D-loop formation of RAD51-dependent ssDNA and double-strand DNA (Thompson and Schild, 2001). In higher eukaryotic cells, RAD51 plays a dominant role in this pathway. Recent data indicate the important role of BRCA2 as well as RAD51 paralogues, which are co-factors of RAD51, in facilitating the assembly of RAD51 nucleoprotein filaments (Galkin et al., 2005; van Veelen et al., 2005).
The BRCA2 gene was cloned based on an analysis of mutations in families predisposed to breast cancer showing that a large percentage of the kindred had alterations within this locus (Tavtigian et al., 1996). BRCA2 is known to bind RAD51 directly through the eight conserved BRC repeats in BRCA2 (Wong et al., 1997; Galkin et al., 2005) as well as through the C-terminal region of BRCA2 (Esashi et al., 2005). Using a gene knockout method to create mice with BRCA2 mutations, homozygous mutant mice show embryo lethality associated with chromosomal rearrangements and breaks (Sharan et al., 1997; Yu et al., 2000). In addition, the formation of RAD51 foci following DNA damage is altered in brca2 mutant cells (Yu et al., 2000). These results suggest that BRCA2 is required to engage RAD51 in HR repair of DNA damage.
Siaud et al. (2004) reported the presence of two BRCA2-like genes in the Arabidopsis genome. The Arabidopsis BRCA2 proteins interact with RAD51, DMC1, and DSS1 in vitro (Siaud et al., 2004; Dray et al., 2006). Moreover, transgenic Arabidopsis plants transformed with a BRCA2 RNA interference (RNAi) construct driven by the meiosis-specific Arabidopsis DMC1 promoter were sterile and showed aberrant chromosomes in meiosis (Siaud et al., 2004). However, the function of AtBRCA2 in HR repair, especially in somatic cells, i.e. during vegetative growth, was not determined.
To determine the function of AtBrca2a and AtBrca2b in somatic cells, mutants in the genes encoding each of these proteins were isolated and characterized: atbrca2a-1 and atbrca2b-1. Each single mutant exhibited hypersensitivity to genotoxic stresses compared to wild-type plants (Nossen), and the atbrca2a-1/atbrca2b-1 double mutant showed an additive increase in sensitivity to genotoxic stresses compared to each single mutant. Interestingly, atbrca2 mutant plants displayed fasciation and abnormal phyllotaxy phenotypes with low incidence, with the proportion of plants exhibiting these phenotypes being increased by γ-irradiation. Moreover, cell cycle regulation in the atbrca2 mutant was investigated. The relationship between inefficient DSB repair, the abnormal phyllotaxy and/or fasciation phenotype, and cell cycle progression is discussed.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana ecotypes Nossen and Col. were used in this study. The atbrca2a-1 and atbrca2b-1 mutants were found by searching the Ds mutant collection (Nossen background) established by the RIKEN Institute (Fedoroff and Smith, 1993; Kuromori et al., 2004; Ito et al., 2005). Plants were grown on a soil mixture of equal parts of vermiculite and commercial soil (Sakata Super Mix, Sakata Seed Co., Tokyo, Japan) in a growth chamber at 22 °C under 12/12 h (light/dark) cycle conditions. Sterile plants were cultured on Murashige–Skoog medium (Murashige and Skoog, 1962) solidified with 0.25% gelrite (Wako, Tokyo, Japan) (MS gelrite plate) or 0.8% bacto-agar (Difco, Detroit, MI) (MS agar medium) in a growth chamber under 12/12 h (light/dark) cycle conditions at 22 °C.
Isolation of DNA and RNA
Genomic DNA was isolated from 2–4-week-old sterile plants using a DNeasy Plant Maxi kit (Qiagen, Hilden, Germany). Total RNA was isolated from 2-week-old plants and young flower buds using the RNeasy Plant Mini kit (Qiagen) according to the manufacturer's instructions.
DNA sequencing
Sequencing reactions were performed with a BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA). The reaction products were analysed with an automatic DNA sequencer (ABI PRISM 3100-Avant Genetic Analyzer, Applied Biosystems).
PCR genotyping assay
Plant genotypes of AtBRCA2a(–/–) and AtBRCA2b(–/–) mutations were identified by PCR. The wild-type AtBRCA2a locus was identified by PCR with the primer combination F6N15-1 (5′-CAAATCGTTTTCAACTTTCCCGCCGTCT-3′) and F6N15-2 (5′-CATTTGGGGAATTGAGCAATTTGTGTTCC-3′). The mutant locus of AtBRCA2a was identified by PCR with Ds3′-4 (5′-CCGTCCCGCAAGTTAAATATG-3′) and F6N15-2. The wild-type AtBRCA2b locus was identified by PCR with the primer combination F7A7-1 (5′-GGCTTCCCCCGTGTAAATTATAGTTCTCAG-3′) and F7A7-2 (5′-CGTTTGGGGAATTGAGCAATTTGTGTTCT-3′). The mutant locus of AtBRCA2b was identified by PCR with Ds5′-3m (5′-ACCTCGGGTTCGAAATCGATCGG-3′) and F7A7-1. PCR products were examined by direct sequencing.
Production of BRCA2–RNAi plants
To produce a hairpin RNAi construct, the Gateway Cloning System (Invitrogen, USA) was used; AtBRCA2 gene-specific primers fused to attB1 and attB2 sequences, Br2-attB1-F1 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGGCATTTATTTTCCGATTCCAGC-3′) and Br2-attB2-R1 (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTGAAAAAGACTGTTGGGAACACC-3′) were used to amplify a 502 bp fragment of the AtBRCA2b gene by PCR. A BP clonase reaction was carried out to clone the PCR product into the donor vector pDONR221 (Invitrogen, USA). An LR clonase reaction was carried out to transfer DNA fragments from this intermediate clone to the destination vector [pB7GWIWG2(II)] (Karimi et al., 2002). The resulting plasmid, pB7GWIWG2(II)-BRCA2 (BRCA2-RNAi vector) was introduced into Agrobacterium tumefaciens strain EHA105, and the resultant strain (Col. background) was used in RNAi silencing experiments.
