Abstract
Tubulin genes are intimately associated with cell division and cell elongation, which are central to plant secondary cell wall development. However, their roles in pollen tube polar growth remain elusive. Here, a TUA1 gene from Picea wilsonii, which is specifically expressed in pollen, was isolated. Semi-quantitative RT-PCR analysis showed that the amount of PwTUA1 transcript varied at each stage of growth of the pollen tube and was induced by calcium ions and boron. Transient expression analysis in P. wilsonii pollen indicated that PwTUA1 improved pollen germination and pollen tube growth. The pollen of transgenic Arabidopsis overexpressing PwTUA1 also showed a higher percentage of germination and faster growth than wild-type plants not only in optimal germination medium, but also in medium supplemented with elevated levels of exogenous calcium ions or boron. Immunofluorescence and electron microscopy showed α-tubulin to be enriched and more vesicles accumulated in the apex region in germinating transgenic Arabidopsis pollen compared with wild-type plants. These results demonstrate that PwTUA1 up-regulated by calcium ions and boron contributes to pollen tube elongation by altering the distribution of α-tubulin and regulating the deposition of pollen cell wall components during the process of tube growth. The possible role of PwTUA1 in microtubule dynamics and organization was discussed.
Keywords: Microtubules, Picea wilsonii, pollen tube, PwTUA1, transgenic Arabidopsis, α-tubulin
Introduction
Three classes of fibres comprise the cytoskeleton of eukaryotic cells: microtubules (MTs), intermediate filaments, and microfilaments (MFs) (Kost and Chua, 2002). As an important component of the cytoskeleton of eukaryotic cells, MTs are involved in many crucial functions during cellular processes (Mayer and Jurgens, 2002), such as the maintenance of cell shape, cell motility, intracellular transport, cell division, and expansion (Mayer and Jurgens, 2002). Unusual MT arrays are found in cells undergoing tip growth such as pollen tubes, root hairs, and other specialized cells such as sperm cells (Pierson and Cresti, 1992). MT arrays of pollen tubes are approximately parallel to the direction of growth (Derksen et al., 1985; Lancelle et al., 1987; Raudaskoski et al., 1987) and persist during pollen tube growth, with new tubulin continuously added at nucleation sites near the tube apex (Heslop-Harrison and Heslop-Harrison, 1988). Recent identification of pollen-specific MT motor kinesin (Cai et al., 2000; Romagnoli et al., 2003) and MT-associated protein (Huang et al., 2007) indicates that MTs may play a role, with the participation of actin filaments, in directing vesicle and organelle transport and the deposition of pollen cell wall components, and maintaining the shape of the pollen tube during pollen tube growth (Cai et al., 2005). However, the role of MTs in pollen development remains elusive (Oakley et al., 2007), and is poorly understood compared with the function of MFs.
The α-tubulin (TUA) and β-tubulin (TUB) proteins, identified as the primary components of MTs (Weisenberg et al., 1968), are continually reassembling according to developmental cues and environmental signals (Wasteneys, 2004). Recent experimental evidence indicates that post-translational modifications of the tubulin building blocks contribute to the functional diversity of MTs (Hammond, 2008). In most plants, tubulins are encoded by multigene families, and tubulin genes are generally regarded as housekeeping genes. However, recent research shows that the function of individual tubulin genes is distinct within species, organs, and even cells, and the existence of pollen-specific tubulin genes has been demonstrated in several species (Carpenter et al., 1992; Cheng et al., 2001; Yoshikawa et al., 2003; Oakley et al., 2007), suggesting an important role in pollen tube growth. For example, AtTUA1 is expressed preferentially in pollen (Carpenter et al., 1992) among the six α-tubulin genes of Arabidopsis thaliana (Kopczak et al., 1992), while the other five are expressed in vegetative tissues (Kopczak et al., 1992; Abe et al., 2004). Of the nine Arabidopsis β-tubulin genes, AtTUB9 accumulates specifically in pollen (Carpenter et al., 1992; Cheng et al., 2001). Rice (Oryza sativa) also contains one pollen-specific isoform (OsTUB8) and seven other TUB genes expressed variably during development (Yoshikawa et al., 2003). Eight α-tubulin and 20 β-tubulin genes are expressed in the woody perennial Populus tremuloides Michx., of which PtTUA6 and PtTUA8 are abundant only in pollen (Oakley et al., 2007). However, further functional analysis of tubulin genes and proteins in plant pollen tubes is lacking.
Vegetative pollen tube growth occurs only at the apex of the cell through the fusion of vesicles to the apical plasma membrane. Simultaneously, an intricate MT array assembles in the vegetative cell, forming a network with actin, the endoplasmic reticulum, and the plasma membrane (Lancelle et al., 1987). The system in conifers differs from that of angiosperms in the growth rate of the pollen tube, the tendency to ramify, and the absence of a tip-to-base zonation of organelles (Win et al., 1996). To our knowledge, all of the pollen-specific tubulin genes identified are currently attributed to angiosperms, and the possible roles of these genes during pollen development have been mainly investigated in Arabidopsis. Gymnosperms, an economically important plant group, represent a major evolutionary divergence with respect to the development of the male gametophyte (Fernando et al., 2005). Little information exists about tubulin genes in gymnosperms, and whether current theories regarding angiosperms are valid remains questionable.
As part of a long-term aim to elucidate the complex functions of MTs during gymnosperm pollen development, mainly focusing on the roles of individual tubulin gene products in different MT arrays, here the characterization of a pollen-specific TUA from Picea wilsonii is reported. Semi-quantitative RT-PCR analysis revealed that the expression of PwTUA1 is up-regulated by calcium ions and boric acid treatments during pollen tube growth. Ectopic expression of PwTUA1 in Arabidopsis suggested that PwTUA1 not only improved pollen germination and pollen tube growth even in suboptimal pollen tube germination media, but also altered the subcellular localization of α-tubulin and the ultrastructure of the pollen tube. In addition, the possible functions of PwTUA1 are discussed.
Materials and methods
Plant material
Cones with mature pollen were collected in mid April 2007 from mature trees of P. wilsonii Mast. in the Beijing Botanical Garden of the Institute of Botany, the Chinese Academy of Sciences, and were dried overnight at room temperature. The dry pollen was stored at –80 °C until use.
In vitro P. wilsonii pollen germination
Picea wilsonii pollen grains stored at –80 °C were resuscitated by transfer to 4 °C for 12 h and then to room temperature for another 2 h. The resuscitated pollen was cultured in standard liquid medium for germination. The standard medium for pollen germination and tube growth contained 12% sucrose, 0.03% Ca(NO3)2, 0.01% H3BO3, and 5 mM citrate–phosphate buffer, pH 5.8. Pollen grains were incubated in small dishes at 25±1 °C in a saturated atmosphere (100% relative humidity) and sampled at 6, 12, 18, 24, 30, and 36 h after germination.
RNA extraction
For RNA isolation, the plant tissues were harvested separately, frozen in liquid nitrogen, and stored at –80 °C until use. Total RNA from germinating pollen was isolated using Trizol reagent (Gibco-BRL, Grand Island, NY, USA) according to the manufacturer's instructions. Total RNA from other tissues was extracted by the standard CTAB (cetyltrimethylammonium bromide) extraction and lithium chloride precipitation as described previously(Chang et al., 1993). The quality and quantity of RNA were assessed by agarose gel electrophoresis and UV spectroscopy, respectively.
