SUMMARY
Engrailed is a key transcriptional regulator in the nervous system and in the maintenance of developmental boundaries in Drosophila, and its vertebrate homologs regulate brain and limb development. Here, we show that the functions of both of the Hox cofactors Extradenticle and Homothorax play essential roles in repression by Engrailed. Mutations that remove either of them abrogate the ability of Engrailed to repress its target genes in embryos, both cofactors interact directly with Engrailed, and both stimulate repression by Engrailed in cultured cells. We suggest a model in which Engrailed, Extradenticle and Homothorax function as a complex to repress Engrailed target genes. These studies expand the functional requirements for extradenticle and homothorax beyond the Hox proteins to a larger family of non-Hox homeodomain proteins.
Keywords: Drosophila, Embryogenesis, Homeodomain, Transcription, Repressor, Cofactor, Pbx, Meis
INTRODUCTION
The engrailed (en) gene is a homeobox-containing gene crucial for the establishment and maintenance of compartment boundaries during Drosophila development (Akam, 1987; Blair, 1995). Its homologs in mammals are important in brain and limb development, where they might provide a similar function (Augustine et al., 1995; Joyner, 1996; Wurst et al., 1994). The En gene product regulates several key target genes. It helps to maintain the activities of the hedgehog gene and of its own gene (Heemskerk et al., 1991), and it represses the genes wingless (wg) (Heemskerk et al., 1991) and cubitus interruptus (ci) (Eaton and Kornberg, 1990), all of which are involved in the functions of compartment boundaries and the signals they provide to developing tissues (DiNardo et al., 1994).
En carries out these functions, at least in part, by repressing downstream target genes (Smith and Jaynes, 1996; Tolkunova et al., 1998). It has two well-characterized repression domains, one of which binds the Groucho co-repressor complex (Han and Manley, 1993; Jaynes and O’Farrell, 1988; Jaynes and O’Farrell, 1991; Jimenez et al., 1997; Tolkunova et al., 1998). Like many eukaryotic DNA-binding proteins, the specificity of DNA binding by En alone appears to be lower than that required for highly selective interaction with specific target genes. This suggests that, like many other DNA binding proteins, En interacts with cofactors that increase its binding specificity. En has been shown to be capable in vitro of binding cooperatively to DNA with the Extradenticle protein (Peltenburg and Murre, 1996), although a role for this interaction in vivo has not been demonstrated.
The extradenticle (exd) gene is also a homeobox-containing gene that was initially characterized as a mutation causing multiple homeotic transformations in Drosophila, without affecting the expression patterns of the homeotic genes. These observations suggested that it might serve as a cofactor for Hox gene products (Peifer and Wieschaus, 1990). Homeotic transformations are seen in zygotic mutants but, when the maternal contribution to exd function is also removed, embryos show alterations that suggest a loss of en function, including a loss of en gene expression, at later embryonic stages (Peifer and Wieschaus, 1990; Rieckhof et al., 1997). Molecular studies have confirmed the role of Exd as a cofactor for Hox proteins, including a function in altering their specificity of binding to DNA (reviewed in Mann and Chan, 1996). This function appears to be conserved in the mammalian homologs of Exd, the Pbx proteins (Peltenburg and Murre, 1997; Phelan et al., 1995).
A third homeobox gene, Meis1, was discovered as an ecotropic retroviral insertion site in mice (Moskow et al., 1995), where its overexpression in conjunction with a subset of Hox proteins leads to leukemic transformation of hematopoietic stem cells (Nakamura et al., 1996). Meis1 is a mammalian homolog of the Drosophila homothorax gene (hth), which has been shown to regulate the nuclear localization of Exd (Rieckhof et al., 1997) and to form complexes with Hox proteins, including co-complexes with Exd (Ryoo et al., 1999). As with the cofactor functions of Exd, the interactions and functions of the Meis1 family appear to be largely conserved between Drosophila and mammals (Jacobs et al., 1999; Liu et al., 2001; Saleh et al., 2000; Shanmugam et al., 1999; Shen et al., 1999). In mammals, a Hox-class (Antp-class) protein that is part of an endodermally expressed ParaHox cluster (Brooke et al., 1998; Leonard et al., 1993) has also been shown to interact functionally with mammalian homologs of Exd and Hth (Swift et al., 1998).
In this work, we show that En interacts specifically with Exd and Hth in vitro and in cultured cells, that Exd and En mediate co-operative repression in cultured cells, and that the genetic functions of both exd and hth are crucial to the repression activity of En in embryos. We identify sloppy paired (slp) as a direct target gene of En, and show that exd and hth are required for En effectively to repress both slp and a second key target gene, wg, in developing embryos. The involvement of exd and hth function in direct repression by En, in conjunction with the observed molecular interactions, suggest that they are likely to participate in En repression complexes in vivo.
MATERIALS AND METHODS
Yeast two-hybrid and in vitro binding assays
Yeast two-hybrid assays were performed as previously described (Tolkunova et al., 1998), using a lacZ reporter gene. Briefly, strain Y190 was co-transformed with a pAS2 and a pACT2 derivative, and lacZ activity assays were performed on colonies streaked and grown on filters. The experiments shown in Fig. 5A used pACT2-Exd, in which the complete Exd open reading frame (ORF) was inserted, pACT2-Hth, containing the complete Hth ORF, pACT2-Meis1b and pAS2-Meis1b (Steelman et al., 1997), and pAS2-En (Tolkunova et al., 1998). The p53 controls used pAS2-p53, which contains the mouse p53 ORF. Details of plasmid construction are available on request.
Fig. 5.

Engrailed interacts with Extradenticle and Homothorax in yeast, in vitro and in cultured cells. (A) En can interact with both Exd and Hth. In the yeast two-hybrid system, En was tested for interaction with full-length Exd, Hth and mouse Meis1. In each case, the protein listed first (or by itself) was expressed as a fusion with the Gal4 DNA-binding domain (DBD, in pAS2), whereas that listed second was fused with the Gal4 activation domain (in pACT2). En shows a strong signal with Exd and a weaker, but still specific, signal with both Hth and Meis1 (relative to these proteins either alone or in combination with the negative control P53, as indicated). Exd also generates a consistently strong signal with Meis1. (Hth produces a strong signal by itself when fused with the Gal4 DBD, so that similar experiments using it were uninformative.) (B) En interacts directly with both Exd and Meis1 in vitro and the three appear to form a co-complex. Glutathione-S-transferase (GST) fused to full-length En was produced and affinity purified from bacteria, then mixed with in vitro translated Exd (either labeled or unlabeled) and/or Meis1 (labeled), as indicated, and extracted from the mixture using glutathione agarose beads. Proteins captured by the beads were examined by SDS-PAGE and autoradiography. On the left, the single strong band migrates at the correct molecular weight to be full-length Exd. On the right, labeled Meis1 was mixed with either GST-En, GST-En plus unlabeled, in vitro translated Exd, GST alone or GST plus unlabeled Exd, as indicated. The prominent band migrates at the correct molecular weight to be authentic Meis1. (C) En interacts with Hth and Exd in cultured cells. Drosophila S2 cells were transfected with plasmids expressing Hth (panels I-IV, lanes 1-3), Hth plus En (panel V, lanes 1-3) or Hth plus En and His6Exd (‘tagExd’, lanes 4-6, all panels), and nuclear extracts were prepared (see Materials and Methods). Hth-specific antiserum (+, panels I and II) or preimmune serum control (-) was incubated with Protein-A/agarose beads and then with the nuclear extracts. His6-specific monoclonal antibodies (+, panels III-V) or nonspecific IgG control (-) were incubated with Protein-G/Sepharose beads and then with the nuclear extracts. ‘In’ indicates one-fifth of input extract (except panel III, where lanes 1 and 4 are shown at a shorter exposure to allow the En band to be clearly distinguished from a background band that is detected by the anti-En antiserum); P indicates a pellet (bead) fraction. Lanes 1-3 contain extract from the Hth-only (or Hth plus En, panel V only) transfection. Lanes 4-6 contain extract from Hth plus En and His6Exd transfection, analyzed by SDS-PAGE and western blotting, after incubation with control beads (-), anti-Hth (‘α-Hth’) beads (+, panels I and II) or anti-His6 (‘α-tag’) beads (+, panels III-V), followed by extensive washing, followed by detection of either En (panels I, III and V), Exd (panel II) or Hth (panel IV) with specific antisera. Notice that the background band (‘b.g.’) in panels III and V, which migrates faster than En and is present in both extracts, is not precipitated. This band does not appear in panel I because of the use of monoclonal anti-En antibody, whereas polyclonal anti-En antibody was used in panels III and V.