Cisplatin treatment and γ-irradiation
Sensitivity of wild-type (Nossen and Col.), mutant plants (Nossen background) and RNAi plants (Col. background) to genotoxic stresses was scored based on the number of true leaves produced after genotoxic stress treatment according to our previous study (Osakabe et al., 2005). For the assay of cisplatin sensitivity, seeds in lots of 50 were imbibed by soaking in 200 μl water for 4 d at 4 °C. Presoaked seeds were plated on MS agar medium containing the appropriate concentration of cisplatin. At 14 d after plating on MS agar plates, plants were scored for the production of true leaves. For the assay of γ-ray sensitivity, imbibed seeds were irradiated and immediately plated on MS agar medium. Ten days after planting on MS agar medium, plants were scored for the production of true leaves.
In situ hybridization analysis
In situ hybridization was performed essentially as described by Kouchi and Hata (1993). Plant material was fixed in 4% (w/v) paraformaldehyde in 50 mM sodium phosphate buffer (pH 7.2) for 6 h at 4 °C, dehydrated through a graded ethanol series and tert-butanol series, and finally embedded in Paraplast Plus (Sherwood Medical, St Louis, MO). Microtome sections (6–10 μm thick) were applied to glass slides treated with silane. The cDNA for AtWUS was a kind gift from Dr T Araki. The antisense and sense RNAs of AtWUS were labelled with digoxigenin by in vitro transcription of linearized pBluescript KS+ carrying a fragment of the entire coding sequence of the AtWUS cDNA. Hybridization and immunological detection were conducted according to the methods of Kouchi and Hata (1993).
Histochemical assay of AtCYCB1;1::GUS reporter
Arabidopsis thaliana (ecotype Col.) plants transformed with AtCYCB1;1::GUS (Colon-Carmona et al., 1999) were crossed with atbrca2 mutant and wild-type plants (Nossen). Sterile 1-week-old plants containing the AtCYCB1;1::GUS reporter gene and homozygous for the AtBRCA2a and AtBRCA2b mutation (AtBRCA2a-1–/–/AtBRCA2b-1–/–) were used for histochemical GUS staining. On the other hand, the AtBRCA2–RNAi construct was transformed to Arabidopsis Col. plants with AtCYCB1;1::GUS. Seeds were imbibed by soaking in 200 μl water for 4 d at 4 °C. Presoaked seeds were plated on MS agar medium. At 7 d after plating on MS agar plates, plants were treated with aphidicolin or cisplatin (3 d). After treatment with aphidicolin or cisplatin, plant materials were soaked in GUS staining buffer containing 100 mM sodium phosphate (pH 7.0), 10 mM ethylenediaminetetraacetic acid (EDTA), 0.1% Triton X100, 0.5 mg ml−1 X-Gluc, 2 mM potassium ferrocyanide, 2 mM potassium ferricyanide, 100 μg ml−1 chloramphenicol, and 5% methanol. The enzymatic reaction was performed over 1 d at 37 °C. Tissues were cleared with 70% ethanol to remove pigments. Plants were observed under a dissecting microscope.
Results
Identification of Arabidopsis Ds insertional mutants in AtBRCA2 genes
To investigate the function of the AtBRCA2a and AtBRCA2b genes in somatic cells, a search was made for loss-of-function mutants using a reverse genetic approach. Single mutant lines were identified in the RIKEN Ds insertion collection (Nossen background) (Fedoroff and Smith, 1993; Kuromori et al., 2004; Ito et al., 2005), (http://rarge.gsc.riken.go.jp/dsmutant/), and the corresponding alleles were named atbrca2a-1 and atbrca2b-1. The AtBRCA2a/Ds and AtBRCA2b/Ds junctions were amplified by PCR and sequenced to determine the position of the Ds insertion (see Supplementary Fig. S1A, B at JXB online). In the atbrca2a-1 allele, the transposon is inserted in the first exon of the AtBRCA2a gene (45 bp downstream from the ATG start codon), whereas in the atbrca2b-1 allele of the AtBRCA2b gene, the transposon is inserted within the 5’ untranslated region of the first exon of the AtBRCA2b gene (91 bp upstream of the ATG; see Supplementary Fig. S1A, B at JXB online). Direct sequence analysis of PCR products revealed that the Ds insertions had not introduced any deletions or other modifications in the AtBRCA2a and AtBRCA2b genes.
Since AtBRCA2a and AtBRCA2b encode proteins that are 94.5% identical to each other, and these genes are probably the result of a recent duplication (Siaud et al., 2004), it is possible that the two proteins play a redundant role in Arabidopsis. To investigate redundancy of the two AtBRCA2 genes, genetic crosses between the two homozygous single mutants were performed to generate double mutants.
atbrca2 mutants are associated with hypersensitivity to DNA damaging agents
Previous studies in vertebrates have indicated that hypersensitivity to genotoxic regents such as cisplatin and γ-irradiation is a consistent feature of mutants deficient in HR repair (Liu et al., 1998; Takata et al., 2000; Sasaki et al., 2004). Therefore, it was thought likely that atbrca2 mutants would be more sensitive than wild-type plants to genotoxic agents. To test this hypothesis, sensitivity to cisplatin and γ-irradiation in atbrca2 mutants was examined. γ-Irradiation is known to induce DSBs directly (Dizdaroglu and Bergtold, 1986). Cisplatin (cis-diaminedichloroplatinum-II) is a cross-linking reagent that forms DNA intra- and inter-strand cross-links (Zamble and Lippard, 1995). Inter-strand cross-links are repaired exclusively by HR, and not by NHEJ (Essers et al., 2000; De Silva et al., 2002; Sasaki et al., 2004). In this context, these sensitivity tests can provide evidence for the role of AtBRCA2a and AtBRCA2b in HR processes.
The sensitivity to cisplatin of both single mutants, atbrca2a-1 and atbrca2b-1, and the atbrca2a-1/atbrca2b-1 double mutant was tested first. Cisplatin sensitivity was scored based on the production of true leaves on MS agar medium containing cisplatin. This assay relies on the fact that the embryo of the mature Arabidopsis seed has already produced two fully formed cotyledons but has not yet initiated true leaves (Jiang et al., 1997).
