Abstract
This study examined the relation between a preterm birth and reading ability and whether children born preterm with poorer reading were more likely to show lower cognitive and executive functioning skills compared to children born term with poor reading ability. Participants born at term (n=97) and preterm (n=156) were studied using the Woodcock-Johnson Test of Achievement Word Attack subtest (WJWA), Stanford-Binet Intelligence Scale, Comprehensive Evaluation of Language Fundamentals, and executive function tasks during the 3rd, 5th and 7th grades. Children born prematurely were divided into low (n=94) and high (n=62) risk groups based on severity of neonatal complications. Growth in WJWA scores was used to cluster the sample into three reading ability groups. Contrary to predictions, children born preterm were not more likely to be in the poor reading group. Poorer reading ability was associated with lower language and cognitive scores. The effect of premature birth demonstrated fewer and weaker associations with such scores. A significant interaction between reading ability and birth status indicated that high risk prematurely born children with poor reading ability were more likely than the other groups to perform poorly on executive function tasks. These data suggest that better reading ability is associated with better neuropsychological function independent of prematurity.
Survival of very low birth weight (VLBW) premature neonates has increased over the last two decades due to improvement in perinatal care (Fanaroff, Hack, & Walsh, 2003; Hobar et al., 2002). Brain development of premature neonates is very sensitive to physiological abnormalities and environmental toxins. For example, poor regulation in cerebral blood flow and oxidative injury can result in damage to axons and supportive oligodendrocytes in important white matter tracts (Mewes et al., 2006). As improvements in perinatal management have reduced the severity of periventricular white matter injury, other patterns of more subtle white matter injury are becoming apparent (Back & Rivkess, 2004). Thus, it is not surprising that children born prematurely are more likely to manifest cognitive, behavioral and academic difficulties (Anderson & Doyle, 2003; Bhutta, Cleves, Casey, Cradock, & Anand, 2002; Kirkegaard, Obel, Hedegaard, & Henriksen, 2006; Short et al., 2003; Wolke & Meyer, 1999).
Although premature birth is associated with an increased risk of mental retardation, when children with major neurological disorders are excluded, the majority of premature children have an adequate intelligence quotient (IQ) with IQ proportional to their birth weight (Aylward, 2002). Despite adequate IQ, premature children are still much more likely to require special assistance and to be diagnosed with a verbal or non-verbal learning disability. The higher percentage of premature children with verbal learning disabilities may be surprising when we consider that studies have shown only subtle abnormalities in early language development in children with a history of prematurity (Sansavini et al., 2006, 2007; Stolt et al., 2007).
While variability in language development for children born preterm has been reported (Landry, Smith and Swank, 2002), it is possible that limitations in other cognitive factors may account for verbal learning disabilities in this group of at risk children. Indeed, neuropsychological studies have demonstrated specific cognitive deficits that might account for the ability to learn effectively and efficiently. School age children with a history of premature birth, like other children with acquired (Levin & Hanten, 2005; Reilly et al., 2008) and developmental (Bull et al., 2008; Wåhlstedt et al., 2008) cognitive disorders, have documented difficulties with executive function (i.e., planning, behavioral inhibition, sequencing, and reasoning; Anderson et al., 2004; Aylward, 2002; Bayless & Stevenson, 2007). Children born prematurely have also been shown to demonstrate visuospatial difficulties (Aylward, 2002; Waber & McCormick, 1995). In fact, some studies have argued that these aforementioned neuropsychological limitations fully account for the effect of premature birth on achievement (Taylor et al., 2006).
Reading disability is the most common learning disability in America, affecting up to 15% of children and adults (Shaywitz, 1998), but the academic and cognitive growth of children with reading disabilities is clearly heterogeneous. Different patterns of growth appear to accompany different subsets of children with reading disabilities (Torgesen, 2000) and different subgroups of children with reading disabilities may respond differentially to interventions (Brown-Chidsey & Steege, 2006; Donovan & Cross, 2002).
For young children born premature, mild, but persistent, defects in phonological working memory, an important skill needed for developing reading skills, have been found (Sansavini et al., 2007). In addition, mild periventricular brain injury has been associated with deficits in the basic skills required for reading and spelling (i.e., working memory and phonological processing) during late childhood and early adolescences in children born prematurely (Downie et al., 2005).
What is less well known is the extent to which neuropsychological skills predict different patterns of reading skill for children born preterm as compared to term. Studying reading disability in the context of premature birth may provide some insight into the patterns of growth in decoding skills and predictors of this growth. For example, anatomic neuroimaging has revealed selective disturbances in specific white matter tract microstructure in children born prematurely who do not show obvious brain injury on ultrasound examination in the prenatal, perinatal or neonatal period. These white matter tracts include the corpus callosum and longitudinal tracks that connect the occipital, parietal and temporal areas to the frontal areas (Skranes et al., 2007). These white matter tracts are important for connecting regions of the brain hypothesized to compensate for poor phonological neural systems in individuals with reading disabilities (Brunswick et al., 1999; Ingvar et al., 2002; Shaywitz et al., 2003). If so, children born prematurely may have particular difficulty compensating for weak phonological neural systems and, as a result, continue to evidence difficulties into grades where decoding skills should be well in place.
To better understand variability in reading decoding skills in the context of prematurity, we investigated the relations among reading ability, prematurity, and neuropsychological function. The neuropsychological functions investigated were those associated with reading skill in full term typical and reading disabled individuals. With regards to neuropsychological function associated with typical reading ability, we investigated oral language (Chiappe, Chiappe & Gottardo, 2004; Lindsey, Manis & Bailey, 2003), verbal reasoning (Berninger, Abbott, Vermeulen & Fulton, 2006) and verbal memory skills (Kibby & Cohen, 2008; Steinbrink & Klatte, 2007). Other nonlanguage neuropsychological skills have been inconsistently related to reading skills in reading disability, particularly non-verbal reasoning (Holopainen, Ahonen & Lyytinen, 2001) and executive function, including attention (Heim et al., 2008; Knivsberg & Andreassen, 2008), dysinhibition (Willcutt, Pennington, Olson, Chhabildas, & Hulslander, 2005) and sequencing (Brosnan et al., 2002). If such areas truly compensate for reading function in individuals with reading disabilities, then children born prematurely, particularly those premature children who are at high risk of white matter microstructure abnormalities with reading disability should demonstrate deficits in these non-verbal neuropsychological domains. This would be expected as studies particularly implicate microstructure abnormalities in white matter tracts connecting the frontal and temporal-parietal-occipital areas (Constable et al., 2008; Skranes et al., 2007).
