Summary
Background
Deep vein thrombosis (DVT) and pulmonary embolism are common after stroke. In small trials of patients undergoing surgery, graduated compression stockings (GCS) reduce the risk of DVT. National stroke guidelines extrapolating from these trials recommend their use in patients with stroke despite insufficient evidence. We assessed the effectiveness of thigh-length GCS to reduce DVT after stroke.
Methods
In this outcome-blinded, randomised controlled trial, 2518 patients who were admitted to hospital within 1 week of an acute stroke and who were immobile were enrolled from 64 centres in the UK, Italy, and Australia. Patients were allocated via a central randomisation system to routine care plus thigh-length GCS (n=1256) or to routine care plus avoidance of GCS (n=1262). A technician who was blinded to treatment allocation undertook compression Doppler ultrasound of both legs at about 7–10 days and, when practical, again at 25–30 days after enrolment. The primary outcome was the occurrence of symptomatic or asymptomatic DVT in the popliteal or femoral veins. Analyses were by intention to treat. This study is registered, number ISRCTN28163533.
Findings
All patients were included in the analyses. The primary outcome occurred in 126 (10·0%) patients allocated to thigh-length GCS and in 133 (10·5%) allocated to avoid GCS, resulting in a non-significant absolute reduction in risk of 0·5% (95% CI −1·9% to 2·9%). Skin breaks, ulcers, blisters, and skin necrosis were significantly more common in patients allocated to GCS than in those allocated to avoid their use (64 [5%] vs 16 [1%]; odds ratio 4·18, 95% CI 2·40–7·27).
Interpretation
These data do not lend support to the use of thigh-length GCS in patients admitted to hospital with acute stroke. National guidelines for stroke might need to be revised on the basis of these results.
Funding
Medical Research Council (UK), Chief Scientist Office of Scottish Government, Chest Heart and Stroke Scotland, Tyco Healthcare (Covidien) USA, and UK Stroke Research Network.
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are common complications of admission to hospital for surgery or acute medical problems, and result in many avoidable deaths.1 These complications emphasise the potential importance of measures that might reduce the risk of venous thromboembolism, such as anticoagulation, external compression with graduated compression stockings (GCS), and intermittent pneumatic compression. Up to 42% of patients admitted with stroke develop venous thromboembolism.2 Although use of anticoagulants reduces this risk, the associated excess of intracranial and extracranial haemorrhages largely offsets any benefit.3 Thus, most national stroke guidelines do not recommend routine use of anticoagulants in ischaemic stroke, but instead recommend the use of GCS.4–12 However, guidelines vary considerably, with some recommending anticoagulation and only GCS in patients unsuitable for anticoagulation, and others recommending routine stocking use but avoidance of anticoagulants. The UK National Institute for Health and Clinical Excellence (NICE) has recently drafted guidelines for reducing the risk of venous thromboembolism which included the recommendation: “For patients diagnosed with stroke, offer mechanical VTE prophylaxis (thigh-length anti-embolism stockings, intermittent pneumatic compression devices or foot impulse devices) from admission until the patient's mobility is no longer increasing or until discharge”.13
A systematic review14 identified 17 single-centre randomised controlled trials in patients admitted to hospital in which 2412 patients or legs were randomly assigned to GCS or control. GCS was associated with a 63% (95% CI 52–70) reduction in the odds of (mainly distal) DVT. 15 of the 17 trials were in surgical patients, one was in acute medical patients (n= 80).15 Only one trial was in patients with stroke16 and in this trial, seven of 65 patients (10·8%) allocated GCS and seven of 32 (21·9%) allocated to avoid GCS had DVTs detected on Doppler ultrasound within 10 days of enrolment (odds ratio 0·43, 95% CI 0·14–1·36). 14 of the 17 trials tested thigh-length GCS, two tested below-knee GCS and, in one, the length was not specified. A systematic review of external compression specifically in stroke17 did not identify any other randomised controlled trials investigating GCS. For patients with stroke, unlike surgical patients, external compression cannot be applied before the patient is immobilised. In stroke, leg paralysis and immobility can persist for weeks or months, rather than hours or days. Additionally, patients with stroke have a high risk of concomitant peripheral vascular disease and diabetes, which potentially increases the risk of ischaemic skin damage to the legs resulting from external compression.18,19
The CLOTS (Clots in Legs Or sTockings after Stroke) trials are three multicentre randomised controlled trials that use the same randomisation, data collection, and follow-up systems and that aim to assess the balance of risk and benefit of external compression in patients with acute stroke. All three trials test the effect of the addition of different compression strategies to routine care. The CLOTS trial 1, reported here, tested thigh-length GCS versus avoidance of GCS. The CLOTS trials 2 and 3 are in progress and are testing thigh-length GCS versus below-knee GCS, and intermittent pneumatic compression versus avoidance of intermittent pneumatic compression, respectively.
Methods
Study design and patients
CLOTS trial 1 is a pragmatic, multicentre, international, outcome-blinded, randomised controlled trial. Between March 7, 2001, and Nov 27, 2008, patients were enrolled in 55 centres in the UK, seven in Italy, and two in Australia. Patients who were admitted to hospital within 1 week of an acute stroke and who were immobile (defined as being unable to walk independently to the toilet) were eligible for inclusion and could be randomised between the day of admission (day 0) up to day 3. We excluded patients with peripheral vascular disease, and those with diabetic or sensory neuropathy, when the responsible clinician or nurse judged that GCS might cause skin damage. Patients with stroke due to subarachnoid haemorrhage were also excluded.
The protocol was approved by the multicentre research ethics committees (in Scotland, reference 00/0/28; and England, reference 07/H0907/178) and by the local ethics committees of all contributing centres. We obtained written informed consent from all patients, or for patients without mental capacity, a valid proxy.
Procedures
Having identified an eligible patient, the clinician completed a randomisation form. The clinician entered the patient's baseline data via a web-based or a 24-h telephone randomisation service. After the baseline data had been entered and the computer program had checked for completeness and consistency, the system generated a group allocation—either routine care plus thigh-length GCS (Tyco Healthcare [Covidien], MA, USA) or routine care plus avoidance of GCS. We used a minimisation program to achieve optimum balance within centres for key prognostic factors: delay since stroke onset (day 0 or 1 vs day ≥2); stroke severity with a validated prognostic model;20 leg paresis (able or not to lift both legs off the bed); and prescription of heparin, warfarin, or alteplase. Because simple minimisation within centres can lead to alternation of treatment allocation and thus potential loss of allocation concealment, our system also incorporated a degree of random allocation—ie, patients were allocated to the treatment group that would minimise the difference between the groups with a probability of 0·80.