Glutathione-S-transferase (GST) pull-down assays were performed as described previously (Tolkunova et al., 1998). GST-En contains En amino acids 1-544 in pGEX-5X-1; full-length Exd was transcribed from pET15b-Exd, which contains the complete Exd ORF. Full-length Meis1b was expressed from pET28b-Meis1b (Steelman et al., 1997).
Cell culture assays
Transfections of Schneider line 2 (S2) cells were performed as described previously (Jaynes and O’Farrell, 1991). Plasmids used for Fig. 5C were pRM-HA3-hth (encoding full-length Hth, 3 μg per 60 mm plate), pAc-en (Jaynes and O’Farrell, 1988) (8 μg per plate), pPAc-His6Exd (encoding full-length Exd tagged at its N-terminus, driven by the actin5C promoter, 8 μg per plate) and pCaSpeR-hs as filler (to 19 μg total DNA per plate). Nuclear extracts for co-immunoprecipitation (coIP) experiments were performed as described by Han and Manley (1993). For coIPs, antibodies were immobilized on either Protein-A/agarose or Protein-G/sepharose in Buffer A (20 mM HEPES pH 7.9, 150 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 10% glycerol), blocked with non-transfected nuclear extract for 4 hours, incubated with transfected nuclear extract overnight, washed extensively at 23°C with Buffer A plus 0.2% Triton X-100, eluted in Laemmli buffer, and analyzed by western blotting essentially as described by Han and Manley (Han and Manley, 1993), except that specific proteins were visualized using the ECL detection system (Amersham). All incubations were at 4°C unless otherwise indicated.
Plasmids used for Fig. 6 were: (1) pT3CM6-CAT, a chloramphenicol acetyltransferase (CAT)-expressing plasmid that can be activated by glucocorticoid receptor (GR) and is derived from pT3N6D-33CAT (Jaynes and O’Farrell, 1991) by replacing the N6 homeodomain binding sites with a tandem array of six composite En-Exd binding sites (Peltenburg and Murre, 1996) or by mutated versions of this site (as indicated in the text and in the legend for Fig. 6); (2) pTAT3-CAT (Jaynes and O’Farrell, 1991), the equivalent plasmid without En-Exd sites; (3) pRK232 (Heemskerk et al., 1991), an En expression construct; (4) pRM-HA3-hth, a metallothionein-promoter-driven Hth expression construct; and (5) pLac82SU (Dorsett et al., 1989) as a reference. The metallothionein promoter was induced by adding CuCl2 to 0.7 mM 24 h after transfection; the GR was activated by adding triamcinolone acetonide to 10-7 M 48 hours after transfection. Fig. 6A used 1 μg each of pRK232 and either pTAT3-CAT or pT3CM6-CAT, as indicated (without or with En-Exd sites, or with mutated sites), 0.5 μg pRM-HA3-hth, and 0.5 ng pLac82SU per 60 mm culture dish. Fig. 6B used 1 μg each of pT3CM6-CAT and pRK232, 0.04 μg pPAc-GR (Jaynes and O’Farrell, 1991), and 0.5 ng pLac82SU per 60mm culture dish, and either 0.5 (‘low’) or 1 μg (‘hi’) of pRM-HA3-hth where indicated in the figure. Total DNA was normalized to 6 μg per dish in both Fig. 6A and Fig. 6B. Fig. 6C used 0.36 μg pT3CM6-CAT, 0.5 μg pRM-HA3-hth, 0.014 μg pPAc-GR and 0.18 ng pLac82SU per 35 mm culture dish, and either 0.108 (‘low’) or 0.36 μg (‘high’) pRK232, as indicated in the figure. Total DNA was normalized to 2.16 μg per dish. Three hours prior to transfection, each dish was treated as described by Clemens et al. (Clemens et al., 2000) with 24 μg of double-stranded RNA (dsRNA) encoding either Rad9 or Exd.
Fig. 6.
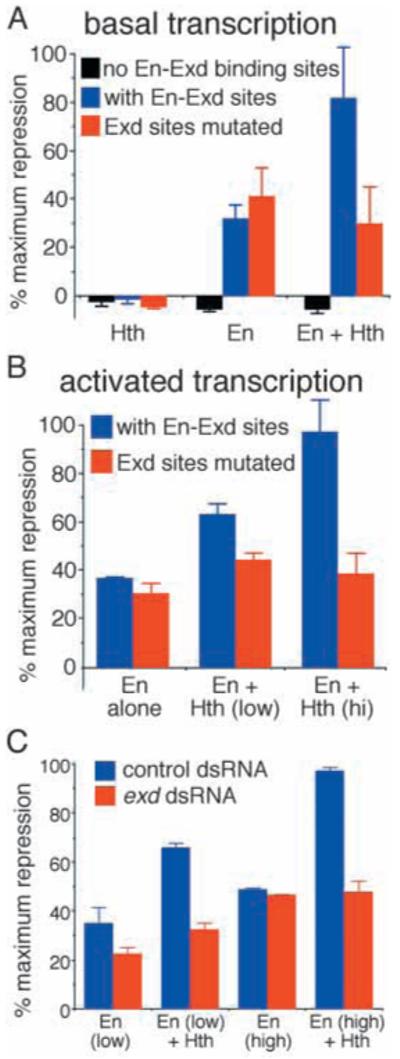
Hth and Exd enhance repression by En in cultured cells. Drosophila S2 cells were transfected with a target gene containing a CAT reporter, binding sites that are bound co-operatively by En and Exd in vitro (oligonucleotide sequence TCGAGTCAATTAAATGATCAATCAATTTCG); or, as indicated in the key, the same plasmid without these sites, or with a two-nucleotide change in an Exd core binding site (oligonucleotide sequence: TCGAGTCAATTAAAGCATCAATCAATTTCG) and activator binding sites. Cells were harvested after 66 hours and assayed for CAT and a co-transfected reference gene. The vertical axis shows results normalized to reference gene activity, as a percentage of the maximum repression observed in each experiment. Maximum repression was eightfold for basal transcription (A) and at least tenfold for activated transcription (B,C). Error bars show the range of values from two parallel transfections. (A) The reporter (either with or without En-Exd sites, or with mutated sites) and reference plasmids were co-transfected with either a Hth expression construct, an En expression construct, or both together. Bars below the baseline indicate the slight activation seen without binding sites. (B) The reporter (with or without Exd sites mutated as indicated in the key), activator and reference plasmids were co-transfected with an En expression construct either alone or in combination with a Hth expression construct at either a low or a higher concentration as indicated (see Materials and Methods for plasmid amounts and other transfection details). There was no effect of Hth alone on activated transcription. (C) The reporter, activator and reference plasmids were co-transfected with the En expression construct (at either a low or higher concentration) either alone or with the Hth expression construct, after a prior treatment of the cells with either control or Exd dsRNA, as indicated (see key).
Drosophila stocks and assays
In situ hybridization was performed as described (Tautz and Pfeifle, 1989). Antibody staining with anti-lacZ, anti-En, anti-Exd (Rieckhof et al., 1997) and female-specific anti-Sex-lethal antibodies (Bopp et al., 1991) was performed as described previously (Kobayashi et al., 2001).
To generate hth mutants with hs-En (Heemskerk et al., 1991), P[hs-En E3D]; hthP2/TM3 Sb ftz-lacZ strains were self-crossed; as controls, P[hs-En E3D]; TM3 Sb ftz-lacZ/+ strains were self-crossed.
To generate exd maternal mutant embryos carrying hs-En, exd1 FRT 18D/FM7-B1 females were crossed with OVOD2 FRT 18D; hs-Flp1 males to generate exd1 FRT 18D/OVOD2 FRT 18D; hs-Flp1/+ females (non-B), which were heat shocked during the wandering larval stage (1 hour at 37°C every 12 hours) to induce mitotic recombination. These females with homozygous mutant germ-line clones were then crossed with either P[hs-En E5] (on the third chromosome) or wild-type (control) males. The exd1 allele (a.k.a. exdXP11) is amorphic (Rauskolb et al., 1995).
To generate exd maternal mutant embryos carrying UAS-en (Tabata et al., 1995), exd1 FRT 18D/FM7-B1;; UAS-en females were crossed with OVOD2 FRT 18D; hs-Flp1; UAS-en males to generate exd1 FRT 18D/OVOD2 FRT 18D; hs-Flp1/+; UAS-en females (non-B), which were heat shocked as above to induce mitotic recombination. These females were then crossed with prd-Gal4/TM3 Sb hb-lacZ males.