This study addressed the following questions and related hypothesis for premature and term born children. To address these questions, we first examined whether reading scores across 3rd, 5th, and 7th grade would result in distinct clusters of reading ability.
Question 1. Are children born premature of low and high medical risk more likely to have more problems in development of reading ability than those born full term? Hypothesis 1. Children born premature are hypothesized to be more likely to have reading scores in the low ability reading cluster and less likely to show average and good scores as compared to those born term, especially those born at the highest medical risk (e.g., bronchopulmonary dysplasia). This is expected because of the greater likelihood that the medical complications of the highest risk group are associated with white matter microstructure injury.
Question 2. Are term and preterm born children with poor reading abilities more likely to have low neuropsychological abilities than children with average and excellent reading and, are these relations more likely to be seen for children born preterm with poor reading skills? Hypothesis 2a. Children born preterm, regardless of reading cluster, are expected to have lower neuropsychological skills than those born term, especially those with higher medical risk during the neonatal period. Hypothesis 2b. Poor readers regardless of birth status are expected to have lower neuropsychological skills than those in the average and good clusters. Hypothesis 2c. Those children born premature with the poorest reading skills are expected to be those most likely to have lower skills.
Methods
Participants
The participants were from an original cohort of 360 children born to lower middle to low socioeconomic status families from Southeast Texas who had continued to participate in the longitudinal study through the 7th grade assessment point. In 1991 and 1992, this cohort was recruited into a longitudinal study of parenting behaviors and development of preterm children from three hospitals in the Houston and Galveston, Texas area. Term children were recruited to be demographically similar to those born preterm and had gestational ages from 37 to 42 weeks, birth weight appropriate for gestational age, Apgar scores of >= 8 at 5 minutes, normal pregnancy history and physical examination at birth and hospital discharge within 5 days of birth. Children were excluded if they were diagnosed with significant sensory impairments, meningitis, encephalitis, symptomatic congenital syphilis, congenital abnormalities of the brain, including congenital hydrocephalous, short bowel syndrome, or if they were found to be positive for the HIV antibody. Families were also excluded if the mother was less than 16 years of age, English was not the primary language, or the mother tested positive for drugs at the time of birth. Spanish speaking families were not recruited as translations for tests were not available when the study began. Eleven percent of parents declined to participate in the study. No differences were found between families that agreed to participate as compared to those who did not choose to participate on several demographic variables.
Children born preterm (n = 156) were defined as neonates, weighing <= 1600g and having a gestational age of ≤ 36 weeks. These children were divided into low risk (n = 94) and high risk (n = 62) groups based on the severity of their neonatal complications. Participants in the low-risk cohort had less severe complications, including respiratory distress syndrome only (31%), transient respiratory distress only (43%), grade I or II intraventricular hemorrhage (IVH) only (14%), and respiratory distress syndrome with grade I or II IVH (12%). Participants in the high-risk cohort had more severe medical complications, such as bronchopulmonary dysplasia only (76%), periventricular leukomalacia (PVL) only (3%), bronchopulmonary dysplasia with PVL (4%), grade III or IV IVH only (8%) and bronchopulmonary dysplasia with grade III or IV IVH (9%). However, participants with a history of grade III or IV IVH or post-hemorrhagic hydrocephalus were excluded from this report. Bronchopulmonary dysplasia was defined as an oxygen requirement at 28 days of age. IVH was defined using the criteria of Papile et al. (1978). The diagnoses of IVH, PVL and hydrocephalus were made by ultrasound.
In order to ensure that participants demonstrated normal intelligence, participants with two or more of the Stanford-Binet 4th Edition (SB-4) quantitative skill scores below 85 were eliminated. Although quantitative skills are not derived from a purely non-verbal measure, we chose quantitative, rather than visual reasoning (a purely non-verbal measure), due to the fact that visuo-spatial abilities can be compromised in premature children and the fact that the quantitative area has a relatively lower language load than verbal reasoning measures. This eliminated sixteen participants (5%). In addition, ninety-one participants (25%) could not be assigned a reading group because they did not have two or more Woodcock-Johnson Revised Edition Word Attack (WJWA) scores. This resulted in a total of 253 participants in this report, 70.3% of the original cohort; (term, n = 97; low risk, preterm n = 94; high risk, preterm, n = 62).
Most participants were African-American (63.0%) with fewer participants being of Caucasian (20.1%) and Hispanic (15.0%) ethnicity. The sample contained predominately lower social economic status (SES) participants and the mean SES in this study was consistent with other studies of VLBW children, M = 28.36, SD = 10.68. There were slightly more females (n=142) than males (n=111). The quality of schooling, SES, gender and ethnicity were not different across the three birth groups.
Procedure
Language, cognitive, and executive function measures were obtained in the 3rd, 5th and 7th grades. The age of the children at those times were 3rd grade, M = 8.2 years (SD = .39), 5th grade M = 10.7 years (SD = .31), and 7th grade, M = 12.7 years (SD = .45). At each grade data were obtained either in the families' homes (majority) or children's schools, which ever was more convenient for the family, during a visit by trained research assistants blind to children's birth status. Families were paid $50 for this visit.
Measures
Because of the longitudinal nature of our research, the same test version was used over time even when new versions became available (e.g., Stanford-Binet, 5th Ed.). This was necessary to avoid introducing bias into the longitudinal results that can occur when shifting from an older to newer version of a test.
Language
Children's language development was evaluated with the Clinical Evaluation of Language Fundamentals, 3rd Ed. (CELF-3; Semel, Wig & Secord, 1995) and the standardized receptive and expressive language skill scores were used in data analyses for the language domain. For the ages evaluated, test-retest (1-4 weeks) reliability ranges from .77 to .84 for the receptive scale and .77 to .92 for the expressive scale. Internal consistency ranged from .86 to .95 for the two subscales.
Cognitive
The Stanford-Binet, 4th Ed. (SB-4; Thorndike, Hagen, & Sattler, 1986) was used to obtain measures of verbal reasoning (i.e., vocabulary, comprehension, absurdities), visual reasoning area (i.e., quantitative, pattern analysis), and memory (i.e., memory for sentences, bead memory). The SB-4 has well documented internal consistency and test-retest reliability (Thorndike, Hagen, & Sattler, 1986). Standardized subscale scores were divided into three domains for data analysis, verbal reasoning, visual reasoning, memory.