For patients allocated to thigh-length GCS, stockings were to be applied to both legs as soon as possible after randomisation and then worn day and night until either the patient was independently mobile around the ward; they were discharged from the recruiting centre; the patient refused to wear them; or the staff became concerned about the patient's skin. We asked centres to record their use of GCS on the medication chart in the same way as for prescribed drugs to help increase compliance and to help with monitoring. The date of, and reason for, early removal of GCS was collected. Patients allocated to avoid GCS were not to be fitted with stockings unless a clear indication for their use developed. Both groups were to be given the same routine care that could have included, depending on local protocols, early mobilisation, hydration, and antiplatelet and anticoagulant drugs. We monitored antiplatelet and anticoagulant use throughout follow-up.
The primary outcome was a definite or probable symptomatic or asymptomatic DVT in the popliteal or femoral veins detected on a screening compression Doppler ultrasound (CDU) or any symptomatic DVT in the popliteal or femoral veins, confirmed on imaging, within 30 days of randomisation. Evidence suggests that occurrence of DVT within the first month is associated with poorer functional outcomes at 6 months, even after adjustment for age, stroke severity, and leg weakness.21 Secondary outcomes were, within 30 days, death, any DVT (including calf, popliteal, or femoral), symptomatic DVT, PE confirmed by imaging or autopsy, medical complications of GCS (eg, skin necrosis), and compliance with allocated treatment.
We aimed to undertake a CDU of the veins in both legs between days 7 and 10, and between days 25 and 30 after randomisation when practical. The decision not to do the second CDU was made and captured securely before randomisation—eg, if the patient was likely to be discharged home to another region or transferred to a rehabilitation facility that did not have Doppler facilities and was remote from the randomising centre. GCS were removed before the CDU to ensure optimum blinding of the investigators to the primary outcome measure. We obtained a hard copy of positive scans for central verification. We also accepted the results of any screening CDU done after 30 days; if these scans showed a popliteal or femoral DVT they were included in the primary outcome. Symptomatic DVTs detected more than 30 days after enrolment, other than on the planned screening CDU, were not included in the primary outcome to reduce ascertainment bias. A discharge form detailing in-hospital outcomes was completed for all randomised patients on discharge from the randomising centre or after death in hospital. We sent a postal questionnaire to every patients' family doctor about 5·5 months after enrolment to establish the patients' vital status, occurrence of DVTs or PE since hospital discharge, and any current use of oral anticoagulants. We followed up patients at 6 months after enrolment by postal questionnaire or telephone to ask whether they had had a DVT or PE after hospital discharge, and later secondary outcomes (to be reported elsewhere).
Statistical analysis
Initially we planned to recruit patients until we had identified 175 with our primary outcome. We estimated that we would need about 1500 patients to provide 90% power (α=0·05) to identify a 6% absolute reduction (from 15% to 9%) in our primary outcome. In 2006, the steering committee, which was blind to any interim analysis that split patients by group allocation, decided to increase the power of the trial to detect a smaller treatment effect of 4% that was judged to be the smallest clinically-worthwhile benefit. The decision was made to recruit until December, 2008, which was the latest that we could continue until and still complete follow-up within our funded period. By that time we expected to have recruited at least 2200 patients, but had actually recruited 2518. The completed trial had 90% power to detect a 4% absolute risk reduction in our primary outcome.
The absolute difference in the proportion of patients with a primary outcome between our allocated groups was calculated with 95% CIs. The proportions with a primary or secondary outcome were compared with odds ratios and 95% CIs. Results relating to the primary outcome were adjusted for three variables included in our minimisation algorithm (predicted stroke outcome, delay from stroke onset to randomisation, and ability of the patient to lift both legs off the bed), and all analyses were based on intention to treat. Analyses of accumulating data were prepared by the trial statistician and reviewed at least once per year in strict confidence by the independent data monitoring committee. Preplanned subgroup analyses included our primary outcome subdivided by key baseline variables: time from stroke onset to randomisation (day 0 or 1 vs ≥2); use of antithrombotic agents (heparin, warfarin, or alteplase vs none); and paralysis of leg (able to lift both legs or not).
This study is registered, number ISRCTN28163533.
Role of the funding source
The sponsors of the study had no role in data collection, data storage, data analysis, preparation of this report, or the decision to publish. The UK Stroke Research Network (study ID 2133) adopted the trial in 2005, and much of the enrolment and data collection was undertaken by staff funded by the network or working for associated NHS organisations after that date. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Figure 1 shows the trial profile and table 1 shows the patients' baseline characteristics, which were well balanced between treatment groups.
Figure 1.
Trial profile
The number screened for eligibility was not collected. GCS=graduated compression stockings. CDU=compression Doppler ultrasound. LMWH=low-molecular-weight heparin.
Table 1.