Amplification and cloning of the PwTUA1 gene
Degenerate primers were designed based on conserved regions of TUA sequences from Pseudotsuga menziesii, Gossypium hirsutum, Oryza sativa, and A. thaliana (Table 1). Total RNA was isolated from P. wilsonii pollen after incubation (0, 6, 12, 18, 24, and 36 h) using Trizol reagent (Gibco-BRL). Reverse transcription of the pooled RNA was carried out with oligo(dT) primers using M-MLV reverse transcriptase (Promega) according to the manufacturer's instructions. Subsequently, PCR was performed with 35 cycles of 94 °C for 1 min, annealing at 68 °C for 1 min, and extension at 72 °C for 1 min in a Tgradient (Biometra). After sequencing the specific PCR fragment, 5′- and 3′-RACE (rapid amplification of cDNA ends) were performed to achieve full-length cDNA using the Gibco-BRL kit (Gibco-BRL).
Table 1.
The primers used in this study
| Primer | Purpose | Primer sequence(5′→3′) |
| F1 | Full-length cDNA | GATGCA(T/C)TTCA(T)ACACA(T/C)TTC(T)TTC(T)G |
| R1 | Full-length cDNA | AGGGCA(G)GCA(G/C)AGA(G)TCCTCACG |
| AAP | 5′-RACE | GGCCACGCGTCGACTAGTACG(G)14 |
| B26 | 3′-RACE | GACTCGAGTCGACATCGA(T)18 |
| R2 | 5′-RACE | GAACCGAGACCAGAACCAGTTCC |
| F2 | 3′-RACE | CTGGAACACACCGATGTGGC |
| PwTUA1F | Full-length cDNA | GGACGACGAGCTGAGCCCTTCG |
| PwTUA1R | Full-length cDNA | CAAAACACGCGTTTCATTTA |
| EF1-α5 | RT-PCR | AACTGGAGAAGGAACCCAAG |
| EF1-α3 | PT-PCR | AACGACCCAATGGAGGATAC |
| FW | RT-PCR | TGCATGATCTCCAATTCGAC |
| RW | PT-PCR | GCACTGTTCTCAACATGAAG |
Expression analysis
RNA from each tissue was treated with DNase I to remove genomic DNA, and then the cDNA was synthesized. RT-PCR was carried out with 30 cycles of denaturation, annealing, and extension steps for each sample. Gene primer pairs were designed to anneal near the 3’ end of each transcript [usually in the 3′-untranslated region (UTR)] to ensure primer specificity. An elongation factor-α gene (EF1-α) was used as a standard control in the RT-PCRs. The PCR experiment has been carried out at least three times. The forward and reverse primers are shown in Table1.
Particle bombardment-mediated transient expression in pollen and determination of pollen germination and tube growth
Mature pollen grains collected from P. wilsonii plants as described in ‘In vitro P. wilsonii pollen germination’ were used for transient expression using a particle bombardment procedure. Microprojectile bombardment was performed using a helium-driven PDS-1000/He biolistic system (Bio-Rad, Hercules, CA, USA). Tungsten particles (1.1 μm) were coated with plasmid DNA according to the manufacturer's recommendation (Bio-Rad) (Sanford et al., 1993). The pollen grains were then incubated for 12 h or 24 h before observation under a fluorescence microscope (Olympus BX50); 100 tubes were counted and measured for each sample. Pollen grains were considered to be germinated when the pollen tube length was greater than the diameter of the pollen grain (Rodriguez-Riano and Dafni, 2000). Fluorescence and transmitted light images were recorded using a fluorescence microscope (Olympus BX50). Fluorescence images used to analyse pollen tube length (using image analysis software: IMAGE) were taken with exposure times of <500 ms.
Arabidopsis transformation
The pBI121 binary vector containing 35S::PwTUA1 or Lat52::GFP was introduced into Agrobacteriun tumefaciens strain GV3101 and the wild-type Arabidopsis plants were transformed by floral dipping (Clough and Bent, 1998). The transgenic plants were screened on MS medium containing 50 μg ml−1 kanamycin. T0 transgenic Arabidopsis plants were identified by PCR to amplify the PwTUA1 gene with specific primers. The corresponding T1 transgenic seedlings that segregated at a ratio of ∼3:1 (resistant:sensitive) were selected to propagate T2 individuals, which were used for further analysis.
Arabidopsis pollen germination and tube growth measurement
Arabidopsis pollen grains were germinated in vitro and tube growth was measured using a modification of the method of Li et al. (1999).
Indirect immunofluorescence microscopy
For subcellular immunolocalization, the method described by Lin and Yang (1997) was used. Briefly, pollen tubes were incubated in fixative [2% paraformaldehyde in phosphate-buffered saline (PBS), comprising 10 mM phosphate buffer, pH 7.2, 138 mM NaCl, 2.7 mM KCl] for 1 h. After washing with PBS, pollen tubes were incubated at room temperature for 10 min in a solution of 1.5% cellulase R-10 and 1.2% macerozyme R-10 in 15 mM MES, pH 5.5, 400 mM mannitol, 5 mM CaCl2, and 1 mg ml−1 proteinase inhibitors (aprotinin, pepstatin A, chymostatin, and leupeptin; Calbiochem). After washing with PBS, pollen tubes were blocked with 3% bovine serum albumin (BSA) in PBS at room temperature for 1 h and then incubated at 4 °C overnight with purified anti-α-tubulin mouse monoclonal antibody (1:50 diluted with 1% BSA in PBS; Calbiochem). After washing in PBS containing 0.05% Triton X-100, pollen tubes were incubated with a secondary antibody (fluorescein isothiocyanate-conjugated, affinity-purified goat-mouse IgG) at 1:100 dilution in PBS containing 1% BSA for 1 h at 37 °C. As controls, pollen tubes were stained as above except for the omission of the primary antibody. Observations and photography were obtained with a confocal microscope (LSM 510 META, Zeiss).
Protein extraction and western blotting
Arabidopsis and P. wilsonii pollen grains were germinated in vitro. Proteins were extracted using the method of Poulter et al. (2008). Samples were measured using the Bio-Rad protein assay; equal amounts were loaded on a 12% SDS–polyacrylamide gel and separated by electrophoresis. For western blotting, the proteins were electrotransferred onto a nitrocellulose membrane (0.45 mm, Amersham Pharmacia Biotech Ltd). The membrane was blocked for 1 h at room temperature with 3% (w/v) BSA and 15% (v/v) calf serum in TRIS-buffered saline (TBS; 25 mM TRIS-HCl, pH 7.5, 137 mM NaCl, 5 mM KCl) and then incubated with gentle shaking for 2 h at room temperature in mouse anti-α-tubulin antibody diluted 1:500 (Calbiochem) in blocking buffer. After three washes of 5 min each in TBS, the membrane was incubated with alkaline phosphatase-conjugated secondary antibody raised against mouse IgG diluted 1:1000 in blocking buffer. The membrane was stained with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium.