Executive functioning
The Continuous Performance Test (CPT; Halperin, Sharma, Greenblatt, & Schwartz, 1991) is a widely used 12 minute computer based task that requires the child to respond to target stimulus (“A-X” sequence) while inhibiting responses to non-target stimuli. The task presents one of 11 letters, 10 of which are the target, for duration of 200 ms with a 1.5 s interval between letters. A total of 400 letters are presented with each block containing 100 letters. The CPT provides individual measures of hits, misses, false alarms, correct rejections, very late correct responses, and the hit reaction time mean and standard deviation. The inattention score from this task was used in data analysis and it is the sum of the frequency of misses, very late responses, and long-latency to “X-only” false alarms (where reaction times are greater than average hit reaction time). This measure has been found to be age-dependent, reliable, sensitive to decrements in performance over time and resistant to practice effects (Halperin, Sharma, Greenblatt, & Schwartz, 1991). It has been widely used with children in the age range of this study and in clinical and research settings to distinguish children and adolescents with and without attention problems and for those who vary in medical risk (e.g., VLBW, head injury; Barkley, 1997).
A modified version of Shallice's Tower of London (TOL; Shallice, 1982) also was used to evaluate cognitive flexibility and planning (Raizner, Song, & Levin, 2002). In this task the child rearranges three colored beads (blue, red, green) on pegs of three different heights to match the examiner's pattern in a given number of moves with a set of rules (e.g., pick up one bead at a time). The modified TOL had 12 items with three levels of difficulty that children were asked to solve. In planning, children need to remember a set of basic rules and behavioral regulation is required because of the need to shift strategies based on examiner feedback. This task is frequently used to evaluate the executive functions of planning, monitoring, self-regulation and problem solving (Levin et al., 1991). The initial planning time and the number of correct solutions across the 12 trials were included in data analyses.
Reading
The Woodcock Johnson Word Attack subscale (WJWA) was used to evaluate children's phonological word decoding (McGrew, Schrank & Woodcock, 2007). This subtest requires the application of the grapheme-to-phoneme correspondence rules in order to decode a nonword letter string and assemble the phoneme units into a pronunciation word. This test is believed to measure both reading decoding and an aspect of phonemic competence (Ashcraft, 2002). The reliability coefficients for this subtest within the age range tested range from 0.80 to 0.91 (McGrew, Schrank & Woodcock, 2007). Scaled scores were used in data analyses.
Data Analysis
Identification of reading clusters
Investigators have demonstrated that the growth of phonological decoding during early school years and into adolescence appears to differentiate persistent dyslexics from compensated dyslexics (i.e., individuals who eventually attained the ability to read as a young adult) and normal readers (Birch & Chase, 2004; Shaywitz et al 1999, 2003; Svensson & Jacobson, 2006). The growth of phonological word decoding was determined for each participant by calculating the linear change (i.e., slope) in the WJWA scaled score from the 3rd to 7th grade based on the three WJWA scores. Prior to entering these values into the cluster analysis, individual intercepts and slopes were centered to the population mean intercept and slope.
The intercept and slope estimates for each participant were entered into a hierarchical cluster analysis that used Ward's technique (Ward, 1963). Hierarchical analysis starts with n clusters, one for each participant, and then at each step groups the most similar individuals into clusters. Ward's technique defines the distance between clusters in terms of the between cluster variability to the within cluster variability. This procedure continues until there is one cluster containing all respondents. By examining the dendogram and several statistics (pseudo F and t statistics and the cubic clustering criterion), a judgment about the number of clusters is made (Anderberg, 1973). Once the number of clusters is determined, a K-means clustering algorithm is used to reassign members using a non-hierarchical technique. The purpose of this is that once a participant is assigned to a group in the hierarchical analysis, the participant remains in that group even if the centroid changes. The K-means procedure starts with the centroids of the original clusters and then reassigns each data point to the centroid nearest to their response. This continues until the solution stabilizes. Profiles of the clusters are then examined to ensure that the clusters make sense. This ensures that each observation remained in the appropriate cluster even after adding new observations. The clustering procedure identified three reading clusters that were used in subsequent analyses. These were labeled as poor, average, and excellent readers.
Association of prematurity and reading clusters, with change in WJWA scores and neuropsychological skills
To address Question 1, the association of prematurity and reading cluster on the change in WJWA reading scores, an omnibus analysis was performed using the WJWA scores as the dependent variable using a mixed-model. The intercept, or level of skill over the three time periods, slope and quadratic parameter estimates were included in the model as random effects to statistically describe the change in the dependent variable over time. Analyses were implemented using the `mixed' procedure of SAS 9.1 (SAS Institute Inc., Cary, NC). Level two, or predictor, variables included reading cluster (poor, average, excellent) and birth group (term, low risk preterm, high risk preterm), coded as categorical variables and quadratic (curvilinear) parameter. Omnibus analyses with all effects and interactions were calculated initially. A final reduced model was produced by removing effects and interactions that were not significant and not dependent on higher order interactions in order to avoid biasing the model results. Significant omnibus tests were followed up with pair-wise comparisons to determine which groups differed. A chi-square analysis also was conducted to determine if the percentage of children in each reading cluster varied by prematurity.
To address Question 2, mixed-model analyses similar to those described for addressing Question 1, were conducted. In these analyses, the dependent measures were growth in the 12 neuropsychological skills (CELF-3: Receptive, Expressive; SB-4 Verbal: Vocabulary, Comprehension, Absurdities; SB-4 Non-verbal: Quantitative, Pattern Analyses; SB-4 Memory: Sentences, Beads; Executive Function: CPT inattentiveness, TOL initial planning time and total solutions). In order to control for the multiple analysis of correlated dependent measures, we used an alpha probability of 0.01 for the effects in the omnibus analyses.