Cuticle preparations were performed as described previously (Fujioka et al., 1999). For Fig. 4, embryos were collected and aged for 22 hours at 25°C. Cuticles were prepared and categorized by severity of defects as follows. The number of abdominal denticle bands fused was determined in at least 120 embryos (for each data point) of the genotypes indicated in Fig. 4 and its legend. The categories, from least to most severe, had the following number of denticle bands fused (two bands completely fused together were counted as two bands fused, partial fusions were counted as one band fused): 1-3, 4, 5, 6, 7-8.
Fig. 4.
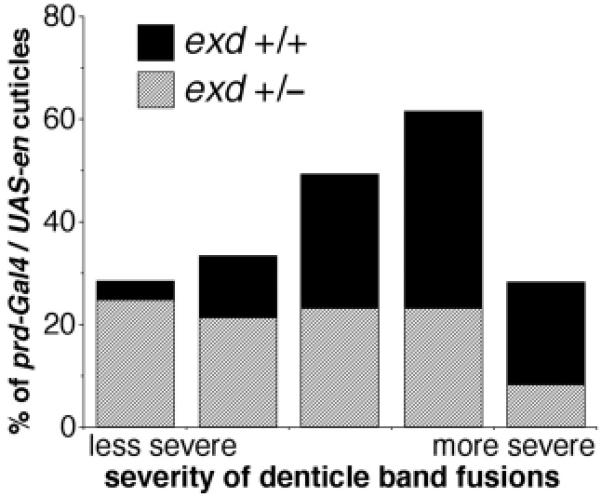
Flies with exd mutations show dose-dependent interactions with ectopically patterned En in embryos. Embryos collected as in Figs 2 and 3 were allowed to develop to the end of embryogenesis, and cuticles were prepared and analyzed. Defects in abdominal denticle bands (mostly pairwise fusions of varying severity) were categorized and tabulated, and the results are shown in a stacked bar graph. As indicated by the percentage of defective cuticles in both the exd+/+ population and the population derived from exd mutant germline clones (data not shown), those embryos that did not receive a copy of the prd-Gal4 driver showed no defects other than those expected from the complete absence of exd function (embryonic cuticles from exd germline clones are completely rescued by one wild-type gene from the father). We did not attempt to analyze exd-null cuticles (which were clearly distinguishable from the exd heterozygotes) for effects of En ectopic expression induced by the driver. Rather, the denticle band fusions caused by ectopic En expression in the population that received a wild-type exd allele from their father were analyzed, and the graph shows the percentage of defects in each category among this population (which are exd+/-, as indicated in the key). Thus, the percentages add to 100% in each case because they include only those cuticles that showed defects caused by ectopic En expression that were of the indicated exd genotype (in each case, these represented the expected overall percentage of cuticles). Notice that when the maternal contribution of exd is removed and the zygotic contribution is simultaneously reduced by half (exd+/-), the overall severity of abdominal cuticle defects in the population caused by ectopic En expression is significantly reduced, indicating a substantial requirement for Exd in En function in the developing abdomen.
RESULTS
exd and hth cooperate with En to repress the direct target gene slp
In the absence of exd or hth function, expression of the endogenous en gene in embryos is unstable and is progressively lost during the middle and later stages of development (Peifer and Wieschaus, 1990; Rieckhof et al., 1997). Therefore, although there is loss of en activity in the absence of exd, this might be secondary to the loss of en expression. To circumvent this ambiguity and to test whether exd and hth are required for the function of En in vivo, we expressed En from transgenes that are independent of exd function. We assayed en activity by examining expression of its target genes, and by examining the consequences for the pattern of embryonic cuticular structures, which is a sensitive function of en activity.
First, we expressed En ubiquitously using a heat shock promoter-driven transgene. This allowed us to focus on a direct target gene of En, based on the immediate response to ectopic En expression. We examined the responses of slp and wg, which are repressed by en, and of the en gene, which is ectopically activated by ubiquitously expressed En (Heemskerk et al., 1991). The gene that responded most rapidly to En was slp, which was noticeably repressed relative to control heat-shocked embryos within 7 minutes of a 5 minute heat pulse (data not shown). Such a rapid response makes it very likely that the response is direct (Manoukian and Krause, 1992; Saulier-Le Drean et al., 1998), and strongly suggests that slp is a direct target of repression by En.
To determine whether repression of slp by En was affected by a loss of hth or exd function, we examined the effect of the strong hth allele hthP2 (Kurant et al., 1998) in combination with a hs-En transgene. As shown in Fig. 1A-E, the loss of hth function clearly reduced the repression of slp by En. This repression of slp expression persisted to later stages of embryogenesis (data not shown), although the degree of persistence varied from embryo to embryo because of the relatively mild induction of ectopic En used here. This mild induction resulted in a level of expression comparable to or less than that in the endogenous En stripes. We also tested whether loss of hth function affected the expression of En from the hs-En transgene by staining embryos with En-specific antiserum. We detected no difference in the strength or persistence of the ectopic En signal between wild-type and hth mutant embryos (data not shown), showing that the repression activity of En is specifically affected in the hth mutants.
Fig. 1.
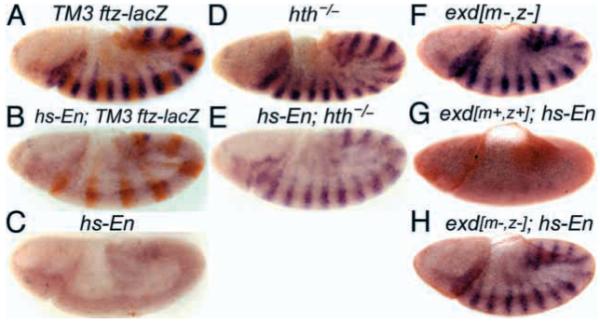
Ubiquitously expressed Engrailed requires exd and hth to repress slp in vivo. Repression of slp by En is abrogated by loss of either hth or exd function. Embryos were collected for 1 hour, aged for 3.5 hours and heat shocked at 37°C for 10 minutes to induce En expression in hs-En embryos, which carry a hs-inducible transgene expressing full-length En). They were then aged for an additional 15 minutes, fixed and stained for slp RNA (dark blue, by in situ hybridization) and for β-galactosidase protein (A,B,D,E) (orange, anti-horseradish-peroxidase stain). lacZ is expressed from a ftz-lacZ transgene on the TM3 balancer chromosome. (A) Wild type (no hs-En, genotype TM3/hthP2 or TM3/TM3, which are indistinguishable). (B) hs-En, TM3 containing (TM3 carries a wild-type hth allele); notice the almost complete repression of slp by hs-En. (C) Control to show that TM3 itself does not affect repression of slp by hs-En (genotype hs-En; +/+, stained in parallel with the others). (D) An hth mutant (genotype hthP2/hthP2, distinguishable in the same population as in A by the absence of β-galactosidase staining). (E) An hth mutant with hs-En (genotype hs-En; hthP2/hthP2, inferred from the absence of β-galactosidase staining; this and the embryo in B were stained together in the same tube). Notice the weak repression of slp relative to B. (F-H) Embryos in F and H, designated ‘mat.exd-’ below (‘m-’ in the figure), were derived from germline clones in the mother that were homozygous for the null exd allele exd1; therefore, no maternally derived Exd was present. These females with exd- germlines were crossed either to wild-type males, to generate the embryo in F, or to males homozygous for a hs-En transgene, to generate the embryo in H. Wild-type females were crossed to the hs-En males to generate the embryo in G. (F) mat.exd-, without hs-En. (G) exd+, with hs-En. (H) mat.exd-, with hs-En. Notice the increase in slp RNA when Exd is missing (H versus G), indicating a requirement for Exd in order for En to efficiently repress slp. These embryos were also stained for the female-specific protein Sex-lethal, to indicate whether they were male or female. Because exd is on the X chromosome, females received a wild-type copy of the exd locus from their fathers, whereas males did not. The embryos shown were male, and so they received their only exd allele from their mother (‘z-’ or ‘z+’). A paternal-only contribution to exd function (that is, in females derived from females with exd- germlines) significantly increased the ability of hs-En to repress slp (data not shown).
Although repression of slp was strongly reduced in hth mutants, it was not abolished. With longer inductions of hs-En expression, slp was more effectively repressed, again consistent with a residual activity in hth mutants (data not shown). A possible explanation for this residual activity is that the hthP2 allele is not a complete null. Alternatively, En might retain some repression activity even in the absence of Hth.