Electron microscopy
For electron microscopy, pollen tubes were fixed for 2 h in 2.5% glutaraldehyde in 100 mM phosphate buffer (pH 7.2) with 2% (w/v) sucrose. Samples were washed with 100 mM phosphate buffer, post-fixed in 2% osmium tetroxide for 2 h, dehydrated in an ethyl-alcohol series, and finally embedded in Spur resin. Semi-thin sections were cut and the pollen tubes were localized. Sections were cut using an LKB-V ultromicrotome, and examined with a JEM-1200 electron microscope (JEOL Ltd, Tokyo, Japan).
Results
Isolation and characterization of the cDNA clone encoding α-tubulin from P. wilsonii
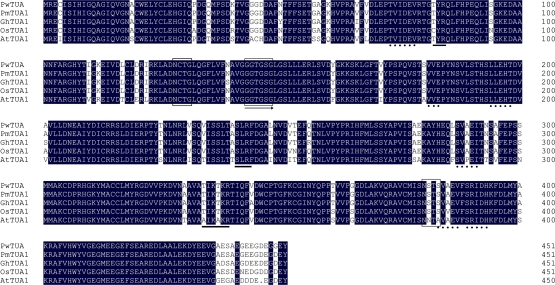
The TUA1 cDNA fragment was obtained from P. wilsonii by RT-PCR using primers designed according to conserved regions of known plant TUAs. After extension by 5′- and 3′-RACE-PCR, the complete open reading frame (ORF) was obtained. This gene was designated as PwTUA1 with the GenBank accession number EU268195. The full-length cDNA sequence was 1724 bp and contained an ORF of 1356 bp that tentatively encoded a PwTUA1 protein of 451 amino acids with a calculated mol. wt of 49.6 kDa and an isoelectric point (pI) of 4.97. A 207 bp 3′-UTR followed the stop codon (TGA) and a 161 bp 5′-UTR was upstream from the start codon (ATG). A BLAST search (http://www.ncbi.nlm.nih.gov/BLAST) and multialignment analysis revealed that PwTUA1 was highly related to other plant TUAs, sharing homology of 88.47% with AtTUA1, 97.78% with GhTUA1, 96.9% with OsTUA1, and 99.78% with PmTUA1 (Fig. 1). The predicted protein sequence contained a GTP nucleotide-binding site (GGGTGSG), which was conserved and necessary for the polymerization of α-/β-tubulins (Kirschner and Mitchison, 1986). An MRECI domain, implicated in autoregulated post-transcriptional mechanisms (Yen et al., 1988a, b; Cleveland, 1989), was found at the N-terminus of PwTUA1. Phosphorylated residues were found at R79 and K336, and an acetylated residue at K40. PwTUA1 contained a C-terminal glutamic acid residue (referred to as an E-type), which was different from the more typical α-tubulins that contain a tyrosine at the C-terminus.
Fig. 1.
Alignment of the deduced PwTUA1 protein sequence (EU268195) with other plant TUA1s. Amino acid sequences used in the analysis are from Pseudotsuga menziesii (PmTUA1, AAV92379), Gossypium hirsutum (GhTUA1, AY345603), Oryza sativa (OsTUA1, Os03g0726100), and Arabidopsis thaliana (AtTUA1, At1g64740). Identical amino acid residues in this alignment are shaded in black. Possible phosphorylation sites were obtained with a PROSITE motif search: boxed amino acids are N-glycosylation sites, underlined amino acids are protein kinase C phosphorylation sites, amino acids underlined with dotted lines are casein kinase II phosphorylation sites, and the arrow indicates tubulin subunits α, β, and γ signatures. (This figure is available in colour at JXB online.)
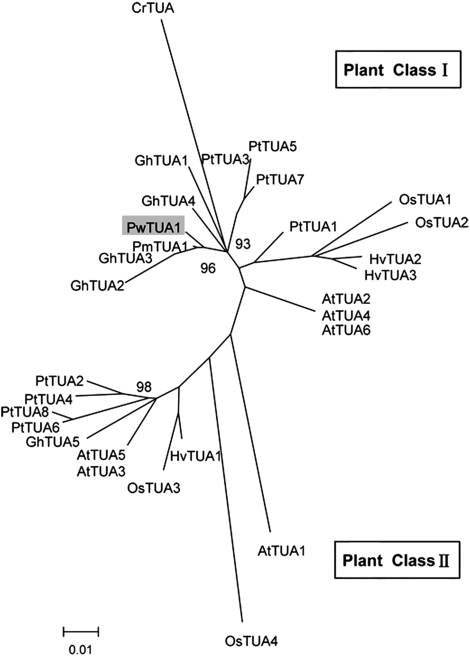
Phylogenetic analysis of TUAs
To elucidate the evolutionary relationships among TUA proteins, a phylogenetic tree was generated in which representative algal and plant full-length TUA proteins were analysed (Fig. 2). The bootstrap values for most nodes were low (data not shown) because of the high degree of sequence similarity. Plant TUAs make up two distinct classes. Although angiospermous TUAs exist in both class I and class II, the known gymnospermous TUAs are currently attributed to class I. Class I contains TUAs involved in secondary cell wall development, including PtTUA1 originating from Populus xylem and fibre-specific GhTUA2, GhTUA3, and GhTUA4 from cotton. Class I also includes a third distinct branch containing three Arabidopsis isoforms (AtTUA2/4/6). In class II, AtTUA1 (Carpenter et al., 1992), PtTUA6, and PtTUA8 (Oakley et al., 2007) were found to be preferentially expressed in pollen.
Fig. 2.
Phylogenetic tree of Picea wilsonii α-tubulin 1 (PwTUA1) and previously characterized α-tubulins. A Neighbor–Joining tree based on the deduced amino acid sequences of the TUAs. CrTUA was used as an outgroup. This bootstrap consensus tree was based on 1000 replicates. Numbers on nodes are bootstrap values. The accession numbers in GenBank and sources of the proteins are as follows: AtTUA1(At1g64740), AtTUA2 (At1g50010), AtTUA3 (At5g19770), AtTUA4 (At1g04820), AtTUA5 (At5g19780), and AtTUA6 (At4g14960) from Arabidopsis thaliana; CrTUA (AY182002) from Chlamydomonas reinhardtii; GhTUA1 (AY345603), GhTUA2(AY345604), GhTUA3 (AF106569), GhTUA4 (AY345605), and GhTUA5 (AF106571) from Gossypium hirsutum; HvTUA1 (X99623), HvTUA2 (Y08490), and HvTUA3 (AJ132399) from Hordeum vulgare; OsTUA1 (Os03g0726100), OsTUA2 (Os11g0247300), OsTUA3 (Os07g0574800), and OsTUA4 (Os03g0219300) from Oryza sativa; PmTUA1 (AY832610) from Pseudotsuga menziesii; and PtTUA1(AY229881), PtTUA2(AY229882), PtTUA3(EF583813), PtTUA4(EF584828), PtTUA5(EF583814), PtTUA6(EF583816), and PtTUA7(EF584829) from Populus tremuloides.