Results
Question 1. Association of Prematurity and Reading Clusters With Change In WJWA Scores
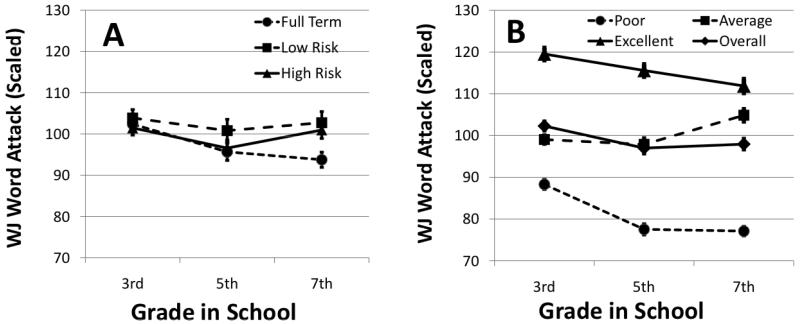
A significant difference in change in WJWA scores was found for prematurity but only for the slope F(2,189) = 3.10, p < .05. As illustrated in Figure 1A, the low risk participants born prematurely demonstrated a less negative slope, or rate of decline in scores, than the participants born at term, t(189) = 2.46, p = .015, essentially negating the decreasing trend in WJWA scores. Significant differences were found for reading clusters on all time parameters, intercept F(2,189) = 169.08, p < .001, slope F(2,189) = 49.21, p < .001, and quadratic (change in slope) parameters, F(2,189) = 5.74, p < .01. At the intercept, excellent readers demonstrated a 37.5 point higher WJWA level as compared to poor readers, t(189) = 18.78, p < .0001, and a 17.5 point higher WJWA score as compared to average readers, t(189) = 9.45, p < .0001. The average readers demonstrated a 19.9 point higher WJWA score as compared to poor readers, t(189) = 10.39, p < .0001. Excellent, t(189) = 7.27, p < .0001, and poor, t(189) = 8.96, p < .001, readers demonstrated a greater decline in WJWA scores as compared to average readers (i.e., slope parameter). Differences in the quadratic parameter estimate were due to the excellent readers continued decline in WJWA scores from 5th grade to 7th grade, t(189) = 2.35, p < .05, while the poor readers demonstrated little change in WJWA scores from 5th grade to 7th grade, t(189) = 2.82, p < .01 (Figure 1B).
Figure 1.
Change in Woodcock-Johnson Word Attack score from 3rd to 7th grade by (a) birth group and (b) reading clusters.
Table 1 shows the total number of participants in each of the three reading clusters by the number in each of the three birth groups. Contrary to our hypothesis, but consistent with the mixed-model analyses, only a trend for birth groups to be unequally distributed across reading clusters was found, X2(4) = 9.2, p = .056. The trend showed that children born high risk, preterm were more likely to be in the average, rather than excellent or poor reading cluster compared to the low risk preterm and term children. Those born term were more equally distributed across the three reading clusters and the low risk preterm group showed a somewhat lower proportion in the poor reading cluster as compared to the average and excellent clusters.
Table 1.
Association of Prematurity with Reading Clusters and Word Attack Scores
| Full Term | Low Risk Premature | High Risk Premature | Total | |
|---|---|---|---|---|
| Reading Group (%) | ||||
| Poor | 37 | 26 | 23 | 29 |
| Average | 33 | 38 | 53 | 40 |
| Excellent | 30 | 36 | 24 | 31 |
| Word Attack Scores M (SD) | |||
|---|---|---|---|
| Grade | |||
| 3rd | 102.07 (16.84) | 103.87 (15.61) | 101.46 (15.42) |
| 5th | 95.63 (18.79) | 100.81 (18.24) | 96.58 (21.37) |
| 7th | 93.53 (17.29) | 102.74 (19.17) | 100.97 (20.77) |
|
| |||
| n | 97 | 94 | 62 253 |
Question 2. Association of Prematurity and Reading Clusters with Neuropsychological Skills
Significant results are presented for the main effects of prematurity and reading group as well as their interaction.
Main effect of birth group
Main effects for birth group were found for the intercept for cognitive skills including absurdities, F(2,193) = 9.49, p < .001, pattern analysis, F(2,193) = 5.34, p < .01, bead memory, F(2,191) = 7.00, p < .01. Differences also were seen for the executive function skills including CPT inattentiveness, F(2,162) = 5.82, p < .01, TOL initial planning time IPT, F(2,205) = 7.53, p < .001, and TOL solutions, F(2,205) = 6.24, p < .01. The follow-up analyses are summarized in Table 2 and show that the cognitive skills for those born term had higher skill levels than those born either low or high risk, preterm. For executive functioning, similar differences were found as for cognitive skills with the exception of TOL total solutions where differences were only apparent between the low risk and term participants and none of the pair-wise comparisons for inattentiveness were significant.
Table 2.
Follow-up t-tests for Significant Main Effects of Prematurity and Reading Clusters on Neuropsychological Skills
| Birth Status | Reading Clusters | ||||
|---|---|---|---|---|---|
| Term vs Low Risk | Term vs High Risk | Excel vs Average | Excel vs Poor | Average vs Poor | |
| Language | |||||
|
| |||||
| Receptive | t(191) = 5.16++ | t(191) = 9.60++ | t(193) = 4.95++ | ||
| Slope | t(191) = 2.14* | t(191) = 3.83++ | |||
| Expressive | t(191) = 5.67++ | t(191) = 8.89++ | t(193) = 3.75++ | ||
|
| |||||
| Verbal Cognitive | |||||
|
| |||||
| Vocabulary | t(193) = 5.97+++ | t(193) = 8.18+++ | t(193) = 2.77+ | ||
| Comprehension | t(193) = 5.32+++ | t(193) = 7.14+++ | t(193) = 2.25* | ||
| Absurdities | t(193) = 3.86++ | t(193) = 3.57+++ | t(193) = 4.07+++ | t(193) = 5.83+++ | t(193) = 2.15* |
|
| |||||
| Nonverbal Cognitive | |||||
|
| |||||
| Pattern Analysis | t(193) = 2.69+ | t(193) = 2.88+ | t(193) = 3.56++ | t(193) = 6.09+++ | t(193) = 2.88+ |
| Quantitative | t(193) = 6.15+++ | t(193) = 7.37+++ | |||
| Slope | t(193) = 3.03+ | t(193) = 3.79* | |||
|
| |||||
| Memory | |||||
|
| |||||
| Sentences | t(194) = 3.39++ | t(194) = 5.17+++ | t(194) = 2.13* | ||
| Beads | t(191) = 3.09+ | t(191) = 3.29++ | t(191) = 2.57+ | t(191) = 4.41+++ | t(191) = 2.09* |
|
| |||||
| Executive Functioning | |||||
|
| |||||
| Inattentiveness | t(162) = 2.30** | t(162) = 5.45+++ | t(193) = 2.72+ | ||
| Planning Time | t(205) = 3.56++ | t(205) = 2.98+ | |||
| Total Solutions | t(205) = 2.46** | t(205) = 3.85++ | |||
Note. Language = Clinical Evaluation of Language Fundamentals; Cognitive/Memory = Stanford-Binet, 4th Ed., Inattentiveness = Continuous Performance Test; Initial Planning Time and Total Solutions = Tower of London; Significant effects are for intercept parameters unless otherwise indicated
p < .05
p < .02
p < .01
p < .001
p < .0001
Main effect of reading cluster
A significant main effect of the reading groups for the intercept, or level of skill, was found for the CELF receptive language, F(2,191) = 46.13, p < .001, CELF expressive language scores, F(2,191) = 40.39, p < .001, the SB-4 Vocabulary, F(2,193) = 35.51, p < .001, the SB-4 Comprehension, F(2,193) = 27.46, p < .001,SB-4 Absurdities, F(2,193) = 17.81, p < .001, SB-4 Pattern Analysis, F(2,193) = 18.71, p < .001, SB-4 Memory for Sentences, F(2,194) = 13.71, p < .001, and Bead Memory, F(2,191) = 9.79, p < .001, CPT inattentiveness, F(2,162) = 16.62, p < .001, and TOL total number of solutions, F(2,205) = 9.37, p < .001. Significant main effects for the linear change, or slope, (i.e., increase /decrease) was also found for the CELF receptive language, F(2,191) = 7.35, p < .001, and Quantitative, F(2,193) = 7.99, p < .001.