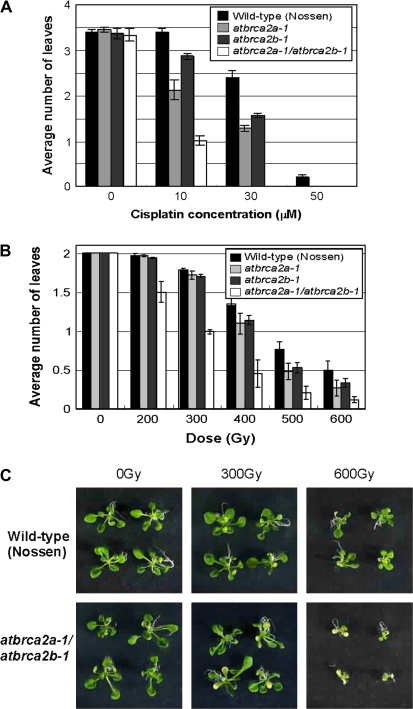
Both single mutants were hypersensitive to cisplatin compared to wild-type (Nossen) (Fig. 1A). Moreover, the atbrca2a-1/atbrca2b-1 double mutant showed an additive increase in sensitivity to cisplatin treatment compared with that of the corresponding single mutants and wild-type plants (Fig. 1A). The effect was particularly apparent following treatment with 30 μM cisplatin, when the number of true leaves appearing in the double mutant plant was close to zero, single mutant plants produced an average of about 1.2–1.5 leaves, and wild-type plants about 2.4 leaves (Fig. 1A).
Fig. 1.
(A) Sensitivity to cisplatin of single mutants atbrca2a-1 and atbrca2b-1, and the double mutant atbrca2a-1/atbrca2b-1. Imbibed seeds were plated on MS agar medium containing 0–50 μM cisplatin. The numbers of true leaves in wild type (Nossen) and mutant plants were counted 14 d after plating. Data represent the mean ±SE of 50 plants in each group from three experiments. (B, C) Sensitivity of AtBRCA2 mutants to γ-irradiation. (B) Imbibed seeds (4 d at 4 °C) were irradiated with increasing doses of 60Co γ-rays. After γ-irradiation, the seeds were immediately plated on MS agar medium. The number of true leaves was counted 10 d after irradiation, and the average number of leaves was calculated according to Harlow et al. (1994). Data represent the mean ±SE of 34 plants in each group from three experiments. (C) Fourteen-day-old plantlets [upper panels: wild type (Nossen), lower panels: atbrca2a-1/atbrca2b-1 double mutant plants] after γ-irradiation (0, 300, 600 Gy).
Next, to confirm cosegregation of AtBRCA2a or AtBRCA2b genotypes with sensitivity to cisplatin, the sensitivity to cisplatin of plants heterozygous for one or both of the AtBRCA2a and AtBRCA2b genes was tested. Accordingly, it was confirmed that sensitivity to cisplatin in AtBRCA2a-1+/–/AtBRCA2b-1–/– heterozygous plants (a-1+/–/b-1–/–) was the same as that in atbrca2b single mutant plants (a-1+/+/b-1–/–) (see Supplementary Fig. S2 at JXB online). Similarly, sensitivity to cisplatin in AtBRCA2a-1–/–/AtBRCA2a-1+/– heterozygous plants (a-1–/–/b-1+/–) was the same as that in atbrca2a single mutant plants (a-1–/–/b-1+/+) (see Supplementary Fig. S2 at JXB online). These results strongly suggest that atbrca2a-1 and atbrca2b-1 are recessive mutants, that the mutant phenotype is caused by the reduced activity of AtBRCA2a or AtBRCA2b products, and that both AtBRCA2a and AtBRCA2b are involved in DSB repair in somatic cells.
To confirm that the Ds insertion was responsible for the observed phenotypes of the brca2a-1 and brca2b-1 mutants, an RNAi construct designed to silence both genes simultaneously was cloned. This RNAi construct was placed under the control of a constitutive plant promoter (CaMV 35S promoter). Most plants transformed by the p35S::AtBRCA2–RNAi construct survived (95%), and exhibited a partially sterile phenotype under standard growth conditions. The sensitivity to cisplatin of AtBRCA2–RNAi plants was tested. AtBRCA2–RNAi plants show increased sensitivity to cisplatin compared to wild-type (Col.) (see Supplementary Fig. S2 at JXB online). This result strongly supports the hypothesis that the cisplatin sensitivity of these mutants is associated with the Ds insertion.
The sensitivity of atbrca2a-1, atbrca2b-1, and atbrca2a-1/atbrca2b-1 mutants to γ-irradiation was examined next. Seeds were irradiated with γ-rays as described in a previous study (Osakabe et al., 2005). Prior to γ-irradiation, seeds were imbibed in water for 4 d at 4 °C. The seeds were then irradiated with 60Co γ-rays at doses ranging from 100 to 700 Gy and immediately plated on MS agar medium. Four days after irradiation, germination rates were measured. Germination rates after each dose of γ-irradiation varied from 90–100% for wild type (Nossen), atbrca2a-1, atbrca2b-1, and atbrca2a-1/atbrca2b-1 plants. Ten days after γ-irradiation, the plants were scored for the appearance of true leaves. Following doses of γ-irradiation of 400–600 Gy, both atbrca2a-1 and atbrca2b-1 were hypersensitive to γ-irradiation compared to wild-type (Nossen) regarding the formation of new leaves (Fig. 1B). Moreover, the atbrca2a-1/atbrca2b-1 double mutant showed an additive increase in sensitivity to γ-irradiation compared to that of the single mutants (Fig. 1B, C). These data support the results of our sensitivity to cisplatin test.
Morphology of atbrca2a-1, atbrca2b-1, and atbrca2a-1/atbrca2b-1 mutants
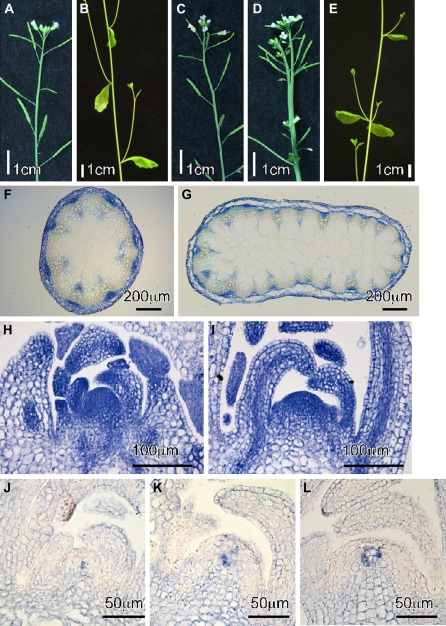
Both atbrca2a-1 and atbrca2b-1 single mutant and homozygous atbrca2a-1/atbrca2b-1 double mutant plants showed substantially normal vegetative development when compared to wild-type plants (Nossen) (cf. Fig. 2A, C), although abnormal phyllotaxy was occasionally observed (Figs 2B, D, 3). In addition, these mutant plants were fertile. By contrast, Siaud et al. (2004) reported that most plants transformed with an AtBRCA2–RNAi construct under the control of the DMC1 promoter were affected in their fertility (Siaud et al., 2004). Although at first glance this report appears inconsistent with our observations, if our mutants are partially deficient mutants, it is possible for the atbrca2a-1/atbrca2b-1 double mutant to be partially fertile. However, all our atbrca2 single and double mutants were as fertile as the wild type (data not shown). Next, to assess pollen grain viability, anthers were dissected from wild-type (Nossen) and mutant flower buds and stained with Alexander's solution (Alexander, 1969). As shown in Supplementary Table S1 and Fig. S3A at JXB online, more than 95% of wild-type anthers were full of red-coloured, viable pollen grains. On the other hand, although about 80% of double mutant anthers were similar to that of the wild type, about 20% of anthers in double mutant plants contained many green-coloured, non-viable pollen grains (see Supplementary Fig. S3B at JXB online). These results suggested that our atbrca2a-1/atbrca2b-1 double mutant is partially defective in male gamete development, but that this defect does not affect overall fertility.