Baseline characteristics of patients enrolled into the CLOTS 1 trial
| Thigh-length GCS (n=1256) | Avoid GCS (n=1262) | |
|---|---|---|
| Overall | ||
| Age (years)* | 76 (68–83) | 76 (68–83) |
| Men | 620 (49·4%) | 622 (49·3%) |
| Final diagnosis at hospital discharge | ||
| Ischaemic stroke | 1058 (84·2%) | 1087 (86·1%) |
| Haemorrhagic stroke | 132 (10·5%) | 100 (7·9%) |
| Uncertain | 39 (3·1%) | 38 (3·0%) |
| Non-strokes (included in primary analysis) | 24 (1·9%) | 35 (2·8%) |
| Missing (no discharge form) | 3 (0·2%) | 2 (0·2%) |
| Past history and risk factors | ||
| Previous DVT or PE | 55 (4·4%) | 56 (4·4%) |
| Diabetes | 191 (15·2%) | 165 (13·1%) |
| Peripheral vascular disease | 36 (2·9%) | 26 (2·1%) |
| Overweight | 320 (25·5%) | 343 (27·2%) |
| Cigarette smoker | 235 (18·7%) | 235 (18·6%) |
| Independent in daily activities before stroke* | 1145 (91·2%) | 1152 (91·3%) |
| Lives alone before stroke* | 443 (35·3%) | 461 (36·5%) |
| Indicators of stroke severity | ||
| Able to lift both arms off bed* | 547 (43·5%) | 558 (44·2%) |
| Able to talk and oriented in time, place, and person* | 898 (71·5%) | 890 (70·5%) |
| Able to lift both legs off bed† | 542 (43·1%) | 550 (43·6%) |
| Able to walk without help* | 0 (0%) | 0 (0%) |
| Prescribed heparin, warfarin, or alteplase at baseline† | 98 (7·8%) | 109 (8·6%) |
| Delay since stroke onset to randomisation (0–1days)† | 525 (41·8%) | 511 (40·5%) |
| Stroke severity: probability of being alive and independent in daily activities 0–0·15† | 660 (52·5%) | 684 (54·2%) |
| Stroke severity: probability of being alive and independent in daily activities | 0·14 (0·04–0·38) | 0·12 (0·03–0·40) |
| Compression Doppler ultrasound at 30 days considered unlikely to be practical at time of randomisation | 351 (27·9%) | 330 (26·1%) |
Data are median (IQR) or number (%). GCS=graduated compression stockings. DVT=deep vein thrombosis. PE=pulmonary embolism.
Factors included in model to predict probability of being alive and independent at 6 months.20
Variables included in minimisation.
Table 2 shows patient outcomes with respect to the primary and secondary endpoints. The use of thigh-length GCS was associated with a non-significant 0·5% (95% CI −1·9 to 2·9) absolute reduction in the primary outcome. The 95% CIs reliably exclude the smallest benefit that the trial steering committee considered clinically worthwhile. No important differences occurred in any DVT (including symptomatic, asymptomatic, and below knee DVT) or in confirmed PE (table 2). Adverse effects (eg, skin breaks, ulcers, blisters, and skin necrosis) were significantly more common in patients allocated to GCS than in those allocated to avoid GCS, and more patients allocated to GCS had lower limb amputation (seven vs two; table 2). Although most of these adverse events were probably directly caused by the GCS, the few amputations that arose usually resulted from apparent embolism causing acute limb ischaemia rather than being attributed to the direct effect of stockings. However, the reporting of adverse effects was based on case-note review and was not blinded to treatment allocation. These data are therefore prone to ascertainment bias.
Table 2.
Primary and secondary outcomes
| Thigh-length GCS (n=1256) | Avoid GCS (n=1262) | Odds ratio (95% CI) | |
|---|---|---|---|
| Primary outcome | |||
| Proximal DVT | 126 (10·0%) | 133 (10·5%) | .. |
| Alive and free of primary outcome | 974 (77·5%) | 1000 (79·2%) | .. |
| Dead before any primary outcome | 115 (9·2%) | 101 (8·0%) | .. |
| Missing | 41 (3·3%) | 28 (2·2%) | .. |
| Unadjusted (dead and missing excluded) | .. | .. | 0·97 (0·75-1·26) |
| Adjusted* (dead and missing excluded) | .. | .. | 0·98 (0·76-1·27) |
| Secondary outcomes by 30 days or later second compression Doppler ultrasound | |||
| Dead by 30 days | 122 (9·7%) | 110 (8·7%) | 1·13 (0·86–1·48) |
| Symptomatic proximal DVT | 36 (2·9%) | 43 (3·4%) | 0·84 (0·53–1·31) |
| Asymptomatic proximal DVT | 90 (7·2%) | 90 (7·1%) | 1·01 (0·74–1·36) |
| Symptomatic DVT (proximal or distal) | 55 (4·4%) | 61 (4·8%) | 0·90 (0·62–1·31) |
| Any DVT (proximal or distal) | 205 (16·3%) | 224 (17·7%) | 0·90 (0·73–1·11) |
| PE confirmed on imaging or autopsy | 13 (1·0%) | 20 (1·6%) | 0·65 (0·32–1·31) |
| PE on autopsy | 1 (0·1%) | 1 (0·1%) | 1·00 (0·06–16·08) |
| Any DVT or PE | 213 (17·0%) | 232 (18·4%) | 0·91 (0·74–1·11) |
| Skin breaks/ulcers/blisters/skin necrosis | 64 (5·1%) | 16 (1·3%) | 4·18 (2·40–7·27) |
| Lower limb ischaemia/amputation | 7 (0·6%) | 2 (0·2%) | 3·53 (0·73–17·03) |
| Primary outcomes within 14 days | |||
| Post-hoc analysis restricting follow-up to 14 days† | 87 (6·9%) | 95 (7·5%) | .. |
| Unadjusted (dead and missing excluded) | .. | .. | 0·95 (0·70–1·28) |
| Adjusted* (dead and missing excluded) | .. | .. | 0·95 (0·70–1·29) |
Data are number (%) unless otherwise indicated. GCS=graduated compression stockings. DVT=deep vein thrombosis. PE=pulmonary embolism.
Adjusted for delay from onset to randomisation, stroke severity, and leg strength at baseline.
Full compliance by 14 days was 79·4% compared with 73·1% by 30 days.
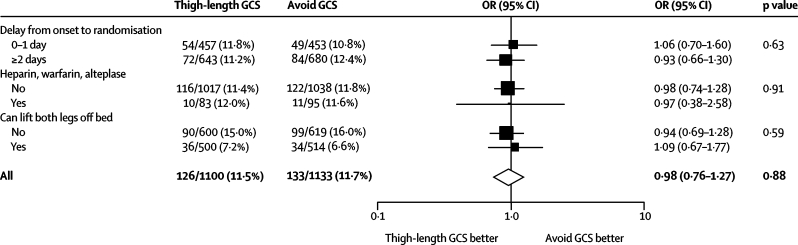
The results of our preplanned subgroup analyses do not suggest any significant interaction between subgroups and treatment effect (figure 2). Table 2 shows the results of some post-hoc analyses restricting follow-up to 14 days, which we undertook to try to understand why the stockings seemed to be ineffective in this acute stroke patient population. The risk of our primary outcome did not differ significantly between treatment groups when follow-up was limited to 14 days (the maximum duration in previous trials in surgical patients), during which time compliance with the thigh-length GCS was better than it was at 30-day follow-up (985 [79·4%] were fully compliant—ie, wore full-length GCS from randomisation to discharge from centre or earlier death or regained mobility—up to 14 days vs 916 [73·1%] up to 30 days).