For all significant cognitive skills, excellent readers had better intercept scores than either the average or poor readers and the average readers were better than those in the poor reading cluster. The same was true for the two significant slope findings. For the executive functioning skills, inattention scores were better for the excellent as compared to the average and poor readers. However, for the TOL total solutions solved only the excellent and poor readers differed, t(205) = 3.85, p < .001.
Interaction of reading cluster and birth group
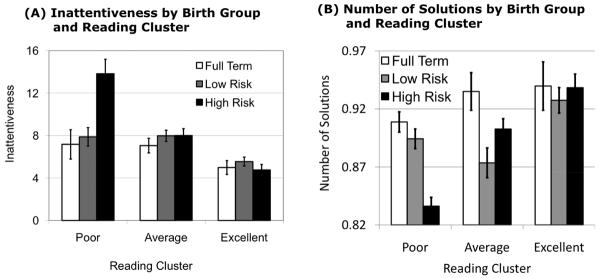
The intercept of the CPT inattentiveness score, F(4,162) = 4.2, p < .01, and the number of solutions on the TOL, F(4,205) = 6.24, p < .01, were dependent on the reading cluster by birth group interaction. The hypothesized interaction was significant for both inattentiveness scores on the CPT, t(162) = 3.62, p < .001, and number of solutions on the TOL, t(205) = 2.87, p < .01. Specifically, inattentiveness was disproportionately high and the number of solutions was disproportionately low for the poor reading high risk premature children as compared to the other high risk readers relative to the relationship between poor and other readers in the other birth groups (Figure 2).
Figure 2.
Inattentiveness scores from the Continuous Performance Task (a) and number of correct solutions from the Tower of London (b) by birth status and reading group.
Discussion
In this study we examine the association between reading skill and the development of cognitive, executive function, and language skills over a five-year interval in a cohort of children born at term and prematurely. Prematurely born children were divided into low and high risk groups depending on the presence and severity of neonatal medical complications. We show that a measure of phonological decoding, WJWA, can be used to separate the population, as a whole, into three reading clusters. These three reading clusters are different on a wide range of cognitive skills. Conversely, prematurity was related to more modest differences in specific skills. Most importantly we demonstrate that high risk prematurely born children with poor reading skills may be especially susceptible to executive dysfunction. This suggests that this particular subgroup may be less likely to use the same neurobiological and neuropsychological mechanisms as children born at term to compensate for their reading disability. Indentifying these different patterns of cognitive strengths and weaknesses may help in the design of interventions tailored to target specific cognitive deficits.
Reading Clusters
Three reading clusters were differentiated based on the change in WJWA scores from the 3rd grade to the 7th grade. All three reading clusters demonstrate a change in reading scores from 3rd to 7th grade with the poor and excellent readers demonstrating decreases in WJWA scores. Excellent readers moved from above average to solidly average levels while poor readers changed from low average to below average. In contrast, the average readers demonstrate an increase in WJWA scores over the three measurement periods. The characteristics of the change in WJWA scores for the poor reading cluster appears to be consistent with both behavioral and functional imaging data (Birch & Chase, 2004; Miller-Shaul, 2005; Shaywitz et al., 2003; Svensson & Jacobson, 2006).
Children in the poor reading cluster demonstrated persistently poor, and possibly worsening, phonological decoding skill from 3rd to 7th grade. This cluster of readers may be analogous to the groups of individuals that manifest persistently poor reading skills through adolescence into adulthood. Svensson and Jacobson (2006) found that poor phonological awareness was the most stable indicator of persistent dyslexia in a group of 40 individuals followed from the 3rd to the 12th grade, and Birch and Chase (2004) found that persistently poor dyslexics continued to show deficits in phonological awareness into adulthood. Miller-Shaul (2005) found that the difference in phonological skills between persistent dyslexics and normal readers was larger for adults than 5th graders, suggesting a widening gap in phonological skills as individuals develop from childhood to adulthood.
Poor phonological skill in adults with persistently poor dyslexia is also consistent with functional neuroimaging findings. For example, functional neuroimaging data suggest that persistently poor readers probably utilize a whole word memory pathway, through occipital-frontal connections, when challenged with a non-word decoding task (Shaywitz et al., 2003). These data are consistent with the fact that WJWA scores in the current study remain low and decrease over time in the poor readers. A lack of growth in word decoding skill could explain the underlying academic weakness that prevents the development of reading skills in individuals with persistently poor reading skills. Further study of identifying characteristics of persistently poor readers is of the upmost importance, as early remediation may be the key to preventing the disengagement of the phonological decoding system.
The WJWA scores in the 3rd grade average reading cluster were solidly average and improving over the study period and this is in contrast to the excellent cluster that clearly decreases. In fact, the WJWA scores for the average and excellent readers in this study would be predicted to converge with time. The continually improving reading skills of the average cluster is consistent with recent neuroimaging findings that suggest that occipitotemporal brain regions, in and around the so-called visual word form area, continually develops into adulthood in dyslexic and typically developing individuals, albeit with slightly different lateralization and anatomic localization (Shaywitz et al., 2007). The average reading cluster is definitely unique and requires further investigation. One of the limitations of our data set is a lack of measurements of phonological decoding earlier than the 3rd grade. Such measurements would allow us to indentify the patterns of phonological decoding growth within our reading clusters in more detail at earlier periods of development.