The expression of PwTUA1 is specific to pollen and is up-regulated by Ca2+ and boron
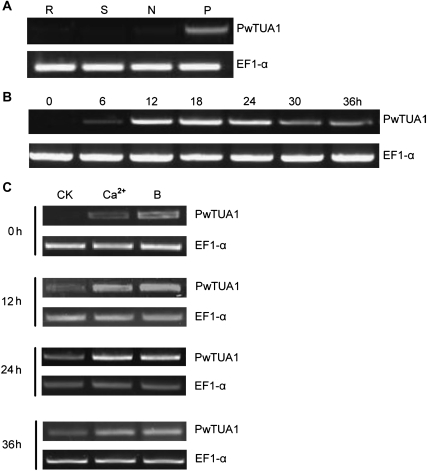
To obtain a profile of PwTUA1 expression patterns, total RNA was isolated from needles, stems, and roots, as well as from germinating P. wilsonii pollen mixtures incubated at 6 h intervals, and transcript accumulation was examined by semi-quantitative RT-PCR using gene-specific primers (Table 1). The RW primer was designed based on the 3′-UTR downstream from the stop codon, as this region was hypervariable. The results showed that PwTUA1 was expressed specifically in germinating pollen but was not detected in needles, stems, and roots (Fig. 3A). RT-PCR was next used to examine the expression of PwTUA1 during pollen development. As shown in Fig. 3B, the expression of PwTUA1 in pollen was first detected at 6 h after incubation (just germinated); it was abundant during the middle developmental stages (12, 18, and 24 h after incubation) and then continued weakly at later stages (30 h and 36 h after incubation).
Fig. 3.
Expression of PwTUA1 in different tissues of Picea wilsonii. (A) Tissue-specific expression of PwTUA1 in P. wilsonii. Total RNA was isolated from needles (N), stems (S), roots (R), and pollen (P) (incubated for 0, 6, 12, 18, and 24 h). (B) Expression of PwTUA1 in pollen at different growth stages. (C) PwTUA1 gene expression induced by 0.1% (w/v) Ca2+ and 0.1% (w/v) H3BO3 at different time points.
Boron was reported to have effects on pollen grain germination and pollen tube growth in Picea meyeri (Wang et al., 2003) and several angiosperm species (Dickinson, 1978). It was speculated that boron acts as an essential element in the induction or repression of PwTUA1 expression. Gymnosperm pollen germination and tube growth were previously reported to be severely inhibited by 0.1% H3BO3 (Wang et al., 2003) and 0.1% CaCl2 in the medium (Hao et al., 2005), respectively. Given that Ca2+ has been appreciated for many years as an essential requirement for pollen tube growth (Franklin-Tong, 1999), the level of PwTUA1 expression was evaluated during different pollen tube developmental stages in boron-stressed (H3BO3 concentration, 0.1%) and Ca2+-stressed medium (Ca2+ concentration, 0.1%). As shown in Fig. 3C, PwTUA1 was effectively induced by boron and Ca2+ during all phases tested. These results indicated that PwTUA1 was a boron/Ca2+-responsive gene that may reasonably be expected to contribute adaptively to pollen development including pollen germination, pollen tube growth, and orientation.
Transient expression of PwTUA1 improves pollen germination and pollen tube growth in P. wilsonii
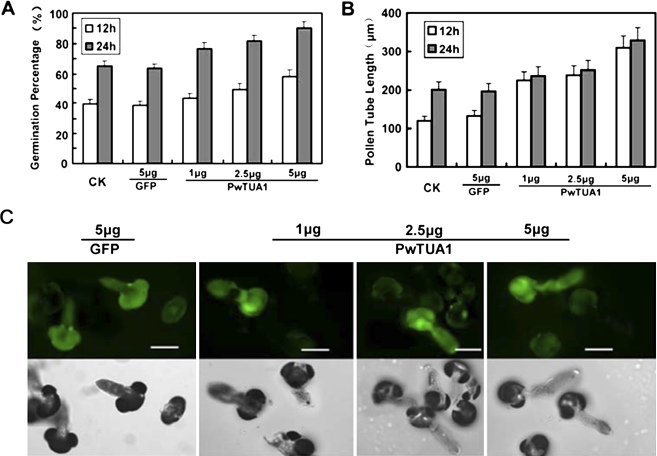
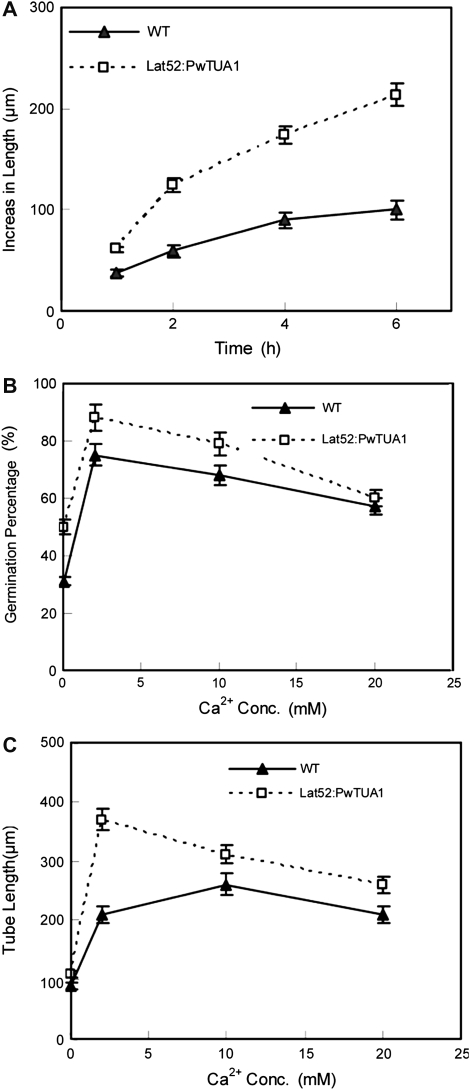
Microprojectile bombardment was performed to investigate the role of PwTUA1 in pollen germination and pollen tube growth. Considering that green florescence protein (GFP) fused to α-tubulin could affect the polymerization of α-tubulin and β-tubulin directly, PwTUA1 was transiently co-expressed under the pollen-specific promoter Lat52 (Twell et al., 1990) with Lat52::GFP as an indicator. No significant difference was observed in pollen germination percentage and tube growth rate between the samples transformed with GFP and those left untreated (Fig. 4A, B), suggesting that neither the microprojectile bombardment itself nor the GFP had any significant effect on pollen germination and pollen tube growth. GFP expression acted as an indicator of co-transformed pollen tubes as it had previously been established that the level of transgene expression is linearly correlated with the amount of input DNA (Chen et al., 2002). All pollen grains were co-bombarded with 5 μg of Lat52::GFP as a marker for transformation and to assess for comparable transgene expression in all samples. Less than 5 μg of experimental chimeric gene was used in the samples, and Lat52-β-glucuronidase (GUS) DNA was added to make up to 10 μg of total DNA used for every transformation. This ensured that equal amounts of DNA were coated onto the tungsten particles and introduced into the pollen samples. The data presented in Fig. 4A and B show that increasing amounts of Lat52::PwTUA1 introduced into these pollen tubes were associated with gradually increasing pollen germination percentages and pollen tube growth. The length of pollen tubes growing for 24 h was increased by the overexpression of PwTUA1: the average length was 239.1±24 μm (n ≥100) at lower PwTUA1 concentrations and 328.7±33 μm at higher PwTUA1 concentrations, which was significantly longer than the tubes transformed with GFP (197.6±20 μm) (Fig. 4C). No significant difference was seen in the pollen tube length at 12 h compared with 24 h, indicating that expression of PwTUA1 exerted its greatest effect in the first 12 h (Fig. 4B). Although the percentage germination of each sample at 12 h was lower than that at 24 h (Fig. 4A), as pollen germination is related to the time of incubation in P. wilsonii, the percentage pollen germination increased significantly with increasing amounts of Lat52::PwTUA1. These results showed that overexpression of PwTUA1 could improve pollen germination and accelerate pollen tube growth in P. wilsonii.