Figure 2.
Frequency of the primary outcome by allocated treatment in the three prespecified subgroups
The figure shows the point estimates of the odds ratio (adjusted for baseline factors) for each subgroup as a square (with size proportional to the amount of information) and the horizontal line depicts the 95% CIs. The open diamond indicates the adjusted odds ratio with 95% CI for all patients enrolled. The vertical line, at the odds ratio of unity, corresponds to the line of no effect. Odds ratio values of less than unity correspond to a reduction in the primary outcome with graduated compression stockings (GCS). p values are for the interaction between the treatment effect and the subgroup. Patients who died without previous deep vein thrombosis (DVT) and those without either Doppler are excluded from the denominators, which are therefore different to the total number allocated to each treatment group.
Discussion
Findings from this study have shown that thigh-length GCS are not clinically effective at reducing the risk of proximal DVT after stroke and are associated with some adverse effects. This large trial included more patients and outcome events (proximal DVTs) than all previous randomised controlled trials of GCS combined. Patients were enrolled by 64 hospitals in three countries, and had similar baseline characteristics to those of patients admitted to hospitals in many different hospitals and countries, suggesting that our results have good external validity. Central randomisation, outcome-blinded assessment of our primary outcome, low losses to follow-up, and intention-to-treat analysis have kept bias to a minimum. Serious complications due to the GCS were rare, but their inconvenience and associated minor problems suggest these stockings should not be used unless they are associated with clinically significant benefits. Recruitment in CLOTS trial 2, which was designed to establish whether thigh-length GCS are more effective than below-knee GCS, has now been stopped because the results of this study suggest that exposure of patients with acute stroke to the discomfort, inconvenience, and risk of an ineffective treatment is not reasonable.
What might account for the absence of effect in patients with stroke compared with the apparent effectiveness shown by trials in patients undergoing surgery? The precision of our trial result is sufficient to make it very unlikely that we have missed a clinically worthwhile treatment effect. We can reliably exclude an absolute reduction of 3% or greater, in a group of patients with an overall risk of proximal DVT of about 10% in untreated controls.
Trials in patients undergoing surgery have shown fairly convincingly that GCS applied before, during, and after a brief insult to the deep veins of the legs prevent DVTs, mainly in the calf.14 Only eight of the 17 trials in the systematic review reported the frequency of proximal DVT.14 Only nine of 435 (2%) patients allocated GCS and 21 of 402 (5%) allocated to avoid GCS had a proximal DVT (odds reduction 60%; 95% CI 17–81; p=0·014).14 However, although in the CLOTS trial 1 we did not systematically screen for DVTs in the distal veins, we did not record any evidence that GCS were more effective in prevention of distal than proximal DVTs.
In patients with stroke, and those with other acute medical disorders, GCS can be applied only after the patient has become immobile. Immobility might then persist for weeks or even be permanent in such patients. DVTs can develop rapidly and cannot then be prevented as effectively by a treatment starting a few days after the onset of paralysis and immobility. Clearly, we cannot test the effectiveness of GCS applied before stroke onset; however, the absence of heterogeneity (figure 2) between patients enrolled on day 0–1 after stroke onset versus day 2 and after does not provide evidence that this delay is crucial to the effectiveness of GCS.
GCS are thought to reduce the risk of DVT by several mechanisms: by increasing the velocity of venous blood by producing a pressure gradient in the leg, by reducing the cross-sectional area of the deep veins, and by making the calf muscle pump more effective. This last mechanism might not operate in patients with stroke who have severe leg weakness, meaning that we might see less effect in stroke patients with weak legs than in those with residual power. Although the absolute risk of proximal DVT was lower in the 1014 patients who were able to lift both their legs off the bed at baseline than in those who could not (6·9% vs 15·5%), our findings did not show any significant interaction between the effectiveness of GCS and these subgroups (figure 2).
We could argue that patients with stroke but without significant leg weakness are more similar to immobile medical patients than are those undergoing surgery. The absence of effect of GCS, even in the presence of some residual leg movement, suggests that the effectiveness of GCS in acute medical patients cannot be assumed. Further trials in such patients are probably warranted.
Incorrect use and poor compliance with GCS might have reduced their effectiveness. The manufacturers of the GCS used in this trial stress the importance of proper sizing and fitting of their stockings. Sizing depends on measurements of the calf and thigh circumferences and the leg length. There are 18 different sizes of thigh-length GCS and an additional ten with a suspender belt. In view of the complexity of achieving a good fit, a proportion of patients might have been given poorly fitting GCS in the trial. Compliance was good initially but reduced over time, mainly because patients found the GCS uncomfortable or staff became concerned by the condition of the patient's skin. Nonetheless, overall compliance with the thigh-length GCS in this trial was reasonable, and we attempted to continue their use until the patient was discharged or regained mobility. Because of the training delivered to centres and the methods that we used to monitor compliance, we are confident that sizing, fitting, and compliance within the trial were at least as good as in routine practice. In the randomised controlled trials in patients undergoing surgery, GCS were applied only for a few days, and screening for DVT was only done for a maximum of 14 days. Compliance in the CLOTS trial 1 was marginally better over the first 14 days than over 30 days, but we did not note any greater effect when we restricted our analyses to events occurring in the first 14 days (table 2).
With the assumption that the results of the CLOTS trial 2 (which will be available in late 2009) will not show an unexpected result (that below-knee stockings are more effective than are thigh-length ones), this trial provides no evidence to support the routine use of GCS in immobile, hospitalised patients with acute stroke. National stroke guidelines that recommend their use might now need to be updated as a result of this new evidence. In view of the absence of net benefit from heparin or low-molecular-weight heparin in ischaemic stroke,3 future research with data available from this and other trials needs to establish whether there are specific subgroups of patients, who are at greater than average risk of venous thromboembolism and low risk of bleeding complications, who might gain net benefit from anticoagulation. Hopefully, the CLOTS trial 3 will establish whether intermittent pneumatic compression, which does not have associated bleeding risks and can be therefore used in haemorrhagic and ischaemic stroke, is effective in reducing the risk of DVT after stroke.