Relation of Reading Cluster to Language, Intellectual and Executive Function Measures
This study confirms previous research linking oral language skills (Chiappe et al., 2004; Lindsey et al., 2003) and cognitive skills (Shaywitz et al., 2003; Birch & Chase, 2004) to basic reading skills. However, the relationship between executive function deficits and reading skills in children is more uncertain. Studies have reported deficits in CPT performance, inhibiting irrelevant stimuli, sequencing events, and verbal and phonological working memory (Brosnan et al., 2002; Jeffries & Everatt, 2004; Reiter et al., 2005; Taroyan et al., 2007). However, the relation between executive function skill deficits and dyslexia may be complex. For example, some have found no deficits in CPT performance when `pure' dyslexic individuals without attention deficit hyperactivity symptoms were specifically selected for the experimental group, while others have suggested that inhibitory control deficits in dyslexia may be dependent on the demands of the task (Reiter et al., 2005; Taroyan et al., 2007). Interestingly, Helland and Asbjørnsen (2000) found two subgroups of dyslexic individuals defined by their receptive language ability who demonstrated different performance on the Stroop and Wisconsin Card Sorting Test. Clearly, the data from the current study supports that notion that the relationship between reading ability and executive function is not simple. This study suggests that a predisposition for poor reading skills is only one factor associated with poor executive function. It appears that individuals may need to possess other risk factors, in this case prematurity, in order for the association between poor reading and executive dysfunction to occur.
Relationship of Prematurity to Language, Intellectual and Executive Function Measures
Prematurity was not associated with differences in language measures (i.e., receptive and expressive), most verbal cognitive skills (vocabulary, comprehension) or nonverbal reasoning skills (i.e., quantitative reasoning) but was associated with differences in a cognitive skill that required reasoning around visually presented information (i.e., absurdities), two non-verbal skills (i.e., pattern analysis, bead memory) and some executive function skills (i.e., TOL). Overall, these data are consistent with other studies that find significant, but mild, deficits in intelligence, visual perceptual and visual motor skills and executive function in children and adolescents born prematurely (Caravale et al., 2005; Rushe et al., 2001; Sansavini et al., 2006; Stolt et al., 2007).
Difference in Poor Readers across Birth Groups
The data from this study suggest that individuals born prematurely with significant medical complications during the neonatal period (e.g., bronchopulmonary dysplasia) with poor reading skill appear to have weak executive function skills. In general, weak executive function in high risk premature children with poor reading ability may be an indication of a failure of the frontal cortical areas to properly engage with other brain areas. Functional imaging studies confirm that frontal brain areas, including the inferior, middle superior, orbital areas, participate in an extensive brain-wide neural networks during the Tower of London task (van den Heuvel et al., 2003; Wagner, Koch, Reichenbach, Sauer, & Schlösser, 2006) and CPT (Ogg et al., 2008). This notion would be consistent with neuroimaging data that demonstrates abnormal microstructure in white matter pathways connecting frontal and parietal, temporal and occipital areas (Constable et al., 2008; Skranes et al., 2007).
The notion that individuals born prematurely may find it difficult to recruit frontal brain areas during reading is consistent with findings from functional neuroimaging studies. Ment et al. (2006) studied language skills as well as brain activation using fMRI during a passive auditory language listening task in term and VLBW premature children. Prematurely born children, as compared to full term children, demonstrated greater modulation of posterior parietal-temporal areas and little modulation of frontal areas, during the task as compared to resting baseline. These neuroimaging results may represent a general failure in the engagement of frontal systems during language tasks in children born prematurely.
These data raise the question regarding how premature children can compensate for weak phonological processes if frontal brain areas purported to play a compensatory role cannot be engaged. If frontal areas were truly compensatory it would be expected that preterm children, especially those at high risk, would be more likely to poor readers. However, our data does not support this notion - in fact it suggests that children born prematurely, particularly those at high risk, are more likely to be average readers! This suggests that children born prematurely may use alternative neural mechanisms to compensate for weak phonological neural systems or that frontal activity described as compensatory in term reading disabled children is, in fact, not compensatory but merely epiphenomenal.
Over the past two decades, the relationship between reading and brain function has been studied in normal and reading disabled individuals using various neuroimaging techniques (Richards et al., 2006; Simos et al., 2006; Shaywitz et al, 2006). Such research has demonstrated that adults with a history of reading disability demonstrate activation of atypical neural pathways despite apparent adequate reading skills and individuals with different levels of reading disability may use different atypical neural pathways (Brunswick et al., 1999; Ingvar et al., 2002; Shaywitz et al., 2003). One hypothesis is that activation of these atypical neural pathways may represent the recruitment of compensatory neural mechanisms that help process written language in individuals with early reading disability. In this way, brain areas that are not typically used for language processing in individuals without reading disability are believed to be recruited to help with word decoding. At least two compensatory neural pathways have been proposed to develop by young adulthood in individuals with a history of reading disability. Shaywitz et al. (2003) suggested that young adults with a history of reading disability who developed an adequate ability to read, so-called `accuracy improved' dyslexics, recruit areas in the frontal lobes, particularly the left and right inferior frontal gyri, and right temporal-parietal area to process language information, while individuals who continued to manifest a poor reading ability, so-called `persistently poor' dyslexics, recruit connections between the left occipital and superior frontal areas.
Although neuroimaging studies have provided intriguing results that have generated interesting hypotheses, for the most part, results from neuroimaging studies only provide correlational brain-behavior relationships. For example, although many authors have hypothesized that activation of the right temporal-parietal and bilateral frontal areas represent compensatory activity, the data supporting this hypothesis is mixed. Following remediation, right temporal-parietal activity has been reported to both increase (Temple et al., 2003) and decrease (Simos et al., 2006), and right temporal-parietal activity relative to the left temporal-parietal activity has been positively (Simos et al., 2000) and negatively (Rumsey et al., 1997) correlated with performance on visual phonological decoding tasks. Similar mixed results have been also reported for inferior frontal gyrus activity. For example, inferior frontal gyrus activity has been associated with chronological age (Brunswick et al., 1999; Shaywitz et al., 1998), degree of compensation (Milne et al., 2002; Shaywitz et al., 2003), remediation (Shaywitz et al., 2004; Temple et al., 2003; Richards et al., 2002), and phonological task difficulty (Milne et al., 2002; Shaywitz et al., 1998).
Further research will be required to examine the exact neural mechanisms involved in compensation for weak phonological neural systems in premature children. Intervention studies have examined changes in the recruitment of cortical areas involved in language tasks with specific interventions in term children (Simos et al., 2006; Temple et al., 2003). Such a repeated measures interventional approach would be most helpful for understanding which specific cortical areas may be recruited in the premature brain to compensate for weak phonological systems.
Conclusions
This study suggests that children born prematurely are not at an increased risk for persistently poor reading skills. However, high risk premature readers may be more likely to be average readers. In addition, the neuropsychological profiles of premature children, particular those with poor reading skill, suggest that they may be at particular risk for executive dysfunction. This study highlights the unique neuropsychological profiles associated with prematurity and may suggest that remediation programs may need to be tailored to individuals with prematurity. Clearly more research is needed to investigate the significance of the different neuropsychological profiles and their potential influence on academic achievement. Phonological decoding and awareness skills need to be followed from pre-reading through adolescence in order to better define the growth in reading skills. Such information regarding growth needs to be correlated with anatomic and functional neuroimaging measures of brain function.