Fig. 4.
Transient expression of PwTUA1 improves Picea wilsonii pollen germination and pollen tube growth. (A) The effect of PwTUA1 on pollen germination using microprojectile bombardment. After 12 h and 24 h of incubation, the percentage germination of pollen bombarded with the GFP-only construct showed no significant difference compared with the untreated control pollen (P <0.05). Significant differences were observed in the percentage of germination of pollen bombarded with 2.5 μg and 5 μg of the PwTUA1 gene construct compared with untreated pollen or GFP-transformed pollen (P <0.05). Error bars indicate averages from the number of pollen tubes measured (n≥81). (B) The effect of PwTUA1 on pollen tube length using microprojectile bombardment. After 12 h and 24 h of incubation, no significant differences were detected in the length of pollen tubes bombarded with GFP compared with untreated pollen tubes (P=0.47). Significant differences were seen in the length of pollen tubes bombarded with 5 μg of PwTUA1 compared with untreated pollen or GFP-transformed pollen (P <0.05). Error bars indicate averages from the number of pollen tubes measured (n≥65). (C) Microscopic analysis of pollen tube co-expression 24 h after gene transfer of different doses of PwTUA1 with GUS and GFP. Upper panel: fluorescence images. Lower panel: transmitted light images. The data were obtained from three independent experiments, and every condition was tested three times. Bars=100 μm.
PwTUA1 overexpression accelerates pollen tube growth and early germination in transgenic Arabidopsis
To investigate the function of PwTUA1 during pollen development, this gene was ectopically expressed in Arabidopsis pollen exclusively by using the pollen-specific promoter Lat52. The development of transgenic Arabidopsis pollen was consistent with the results observed for the transformed P. wilsonii pollen grains. Both pollen germination and pollen tube growth were enhanced in the transgenic lines when compared with wild-type Arabidopsis, even at the non-optimal boron or Ca2+ concentrations present in the culture medium (Figs 5, 6A). Pollen expressing the PwTUA1 gene exhibited earlier germination than wild-type pollen exposed to 0.1% H3BO3 (w/v), but resulted in the same germination rate as observed for wild-type pollen. However, pollen germination of the transgenic and wild-type plants was severely and completely inhibited in 1% H3BO3 (data not shown). Furthermore, the pollen tube growth rate of the wild-type plants showed a significant decrease compared with that of transgenic plants. The mean growth rate of wild-type plant pollen tubes was 16.7 μm h−1 compared with 35.6 μm h−1 for pollen tubes of transgenic plants cultured in medium containing 0.1% H3BO3 (Fig. 6A). In the above medium, the concentration of Ca2+ was 0.2 mM.
Fig. 5.
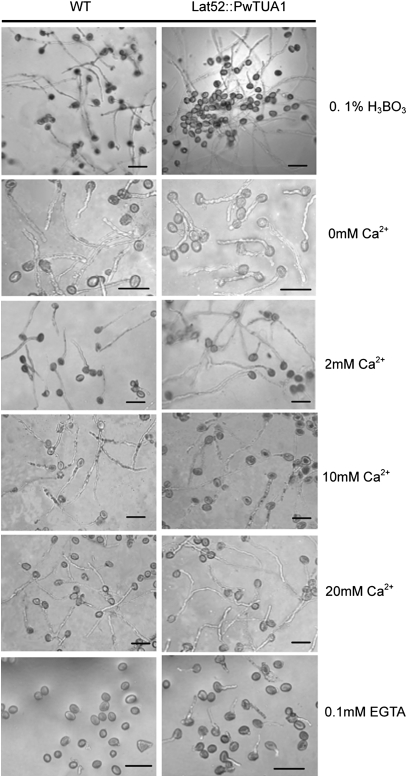
Pollen tubes of wild-type (WT) and transgenic Arabidopsis cultured in different media. Pollen from wild-type Arabidopsis and transformants was germinated on medium containing 0.1% H3BO3, different concentrations of Ca2+, and 0.1 mM EGTA for 6 h and examined under a light microscope. Bars=50 μm.
Fig. 6.
Effects of PwTUA1 overexpression on plant pollen germination and pollen tube growth exposed to different concentrations of boron and Ca2+. (A) Comparison of tube growth of wild-type (WT) and transgenic plant pollen cultured in medium with 0.1% H3BO3 for 6 h. Only the pollen tubes of germinated pollen grains were measured. (B) Arabidopsis pollen was incubated in the germination medium with the indicated amounts of Ca2+. After 6 h of incubation, the percentage of germinating pollen grains was determined. To establish the pollen germination percentage, the percentage germination of pollen grains in at least 10 aliquots per treatment was determined and experiments were repeated three times. (C) Statistical analysis of pollen tube length after 6 h of incubation. Only the pollen tubes of germinated pollen grains were measured. Average lengths of >100 tubes from each line are shown. The experiments were repeated three times, and consistent results were obtained in all repeat experiments.
The effect of varying Ca2+ influxes on pollen tube growth of the transgenic plants was further determined. Pollen tubes were grown on medium containing various amounts of Ca2+. Optimal growth of wild-type Arabidopsis pollen tubes required nearly 2 mM extracellular Ca2+ (Li et al., 1999). Pollen from wild-type plants and transgenic plants homozygous for the PwTUA1 gene was germinated on medium containing 0, 2, 10, or 20 mM Ca2+. The percentage of pollen germination and tube elongation rates of transgenic plants were higher than those of wild-type plants when extracellular Ca2+ levels increased from 0 mM to 20 mM (Fig. 6B, C). The pollen tubes of wild-type plants showed significantly slower growth rates than transgenic plants when the extracellular Ca2+ concentrations were <10 mM (Fig. 6C). Note that when 0.1 mM EGTA (a chelating agent with a high affinity for Ca2+) was added into the medium, almost none of the wild-type pollen germinated. In contrast, the mean pollen germination percentage of the transgenic plants reached 21% (Fig. 5). These observations suggest a general and important role for PwTUA1 in the regulation of pollen development.