We used the same approach to test whether En activity in embryos requires Exd, using a null allele of exd. As shown in Fig. 1F-H, complete removal of both maternal and zygotic exd function caused a dramatic drop in En repression activity on the slp gene. However, as with hth, there appeared to be residual activity even in the absence of exd function. Thus, although En retains a residual repression activity in vivo without Hth and Exd, its effectiveness is severely reduced in the absence of either.
Repression of both wingless and slp by En is facilitated by exd
To examine further the requirements for exd and hth by En, we expressed En ectopically using the Gal4-UAS system (Brand and Perrimon, 1993), in a pattern that partially overlaps with the expression of two En target genes, wg and slp. This patterned ectopic expression provides an internal control within individual embryos of unrepressed target gene expression, as well as avoiding the potential complicating effects of heat shock. In this system, a transgene (the driver) expresses the Gal4 activator protein in a pattern, and this drives the expression of a responding transgene, in this case UAS-en. Using the prd-Gal4 driver [made by L. Fasano and C. Desplan (see Yoffe et al., 1995)], UAS-en is activated in a striped pattern (Fig. 2B) that covers every other stripe of both wg and slp in the segmented portion of the embryo (wg is expressed within the slp domain, just anterior to each normal en stripe). The effects of this ectopic En expression were assayed in wild-type and exd mutant embryos. The effects on wg expression are shown in Fig. 2. Alternate wg stripes (in even-numbered parasegments) were repressed completely in wild-type embryos (Fig. 2D), whereas repression of these stripes was less complete in embryos that lacked a maternal supply of Exd protein and also had a reduced zygotic gene dose, and appeared to be delayed relative to the wild-type controls (Fig. 2F, and data not shown; notice that some expression remains, particularly in parasegments 0, 2 and 4). In embryos that completely lack exd function (both maternally and zygotically), the situation is complicated by the fact that continued wg expression requires exd. The weakening of wg stripes in exd null embryos might be secondary to the loss of en expression, because en is necessary to maintain hedgehog and wg transcription (Heemskerk et al., 1991; Ingham, 1993). Thus, even without ectopic En expression, wg expression is weaker, particularly in odd-numbered parasegments such as 3 and 5 (Fig. 2G,I). Nevertheless, against this ‘background’ of weakened odd-numbered wg stripes, the ability of prd-Gal4-driven En (expressed only in even-numbered parasegments) to repress wg was significantly reduced. Although this effect was also seen in more-posterior parasegments, the effect was noticeably stronger anterior to the abdomen (particularly in parasegments 0, 2 and 4), where there appeared to be very little repression of wg compared with exd mutant embryos in which En was not ectopically expressed (Fig. 2G-J).
Fig. 2.
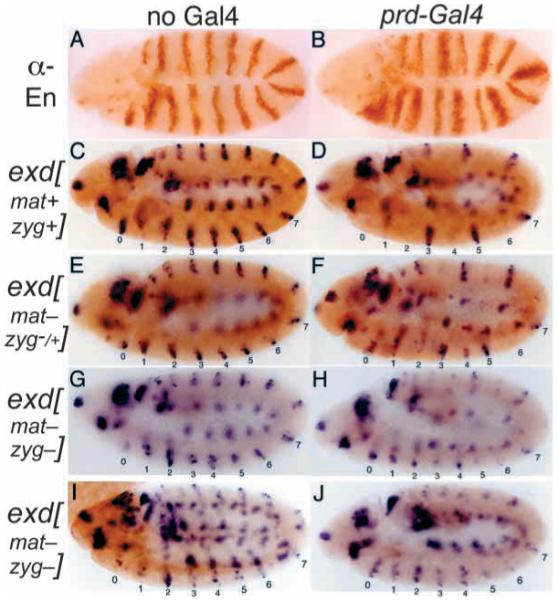
Ectopically patterned En uses exd to repress wg in vivo. All embryos carry a UAS-en transgene. (A) Wild-type embryo stained for En. (B) Embryo carrying a prd-Gal4 driver transgene, in addition to UAS-en. Notice the anteriorly expanded alternate En stripes, which overlap alternate wg and slp stripes (Fig. 3). (C-J) Embryos were derived from either exd+/+ females (C,D) or from females with exd mutant germlines (E-J), and received either a wild-type exd allele (E,F) or no exd allele (G-J; exd is on the X chromosome) from their father. Thus exd[mat+ zyg+] refers to either a homozygous or hemizygous wild-type exd genotype; exd[mat- zyg-/+] refers to heterozygous females that lack a maternal contribution; and exd[mat- zyg-] refers to hemizygous mutants that also lack a maternal contribution. These embryos were double-stained for wg RNA by in situ hybridization and either for Exd (C-H), indicating whether they do or do not have a wild-type exd allele, or for a balancer marker (I,J; hb-lacZ, in brown, indicating absence of the prd-Gal4 driver transgene; lacZ-negative indicates the presence of the driver). Notice that, in exd wild-type embryos, wg is completely repressed by the ectopic En expression within alternate (even-numbered) stripes induced by prd-Gal4, whereas, in heterozygous exd embryos (which lack a maternal exd contribution), this repression is reduced. This effect was greater in embryos lacking all exd function (H,J). J is inferred to be exd mutant because of the weak and incomplete odd-numbered wg stripes that characterize them, as seen in H; H is inferred to contain prd-Gal4 because of the repression of even-numbered wg stripes in the abdomen, as seen in J. Notice the lack of repression of even-numbered stripes (particularly stripes 0, 2 and 4) in H and J, and, to a lesser extent, in F. Staining for En showed no difference in either the pattern or extent of ectopic expression between the wild-type and exd mutant populations (not shown).
In similar collections of embryos, we examined the consequences of loss of exd function on the ability of En to repress slp. As with wg, alternate slp stripes were strongly repressed by prd-Gal4-driven En (Fig. 3A,B). Simultaneously eliminating the maternal contribution and reducing the zygotic dose of exd+ caused a delay in repression and an ultimately less-complete reduction of slp expression (Fig. 3E,F). In embryos completely lacking both maternal and zygotic exd function, repression activity was strongly reduced (Fig. 3G,H), consistent with the results involving ubiquitously expressed En (Fig. 1). As with repression of wg, the repression of slp by En was more dependent on exd function in the gnathal-thoracic region than in the abdomen (Fig. 3H, parasegments 0, 2 and 4 vs 6, 8, 10 and 12). However, even in the abdomen, repression by En was significantly reduced in the absence of exd (Fig. 3G,H vs A,B).
Fig. 3.

Ectopically patterned Engrailed uses exd to repress slp in vivo. Embryos collected as in Fig. 2 were double stained for a balancer marker (hb-lacZ, in brown in C,D, indicating absence of the prd-Gal4 driver transgene; all others are lacZ negative, indicating that they carry the driver) and for slp RNA by in situ hybridization. Notice that, in exd wild-type embryos (A,B), slp is progressively repressed by the ectopic En expression within alternate stripes; in heterozygous exd embryos, which lack a maternal exd contribution (E,F), the repression is delayed and reduced; in embryos that lack all exd function both maternally and zygotically (G,H), this effect is stronger. Because repression is progressive, mutant embryos are shown that are at least as old as the corresponding wild-type controls.
Activity of En in pattern formation is dependent on both exd and hth
To test the significance of the changes in the specific En target genes described above for the overall ability of En to regulate its target genes, we examined the extent to which reduced hth and exd function affected the ability of ectopically expressed En to alter the pattern of structures produced at the end of embryogenesis in the external larval cuticle. First, in hth mutant embryos, we examined the severity of cuticle defects produced by hs-En. As Table 1 shows, the ability of hs-En to cause severe cuticle defects was reduced in hth heterozygotes and homozygotes, suggesting that the observed reductions in En repression activity on the specific target genes wg and slp accurately reflects its overall ability to regulate the target genes responsible for cuticle pattern.