Acknowledgments
Acknowledgments
The trial was funded by research grants from the Chief Scientist Office of the Scottish Government (reference CZH/4/7), Chest Heart and Stroke Scotland (reference 03/01), and the Medical Research Council of the UK (reference G0200531). The graduated compression stockings were donated to centres by Tyco Healthcare (Covidien) who also trained nursing staff in their sizing, fitting, and monitoring. We thank all the patients and their families who participated in CLOTS, the nursing and radiology staff who assisted at collaborating sites, and the UK stroke research network staff without whom the trial would not have been possible.
Contributors
M Dennis (University of Edinburgh, Edinburgh, UK) participated in the steering committee of the CLOTS trial, commented on a draft of this report, was involved in the design of the trial, and collected, verified, and analysed data. P Sandercock (University of Edinburgh, Edinburgh, UK) participated in the steering committee of the CLOTS trial, commented on a draft of this report, and was involved in the design of the trial. J Reid (Borders General Hospital, Galashiels, UK) participated in the steering committee of the CLOTS trial, commented on a draft of this report, and collected and verified data. C Graham (University of Edinburgh, Edinburgh, UK) participated in the steering committee of the CLOTS trial, commented on a draft of this report, was involved in the design of the trial, and collected, verified, and analysed data. G Murray (University of Edinburgh, Edinburgh, UK) participated in the steering committee of the CLOTS trial, commented on a draft of this report, and was involved in the design of the trial. G Venables (University of Sheffield, Sheffield, UK) participated in the steering committee of the CLOTS trial and commented on a draft of this report. A G Rudd (St Thomas' Hospital, Kings College, London, UK) participated in the steering committee of the CLOTS trial and commented on a draft of this report. G Bowler (Western General Hospital, Edinburgh, UK) participated in the steering committee of the CLOTS trial and commented on a draft of this report. All members of the writing committee listed here have seen and approved the final version of the report.
The CLOTS trials collaboration
Chief Investigator M Dennis.
CLOTS Trial Co-ordinating Centre G Cranswick, A Deary, A Fraser, C Graham, S Grant, A Gunkel, J Hunter, A MacRae, D Perry, V Soosay, C Williams, A Williamson, A Young.
Writing Group M Dennis (Chair), P A G Sandercock, J Reid, C Graham, G Murray, G Venables, A Rudd, G Bowler.
Trial Steering Committee G Cranswick, M Dennis, C Graham, S Lewis, G Murray, J Reid, A Rudd, P A G Sandercock, G Venables (Chair), G Bowler, and observers from the MRC and Tyco Healthcare (Covidien).
National Co-ordinators M G Celani, S Ricci (Italy); R Lindley (Australia).
Auditors/Translators M G Celani (translation), M Hautvast (data audit) M Paterson (data audit), J Reid (audit of positive DVT evidence), T Ting (translation).
Independent Data Monitoring Committee C Baigent (Oxford), J Bamford (Leeds, Chair), J Slattery (London).
Participating centres
We have listed each hospital with the names of the local principal investigator, local coordinator, and other significant contributors who have enrolled patients into the CLOTS trials. The hospitals are grouped by country and ordered depending on the numbers recruited into trial 1. The figure in brackets represents number of patients recruited into trial 1. A (0) indicates centres who only recruited patients into trial 2, which were continuing to recruit at the time of submission.
Australia (24 patients): Westmead Hospital, Westmead (21) N Beydoun, R Lindley, M Romerosa; John Hunter Hospital, New Lambton (3) A Royan, M Russell; St George Hospital, Kogarah (0) P Boers, R Millar; Royal Perth Hospital, Perth (0) A Claxton, G Hankey. Canada (trial 2 centre only): QEII Health Sciences Centre, Halifax Infirmary, Halifax (0) G Gubitz, J Jarrett, K Legg, M MacKay, S Nearing, S Phillips. Czech Republic (trial 2 centre only): District Hospital Pardubice, Pardubice (0) E Ehler, P Geier, M Mrklovský. India (trial 2 centre only): St John's Medical College Hospital, Bangalore (0) A M Anandan, G Kusumakar, J Rosario, A K Roy. Ireland (trial 2 centres only): Midland Regional Hospital at Mullingar, Mullingar (0) C Duffy, E Farrelly, M Jadrnickova, L Masterson, J Morris, S Murphy St Vincent's University Hospital, Dublin (0) M Crowe, I Noone. Italy (214 patients): Universita di Sassari, Sassari (59) M A Fancello, L D Parish, P Pileri, M Pinna, M P Piras, A Pirisi, C Scodino, M L Zedde; Ospedale Beato Giacomo Villa—Citta' della Pieve, Citta della Pieve (45) V Bondo, D Capecchi, M G Celani, S Cupella, L Guerra, A Macchitella, C Ottaviani, E Righetti, C Rossi, M G Scucchi, V Stefanini, A Tufi; Ospedale Citta di Castello—Perugia, Perugia (37) A Barilaro, S Cenciarelli, S Dioguardi, E Gallinella, L Greco, A Mattioni, T Mazzoli, S Ricci Ospedale Maggiore—Bologna, Bologna (35) G Procaccianti; Ospedale A Segni, Ozieri (24) P Beccu, A Del Rio, M Fresu, M A Musselli, A Pala, S Traccis; Policlinico Universitario G Martino, Messina (13) M R Musolino; San Matteo Degli Infermi—Spoleto, Spoleto (1) G S Grasselli; Azienda Ospedaliera Sant' Andrea—Roma, Rome (0) M Rasura; Ospedale di Cattinara—Trieste, Trieste (0) S Chiarandini, F Chiodo Grandi, B Ziani, L Zugna; Ospedale