PwTUA1 overexpression changes the subcellular localization of the α-tubulin protein in Arabidopsis pollen tubes
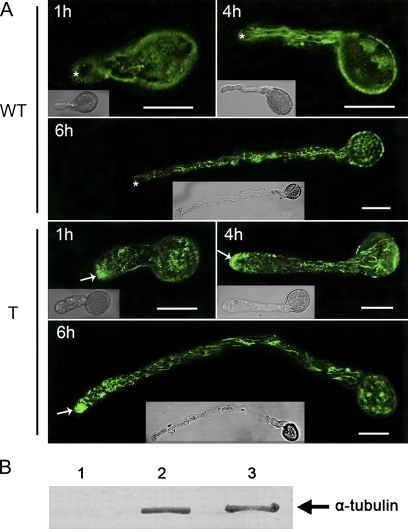
The subcellular localization of α-tubulin in pollen tubes of wild-type and transgenic Arabidopsis was investigated using anti-α-tubulin serum and indirect immunofluorescence microscopy. A series of experiments conducted at different growth stages showed that the α-tubulin protein existed throughout the tubes of wild-type Arabidopsis, excluding the tip or apical region (Fig. 7A). However, highly intense fluorescence was observed in the apical region of transgenic Arabidopsis pollen tubes, and the fluorescence in the pollen grains and pollen tubes exhibited decreasing intensities compared with that observed in the tube tips (Fig. 7A) in accordance with the observations in P. wilsonii pollen tubes (data not shown) as well as the findings reported for pollen tubes in several other gymnosperm species (Lazzaro, 1999). Note that the distribution of MTs in transgenic Arabidopsis pollen tubes was very different from that in angiospermous pollen tubes (Pierson and Cresti, 1992; Li et al., 1997), where MTs are absent in the tip regions. Moreover, no fluorescence was found in any of the controls not treated with the antiserum or pre-immune serum controls, indicating that the antiserum was specific and that non-specific labelling was negligible.
Fig. 7.
Subcellular localization of the α-tubulin protein in Arabidopsis pollen tubes. In vitro cultured pollen tubes were fixed, incubated with anti-α-tubulin antiserum, and detected with goat anti-mouse secondary antibody as described in the Materials and methods. The fluorescence of stained pollen tubes was observed under a confocal microscope. (A) Fluorescence from immunolabelling of Arabidopsis pollen tubes harvested at 1, 4, and 6 h after germination. WT indicates pollen of wild-type Arabidopsis; T indicates pollen of transgenic Arabidopsis. The transgenic tubes tip (arrows) were strongly labelled with antibody for α-tubulin, whereas wild-type pollen tubes showed a much weaker signal in the apical region (asterisks). These data were obtained from three independent experiments, and every condition was tested three times. Bars=20 μm. (B) Western blot of extracts (equal loading of samples) from Arabidopsis pollen tubes (lane 2) and P. wilsonii pollen tubes (lane 3) probed with anti-α-tubulin antibody. Lane 1 is the blank control.
PwTUA1 overexpression affects the ultrastructure of pollen tubes in transgenic Arabidopsis
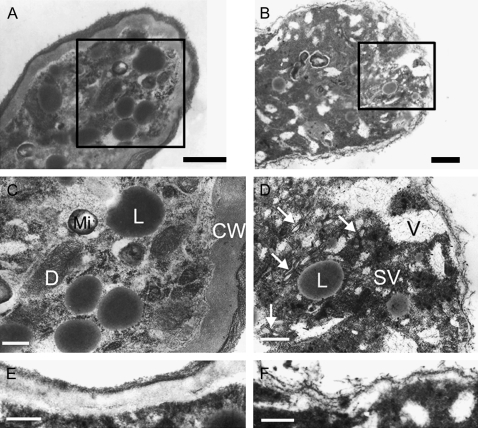
As the PwTUA1 gene not only improved pollen germination and tube growth, but also altered the distribution of α-tubulin protein in transgenic Arabidopsis, the ultrastructure of the pollen grains and tubes was investigated. Note that the pollen tube wall of transgenic Arabidopsis was only slightly detectable (Fig. 8F), while the wall of wild-type Arabidopsis was much thicker (Fig. 8E). Additionally, many short, solitary cytoplasmic MTs were observed in the tip-most portion of the transgenic pollen tubes where the vesicles accumulated (Fig. 8D), but were rarely observed in the tip region of wild-type pollen tubes (Fig. 8C). It was also determined that more lipid bodies were in the wild-type pollen tube apex than in transgenic pollen, while many more vacuoles were in the transgenic pollen tube apex compared with wild-type plants (Fig. 8C, D).
Fig. 8.
Effects of PwTUA1 on the ultrastructure of pollen tubes in transgenic Arabidopsis. Pollen from wild-type and transgenic plants was cultured in vitro for 6 h. Bars=0.2 μm (A, B, E, F) or 0.5 μm (C, D). (A) Tip region of wild-type pollen tube showing organelles at the tip. (B) Tip region of a pollen tube expressing PwTUA1; MTs (arrowhead) appeared and the number of vacuoles and secretory vesicles increased. (C and D) Magnified images of boxed sections in A and B. (E) Pollen tube wall of wild-type Arabidopsis. (F) Pollen tube wall of transgenic Arabidopsis. For Arabidopsis expressing PwTUA1, analyses of two independent transgenic lines showed a similar ultrastructure of pollen tubes. Data from one transgenic line were used. CW, cell wall; Mi, mitochondria; D, dictyosome; SV, secretory vesicle; V, vacuole; L, lipid body.
Discussion
Controversy exists regarding the role of MTs during pollen tube growth. MTs have been suggested not to be strictly required for tube extension because the depolymerization of MTs by specific inhibitors did not inhibit the rate of pollen tube growth to the same extent (Mascarenhas, 1993; Åström et al., 1995). In contrast, MTs have been reported to be essential in imposing the typical tubular shape and directionality upon expansion in Pinus (Terasaka and Niitsu, 1994). However, research on the function of MTs has focused on protein depolymerization rather than gene transcriptional regulation during pollen germination and tube growth.
PwTUA1 is a pollen-specific gene and induced by Ca2+ and boron
In the present study, a full-length α-tubulin cDNA was obtained from P. wilsonii pollen using PCR and RACE. Amino acid sequence alignment showed that the deduced PwTUA1 protein had high identity with other previously characterized plant α-tubulins. In addition, important evolutionarily and functionally related structural features were conserved (Fig. 1). Note that the C-terminal glutamate residue of PwTUA1 is thought to be involved in the detyrosination reaction, which is a required event in animal cell proliferation (Phung et al., 2006). This is in contrast to the angiospermous pollen-specific and pollen-preferential TUAs that contain a typical C-terminal tyrosine (Oakley et al., 2007). Furthermore, analysis of the phylogenetic tree shows that PwTUA1 is more closely related to the α-tubulins involved in secondary cell wall development than to the α-tubulins from other angiospermous species that are expressed in pollen preferentially. However, in contrast to observations that AtTUA1 exhibited low levels of expression in other tissues (Carpenter et al., 1992) and that PtTUA6/8 was detected in roots in addition to pollen (Oakley et al., 2007), the research demonstrated that PwTUA1 was expressed specifically in pollen only (Fig. 3A).
It has been demonstrated that boron and Ca2+ are essential for pollen germination and tube growth (Dickinson, 1978; Franklin-Tong, 1999; Wang et al., 2003). The present results showed that PwTUA1 was up-regulated by Ca2+/boron (Fig. 3C), and PwTUA1 overexpression improved pollen germination and tube growth both in P. wilsonii and in Arabidopsis (Figs 4–6). It is hypothesized that boron and Ca2+ promote pollen germination and tube growth through up-regulating the expression of PwTUA1. However, pollen germination and tube growth did not show consistent promotion with increasing concentrations of Ca2+ or boron (data not shown). This is due to the complicated signalling pathways regulated by Ca2+/boron to promote pollen germination and tube growth. As one of these multiple complex pathways, up-regulated expression of PwTUA1 by Ca2+/boron just plays a part in this process.