Table 1. hth mutants show dose-dependent interactions with ubiquitous En in embryos*.
| Genotype of parent stock | Defective cuticles (%) | hth-like defects (%) | Severely defective (%) |
|---|---|---|---|
| Wild type (300) | 1 | 0 | 0 |
| hth/TM3 (440) | 22 | 22 (3) | 0 |
| hs-En; +/TM3 (254) | 36 | 0 | 23 |
| hs-En; hth/TM3 (392) | 37 | 34 (16) | 7 |
Cuticle defects caused by ectopically expressed En are abrogated by loss of hth function. Cuticles were prepared after a mild heat shock (7 minutes at 37°C) of embryos from crosses of stocks of the indicated genotype. Those showing defects were categorized as follows: ‘defective’ means having clear-cut fusions or deletions in ventral denticle bands (shortened dorsal cuticles resulting in an abnormally curved overall shape, characteristic of most of the hs-En embryos, were not included); ‘hth-like’ refers to characteristic denticle-band defects caused by loss of hth function, which include posteriorward transformations and loss of denticle diversity, particularly loss of row-1 denticles; ‘severely defective’ means multiple fusions or deletions of ventral denticle bands. Numbers in parentheses under ‘genotype’ indicate the number of embryos examined for each. Numbers in parentheses under ‘hth-like defects’ refer to the percentage showing denticle-band fusions in addition to the ‘hth-like’ defects described above.
The 22% defective cuticles with hth alone is in line with the 1/4 expected to be hth/hth, lacking hth function. Comparing hth alone with hth in combination with hs-En, the defective population increased by only 15%, from 22% to 37% (compared with an increase of 35% for hs-En relative to wild type), and half of this 37% (34%-16%=18%) showed only hth-like defects. Strikingly, the percentage of severely defective cuticles was strongly reduced in the hs-En lines by the removal of hth function. Only 7% of hs-En; hth/TM3 progeny showed severe defects, compared with 23% when hth function was wild type. Overall, it appears that hs-En defects are reduced in severity not only in hth homozygotes but in heterozygotes as well, because a reduction in homozygotes would be expected to reduce the percentage of defects by only 25% (in this case 1/4 of 23%, or ∼6%), whereas the reduction of severe defects is from 23% to 7%, a reduction of about 3/4, consistent with a significant reduction in both homozygotes and heterozygotes. The increase in ‘hth-like’ defects is probably due to the partial overlap in the consequences of low-level, ubiquitous En induction and reduced hth or exd function. For example, as seen in Fig. 2, both loss of exd and ectopic En induction result in decreased wg expression. In spite of this, the combination of the two produced fewer severely defective cuticles than hs-En alone, consistent with the conclusion that hth function is required for hs-En to be fully effective. (This experiment was done using a mild heat shock, to test the effect of hth under conditions expected to be sensitive to modification. Stronger heat shocks produce a much higher percentage of ventral cuticle defects in hs-En embryos, without producing defects in wild-type embryos, data not shown.)
We also quantified the severity of cuticle defects caused by ectopically patterned En expression and compared the results between the exd+/+ and exd+/- populations, which are both wild type in the absence of hs-En. The analysis (Fig. 4) showed that, in the embryo, the reduction in exd activity caused by simultaneously removing maternal exd function and reducing the zygotic gene dose had a significant effect on the ability of prd-Gal4-driven En expression to disrupt the proper development of abdominal cuticular structures. This confirms the developmental impact of the changes in target gene expression characterized above. These results also confirm that En has a significant requirement for exd in the abdomen, as well as in more anterior regions.
En interacts directly with both Exd and Hth
As a first step in determining the mechanisms whereby exd and hth contribute to repression by En in vivo, we examined the possibility of direct interaction. Previous studies had shown that En can bind co-operatively with Exd in vitro to artificial DNA sites (Peltenburg and Murre, 1996). We tested whether a direct En-Exd interaction could also occur in other contexts, using yeast two-hybrid and in vitro assays. We also tested whether En could interact similarly with Hth. Fig. 5A shows the results of two-hybrid assays in yeast. En appeared to interact robustly with Exd in this system, because the signal strength observed with both isolated colonies (data not shown) and colony streaks (Fig. 5A) was consistently higher than that seen with some of our positive controls, including the functionally important interaction between En and Groucho (data not shown) (Tolkunova et al., 1998). This signal was also comparable to that seen with Exd and the mouse homolog of Hth, Meis1 (Fig. 5A; Hth fused with the Gal4 DNA-binding domain gave a high background signal, so that parallel results using Hth with Exd were uninformative). En also gave a somewhat weaker, but apparently specific, signal in combination with either Hth or Meis1 (Fig. 5A).
In vitro, En also interacts specifically with both Exd and Meis1 (Fig. 5B). Here, En fused with GST, but not GST alone, effectively pulls down either Exd or Meis1. Meis1 was used in these studies because of the high level of non-specific interaction observed with in-vitro-translated Hth, perhaps owing to the heterologous nature of the translation system. In this system, it is unlikely that the interactions are due to cooperative binding to DNA, and we interpret these results to mean that these interactions can occur in solution. Furthermore, Meis1 appears to interact more strongly with En in the presence of Exd, suggesting that the three proteins form a co-complex.
We tested whether these molecular interactions can also occur in cultured Drosophila cells (S2 cells). We transfected these cells with a Hth expression plasmid, which induces nuclear localization of endogenously expressed Exd (Rieckhof et al., 1997), either alone or with expression plasmids for En and for a tagged form of Exd (His6Exd, see Materials and Methods). In nuclear extracts from these cultures, anti-Hth antiserum specifically precipitated both En and endogenous Exd, as well as the tagged form of Exd (Fig. 5C). Conversely, anti-His6 antibodies specifically precipitated both En and Hth (Fig. 5C). This ability of either Hth or His6Exd to mediate precipitation of En occurs at a salt concentration of 150 mM and with 0.2% Triton (see Materials and Methods), suggesting that the complexes involved are reasonably stable. However, the interaction of Hth with Exd, which is known to be functionally important, might be somewhat more stable, because the background seen under conditions needed to generate a readily detectable signal is somewhat lower between Hth and endogenous Exd, and between His6Exd and transfected Hth, than that seen with En (notice the slight background visible in the ‘- antibody’ lane at the positions of En and tag-Exd in Fig. 5C, and the lack of any background at the positions of endogenous Exd and Hth). Although the interactions seen with En might be weaker than those between Exd and Hth, they are nonetheless strong enough to suggest the direct involvement of Exd and Hth in repression by En in vivo.
Hth and Exd augment repression by En in cultured cells
As a first step in assessing the influence of the physical interactions identified above on the transcriptional activity of En, we used a previously constructed co-operative binding site for En and Exd (Peltenburg and Murre, 1996) as a target site in transfection assays. Repression by En has been extensively studied in Drosophila S2 cells, which have been shown to express Exd, but not Hth, constitutively (Rieckhof et al., 1997). Co-operative binding sites were inserted into a reporter vector previously shown to be unresponsive to En in the absence of inserted sites (Jaynes and O’Farrell, 1991) and their responses to En and to En in combination with Hth were measured. Consistent with previous results, En was able to repress this artificial target gene only when it contained the inserted sites and, without these sites, neither Hth alone nor En plus Hth caused any repression (Fig. 6A). Importantly, with the binding-site-containing reporter, the presence of Hth significantly increased the effectiveness of repression by En, either on the basal expression level of the target gene (Fig. 6A) or, in an established assay for active repression, when this gene was activated from separate activator binding sites (Fig. 6B). Because Hth has been shown to induce the nuclear localization of endogenously expressed Exd in these cells (Abu-Shaar et al., 1999), this might be due to an increased nuclear concentration of En-Exd complexes, to a Hth-En interaction or to a combination of the two.
In order to test whether the increase in repression by En caused by expression of Hth was mediated by cooperative binding between En and Exd, we tested mutated versions of the cooperative binding site. A two-nucleotide change in an Exd core consensus binding sequence (TGAT) within the 30-nucleotide oligonucleotide has previously been shown to abolish co-operative binding by En and Exd (van Dijk, 1994). As shown in Fig. 6, this Exd-site-mutated target gene still responded to En, but the increased repression caused by Hth was largely absent. Although there was no apparent increase in repression of basal transcription caused by Hth with this mutated target gene, some residual increase in repression of activated transcription appeared to persist. This was true even when the other core Exd consensus binding sequences (ATCA) within the oligonucleotide were also mutated (data not shown). Thus, eliminating co-operative binding between Exd and En significantly reduced the cooperative repression caused by Hth expression, suggesting that this effect of Hth is mediated at least in part by co-operative binding between Exd and En, whereas a secondary effect of Hth expression on repression of activated transcription might be independent of such cooperative binding. Consistent with previous results (Jaynes and O’Farrell, 1991), when the En core consensus sequence ATTA was mutated to AGGA, repression by En, or by En plus Hth, was eliminated (data not shown).