S. Giovanni Battista—Foligno Perugia, Perugia (0) S Stefanucci. Mexico (trial 2 centre only): Instituto Nacional de Neurologia y Neurojrugia MVS, Mexico City (0) A Leyva. Portugal (trial 2 centre only): Hospital de Santa Maria, Lisbon (0) P Canhão, F Falcão, T P Melo. UK (2280 patients): Western General Hospital, Edinburgh (369) M Dennis, E Eadie, S Keir, L Smith, P Taylor, A Thomas, J Wardlaw; Mid Yorkshire Hospitals, Wakefield (123) J Anthony, A Boynes, R Clough, C Colabella, J Collins, K Godson, A McGuiness, K Malinder, A Needle, S Robertshaw, A Sharp, D Skelton, A Stanners, S Tempest, T White, J Wiehl, H Wilkns; Leeds General Infirmary, Leeds (120) J Cooper, A Hassan, M Keeling, P Wanklyn, H Wild; Eastbourne District General Hospital, Eastbourne (104) A Conrad, N Cornford, J Gallagher, F Kirrage, A Mason; St Thomas' Hospital, London (89) F Asare, H Audebert, S Banfield, J Birns, G Cluckie, N Iles, J Leon, A Reindorf, O Roncale, A Roots, A G Rudd, R Sankoh, V Scott, N Smyth; Calderdale Royal Hospital, Halifax (81) L Bury, J Hodgson, N Murray, P Rana, G Seebass, I Shakir, C Whitworth, R Sykes-Elwers; Luton and Dunstable Hospital, Luton (80) G Jutlla, S Ramkumar, L Sekaran, S Sethuraman; Royal Infirmary of Edinburgh, Edinburgh (67) B Chapman, A Cormack, A Coull, S Hart, G Mead, B Morrow; North Tyneside General Hospital, North Shields (66) M Badanhatti, J Cobb, R Curless, J Dickson, K Greenwell, C Price, H Rodgers, M Sudlow; Borders General Hospital, Melrose (63) A Brown, S Haines, M Mckay, A McLaren, J Reid; West Cumberland Hospital, Whitehaven (62) J Frazer, L Haslop, R Jolly, E Lavery, O Orugun, J Starkey, A Young; Weston General Hospital, Weston-super-Mare (53) J Chambers, N Devitt, H Dymond, F Henchie, C Ramsey, G Saunders; Harrogate District Hospital, Harrogate (53) S Boland, S Brotheridge, J Crabtree, C Hare, S Lee, J Strover, G Whil, L White; Bradford Teaching Hospitals NHS Foundation Trust, Bradford (50) I Green, L Johnston, K Lomas, S Maguire, C Patterson, S Riley, S Williamson; Broomfield Hospital, Chelmsford (49) J Blackwell, V Umachandran; University Hospital Aintree, Liverpool (49) J Atherton, E Bacabac, R Durairaj, R Kumar, M Koufali, H Martin, A Sharma, V Sutton; Countess of Chester Hospital, Chester (49) G Abbott, K Chatterjee, C Kelly; Royal Gwent Hospital, Newport (47) K Crook, E A Freeman, C Watkins; Scarborough Hospital, Scarborough (47) S Jamieson, J Paterson, R Rose; Watford General Hospital, Watford (46) M Cottle, D Collas, P Jacob; University Hospitals Coventry and Warwickshire NHS Trust, Coventry (45) P Ray, S Thelwell; Norfolk and Norwich University Hospital, Norwich (43) H Bennett, M Downing, R Fulcher, P McCarthy, K Metcalf, N Wyatt; Gloucestershire Royal Hospital, Gloucester (41) V Cannon, T Chambers, D Dutta, K Harvey, V Wager, G Ward; Hairmyres Hospital, East Kilbride (39) M Dobbin, E Feeley, L Forsyth, F Gardner, B MacInnes, B Martin, C Stirling, B Yip; Worcestershire Royal Hospital, Worcester (34) K Law, P Sanmuganathan, E Stratford; Airedale General Hospital, Keighley (29) A Catto, D Karanwal, K Lindsay, S Mawer, C Orgles, K Smith, K Spence, M White, S Williamson; Ashford and St Peter's Hospitals NHS Trust, Chertsey (29) E Caldwell, C Long, H Ramsay, M J Wrigley; Derbyshire Royal Infirmary, Derby (24) N Brain, R Donnelley, P Gorman, K McLean, K Muhidden, C Roe, F Ryan, N Palin, G Powell, R Wells, E Wright; Royal Bournemouth and Christchurch NHS Trust, Bournemouth (23) O David, D Jenkinson, J Kwan, A Orpen, C Ovington; Queen Elizabeth Hospital, Kings Lynn (20) A Lankester, J Phillips; Lorn and Islands District General Hospital, Oban (20) H Hamilton, F Johnson, S Reilly, K Smeaton; William Harvey Hospital, Ashford (20) L Cowie, D G Smithard; Salford Royal Hospital Foundation NHS, Salford (20) J Barber, A Ingham, C Levick, B Simpson, J Stevens, L Swindells, P Tyrrell, J Wainwright, T Whittle; Royal Preston Hospital, Preston (20) S Duberley, S Punekar; South Manchester University Hospitals NHS Trust, Manchester (19) F Kelly, G E Gamble, S J Welsh; Blackpool Victoria Hospital, Blackpool (18) J McIlmoyle, M J O'Donnell, J Howard, H Goddard; Selly Oak Hospital, Birmingham (18) E Jones, K Law, D Sims; Torbay Hospital, Torquay (16) D Kelly, S Szabo; James Cook University Hospital, Middlesbrough (15) A Atkinson, P Bond, D Broughton, I Tullo; Royal London Hospital, London (15) K Crilly, E Friedman, P Gompertz, K Kee, E Klaasen, T Sachs, M Stoneman, K Upton, T Waters; Queen Margaret Hospital NHS Trust, Dunfermline (13) N Chapman, K McCormick; Queen's Hospital Burton, Burton on Trent (13) B Mherjee, P Tari; Mid Staffordshire NHS FoundationTrust, Stafford (12) B Clamp, A Oke; Queen Elizabeth The Queen Mother Hospital, Margate (12) G Gunathilagan, J Idris, S A Jones, D G Smithard, G Thomas; Southport & Ormskirk Hospital, Southport (11) J Horsley, R Lawrence; Salisbury District Hospital, Salisbury (8) J Cronan, L Harris, D Walters; Hexham General Hospital, Hexham (8) J Robson, A Wright; South Tyneside General Hospital, South Shields (7) J A Graham, J Scott; Stobhill NHS Trust, Glasgow (5) P Fraser, R Graham, C McAlpine, M Shields; Charing Cross Hospital, London (4) M Ellis, H Jenkins, P Sharma, J Slark, N Wilson; Derby City General Hospital, Derby (3) K Muhidden, P Thornton; Raigmore Hospital, Inverness (3) L Campbell, P Findlay; Kent & Canterbury Hospital, Canterbury (3) H S Baht, C Collins; Chesterfield Royal