PwTUA1 contributes to pollen tube growth based on altering the distribution of α-tubulin and ultrastructure of pollen tubes
By indirect immunofluorescence and electron microscopy, it was confirmed that a relationship exists between the cellular structure of germinating pollen and PwTUA1 expression. In most angiosperms, a common feature of all pollen tubes is the absence of MTs from the actual tip region and the short and randomly oriented MTs in the subapical region (Raudaskoski et al., 2001). In contrast, gymnosperm MTs have been reported to be enriched at the elongating tip, where they form an array beneath the plasma membrane that is perpendicular to the direction of tube growth (Lazzaro, 1999). Overexpression of the PwTUA1 gene alters the distribution of α-tubulin in germinating transgenic Arabidopsis pollen, which is enriched in the apex region instead of being absent altogether (Fig. 7). With the occurrence of short, solitary cytoplasmic MTs near the pollen tube apex region in transgenic Arabidopsis plants (Fig. 8), the existence of α-tubulin in the tube tip may contribute to the distribution and structural stability of pollen tube MTs in concert with microtubule-associated proteins (MAPs), actin filaments, or other protein factors.
In most plant species, the pollen tube cell wall consists of two layers, the inner sheath of callose and outer coating containing mainly pectin with cellulose and hemicellulose. The pollen tube grows exclusively at its tip, where the newly synthesized cell wall is continually forming (Taylor and Hepler, 1997; Krichevsky et al., 2007). The digestion of pectin at the apex is sufficient to destabilize the cell wall, with the result that the turgor was able to expand the wall (Parre and Geitmann, 2005). In the present study, the cell wall of the tip region mainly composed of pectin was infrequently observed in the apex of transgenic pollen tubes (Fig. 8). This result was consistent with the observation that the earliest visible cell wall alteration upon emergence of the pollen tube is a loss of pectic material (Taylor and Hepler, 1997). Additionally, MTs contribute to the deposition of pollen cell wall components (Cai et al., 2005) and, in conifers, the MTs at the tip may orient the deposition of cellulose microfibrils (Lazzaro, 1999). Taken together, it is suggested that PwTUA1 plays a crucial role in regulating the deposition of pollen cell wall components in such a manner that it was able to promote pollen germination and tube growth.
In Nicotiana tabacum (Derksen et al., 1995) and Lilium longiflorum pollen tubes (Lancelle et al., 1997), the cell wall of the tip extremity has been demonstrated to be slightly thicker than the cell wall below the tip region, and the former region shows slow expansion, whereas the maximum growth rate occurs in the thin wall layer region below the tip extremity (Raudaskoski et al., 2001). Visualization studies (Fig. 8) show directly that the pollen tube wall of transgenic Arabidopsis plants is thinner than that of the Arabidopsis plants not expressing PwTUA1. Considering that PwTUA1 improves pollen tube growth in transgenic Arabidopsis, it is postulated that fast elongation preferentially takes place in regions where the tube wall is thinner. The balance between intracellular turgor pressure (hydrostatic pressure, reflected by the cell volume) and cell wall loosening is a critical factor driving pollen tube growth (Zonia et al., 2006). The thinner pollen tube cell wall observed in Arabidopsis plants overexpressing PwTUA1 suggests that cell wall rigidity of transgenic pollen tubes may decrease compared with wild-type Arabidopsis. Thus, the transgenic Arabidopsis pollen tubes displayed a faster growth rate than wild-type pollen tubes in vitro.
Pollen tubes grow by the deposition of cell wall components at the tip (Derksen et al., 1995; Taylor and Hepler, 1997), and these cell wall components are carried by vesicles, which are transported to the tip via highly active cytoplasmic streaming and incorporated into a zone of elongation in the apical dome of the pollen tube tip (Franklin-Tong, 1999). In gymnosperms, the cell wall may develop in the terminal 20 mm but may be mature in older parts of the tube (Lazzaro, 1999). Thus, the tube wall is not always thick in the region where cell wall components deposit. Within the transgenic Arabidopsis, vesicles accumulated in the tip region of pollen tubes were frequently observed. In contrast, empty compartments were hardly ever detected in the wild-type Arabidopsis tube tip. These results suggest that the contribution of PwTUA1 to pollen tube growth is supported by the presence of vesicles localized in the tip of transgenic Arabidopsis pollen tubes.
Taken together, all these observations suggest that PwTUA1 expression plays an important role in maintaining rapid pollen tube growth. However, at present, the mechanism underling PwTUA1 gene involvement in the structure of MTs cannot be explained. To address the question conclusively, more experimental evidence is needed.
Acknowledgments
We thank Dr J Haseloff (MRC Laboratory of Molecular Biology, Cambridge, UK) for the GFP construct, pBINmGFP5-ER. This work was supported by the National Natural Science Foundation (grant no. 30671659 to LYZ and 30570144 to CCZ), the Programme for New Century Excellent Talents in University (NCET-06-0121 to LYZ), and the National Key Basic Research Programme (grant no.2007CB947600 to LYZ) in China.
References
- Abe T, Thitamadee S, Hashimoto T. Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant and Cell Physiology. 2004;45:211–220. doi: 10.1093/pcp/pch026. [DOI] [PubMed] [Google Scholar]
- Åström H, Sorri O, Raudaskoski M. Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sexual Plant Reproduction. 1995;8:61–69. [Google Scholar]
- Cai G, Del CC, Romagnoli S, Cresti M. Pollen cytoskeleton during germination and tube growth. Current Science. 2005;89:1853–1860. [Google Scholar]
- Cai G, Romagnoli S, Moscatelli A, Ovidi E, Gambellini G, Tiezzi A, Cresti M. Identification and characterization of a novel microtubule-based motor associated with membranous organelles in tobacco pollen tubes. The Plant Cell. 2000;12:1719–1736. doi: 10.1105/tpc.12.9.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JL, Ploense SE, Snustad DP, Silflow CD. Preferential expression of an alpha-tubulin gene of Arabidopsis in pollen. The Plant Cell. 1992;4:557–571. doi: 10.1105/tpc.4.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Purgear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11:117–121. [Google Scholar]
- Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu HM, Cheung AY. The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. The Plant Cell. 2002;14:2175–2190. doi: 10.1105/tpc.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Snustad DP, Carter JV. Temporal and spatial expression patterns of TUB9, a beta-tubulin gene of Arabidopsis thaliana. Plant Molecular Biology. 2001;47:389–398. doi: 10.1023/a:1011628024798. [DOI] [PubMed] [Google Scholar]
- Cleveland DW. Autoregulated control of tubulin synthesis in animal cells. Current Opinion in Cell Biology. 1989;1:10–14. doi: 10.1016/s0955-0674(89)80030-4. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Derksen J, Pierson ES, Traas JA. Microtubules in vegetative and generative cells of pollen tubes. European Journal of Cell Biology. 1985;38:142–148. [Google Scholar]
- Derksen J, Rutten T, Lichtscheidl IK, de Win AHN, Pierson ES, Rongen G. Quantitative analysis of the distribution of organelles in tobacco pollen tubes: implications for exocytosis and endocytosis. Protoplasma. 1995;188:267–276. [Google Scholar]