Fig. 2.
Morphological phenotypes of atbrca2 mutants. (A, B) Five-week-old wild-type plants (Nossen), (C, D, E) atbrca2a-1/atbrca2b-1 double mutant plants. (F, G) Cross-sections of stems from 5-week-old plants: wild type (Nossen) (F) and atbrca2a-1/atbrca2b-1 double mutant (G). (H, I) Longitudinal-sections of 2-week-old plants: wild type (Nossen) (H) and atbrca2a-1/atbrca2b-1 double mutant plants (I). (J, K, L) In situ hybridization analysis of the AtWUS gene in wild-type (Nossen) (J) and atbrca2a-1/atbrca2b-1 double mutant (K, L) plants.
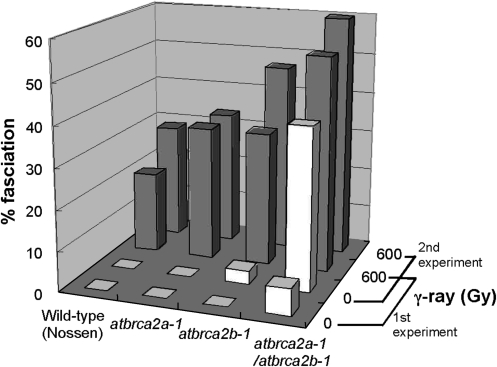
Fig. 3.
γ-irradiation induces fasciation in wild-type (Nossen), atbrca2a-1 and atbrca2b-1 single mutants, and the atbrca2a-1/atbrca2b-1 double mutant.
Homozygous atbrca2a-1/atbrca2b-1 plants grew a little slower than the wild type (Nossen) (data not shown), and showed stem fasciation (flattened and thick stems and fused organs; Fig. 2D, G) and/or abnormal phyllotaxy (resulting in an irregular branching pattern; Fig. 2E) in approximately 5–40% of the double mutant plants. Figure 2G shows a cross-section of the fasciated stem of the double mutant. The fasciation phenotype represents disintegration in the pattern of organogenesis and is associated with an enlargement of the shoot apical meristem (SAM) (Leyser and Furner, 1992). In double mutant plants, the SAM occasionally developed into an enlarged mass compared to wild-type (Fig. 2H, I). Fertility of the double mutant plant was similar to that of the wild type and corresponding single mutant plants.
γ-Ray-induced fasciation phenotype in atbrca2 mutants
The long-term effects of γ-irradiation on development in atbrca2 mutants transplanted in soil were investigated further. After high doses of γ-irradiation, although seedling growth of the wild type (Nossen), and atbrca2 single and double mutants was retarded (Fig, 1B, C), all these seedlings were able to survive and growth of these plants recovered (approximately 40–70% of control). One month after irradiation, shoot meristems underwent a transition from vegetative to inflorescence development, and the plants formed stems and floral meristems. It was found that γ-irradiation (600 Gy) accelerated the stem fasciation phenotype (including abnormal phyllotaxy) in atbrca2a-1 and atbrca2b-1 single mutants and in the double mutant plants (Nossen) (Fig. 3). Most interestingly, these phenotypes were also induced by γ-irradiation in wild-type plants (Nossen) (Fig. 3). Without γ-irradiation, the double mutant exhibited stem fasciation in 6.6% and 40% of plants transplanted to soil in the first and second experiments, respectively. Thus, the proportion of plants exhibiting stem fasciation differed markedly between the first and second experiments. Although every attempt was made to grow all the Arabidopsis plants in both experiments under identical conditions, even with sophisticated environmental control systems, subtle variations in conditions may occur. It is possible that such slight variations could have an effect on the ratio of plants that show stem fasciation. When imbibed seeds of the double mutant plants were irradiated with a 600 Gy dose of γ-rays, the frequency of stem fasciation increased by more than 20%. The frequency of stem fasciation was further increased by irradiation with a γ-ray dose of 700 Gy (data not shown). Thus, the proportion of plants showing stem fasciation increased with γ-irradiation in a dose-dependent manner. Each single mutant also showed stem fasciation at a low frequency without γ-irradiation, while γ-irradiation induced stem fasciation. Importantly, in wild-type plants, the frequency of stem fasciation was very low (<0.1%) under the growth conditions used, but these phenotypes were induced by γ-irradiation. In this study, the Nossen ecotype was used as a control plant since the mutant background is the Nossen ecotype. Imbibed seeds of several other ecotypes were also irradiated, and it was found that Nossen was one of ecotypes with relatively higher sensitivity to γ-irradiation. Ecotype Columbia was less sensitive to γ-irradiation than the other ecotypes (data not shown). These data suggest that the fasciation phenotypes in the atbrca2 single and double mutants could be linked to sensitivity to γ-irradiation.
To elucidate the relationship between the fasciation phenotype and the sensitivity to γ-irradiation further in the atbrca2a-1/atbrca2b-1 double mutant, in situ hybridization analysis of the WUSCHEL (WUS) gene was performed with wild-type (Nossen) and atbrca2a-1/atbrca2b-1 plants. WUS is one of the regulatory factors required for maintenance of stem cell identity, and the WUS gene is expressed in a small group of cells in the centre of the SAM (Mayer et al., 1998). It was found that the region in which the WUS gene is expressed expanded laterally but not uniformly in double mutant plants (Fig. 2K, L). Occasionally, the WUS expression region was also shifted to the peripheral zone in the double mutant. These observations suggested that the defect in DSB repair in the atbrca2a/atbrca2b double mutant might lead to SAM disorganization via ectopic expression of the WUS gene.