To test the influence of Exd more directly, we turned to RNA interference (Clemens et al., 2000) to reduce endogenous Exd levels in S2 cells. As shown in Fig. 6C, treatment of S2 cells with Exd dsRNA reduced En-mediated repression from the cooperative binding sites significantly, relative to non-specific dsRNA. This effect was not seen at higher concentrations of En, possibly owing to saturating levels of En. However, the increase in repression by Hth was clearly reduced by Exd dsRNA at both high and low En concentrations, suggesting that Hth and Exd cooperate to increase the repression activity of En.
Although these results might be accounted for by a combination of Hth-induced nuclear localization of Exd and co-operative binding by Exd and En to the target gene, the observed ability of En to form complexes with both Hth and Exd suggest the simple model that repression by En alone is less effective than repression by a trimolecular complex of En, Exd and Hth.
DISCUSSION
We show here that En, a non-Hox homeodomain protein with multiple roles in development, is dependent on the functions of the Hox cofactors exd and hth to repress target genes in vivo. Molecular studies demonstrate the ability of En to form complexes with Hth and Exd in vitro, in yeast and in Drosophila cells, leading to the model that complexes among these genetic partners carry out repression in vivo. This model is supported by the ability of Exd and Hth to facilitate repression by En from co-operative binding sites in co-transfection assays. Previously, Hth and Exd were known to act as cofactors only for Hox proteins. This work therefore sheds new light on how En might achieve target specificity in vivo, and expands the diversity of transcriptional regulators that require hth and exd function. Below, we discuss the evidence supporting this model, as well as its implications.
Exd and Hth form complexes with Engrailed
En can form complexes with both Exd and Hth (as well as its mammalian homolog Meis1) in vitro, in yeast and in cultured cells (Fig. 5). Interestingly, rather than the two-way interactions being mutually exclusive, En appears to be able to interact simultaneously with Exd and Meis1 in vitro (Fig. 5B). In extracts from transfected cultures, En is specifically precipitated by antisera against both Exd and Hth (Fig. 5C). Therefore, En might interact with these cofactors similarly to Hox proteins, which have been shown to form such three-way complexes (Ferretti et al., 2000; Jacobs et al., 1999; Ryoo et al., 1999).
In cultured Drosophila cells, Exd and Hth cooperate with En to repress transcription. Using a co-operative binding site for Exd and En (Peltenburg and Murre, 1996) to construct an En-responsive target gene, we found that both Exd and Hth are required for full repression activity (Fig. 6). When a mutation was introduced into an Exd consensus binding sequence that eliminates co-operative binding, co-operative repression was largely eliminated, whereas mutating the En consensus binding sequence eliminated repression. This, along with the fact that RNA interference directed against Exd mRNA also largely eliminated co-operative repression (Fig. 6C), suggests that a complex containing Exd and En is responsible for the cooperative repression caused by coexpression of Hth and En (Exd is constitutively expressed in these cells). Because Hth regulates the nuclear localization of Exd, it can allow Exd-En repression complexes to form in the nucleus. In addition, the observed molecular interactions suggest that the fully active repression complex might include all three proteins.
Exd cooperate s with En to repress target genes and to pattern embryos
Loss of exd function has been shown to result in a loss of en expression at later embryonic stages (Rieckhof et al., 1997). Because en function is required to maintain its own expression (Heemskerk et al., 1991), the loss of en expression could be a downstream effect of a loss of en function, or it could be due to some other consequence of the lack of exd. This ambiguity concerning the role of exd in en function led us to investigate whether the activities of ectopically expressed En (Heemskerk et al., 1991) are dependent on exd function. We expressed En ectopically in two ways: from a heat-shock promoter and using a patterned Gal4 ‘driver’ transgene. An advantage of the former approach is that one can often distinguish between immediate and secondary downstream effects based on how rapidly they occur following heat induction. Advantages of the second approach include having normal and altered expression in parts of the same embryo, providing a rigorous internal control. Both of these approaches led to similar conclusions (Figs 1-4), that exd function is important for the repression by En of its direct target gene slp, that wg also shows a strong dependence on exd function for its repression by En and that the ability of En to alter the pattern of embryonic cuticles is sensitive to the gene dosage of exd. Further, in each set of experiments, the observed dependence of repression on exd was accompanied by a residual repression activity when exd function was removed both maternally and zygotically. This residual exd-independent repression activity might be due to the ability of En to bind to target sites independently of exd but with a reduced affinity, or it could be accounted for by the existence of two classes of binding sites, one exd dependent and the other exd independent. This possibility is paralleled by the relationship of Exd with Ubx, which has been shown to function either co-operatively with Exd or alone on multiple binding sites in target genes (Galant et al., 2002). Alternatively, exd might be exerting an indirect effect on repression by En. However, because Exd forms complexes with En in yeast and in vitro, and because it appears to facilitate repression by En directly in cultured cells, it seems likely that the dependence of En on exd function in vivo is due at least in part to the direct action of En-Exd complexes. Confirmation of this model will require the analysis of specific regulatory sites, which have not yet been identified, in target genes such as slp. If this model is correct then our results suggest that the repression activity of Exd-En complexes might come exclusively from En repression domains (Han and Manley, 1993; Jaynes and O’Farrell, 1991; Jimenez et al., 1997; Smith and Jaynes, 1996; Tolkunova et al., 1998), because Exd has been shown to act as a cofactor in the activation of target genes in vivo in conjunction with Hox proteins (reviewed in Mann and Chan, 1996; Grieder et al., 1997; Inbal et al., 2001; Liu et al., 2001; Pinsonneault et al., 1997; Ryoo et al., 1999).
The effects of eliminating exd function on repression by En appear to be different in the abdomen and the more-anterior regions (Figs 2, 3), in that En is less dependent on exd in the abdomen (parasegments 6-12). Similar results have recently been obtained by Alexandre and Vincent (Alexandre and Vincent, 2003), as described in their accompanying paper. One possible explanation is that hth can provide the observed exd-independent activity. However, in exd mutants, Hth levels are reduced, probably because Hth protein is less stable without Exd (Abu-Shaar et al., 1999). Nevertheless, our data are consistent with the possibility that, on their own, either Hth or Exd might provide partial cofactor activity, whereas both together might be required for full activity. The latter possibility is suggested by our observation that maximal repression activity in S2 cells requires all three gene products.
An additional possibility to account for the residual exd- and hth-independent repression activity of En in the abdomen is that other cofactors assist En in binding to its target genes in the abdomen. If there are other cofactors at work, it is likely that their activity (or expression) is dependent, either directly or indirectly, on the Hox genes Ubx and abd-A, because these genes are responsible for all known aspects of differential segment identity in this region of the embryo. This expectation has been directly confirmed by Alexandre and Vincent (Alexandre and Vincent, 2003).
It is noteworthy that the difference in the dependence of En on exd in the abdomen versus the thorax is seen only after stage 9 (for example, it is not seen in Fig. 1), when the levels of Hth, and the consequent nuclear concentration of Exd, have declined in the abdomen (Rieckhof et al., 1997). Thus, the dependence of En on exd parallels the nuclear concentration of Exd, and might reflect an evolutionary adaptation to the changing levels of Exd in different regions of the embryo.
Requirements for hth and exd in En activity are similar in vivo
Hth has been shown to act in part through its facilitation of the nuclear localization of Exd, and strong hth and exd mutants have very similar phenotypes (Rieckhof et al., 1997). Although Hth can also interact with En independently of Exd (Fig. 5), transfection assays in cultured cells suggest that Hth might depend entirely on Exd for its ability to increase repression by En, at least from artificial En-Exd co-operative binding sites (Fig. 6C). Because Hth forms complexes with En in these cells (Fig. 5C), in addition to increasing its repression activity (Fig. 6A,B), a simple model is that maximal repression activity is due to complexes containing En, Exd and Hth. However, we cannot rule out the possibility that Hth acts solely by making Exd available to interact with En on target sites, through its ability to bring Exd into the nucleus.
We tested whether the repression activity of ectopically expressed En in vivo is dependent on hth function, using assays similar to those used for exd (Fig. 1, Table 1). In each case, we observed a close similarity to results with exd mutants. En activity showed a strong dependence on hth function, although residual activity remained in hth mutants. In addition, En activity showed a sensitivity to the hth gene dose. All of these results are consistent with the effects of Hth being exerted through its effect on Exd nuclear localization, provided that the nuclear targeting of Exd is necessary for its ability to function with En. However, as noted above, Hth might also increase the effectiveness of En repression directly, by forming complexes with En and/or as part of En-Exd complexes. A detailed analysis of a number of in vivo target sites will be necessary to distinguish among these possibilities.