- Dickinson DB. Influence of borate and pentaerythritol concentration on germination and tube growth of Lilium longiflorum pollen. Journal of the American Society for Horticultural Science. 1978;103:263–269. [Google Scholar]
- Fernando D, Lazzaro M, Owens J. Growth and development of conifer pollen tubes. Sexual Plant Reproduction. 2005;18:149–162. [Google Scholar]
- Franklin-Tong VE. Signaling and the modulation of pollen tube growth. The Plant Cell. 1999;11:727–738. doi: 10.1105/tpc.11.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JWC, Dawen Verhey, Kristen J. Tubulin modifications and their cellular functions. Current Opinion in Cell Biology. 2008;20:71–76. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Li Y, Hu Y, Lin J. Inhibition of RNA and protein synthesis in pollen tube development of Pinus bungeana by actinomycin D and cycloheximide. New Phytologist. 2005;165:721–729. doi: 10.1111/j.1469-8137.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Sites of origin of the peripheral microtubule system of the vegetative cell of the angiosperm pollen tube. Annals of Botany. 1988;62:455–461. [Google Scholar]
- Huang S, Jin L, Du J, Li H, Zhao Q, Ou G, Ao G, Yuan M. SB401, a pollen-specific protein from Solanum berthaultii, binds to and bundles microtubules and F-actin. The Plant Journal. 2007;51:406–418. doi: 10.1111/j.1365-313X.2007.03153.x. [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kopczak SD, Haas NA, Hussey PJ, Silflow CD, Snustad DP. The small genome of Arabidopsis contains at least six expressed alpha-tubulin genes. The Plant Cell. 1992;4:539–547. doi: 10.1105/tpc.4.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Chua NH. The plant cytoskeleton: vacuoles and cell walls make the difference. Cell. 2002;108:9–12. doi: 10.1016/s0092-8674(01)00634-1. [DOI] [PubMed] [Google Scholar]
- Krichevsky A, Kozlovsky SV, Tian G-W, Chen M-H, Zaltsman A, Citovsky V. How pollen tubes grow. Developmental Biology. 2007;303:405–420. doi: 10.1016/j.ydbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK. Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma. 1987;140:141–150. [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK. Growth inhibition and recovery in freeze-substituted Lilium longiflorum pollen tubes: structural effects of caffeine. Protoplasma. 1997;196:21–33. [Google Scholar]
- Lazzaro MD. Microtubule organization in germinated pollen of the conifer Picea abies (Norway spruce, Pinaceae) American Journal of Botany. 1999;86:759. [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. The Plant Cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Moscatelli A, Cai G, Cresti M. Functional interactions among cytoskeleton, membranes, and cell wall in the pollen tube of flowering plants. International Review of Cytology. 1997;176:133–199. doi: 10.1016/s0074-7696(08)61610-1. [DOI] [PubMed] [Google Scholar]
- Lin Y, Yang Z. Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. The Plant Cell. 1997;9:1647–1659. doi: 10.1105/tpc.9.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP. Molecular mechanisms of pollen tube growth and differentiation. The Plant Cell. 1993;5:1303–1314. doi: 10.1105/tpc.5.10.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Jurgens G. Microtubule cytoskeleton: a track record. Current Opinion in Plant Biology. 2002;5:494–501. doi: 10.1016/s1369-5266(02)00302-3. [DOI] [PubMed] [Google Scholar]
- Oakley RV, Wang YS, Ramakrishna W, Harding SA, Tsai CJ. Differential expansion and expression of alpha- and beta-tubulin gene families in Populus. Plant Physiology. 2007;145:961–973. doi: 10.1104/pp.107.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parre E, Geitmann A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta. 2005;220:582–592. doi: 10.1007/s00425-004-1368-5. [DOI] [PubMed] [Google Scholar]
- Phung AD, Soucek K, Kubala L, Harper RW, Chloe Bulinski J, Eiserich JP. Posttranslational nitrotyrosination of alpha-tubulin induces cell cycle arrest and inhibits proliferation of vascular smooth muscle cells. European Journal of Cell Biology. 2006;85:1241–1252. doi: 10.1016/j.ejcb.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Pierson ES, Cresti M. Cytoskeleton and cytoplasmic organization of pollen and pollen tubes. International Review of Cytology. 1992;140:73–125. [Google Scholar]
- Poulter NS, Vatovec S, Franklin-Tong VE. Microtubules are a target for self-incompatibility signaling in Papaver pollen. Plant Physiology. 2008;146:1358–1367. doi: 10.1104/pp.107.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudaskoski M, Åström H, Laitiainen E. Pollen tube cytoskeleton: structure and function. Journal of Plant Growth Regulation. 2001;20:113–130. [Google Scholar]
- Raudaskoski M, Åström H, Pertillä K, Virtanen I, Louhelian J. Role of the microtubule cytoskeleton in pollen tubes: an immunocytochemical and ultrastructural approach. Biology of the Cell. 1987;61:177–188. [Google Scholar]
- Rodriguez-Riano T, Dafni A. A new procedure to assess pollen viability. Sexual Plant Reproduction. 2000;12:241–244. [Google Scholar]
- Romagnoli S, Cai G, Cresti M. In vitro assays demonstrate that pollen tube organelles use kinesin-related motor proteins to move along microtubules. The Plant Cell. 2003;15:251–269. doi: 10.1105/tpc.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JC, Smith FD, Russell JA. Optimizing the biolistic process for different biological applications. Methods in Enzymology. 1993;217:483–509. doi: 10.1016/0076-6879(93)17086-k. [DOI] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK. Pollen germination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- Terasaka O, Niitsu T. Differential roles of microtubule and actin–myosin cytoskeleton in the growth of Pinus pollen tubes. Sexual Plant Reproduction. 1994;7:264–272. [Google Scholar]
- Twell D, Yamaguchi J, McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development. 1990;109:705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lu L, Wu X, Li Y, Lin J. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiology. 2003;23:345–351. doi: 10.1093/treephys/23.5.345. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO. Progress in understanding the role of microtubules in plant cells. Current Opinion in Plant Biology. 2004;7:651–660. doi: 10.1016/j.pbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Weisenberg RC, Borisy GG, Taylor EW. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968;7:4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Win A, Knuiman B, Pierson E, Geurts H, Kengen H, Derksen J. Development and cellular organization of Pinus sylvesfris pollen tubes. Sexual Plant Reproduction. 1996;9:93–101. [Google Scholar]
- Yen TJ, Gay DA, Pachter JS, Cleveland DW. Autoregulated changes in stability of polyribosome-bound beta-tubulin mRNAs are specified by the first 13 translated nucleotides. Molecular and Cellular Biology. 1988b;8:1224–1235. doi: 10.1128/mcb.8.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Machlin PS, Cleveland DW. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988a;334:580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Yang GX, Kawaguchi K, Komatsu S. Expression analyses of beta-tubulin isotype genes in rice. Plant and Cell Physiology. 2003;44:1202–1207. doi: 10.1093/pcp/pcg150. [DOI] [PubMed] [Google Scholar]
- Zonia L, Muller M, Munnik T. Hydrodynamics and cell volume oscillations in the pollen tube apical region are integral components of the biomechanics of Nicotiana tabacum pollen tube growth. Cell Biochemistry and Biophysics. 2006;46:209–232. doi: 10.1385/CBB:46:3:209. [DOI] [PubMed] [Google Scholar]