Cell cycle regulation in atbrca2 mutants
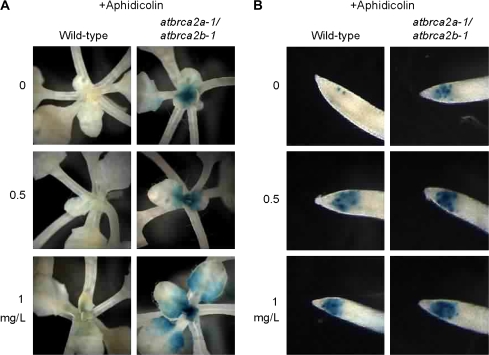
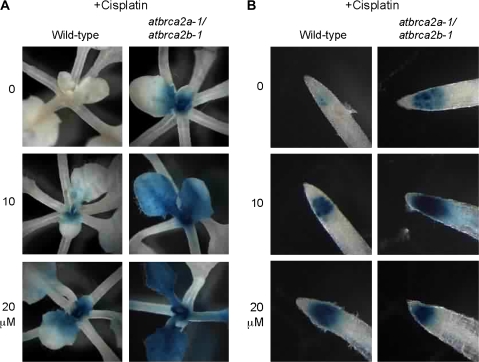
Previous studies have indicated that cell cycle control is essential for normal development of the apical meristem in plants (Endo et al., 2006; Andersen et al., 2008). To study the relationship between disorganization of meristem structure and cell cycle progression, especially tissue-specific effects, expression of the AtCYCB1;1::GUS repoter gene was examined. In this fusion construct, transcription of the GUS gene is driven by a AtCYCB1;1 promoter, and GUS protein degradation is controlled by the Cyclin B mitotic destruction sequence (Colon-Carmona et al., 1999). This GUS fusion protein accumulates on entry into G2, and is degraded just before anaphase during mitosis. Thus, products of this GUS fusion gene accumulate from late G2 until M phase (Colon-Carmona et al., 1999; Culligan et al., 2004, 2006). Arabidopsis Col plants transformed with AtCYCB1;1::GUS (Colon-Carmona et al., 1999) were crossed with the atbrca2a-1/atbrca2b-1 double mutant. As shown in Figs 4 and 5, cells expressing GUS activity appeared sporadically only in the root tip of wild-type plants without any treatment. By contrast, significantly large numbers of cells in the shoot apex, young leaves, and root tips of the atbrca2a-1/atbrca2b-1 double mutant showed strong GUS activity (Figs 4, 5). These results suggest that disorganization of the meristem structure is correlated with the defect in cell cycle progression in the atbrca2a-1/atbrca2b-1 double mutant. In addition, AtBRCA2–RNAi construct was transformed to Arabidopsis Col plants with AtCYCB1;1::GUS. AtBRCA2–RNAi plants show strong GUS activity in the shoot apex, young leaves, and root tips (see Supplementary Fig. S4 at JXB online). This result supports the hypothesis that the strong expression of AtCYCB1;1 in meristematic tissues of the double mutant is associated with the Ds insertion.
Fig. 4.
Histochemical assay of the AtCYCB1;1::GUS reporter gene (Colon-Carmona et al., 1999) in 7-d-old wild-type and atbrca2a-1/atbrca2b-1 mutant seedlings with or without aphidicolin (A, B). GUS activity was detected after 3 d of treatment with aphidicolin. (A) GUS staining of SAMs and leaves. (B) GUS staining of root tips.
Fig. 5.
Histochemical assay of the AtCYCB1;1::GUS reporter gene (Colon-Carmona et al., 1999) in 7-d-old wild-type and atbrca2a-1/atbrca2b-1 mutant seedlings with or without cisplatin (A, B). GUS activity was detected after 3 d of treatment with cisplatin. (A) GUS staining of SAMs and leaves. (B) GUS staining of root tips.
Next, the effect of the atbrca2 mutation on cell cycle regulation in response to aphidicolin was studied. Aphidicolin is an inhibitor of the replicative DNA polymerases δ and ϵ. As shown in Fig. 4, meristematic cells of shoots and roots in aphidicolin-treated wild-type and atbrca2a-1/atbrca2b-1 double mutant plants displayed a substantial increase in the number of AtCYCB1;1::GUS expressing cells (Fig. 4). This property is especially pronounced in the atbrca2a-1/atbrca2b-1 double mutant (Fig. 4). These results indicate that cell cycle progression of dividing cells in wild-type plants was delayed due to the replicational stress caused by aphidicolin, and that the brca2 mutation exacerbates this effect.
In addition, AtCYCB1;1 is strongly induced by DNA damaging agents (Culligan et al., 2006; Endo et al., 2006). The induction of AtCYCB1;1 in response to cisplatin in the atbrca2a-1/atbrca2b-1 double mutant compared to that of the wild type was also studied. As in the case of aphidicolin treatment, double mutant plants particularly displayed an increased level of AtCYCB1;1::GUS-expressing cells in SAMs (Fig. 5). These results suggested that AtCYCB1;1 is strongly induced by cisplatin in wild-type plants and that the AtBRCA2 mutation enhances this induction. As described above, cisplatin is a cross-linking reagent that forms DNA intra- and inter-strand cross-links (Zamble and Lippard, 1995). Inter-strand cross-links are repaired exclusively by HR, and not by NHEJ (Essers et al., 2000; De Silva et al., 2002; Sasaki et al., 2004). Particularly in the S phase of the cell cycle, inter-strand cross-links are repaired by HR using sister chromatids as homologous templates. The brca2 mutation may limit this step in plant meristems.
Discussion
Previously, Siaud et al. (2004) reported the role of AtBRCA2a and AtBRCA2b in meiotic recombination in Arabidopsis. However, the function of AtBRCA2 in HR repair, especially in somatic cells, was not determined. In this study, it has been shown that AtBRCA2a and AtBRCA2b are important for DSB repair in somatic cells. atbrca2a-1 and atbrca2b-1 single mutants exhibited hypersensitivity to genotoxic stresses compared to wild-type plants, and the atbrca2a-1/atbrca2b-1 double mutant showed an additive increase in sensitivity to genotoxic stresses compared to each single mutant (Fig 1). These results suggested that AtBRCA2a and AtBRCA2b play essential roles in DNA repair in somatic cells. Siaud et al. (2004) reported that most plants transformed with an AtBRCA2–RNAi construct under the control of a constitutive plant promoter (p35S) became bleached and died, thus indicating that AtBRCA2 genes are necessary for plant survival. On the other hand, our atbrca2a-1/atbrca2b-1 double mutation is not lethal. In addition, AtBRCA2–RNAi plants were obtained. Taken together, these observations suggest that the production and/or activity of AtBRCA2a and AtBRCA2b are partially deficient in our mutants and that the silencing of both BRCA2 genes also defective in our AtBRCA2–RNAi plants.