New exd and hth functions
Exd and Hth are essential to the correct regulation of target genes by the homeodomain proteins of the Hox clusters (reviewed in Mann and Affolter, 1998). However, their functional interactions have not previously been shown to extend beyond the highly restricted subset of homeodomain proteins that are found within the Hox clusters (the Antp, Abd-B and Labial classes). The identification of functional interactions with En suggests that exd and hth might provide functional specificity in conjunction with other non-Hox-class homeodomain proteins.
The identification of slp as a direct target gene of En has implications for the mechanism by which En helps to maintain the activity of its own and other genes, including hedgehog, within its domains of expression, the posterior compartments. The slp locus produces two closely related, coordinately regulated gene products (Slp1 and Slp2), which have essentially indistinguishable functions (Cadigan et al., 1994; Grossniklaus et al., 1992). They are forkhead-domain transcription factors (Grossniklaus et al., 1992) that repress en expression (Cadigan et al., 1994), and both contain a conserved motif (homology region II) (Grossniklaus et al., 1992) that is similar to the Groucho-binding domain of En (Tolkunova et al., 1998). Slp1 has also been shown to bind the Groucho corepressor in vitro (Kobayashi et al., 2001), suggesting that it is a repressor and therefore that its action on the en gene is likely to be direct. Thus, the mechanism of en autoregulation, as well as the ability of En to activate other target genes, is likely to be due, at least in part, to an indirect effect of repression of slp expression. Similar conclusions have been reached by Alexandre and Vincent (Alexandre and Vincent, 2003), as described in the accompanying paper. In addition, En might activate target genes indirectly by repressing other repressors that are also normally excluded from its expression domain, such as Odd-skipped (Mullen and DiNardo, 1995; Saulier-Le Drean et al., 1998) and the repressor form of Cubitus interruptus (Eaton and Kornberg, 1990).
Although there have been previous suggestions that Exd and Hth might participate in active repression as well as activation complexes (Abu-Shaar and Mann, 1998; Abzhanov et al., 2001; Manak et al., 1994; White et al., 2000), most of the well-characterized direct Exd-Hth-Hox target genes are activated in an exd- or hth-dependent fashion (Manak et al., 1994; Pinsonneault et al., 1997; Ryoo and Mann, 1999; Ryoo et al., 1999). In fact, these observations raised the question of whether Exd and Hth might be dedicated to gene activation (Pinsonneault et al., 1997). Recently, Hth and Exd have been shown to act directly with Ubx to repress the Hox target gene Distalless in the Drosophila abdomen (Gebelein et al., 2002). The partnership with En in repression further argues that these cofactors can increase the target site discrimination of homeodomain proteins without restricting the resulting transcriptional activity to activation alone. Based on these results, we suggest that Hth and Exd increase the target-site discrimination of several classes of homeodomain proteins and that they do so without defining the transcriptional activity of the resulting protein complex.
Acknowledgments
We thank J. Montgomery and A. M. Buchberg for Meis1 plasmids, P. Schedl for anti-Sex Lethal antibodies, C. Girdham and P. O’Farrell for anti-En antibodies, G. L. Yusibova, Jian Zhou, and Y. Emi-Sarker for excellent technical assistance, and A. M. Buchberg and A. Mazo for helpful comments on the manuscript. This work was supported by NIH (GM50231) and NSF (0110856) awards to J.B.J. and by NIH and Leukemia and Lymphoma Society of America awards to R.S.M.
REFERENCES
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Abu-Shaar M, Ryoo HD, Mann RS. Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev. 1999;13:935–945. doi: 10.1101/gad.13.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abzhanov A, Holtzman S, Kaufman TC. The Drosophila proboscis is specified by two Hox genes, proboscipedia and Sex combs reduced, via repression of leg and antennal appendage genes. Development. 2001;128:2803–2814. doi: 10.1242/dev.128.14.2803. [DOI] [PubMed] [Google Scholar]
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. [PubMed] [Google Scholar]
- Alexandre C, Vincent J-P. Requirements for transcriptional repression and activation by Engrailed in Drosophila embryos. Development. 2003;130:729–739. doi: 10.1242/dev.00286. [DOI] [PubMed] [Google Scholar]
- Augustine KA, Liu ET, Sadler TW. Antisense inhibition of engrailed genes in mouse embryos reveals roles for these genes in craniofacial and neural tube development. Teratology. 1995;51:300–310. doi: 10.1002/tera.1420510506. [DOI] [PubMed] [Google Scholar]
- Blair SS. Compartments and appendage development in Drosophila. BioEssays. 1995;17:299–309. doi: 10.1002/bies.950170406. [DOI] [PubMed] [Google Scholar]
- Bopp D, Bell LR, Cline TW, Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991;5:403–415. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brooke NM, Garcia-Fernandez J, Holland PW. The ParaHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature. 1998;392:920–922. doi: 10.1038/31933. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Grossniklaus U, Gehring WJ. Functional redundancy: the respective roles of the two sloppy paired genes in Drosophila segmentation. Proc. Natl. Acad. Sci. USA. 1994;91:6324–6328. doi: 10.1073/pnas.91.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, Dixon JE. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S, Heemskerk J, Dougan S, O’Farrell PH. The making of a maggot: patterning the Drosophila embryonic epidermis. Curr. Opin. Genet. Dev. 1994;4:529–534. doi: 10.1016/0959-437x(94)90068-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D, Viglianti GA, Rutledge BJ, Meselson M. Alteration of hsp82 gene expression by the gypsy transposon and suppressor genes in Drosophila melanogaster. Genes Dev. 1989;3:454–468. doi: 10.1101/gad.3.4.454. [DOI] [PubMed] [Google Scholar]
- Eaton S, Kornberg TB. Repression of ci-D in posterior compartments of Drosophila by engrailed. Genes Dev. 1990;4:1068–1077. doi: 10.1101/gad.4.6.1068. [DOI] [PubMed] [Google Scholar]
- Ferretti E, Marshall H, Popperl H, Maconochie M, Krumlauf R, Blasi F. Segmental expression of Hoxb2 in r4 requires two separate sites that integrate cooperative interactions between Prep1, Pbx and Hox proteins. Development. 2000;127:155–166. doi: 10.1242/dev.127.1.155. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Analysis of an even-skipped rescue transgene reveals both composite and discrete neuronal and early blastoderm enhancers, and multi-stripe positioning by gap gene repressor gradients. Development. 1999;126:2527–2538. doi: 10.1242/dev.126.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant R, Walsh CM, Carroll SB. Hox repression of a target gene: extradenticle-independent, additive action through multiple monomer binding sites. Development. 2002;129:3115–3126. doi: 10.1242/dev.129.13.3115. [DOI] [PubMed] [Google Scholar]
- Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Dev. Cell. 2002;3:487–498. doi: 10.1016/s1534-5807(02)00257-5. [DOI] [PubMed] [Google Scholar]
- Grieder NC, Marty T, Ryoo HD, Mann RS, Affolter M. Synergistic activation of a Drosophila enhancer by HOM/EXD and DPP signaling. EMBO J. 1997;16:7402–7410. doi: 10.1093/emboj/16.24.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus U, Pearson RK, Gehring WJ. The Drosophila sloppy paired locus encodes two proteins involved in segmentation that show homology to mammalian transcription factors. Genes Dev. 1992;6:1030–1051. doi: 10.1101/gad.6.6.1030. [DOI] [PubMed] [Google Scholar]
- Han K, Manley JL. Functional domains of the Drosophila Engrailed protein. EMBO J. 1993;12:2723–2733. doi: 10.1002/j.1460-2075.1993.tb05934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk J, DiNardo S, Kostriken R, O’Farrell PH. Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature. 1991;352:404–410. doi: 10.1038/352404a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbal A, Halachmi N, Dibner C, Frank D, Salzberg A. Genetic evidence for the transcriptional-activating function of Homothorax during adult fly development. Development. 2001;128:3405–3413. doi: 10.1242/dev.128.18.3405. [DOI] [PubMed] [Google Scholar]