Acknowledgments
This study was supported by NIH Grant HD25128 to Dr. Susan Landry and NS046565 to Dr. Richard Frye.
References
- Anderberg M. Cluster analyses for applications. Academic Press; New York: 1973. [Google Scholar]
- Anderson P, Doyle LW, Victorian Infant Collaborative Study Group Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. Journal of the American Medical Association. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Ashcraft MH. Cognition - Third edition. Prentice Hall; Upper Saddle River, NJ: 2002. [Google Scholar]
- Aylward GP. Cognitive and neuropsychological outcomes: More than IQ scores. Mental Retardation & Developmental Disabilities Research Review. 2002;8:234–240. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Seminar in Perinatology. 2004;28:405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–74. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Human Development. 2007;83:247–254. doi: 10.1016/j.earlhumdev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Berninger VW, Abbott RD, Vermeulen K, Fulton CM. Paths to reading comprehension in at-risk second-grade readers. Journal of Learning Disabilities. 2006;39:334–351. doi: 10.1177/00222194060390040701. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. Journal of the American Medical Association. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Birch S, Chase C. Visual and language processing deficits in compensated and uncompensated college students with dyslexia. Journal of Leaning Disabilities. 2004;37:389–410. doi: 10.1177/00222194040370050301. [DOI] [PubMed] [Google Scholar]
- Brosnan M, Demetre J, Hamill S, Robson K, Shepherd H, Cody G. Executive functioning in adults and children with developmental dyslexia. Neuropsychologia. 2002;40:2144–2155. doi: 10.1016/s0028-3932(02)00046-5. [DOI] [PubMed] [Google Scholar]
- Brown-Chidsey R, Steege MW. Response to intervention: Principles and strategies for effective practice. Guilford Press; New York, NY: 2006. [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke's Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Bull R, Espy KA, Wiebe SA. Short-term memory, working memory, and executive functioning in preschoolers: longitudinal predictors of mathematical achievement at age 7 years. Developmental Neuropsychology. 2008;33:205–228. doi: 10.1080/87565640801982312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravale B, Tozzi C, Albino G, Vicari S. Cognitive development in low risk preterm infants at 3-4 years of life. Archives of Disease in Childhood, Fetal Neonatal Ed. 2005;90:F474–9. doi: 10.1136/adc.2004.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe P, Chiappe DL, Gottardo A. Vocabulary, context, and speech perception among good and poor readers. Educational Psychology. 2004;24:825–843. [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Downie AL, Frisk V, Jakobson LS. The impact of periventricular brain injury on reading and spelling abilities in the late elementary and adolescent years. Child Neuropsychology. 2005;11:479–495. doi: 10.1080/09297040591001085. [DOI] [PubMed] [Google Scholar]
- Donovan MS, Cross CR. Minority students in special and gifted education. National Academies Press; Washington, DC: 2002. [Google Scholar]
- Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Seminar in Perinatology. 2003;27:281–287. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Sharma V, Greenblatt E, Schwartz ST. Assessment of the Continuous Performance Test reliability and validity in a nonreferred sample. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:603–608. [Google Scholar]
- Helland T, Asbjørnsen A. Executive functions in dyslexia. Child Neuropsychology. 2000;6:37–48. doi: 10.1076/0929-7049(200003)6:1;1-B;FT037. [DOI] [PubMed] [Google Scholar]
- Heim S, Tschierse J, Amunts K, Wilms M, Vossel S, Willmes K, et al. Cognitive subtypes of dyslexia. Acta neurobiologiae experimentalis. 2008;68:73–89. doi: 10.55782/ane-2008-1674. [DOI] [PubMed] [Google Scholar]
- Hobar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002;110:143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- Holopainen L, Ahonen T, Lyytinen H. Predicting delay in reading achievement in a highly transparent language. Journal of Learning Disabilities. 2001;34:401–413. doi: 10.1177/002221940103400502. [DOI] [PubMed] [Google Scholar]
- Ingvar M, af Trampe P, Greitz T, Eriksson L, Stone-Elander S, von Euler C. Residual differences in language processing in compensated dyslexics revealed in simple word reading tasks. Brain Language. 2002;8:249–267. doi: 10.1016/s0093-934x(02)00055-x. [DOI] [PubMed] [Google Scholar]
- Jeffries S, Everatt J. Working memory: Its role in dyslexia and other specific learning difficulties. Dyslexia. 2004;10:196–214. doi: 10.1002/dys.278. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Cohen MJ. Memory Functioning in Children with Reading Disabilities and/or Attention Deficit/Hyperactivity Disorder: A Clinical Investigation of their Working Memory and Long-Term Memory Functioning. Child Neuropsychology. 2008;31:1–22. doi: 10.1080/09297040701821752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard I, Obel C, Hedegaard M, Henriksen TB. Gestational age and birth weight in relation to school performance of 10-year-old children: A follow-up study of children born after 32 completed weeks. Pediatrics. 2006;118:1600–1606. doi: 10.1542/peds.2005-2700. [DOI] [PubMed] [Google Scholar]
- Knivsberg AM, Andreassen AB. Behaviour, attention and cognition in severe dyslexia. Nordic Journal of Psychiatry. 2008;62:59–65. doi: 10.1080/08039480801970098. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR. Environmental effects on language developments in normal high-risk child populations. Seminars in Pediatric Neurology. 2002;9:192–208. doi: 10.1053/spen.2002.35499. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ, Harward H, et al. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. [Google Scholar]
- Levin HS, Hanten G. Executive functions after traumatic brain injury in children. Pediatric Neurology. 2005;33:79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lindsey KA, Manis FR, Bailey CE. Prediction of first-grade reading in Spanish-speaking English-language learners. Journal of Educational Psychology. 2003;95:482–494. [Google Scholar]
- McGrew KS, Schrank FA, Woodcock RW. Woodcock-Johnson III Normative Update. Riverside Publishing; Rolling Meadows, IL: 2007. Technical Manual. [Google Scholar]
- Ment LR, Peterson BS, Vohr B, Allan W, Schneider KC, Lacadie C, et al. Cortical recruitment patterns in children born prematurely compared with control subjects during a passive listening functional magnetic resonance imaging task. Journal of Pediatrics. 2006;149:490–498. doi: 10.1016/j.jpeds.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewes AU, Huppi PS, Als H, Rybicki FJ, Inder TE, McAnulty GB, et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- Miller-Shaul S. The characteristics of young and adult dyslexics readers on reading and reading related cognitive tasks as compared to normal readers. Dyslexia. 2005;11:132–151. doi: 10.1002/dys.290. [DOI] [PubMed] [Google Scholar]