In addition, it was found that atbrca2 mutant plants displayed fasciation and abnormal phyllotaxy phenotypes. Bundock and Hooykaas (2002) identified mutants of the Arabidopsis MRE11 gene. One of these mutants, mre11-1, is hypersensitive to the alkylating reagent methyl methane sulphonate (MMS) and shows fasciation (Bundock and Hooykaas, 2002). On the other hand, mutants impaired in the NHEJ repair pathway [ku70 (Riha et al., 2002), ku80 (Friesner and Britt, 2003; Gallego et al., 2003; West et al., 2002), ligIV (Friesner and Britt, 2003; van Attikum et al., 2003)] do not cause fasciation phenotypes. Indeed, it was confirmed that abnormal phyllotaxy and stem fasciation were not increased in the ku80 mutant (WS ecotype background; West et al., 2002) compared to the wild type, with or without γ-irradiation. Thus, in contrast to the HR repair pathway, inefficient DSB repair via NHEJ does not seem to induce disorganization of apical meristem cells. Therefore, the fasciation phenotype induced by γ-irradiation may be associated with the type of repair system, and may also depend on the cell cycle because NHEJ repair occurs during G1 to early S-phase, and HR during late S to G2 phase (Essers et al., 2000; Couedel et al., 2004; Bleuyard et al., 2006).
Moreover, it was found that the ratio of plants displaying fasciation and abnormal phyllotaxy phenotypes increased upon γ-irradiation in the atbrca2 mutant. Interestingly, these phenotypes were also induced by γ-irradiation in wild-type plants (Nossen). These results suggest that the fasciation phenotypes in the atbrac2 single and double mutants could be linked to sensitivity to γ-irradiation. The clv1 and clv3 mutants of Arabidopsis are well known as showing stem fasciation. The CLAVATA3 (CLV3) gene encodes a putative ligand for a transmembrane receptor kinase, CLAVATA1 (CLV1). Reddy et al. (2005) reported that a CLV3 RNAi construct induced by dexamethasone also shows stem fasciation (Reddy and Meyerowitz, 2005). Such reports indicate that developmental programmes in plant SAMs can be changed by exogenous factors during development. This notion is in agreement with our results, which clearly demonstrate that developmental programmes in Arabidopsis SAMs are changed by γ-irradiation as an exogenous factor.
In our results, a large number of cells in the shoot apex, young leaves, and root tips of the atbrca2a-1/atbrca2b-1 double mutant showed strong GUS activity upon expression of AtCYCB1;1::GUS, whereas cells expressing GUS activity appeared sporadically only in the root tip of wild-type plants (Figs 4, 5). These results indicate that cell cycle progression is important for the regulation of the pattern of cell division and differentiation in plant development. Other studies support our results. For example, Arabidopsis fas1 and fas2 mutants show stem fasciation (Kaya et al., 2001)—FAS1 (CAF-1 p150) and FAS2 (CAF-1 p60) are subunits of the chromatin assembling factor complex (Smith and Stillman, 1989). The absence of CAF-1 could cause the delay or down-regulation of chromatin assembly. Delayed chromatin assembly might lead to a prolongation of the cell cycle at the S or G2/M phase. Recently, it was found that fas1 and fas2 mutants exhibited an increased level of DSBs and G2 phase retardation (Endo et al., 2006). Our report suggested that DSBs were induced during the late S to G2 phase of the cell cycle in these fas mutants. In teb mutants, whose causative gene encodes a homologue of Drosophila MUS308 and mammalian DNA polymeraseθ, morphological defects such as fasciation, serrated leaves, and short roots are observed (Inagaki et al., 2006). These mutants also show a defect in G2/M cell cycle progression. The mutation and suppression of condensin (AtCAP-E1 and AtCAP-E2), which is involved in chromatin condensation, also causes stem fasciation. Although the functions of AtCAP-E1 and AtCAP-E2 in DSB repair and cell cycle progression have not been determined (Siddiqui et al., 2003), studies in yeast indicate that condensin is required for the arrest caused by DNA replication inhibition, the activation of checkpoints, and DNA repair (Aono et al., 2002). Recently, Andersen et al. (2008) demonstrated that CDKB2;1 and CDKB2;2 are necessary both for cell cycle progression and for meristem organization. Plants transformed with microRNAs of both CDKB2;1 and CDKB2;2 genes show dwarfism, abnormal structure of the shoot meristem and phyllotaxis defects (Andersen et al., 2008). These results indicate directly that progression of the cell cycle is linked closely to the regulation of meristem organization in plants. Reidt et al. (2006) demonstrated that BARD1 plays an important role in the regulation of DNA repair in somatic cells in Arabidopsis (Reidt et al., 2006). Interestingly, Han et al. (2008) suggested that BARD1 regulates SAM organization and maintenance by limiting expression of the WUS gene in plants (Han et al., 2008). Although the latter authors did not discuss any relationship between SAM organization and the cell cycle, it is likely that cell cycle regulation is involved in the regulation of SAM organization by BARD1.
In conclusion, our results demonstrate that AtBRCA2a and AtBRCA2b are important for DSB repair in somatic cells. In addition, extrinsic DSBs and inefficient repair of DSBs induce an abnormal morphological phenotype in Arabidopsis, including stem fasciations. Moreover, disorganization of the meristem structure is connected to the defect in cell cycle progression in Arabidopsis plants.
Supplementary data
The following supplementary data relating to this study are available at JXB online.
Supplementary Fig. S1. Ds-transposon insertion sites of atbrca2 mutants.
Supplementary Fig. S2. Sensitivity to cisplatin of heterozygous plants of both AtBRCA2a and AtBRCA2b genes, and AtBRCA2–RNAi plants.
Supplementary Fig. S3. Anthers of wild-type (A) and atbrca2a-1/atbrca2b-1 double mutant (B) stained with Alexander's solution (Alexander, 1969).