- Ingham PW. Localized hedgehog activity controls spatial limits of wingless transcription in the Drosophila embryo. Nature. 1993;366:560–562. doi: 10.1038/366560a0. [DOI] [PubMed] [Google Scholar]
- Jacobs Y, Schnabel CA, Cleary ML. Trimeric association of Hox and TALE homeodomain proteins mediates Hoxb2 hindbrain enhancer activity. Mol. Cell. Biol. 1999;19:5134–5142. doi: 10.1128/mcb.19.7.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes JB, O’Farrell PH. Activation and repression of transcription by homoeodomain-containing proteins that bind a common site. Nature. 1988;336:744–749. doi: 10.1038/336744a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes JB, O’Farrell PH. Active repression of transcription by the Engrailed homeodomain protein. EMBO J. 1991;10:1427–1433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez G, Paroush Z, Ish-Horowicz D. Groucho acts as a corepressor for a subset of negative regulators, including Hairy and Engrailed. Genes Dev. 1997;11:3072–3082. doi: 10.1101/gad.11.22.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL. Engrailed, Wnt and Pax genes regulate midbrain-hindbrain development. Trends Genet. 1996;12:15–20. doi: 10.1016/0168-9525(96)81383-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Goldstein RE, Fujioka M, Paroush Z, Jaynes JB. Groucho augments the repression of multiple Even skipped target genes in establishing parasegment boundaries. Development. 2001;128:1805–1815. doi: 10.1242/dev.128.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurant E, Pai CY, Sharf R, Halachmi N, Sun YH, Salzberg A. dorsotonals/homothorax, the Drosophila homologue of meis1, interacts with extradenticle in patterning of the embryonic PNS. Development. 1998;125:1037–1048. doi: 10.1242/dev.125.6.1037. [DOI] [PubMed] [Google Scholar]
- Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy MR. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol. Endocrinol. 1993;7:1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- Liu Y, MacDonald RJ, Swift GH. DNA binding and transcriptional activation by a PDX1.PBX1b.MEIS2b trimer and cooperation with a pancreas-specific basic helix-loop-helix complex. J. Biol. Chem. 2001;276:17985–17993. doi: 10.1074/jbc.M100678200. [DOI] [PubMed] [Google Scholar]
- Manak JR, Mathies LD, Scott MP. Regulation of a decapentaplegic midgut enhancer by homeotic proteins. Development. 1994;120:3605–3619. doi: 10.1242/dev.120.12.3605. [DOI] [PubMed] [Google Scholar]
- Mann RS, Affolter M. Hox proteins meet more partners. Curr. Opin. Genet. Dev. 1998;8:423–429. doi: 10.1016/s0959-437x(98)80113-5. [DOI] [PubMed] [Google Scholar]
- Mann RS, Chan SK. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- Manoukian AS, Krause HM. Concentration-dependent activities of the even-skipped protein in Drosophila embryos. Genes Dev. 1992;6:1740–1751. doi: 10.1101/gad.6.9.1740. [DOI] [PubMed] [Google Scholar]
- Moskow JJ, Bullrich F, Huebner K, Daar IO, Buchberg AM. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol. Cell. Biol. 1995;15:5434–5443. doi: 10.1128/mcb.15.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, DiNardo S. Establishing parasegments in Drosophila embryos: roles of the odd-skipped and naked genes. Dev. Biol. 1995;169:295–308. doi: 10.1006/dbio.1995.1145. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Largaespada DA, Shaughnessy JD, Jr, Jenkins NA, Copeland NG. Cooperative activation of Hoxa and Pbx1-related genes in murine myeloid leukaemias. Nat. Genet. 1996;12:149–153. doi: 10.1038/ng0296-149. [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. Mutations in the Drosophila gene extradenticle affect the way specific homeodomain proteins regulate segmental identity. Genes Dev. 1990;4:1209–1223. doi: 10.1101/gad.4.7.1209. [DOI] [PubMed] [Google Scholar]
- Peltenburg LT, Murre C. Engrailed and Hox homeodomain proteins contain a related Pbx interaction motif that recognizes a common structure present in Pbx. EMBO J. 1996;15:3385–3393. [PMC free article] [PubMed] [Google Scholar]
- Peltenburg LT, Murre C. Specific residues in the Pbx homeodomain differentially modulate the DNA-binding activity of Hox and Engrailed proteins. Development. 1997;124:1089–1098. doi: 10.1242/dev.124.5.1089. [DOI] [PubMed] [Google Scholar]
- Phelan ML, Rambaldi I, Featherstone MS. Cooperative interactions between HOX and PBX proteins mediated by a conserved peptide motif. Mol. Cell. Biol. 1995;15:3989–3997. doi: 10.1128/mcb.15.8.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault J, Florence B, Vaessin H, McGinnis W. A model for extradenticle function as a switch that changes HOX proteins from repressors to activators. EMBO J. 1997;16:2032–2042. doi: 10.1093/emboj/16.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C, Smith KM, Peifer M, Wieschaus E. extradenticle determines segmental identities throughout Drosophila development. Development. 1995;121:3663–3673. doi: 10.1242/dev.121.11.3663. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Nuclear translocation of Extradenticle requires homothorax, which encodes an Extradenticle-related homeodomain protein. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Mann RS. The control of trunk Hox specificity and activity by Extradenticle. Genes Dev. 1999;13:1704–1716. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Marty T, Casares F, Affolter M, Mann RS. Regulation of Hox target genes by a DNA bound Homothorax/Hox/Extradenticle complex. Development. 1999;126:5137–5148. doi: 10.1242/dev.126.22.5137. [DOI] [PubMed] [Google Scholar]
- Saleh M, Huang H, Green NC, Featherstone MS. A conformational change in PBX1A is necessary for its nuclear localization. Exp. Cell Res. 2000;260:105–115. doi: 10.1006/excr.2000.5010. [DOI] [PubMed] [Google Scholar]
- Saulier-Le Drean B, Nasiadka A, Dong J, Krause HM. Dynamic changes in the functions of Odd-skipped during early Drosophila embryogenesis. Development. 1998;125:4851–4861. doi: 10.1242/dev.125.23.4851. [DOI] [PubMed] [Google Scholar]
- Shanmugam K, Green NC, Rambaldi I, Saragovi HU, Featherstone MS. PBX and MEIS as non-DNA-binding partners in trimeric complexes with HOX proteins. Mol. Cell. Biol. 1999;19:7577–7588. doi: 10.1128/mcb.19.11.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WF, Rozenfeld S, Kwong A, Kom ves LG, Lawrence HJ, Largman C. HOXA9 forms triple complexes with PBX2 and MEIS1 in myeloid cells. Mol. Cell. Biol. 1999;19:3051–3061. doi: 10.1128/mcb.19.4.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ST, Jaynes JB. A conserved region of engrailed, shared among all en-, gsc-, Nk1-, Nk2- and msh-class homeoproteins, mediates active transcriptional repression in vivo. Development. 1996;122:3141–3150. doi: 10.1242/dev.122.10.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman S, Moskow JJ, Muzynski K, North C, Druck T, Montgomery JC, Huebner K, Daar IO, Buchberg AM. Identification of a conserved family of Meis1-related homeobox genes. Genome Res. 1997;7:142–156. doi: 10.1101/gr.7.2.142. [DOI] [PubMed] [Google Scholar]
- Swift GH, Liu Y, Rose SD, Bischof LJ, Steelman S, Buchberg AM, Wright CV, MacDonald RJ. An endocrine-exocrine switch in the activity of the pancreatic homeodomain protein PDX1 through formation of a trimeric complex with PBX1b and MRG1 (MEIS2) Mol. Cell. Biol. 1998;18:5109–5120. doi: 10.1128/mcb.18.9.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development. 1995;121:3359–3369. doi: 10.1242/dev.121.10.3359. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tolkunova EN, Fujioka M, Kobayashi M, Deka D, Jaynes JB. Two distinct types of repression domain in Engrailed: one interacts with the Groucho corepressor and is preferentially active on integrated target genes. Mol. Cell. Biol. 1998;18:2804–2814. doi: 10.1128/mcb.18.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MA, Murre C. extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell. 1994;78:617–624. doi: 10.1016/0092-8674(94)90526-6. [DOI] [PubMed] [Google Scholar]
- White RA, Aspland SE, Brookman JJ, Clayton L, Sproat G. The design and analysis of a homeotic response element. Mech. Dev. 2000;91:217–226. doi: 10.1016/s0925-4773(99)00306-8. [DOI] [PubMed] [Google Scholar]
- Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120:2065–2075. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- Yoffe KB, Manoukian AS, Wilder EL, Brand AH, Perrimon N. Evidence for engrailed-independent wingless autoregulation in Drosophila. Dev. Biol. 1995;170:636–650. doi: 10.1006/dbio.1995.1243. [DOI] [PubMed] [Google Scholar]