- Milne RD, Syngeniotis A, Jackson G, Corballis MC. Mixed lateralization of phonological assembly in developmental dyslexia. Neurocase. 2002;8:205–209. doi: 10.1093/neucas/8.3.205. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK. Neural correlates of a clinical continuous performance test. Magnetic Resonance Imaging. 2008;26:504–512. doi: 10.1016/j.mri.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. Journal of Pediatrics. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- Raizner RD, Song J, Levin HS. Raising the ceiling: The tower of Londonextended version. Developmental Neuropsychology. 2002;21:1–14. doi: 10.1207/S15326942DN2101_1. [DOI] [PubMed] [Google Scholar]
- Reilly DS, Woollacott MH, van Donkelaar P, Saavedra S. The interaction between executive attention and postural control in dual-task conditions: children with cerebral palsy. Archives of Physical Medicine and Rehabilitation. 2008;89:834–842. doi: 10.1016/j.apmr.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Reiter A, Tucha O, Lange KW. Executive functions in children with dyslexia. Dyslexia. 2005;11:116–131. doi: 10.1002/dys.289. [DOI] [PubMed] [Google Scholar]
- Richards TL, Berninger VW, Aylward EH, Richards AL, Thomson JB, Nagy WE, et al. Reproducibility of proton MR spectroscopic imaging (PEPSI): comparison of dyslexic and normal-reading children and effects of treatment on brain lactate levels during language tasks. American Journal of Neuroradiology. 2002;23:1678–1685. [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Aylward EH, Field KM, Grimme AC, Raskind W, Richards AL, et al. Converging evidence for triple word form theory in children with dyslexia. Developmental Neuropsychology. 2006;30:547–589. doi: 10.1207/s15326942dn3001_3. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Rushe TM, Rifkin L, Stewart AL, Townsend JP, Roth SC, Wyatt JS, et al. Neuropsychological outcome at adolescence of very preterm birth and its relation to brain structure. Developmental Medicine & Child Neurology. 2001;43:226–233. doi: 10.1017/s0012162201000433. [DOI] [PubMed] [Google Scholar]
- Sansavini A, Guarini A, Alessandroni R, Faldella G, Giovanelli G, Salvioli G. Early relations between lexical and grammatical development in very immature Italian preterms. Journal of Child Language. 2006;33:199–216. doi: 10.1017/s0305000905007208. [DOI] [PubMed] [Google Scholar]
- Sansavini A, Guarini A, Alessandroni R, Faldella G, Giovanelli G, Salvioli G. Are early grammatical and phonological working memory abilities affected by preterm birth? Journal of Communication Disorders. 2007;40:239–256. doi: 10.1016/j.jcomdis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Semel, Wig E, Secord W. Clinical Evaluation of Language Fundamentals. 3rd edition Psychological Corp; San Antonio, TX: 1995. [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical transactions of the Royal Society of London. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Current concepts: Dyslexia. New England Journal of Medicine. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Fletcher JM, Holahan JM, Shneider AE, Marchione KE, Stuebing KK, et al. Persistence of dyslexia: the Connecticut Longitudinal Study at adolescence. Pediatrics. 1999;104:1351–1359. doi: 10.1542/peds.104.6.1351. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, SkudlarskI P, Mencl WE, Constable RT, et al. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Lyon GR, Shaywitz SE. The role of functional magnetic resonance imaging in understanding reading and dyslexia. Developmental Neuropsychology. 2006;30:613–632. doi: 10.1207/s15326942dn3001_5. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, et al. Age-related changes in reading systems of dyslexic children. Annals of Neurology. 2007;61:363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: a magnetic source imaging approach. Cerebral Cortex. 2000;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Denton C, Sarkari S, Billingsley-Marshall R, Papanicolaou AC. Magnetic source imaging studies of dyslexia interventions. Developmental Neuropsychology. 2006;30:591–611. doi: 10.1207/s15326942dn3001_4. [DOI] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Klatte M. Phonological working memory in German children with poor reading and spelling abilities. Dyslexia. 2007 doi: 10.1002/dys.357. in press. [DOI] [PubMed] [Google Scholar]
- Stolt S, Klippi A, Launonen K, Munck P, Lehtonen L, Lapinleimu H, et al. Size and composition of the lexicon in prematurely born very-low-birth-weight and full-term Finnish children at two years of age. Journal of Child Language. 2007;34:283–310. doi: 10.1017/s0305000906007902. [DOI] [PubMed] [Google Scholar]
- Svensson I, Jacobson C. How persistent are phonological difficulties? A longitudinal study of reading retarded children. Dyslexia. 2006;12:3–20. doi: 10.1002/dys.296. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. Journal of Developmental & Behavioral Pediatrics. 2006;27:459–469. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- Taroyan NA, Nicolson RI, Fawcett AJ. Behavioural and neurophysiological correlates of dyslexia in the continuous performance task. Clinical Neurophysiology. 2007;118:845–855. doi: 10.1016/j.clinph.2006.11.273. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proceedings of the National Academy of Science. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Guide for administering and scoring the Stanford-Binet Intelligence Scale. Fourth edition Riverside Publishing; Chicago: 1986. [Google Scholar]
- Torgesen JK. Individual responses in response to early interventions in reading: The lingering problem of treatment resisters. Learning Disabilities Research and Practice. 2000;15:55–64. [Google Scholar]
- van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van Dyck R, Veltman DJ. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18:367–374. doi: 10.1016/s1053-8119(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Waber D, McCormick M. Late neuropsychological outcomes in preterm infants of normal IQ: Selective vulnerability of the visual system. Journal of Pediatric Psychology. 1995;20:721–735. doi: 10.1093/jpepsy/20.6.721. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlösser RG. The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44:2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Wåhlstedt C, Thorell LB, Bohlin G. ADHD symptoms and executive function impairment: early predictors of later behavioral problems. Developmental Neuropsychology. 2008;33:160–178. doi: 10.1080/87565640701884253. [DOI] [PubMed] [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. Journal of American Statistical Society. 1963;77:841–847. [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: In search of the common deficit. Developmental Neuropsychology. 2005;27:35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: The Bavarian Longitudinal Study. Developmental Medicine & Child Neurology. 1999;41:94–109. doi: 10.1017/s0012162299000201. [DOI] [PubMed] [Google Scholar]