Supplementary Fig. S4. Histochemical assay of the AtCYCB1;1::GUS reporter gene (Colon-Carmona et al., 1999) in 7-d-old wild-type, atbrca2a-1/atbrca2b-1 mutant and AtBRCA2–RNAi seedlings.
Supplementary Table S1. Pollen grain viability in atbrca2 mutants.
Acknowledgments
We thank Dr T Araki for providing the AtWUS cDNA; Dr CM Bray for providing Arabidopsis seeds of ku80; Dr P Doerner for CYCB1;1::GUS, and Dr S Takeda for critical comments on this manuscript. We also thank K Amagai, R Aoto, C Furusawa, E Ozawa, A Nagashii, and F Suzuki for their technical help. This work was supported by a Grant-in-Aid from PROBRAIN (Program for Promotion of Basic Research Activities for Innovative Biosciences) and by the Ministry of Agriculture, Forestry, and Fisheries of Japan. This study was also financially supported by the Budget for Nuclear Research of the Ministry of Education, Culture, Sports, Science, and Technology, based on the screening and counseling by the Atomic Energy Commission.
References
- Alexander MP. Differential staining of aborted and nonaborted pollen. Stain Technology. 1969;44:117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Andersen SU, Buechel S, Zhao Z, Ljung K, Novak O, Busch W, Schuster C, Lohmann JU. Requirement of B2-type cyclin-dependent kinases for meristem integrity in Arabidopsis thaliana. The Plant Cell. 2008;20:88–100. doi: 10.1105/tpc.107.054676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono N, Sutani T, Tomonaga T, Mochida S, Yanagida M. Cnd2 has dual roles in mitotic condensation and interphase. Nature. 2002;417:197–202. doi: 10.1038/417197a. [DOI] [PubMed] [Google Scholar]
- Bleuyard JY, Gallego ME, White CI. Recent advances in understanding of the DNA double-strand break repair machinery of plants. DNA Repair. 2006;5:1–12. doi: 10.1016/j.dnarep.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Bundock P, Hooykaas P. Severe developmental defects, hypersensitivity to DNA-damaging agents, and lengthened telomeres in Arabidopsis MRE11 mutants. The Plant Cell. 2002;14:2451–2462. doi: 10.1105/tpc.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P. Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. The Plant Journal. 1999;20:503–508. doi: 10.1046/j.1365-313x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Couedel C, Mills KD, Barchi M, et al. Collaboration of homologous recombination and nonhomologous end-joining factors for the survival and integrity of mice and cells. Genes and Development. 2004;18:1293–1304. doi: 10.1101/gad.1209204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan K, Tissier A, Britt A. ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. The Plant Cell. 2004;16:1091–1104. doi: 10.1105/tpc.018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan KM, Robertson CE, Foreman J, Doerner P, Britt AB. ATR and ATM play both distinct and additive roles in response to ionizing radiation. The Plant Journal. 2006;48:947–961. doi: 10.1111/j.1365-313X.2006.02931.x. [DOI] [PubMed] [Google Scholar]
- De Silva IU, McHugh PJ, Clingen PH, Hartley JA. Defects in interstrand cross-link uncoupling do not account for the extreme sensitivity of ERCC1 and XPF cells to cisplatin. Nucleic Acids Research. 2002;30:3848–3856. doi: 10.1093/nar/gkf479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M, Bergtold DS. Characterization of free radical-induced base damage in DNA at biologically relevant levels. Analytical Biochemistry. 1986;156:182–188. doi: 10.1016/0003-2697(86)90171-5. [DOI] [PubMed] [Google Scholar]
- Dray E, Siaud N, Dubois E, Doutriaux MP. Interaction between Arabidopsis Brca2 and its partners Rad51, Dmc1, and Dss1. Plant Physiology. 2006;140:1059–1069. doi: 10.1104/pp.105.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Ishikawa Y, Osakabe K, et al. Increased frequency of homologous recombination and T-DNA integration in Arabidopsis CAF-1 mutants. EMBO Journal. 2006;25:5579–5590. doi: 10.1038/sj.emboj.7601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M, West SC. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature. 2005;434:598–604. doi: 10.1038/nature03404. [DOI] [PubMed] [Google Scholar]
- Essers J, van Steeg H, de Wit J, Swagemakers SM, Vermeij M, Hoeijmakers JH, Kanaar R. Homologous and non-homologous recombination differentially affect DNA damage repair in mice. EMBO Journal. 2000;19:1703–1710. doi: 10.1093/emboj/19.7.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff NV, Smith DL. A versatile system for detecting transposition in Arabidopsis. The Plant Journal. 1993;3:273–289. doi: 10.1111/j.1365-313x.1993.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Friesner J, Britt AB. Ku80- and DNA ligase IV-deficient plants are sensitive to ionizing radiation and defective in T-DNA integration. The Plant Journal. 2003;34:427–440. doi: 10.1046/j.1365-313x.2003.01738.x. [DOI] [PubMed] [Google Scholar]
- Galkin VE, Esashi F, Yu X, Yang S, West SC, Egelman EH. BRCA2 BRC motifs bind RAD51-DNA filaments. Proceedings of the National Academy of Sciences, USA. 2005;102:8537–8542. doi: 10.1073/pnas.0407266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego ME, Bleuyard JY, Daoudal-Cotterell S, Jallut N, White CI. Ku80 plays a role in non-homologous recombination but is not required for T-DNA integration in Arabidopsis. The Plant Journal. 2003;35:557–565. doi: 10.1046/j.1365-313x.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- Han P, Li Q, Zhu YX. Mutation of Arabidopsis BARD1 causes meristem defects by failing to confine WUSCHEL expression to the organizing center. The Plant Cell. 2008;20:1482–1493. doi: 10.1105/tpc.108.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow GR, Jenkins ME, Pittalwala TS, Mount DW. Isolation of uvh1, an Arabidopsis mutant hypersensitive to ultraviolet light and ionizing radiation. The Plant Cell. 1994;6:227–235. doi: 10.1105/tpc.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki S, Suzuki T, Ohto MA, Urawa H, Horiuchi T, Nakamura K, Morikami A. Arabidopsis TEBICHI, with helicase and DNA polymerase domains, is required for regulated cell division and differentiation in meristems. The Plant Cell. 2006;18:879–892. doi: 10.1105/tpc.105.036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Motohashi R, Kuromori T, Noutoshi Y, Seki M, Kamiya A, Mizukado S, Sakurai T, Shinozaki K. A resource of 5814 dissociation transposon-tagged and sequence-indexed lines of Arabidopsis transposed from start loci on chromosome 5. Plant and Cell Physiology. 2005;46:1149–1153. doi: 10.1093/pcp/pci112. [DOI] [PubMed] [Google Scholar]