Hospital, Chesterfield (3) M Ball, A Marsh, A Oldfield, S Potter, S Punnoose, P Rose, T Vaughn; Hemel Hempstead Hospital, Hemel Hempstead (1) K Butchard, R Farag; St Mary's Hospital London, London (0) D Ames, A Amorim, J Chataway; Kettering General Hospital, Kettering (0) K Ayes, H Crockatt, P Das, P Lai, L Lavelle, N Peacock; Royal Cornwall Hospital, Truro (0) K Adie, R Bland, G Courtauld, F Harrington, A James, A Mate, C Schofield, C Wroath; Stirling Royal Infirmary, Stirling (0) F Dick, M MacLeod; University Hospital North Durham, Durham (0) E Brown, C Church, P Earnshaw, S Hunter, E Roberts; Fairfield General Hospital, Bury (0) A Bell, C Boyden, L Corrigan, C Curley, J Howard, K Kawafi; King's College Hospital, London (0) A Davis, M Fitzpatrick, L Kalra, R Pathansali, C Potter; Lincoln County Hospital, Lincoln (0) R Brown, S Leach; Doncaster Royal Infirmary, Doncaster (0) N Betts, D K Chadha, L Holford, J Sayles; Sunderland Royal Hospital, Sunderland (0) H Brew, E Brown, C Church, P Earnshaw, J Foster, D Gulliver, D Hindmarsh, S Hunter, J O'Connell, M Reddick, E Roberts; Southend University Hospital, Westcliffe on Sea (0) P Guyler, C Khuoge, A O'Brien; Derriford Hospital, Derriford (0) C Brown, P Dobson, B Hyams, L March, A Mohd Nor; St John's Hospital, Livingston (0) P Bailey, D French, K Jackson, S G Ramsay, L Spence; St Helens and Knowsley Hospital, Prescot (0) R Browne, S Dealing, D Meek, T Smith; St Mary's Hospital Newport, Isle of Wight, Newport (0) E Hakim, U Sinclair; John Radcliffe Hospital, Oxford (0) C Barker, A Buchan, A Flowers, R Hanna, J Hinkle, J Kennedy, G Littlejohn, C Mayell, A McCulloch, R Teal, H Tinamisan, S Webster, M Westwood; Ayr Hospital, Ayr (0) E Barrie, L Birkhead, A Burinski, M Davisdon, A Denham, T Flanninigan, S Ghosh, J Given, M Gormanely, K Hockings, C Hutton, K Kerr, J McCall, P McLaren, L McLean, R McNeil, M Middleton, A Reid, C Somerville, E Todd, C Wallace, C Wells, K Whyte; Barnsley District General Hospital, Barnsley (0) M K Al Bazzaz, K Elliott, K Hawley, L Smith; Yeovil District Hospital, Yeovil (0) N Beacham, C Buckley, S Bulley, D Gibbons, L Jones, C Lawson, S More, K Rashed, S Savage; University Hospital of North Staffordshire, Stoke on Trent (0) R Miller, C Roffe; Aberdeen Royal Infirmary, Aberdeen (0) P Acheampong, M Bruce, M J Macleod; Cumberland Infirmary, Carlisle (0) P Davies, C Walker; Rotherham General Hospital, Rotherham (0) C Draper, J Harris, J Okwera; Brighton and Sussex University Hospital NHS Trust, Haywards Heath (0) K Ali, J Breeds, C Rajkumar, S Walker; Arrowe Park Hospital, Liverpool (0) H Aitken, J Barrett, V Gott, A Lenfesty, V Little, D Lowe, G Sangster, P Weir; Birmingham Heartlands Hospital, Birmingham (0) J McCormack, R Shinton; Nottingham City Hospital, Nottingham (0) M Adrian, P Bath, J Clarke, F Hammonds; Macclesfield District General Hospital, Macclesfield (0) L Butler, C Davison, M Fairhurst, M Horner, F Hussain, C Loughran, M Sein, D Walker; Royal Liverpool University Hospital, Liverpool (0) G Fletcher, C Kearns, S Loharuka; Royal Berkshire Hospital, Reading (0) M Adamson, W E Barrett, H Gange, A Gonzalez, C Gould, S Heaton, L Le Blanc, S Panchalingam, G Pope, PK Tun, A Van Wyk; Newham General Hospital, London (0) K Darawil; Bishop Auckland General Hospital, Bishop Auckland (0) V Baliga, E Brown, L Burnside, S Clayton, A Mehrzad; Belfast City Hospital, Belfast (0) I Wiggam; Kings Mill Hospital, Sutton in Ashfield (0) M Ball, J Sharma; The County Hospital, Hereford (0) C Jenkins, J Powell, J Smith; Royal Edward Albert Infirmary, Wigan (0) T Donlan, S Herath; North Manchester General Hospital, Manchester (0) U Ahmed, B Simpson; George Eliot Hospital NHS Trust, Nuneaton (0) J Egbuji, K Hotchkiss, A M Lappin; Musgrove Park Hospital, Taunton (0) J Ashcroft, L Caudwell, M Farrar, S Gillam, M Hanley, M Hussain, R Larkham, R Norris; Lister Hospital, Stevenage (0) L Butler, P Ghosh, C O'Brien; Manchester Royal Infirmary, Manchester (0) G Subramanian, L Swart; Hull Royal Infirmary, Hull (0) A Abdul-Hamid, J Greig, P Parker, R Rayessa; Bristol Royal Infirmary, Bristol (0) S Caine; Warwick Hospital, Warwick (0) O Khan, S Mountford; Pilgrim Hospital, Boston (0) M Aslam, R Brown, S Duffy, A Hardwick, J Hull, S Leach, C Logan, H Maltby, D Mangion, A Palmer, T Smith; Morriston Hospital, Swansea (0) L Dacey, R Navaratnasingam, M Wani; Northern General Hospital, Sheffield (0) G Dunn, A Jones; York Health Services NHS Trust, York (0) J Coyle, C Croser, C Rhymes; Queens Park Hospital Blackburn, Blackburn (0) N Roberts; Royal United Hospital Bath, Bath (0) L Abey-Koch, V Davis, K O'Brien, L Shaw; Glasgow Royal Infirmary, Glasgow (0) R Graham, P Langhorne, D Stott, M Shields, F Wright; Perth Royal Infirmary, Perth (0) S Johnston, M Stirling; Royal Shrewsbury Hospital, Shrewsbury (0) F Baig, D Bates, H Brown, K Cool, L Ijewsky, J Long, U Sinha; Basingstoke and North Hampshire NHS Foundation Trust, Basingstoke (0) E Giallombardo; University Hospital of Wales, Cardiff (0) D E McCreery, HGM Shetty, LF Smith; University Hospital of Hartlepool, Hartlepool (0) D Bruce, S Crawford; The Lewisham Hospital NHS Trust, London (0) M Patel; Solihull Hospital, Heart of England NHS Trust, Solihull (0) L Deans, K Elfandi, D Greenway, J McCormack, S Stafford; Ulster Hospital, Belfast (0) A McSorley, M Matthews, M Power, R Wright; University College London Hospital, London (0) V Bassan, M Brown, O Browne.
Conflicts of interest
The writing committee declares that they have no conflicts of interest.
Correspondence to: Prof Martin Dennis, Division of Clinical Neurosciences, University of Edinburgh, Bramwell Dott Building, Western General Hospital, Edinburgh EH4 2XU, UK martin.dennis@ed.ac.uk
References
- 1.House of Commons Health Committee . The prevention of venous thromboembolism in hospitalised patients. Second Report of Session 2004–05 Report, HC 99. The Stationery Office; London: 2005. [Google Scholar]
- 2.Kelly J, Rudd A, Lewis RR, Coshall C, Moody A, Hunt BJ. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke. 2004;35:2320–2355. doi: 10.1161/01.STR.0000140741.13279.4f. [DOI] [PubMed] [Google Scholar]
- 3.Sandercock PAG, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;4 doi: 10.1002/14651858.CD000024.pub3. CD000024. [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, Jr, del Zoppo G, Alberts M. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 5.Royal College of Physicians . National Clinical Guidelines for Stroke 2004. Royal College of Physicians; London: 2004. [Google Scholar]
- 6.Scottish Intercollegiate Guidelines Network . Management of patients with stroke. Rehabilitation, prevention and management of complications and discharge planning. A national clinical guideline. Scottish Intercollegiate Guidelines Network, Royal College of Physicians of Edinburgh; Edinburgh: 2002. [Google Scholar]
- 7.Scottish Intercollegiate Guidelines Network . Management of patients with stroke or TIA: assessment, investigation, immediate management and secondary prevention: a national clinical guideline. Scottish Intercollegiate Guidelines Network; Edinburgh: 2008. [Google Scholar]
- 8.Agence Nationale d'Accr'editation et d'Evaluation en Sant'e . Prise en charge initiale des patients adultes atteints d'accident vasculaire c'er'ebral–aspects m'edicaux 2002. Agence Nationale d'Accr'editation et d'Evaluation en Sant'e.Prise (ANAES); Paris: 2002. http://www.anaes.fr (accessed March 22, 2009). [Google Scholar]
- 9.SPREAD (Stroke prevention and educational awareness diffusion), 5th edn . Italian guidelines for stroke prevention and management, 2007. Hyperphar Group SpA; Milan: 2007. http://old.spread.it/SpreadEng/SPREAD_eng_5th.pdf (accessed March 22, 2009). [Google Scholar]
- 10.The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack. 2008. http://www.eso-stroke.org/recommendations.php?cid=9 (accessed March 22, 2009). [DOI] [PubMed]
- 11.National Stroke Foundation (Australian) Acute stroke management. 2007. http://www.strokefoundation.com.au/images/stories/healthprofessionals/clinical%20guidelines%20for%20acute%20stroke%20management.pdf (accessed March 22, 2009).
- 12.New Zealand Guidelines Group . Life after stroke: New Zealand guideline for management of stroke. Best practice evidence-based guideline. Stroke Foundation New Zealand; Wellington: 2003. [Google Scholar]
- 13.National Institute for Health and Clinical Excellence Venous thromboembolism: reducing the risk—full guideline DRAFT. March, 2009. http://www.nice.org.uk/Guidance/CG/Wave14/26 (accessed March 27, 2009).
- 14.Roderick P, Ferris G, Wilson K. Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis. Health Technol Assess. 2005;9:iii–x. doi: 10.3310/hta9490. [DOI] [PubMed] [Google Scholar]
- 15.Kierkegaard A, Norgren L. Graduated compression stockings in the prevention of deep vein thrombosis in patients with acute myocardial infarction. Eur Heart J. 1993;14:1365–1368. doi: 10.1093/eurheartj/14.10.1365. [DOI] [PubMed] [Google Scholar]
- 16.Muir KW, Watt A, Baxter G, Grosset DG, Lees KR. Randomised trial of graded compression stockings for prevention of deep-vein thrombosis after acute stroke. Q J Med. 2000;93:359–364. doi: 10.1093/qjmed/93.6.359. [DOI] [PubMed] [Google Scholar]
- 17.Mazzone C, Chiodo Grandi F, Sandercock PAG, Miccio M, Salvi R. Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database Syst Rev. 2004;4 doi: 10.1002/14651858.CD001922.pub2. CD001922. [DOI] [PubMed] [Google Scholar]
- 18.Kay TW, Martin FI. Heel ulcers in patients with long standing diabetes who wear antiembolism stockings. Med J Aust. 1986;145:290–291. doi: 10.5694/j.1326-5377.1986.tb101128.x. [DOI] [PubMed] [Google Scholar]
- 19.Merrett ND, Hanel KC. Ischaemic complications of graduated compression stockings in the treatment of deep vein thrombosis. Postgrad Med J. 1993;69:232–234. doi: 10.1136/pgmj.69.809.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute stroke: development and validation of new models. Stroke. 2002;33:1041–1047. doi: 10.1161/hs0402.105909. [DOI] [PubMed] [Google Scholar]
- 21.De Silva DA, Pey HB, Wong MC, Chang HM, Chen CP. Deep vein thrombosis following ischemic stroke among Asians. Cerebrovasc Dis. 2006;22:245–250. doi: 10.1159/000094011. [DOI] [PubMed] [Google Scholar]