- Jiang CZ, Yen CN, Cronin K, Mitchell D, Britt AB. UV- and gamma-radiation sensitive mutants of Arabidopsis thaliana. Genetics. 1997;147:1401–1409. doi: 10.1093/genetics/147.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- Kaya H, Shibahara KI, Taoka KI, Iwabuchi M, Stillman B, Araki T. FASCIATA genes for chromatin assembly factor-1 in arabidopsis maintain the cellular organization of apical meristems. Cell. 2001;104:131–142. doi: 10.1016/s0092-8674(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Kouchi H, Hata S. Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Molecular and General Genetics. 1993;238:106–119. doi: 10.1007/BF00279537. [DOI] [PubMed] [Google Scholar]
- Kuromori T, Hirayama T, Kiyosue Y, Takabe H, Mizukado S, Sakurai T, Akiyama K, Kamiya A, Ito T, Shinozaki K. A collection of 11 800 single-copy Ds transposon insertion lines in Arabidopsis. The Plant Journal. 2004;37:897–905. doi: 10.1111/j.1365.313x.2004.02009.x. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Furner IJ. Characterisation of three shoot apical meristem mutants of Arabidopsis thaliana. Development. 1992;116:397–403. [Google Scholar]
- Liu N, Lamerdin JE, Tebbs RS, et al. XRCC2 and XRCC3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Molecular Cell. 1998;1:783–793. doi: 10.1016/s1097-2765(00)80078-7. [DOI] [PubMed] [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Osakabe K, Abe K, Yamanouchi H, et al. Arabidopsis Rad51B is important for double-strand DNA breaks repair in somatic cells. Plant Molecular Biology. 2005;57:819–833. doi: 10.1007/s11103-005-2187-1. [DOI] [PubMed] [Google Scholar]
- Reddy GV, Meyerowitz EM. Stem-cell homeostasis and growth dynamics can be uncoupled in the Arabidopsis shoot apex. Science. 2005;310:663–667. doi: 10.1126/science.1116261. [DOI] [PubMed] [Google Scholar]
- Reidt W, Wurz R, Wanieck K, Chu HH, Puchta H. A homologue of the breast cancer-associated gene BARD1 is involved in DNA repair in plants. EMBO Journal. 2006;25:4326–4337. doi: 10.1038/sj.emboj.7601313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha K, Watson JM, Parkey J, Shippen DE. Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO Journal. 2002;21:2819–2826. doi: 10.1093/emboj/21.11.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki MS, Takata M, Sonoda E, Tachibana A, Takeda S. Recombination repair pathway in the maintenance of chromosomal integrity against DNA interstrand crosslinks. Cytogenetic Genome Research. 2004;104:28–34. doi: 10.1159/000077463. [DOI] [PubMed] [Google Scholar]
- Sharan SK, Morimatsu M, Albrecht U, Lim DS, Regel E, Dinh C, Sands A, Eichele G, Hasty P, Bradley A. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature. 1997;386:804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- Siaud N, Dray E, Gy I, Gerard E, Takvorian N, Doutriaux MP. Brca2 is involved in meiosis in Arabidopsis thaliana as suggested by its interaction with Dmc1. EMBO Journal. 2004;23:1392–1401. doi: 10.1038/sj.emboj.7600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui NU, Stronghill PE, Dengler RE, Hasenkampf CA, Riggs CD. Mutations in Arabidopsis condensin genes disrupt embryogenesis, meristem organization and segregation of homologous chromosomes during meiosis. Development. 2003;130:3283–3295. doi: 10.1242/dev.00542. [DOI] [PubMed] [Google Scholar]
- Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Takata M, Sasaki MS, Sonoda E, Fukushima T, Morrison C, Albala JS, Swagemakers SM, Kanaar R, Thompson LH, Takeda S. The Rad51 paralog Rad51B promotes homologous recombinational repair. Molecular and Cellular Biology. 2000;20:6476–6482. doi: 10.1128/mcb.20.17.6476-6482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavtigian SV, Simard J, Rommens J, et al. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nature Genetics. 1996;12:333–337. doi: 10.1038/ng0396-333. [DOI] [PubMed] [Google Scholar]
- Thompson LH, Schild D. Homologous recombinational repair of DNA ensures mammalian chromosome stability. Mutation Research. 2001;477:131–153. doi: 10.1016/s0027-5107(01)00115-4. [DOI] [PubMed] [Google Scholar]
- van Attikum H, Bundock P, Overmeer RM, Lee LY, Gelvin SB, Hooykaas PJ. The Arabidopsis AtLIG4 gene is required for the repair of DNA damage, but not for the integration of Agrobacterium T-DNA. Nucleic Acids Research. 2003;31:4247–4255. doi: 10.1093/nar/gkg458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veelen LR, Essers J, van de Rakt MW, Odijk H, Pastink A, Zdzienicka MZ, Paulusma CC, Kanaar R. Ionizing radiation-induced foci formation of mammalian Rad51 and Rad54 depends on the Rad51 paralogs, but not on Rad52. Mutation Research. 2005;574:34–49. doi: 10.1016/j.mrfmmm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- West CE, Waterworth WM, Story GW, Sunderland PA, Jiang Q, Bray CM. Disruption of the Arabidopsis AtKu80 gene demonstrates an essential role for AtKu80 protein in efficient repair of DNA double-strand breaks in vivo. The Plant Journal. 2002;31:517–528. doi: 10.1046/j.1365-313x.2002.01370.x. [DOI] [PubMed] [Google Scholar]
- Wong AK, Pero R, Ormonde PA, Tavtigian SV, Bartel PL. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2. Journal of Biological Chemistry. 1997;272:31941–31944. doi: 10.1074/jbc.272.51.31941. [DOI] [PubMed] [Google Scholar]
- Yu VP, Koehler M, Steinlein C, Schmid M, Hanakahi LA, van Gool AJ, West SC, Venkitaraman AR. Gross chromosomal rearrangements and genetic exchange between nonhomologous chromosomes following BRCA2 inactivation. Genes and Development. 2000;14:1400–1406. [PMC free article] [PubMed] [Google Scholar]
- Zamble DB, Lippard SJ. Cisplatin and DNA repair in cancer chemotherapy. Trends in Biochemical Science. 1995;20:435–439. doi: 10.1016/s0968-0004(00)89095-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.