Abstract
Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in folate metabolism. We ssessed the association between two common MTHFR variants, 677C>T and 1298A>C, and adenoma recurrence in the context of a randomized double blind clinical trial of aspirin use and folate supplementation. We used generalized linear regression to estimate risk ratios and 95% CIs for recurrence, adjusting for age, sex, clinical center, follow-up time, and treatment status. Neither MTHFR polymorphism was associated with overall or advanced adenoma recurrence. Compared to those with 2 wild type alleles, the relative risk for advanced adenoma was 0.75 (95% CI 0.36 to 1.55), for the MTHFR 677 TT genotype and 1.16 (95% CI 0.58–2.33) for the MTHFR 1298 CC genotype. The effect of folate supplementation on recurrence risk did not differ by genotype. Our findings indicate that MTHFR genotype does not change adenoma risk in a manner similar to its effect on colorectal cancer, and does not modify the effect of folate supplementation on metachronous adenoma risk.
Keywords: Colorectal Adenomas, MTHFR, Clinical Trials, Folate, Genetic Analysis, Risk Factors
Introduction
One carbon groups are essential for many physiologic reactions including nucleotide synthesis and DNA methylation (1–4). In humans, virtually all one-carbon groups are carried by folates. Data from human studies support an association between folate deficiency and an increased risk of colorectal neoplasia (5).
Methylenetetrahydrofolate reductase (MTHFR) irreversibly reduces 5,10-methylenetetrahydrofolate (5,10-MTHF) to 5-methyltetrahydrofolate (5-MTHF) in a flavin adenine dinucleotide (FAD)-dependent reaction that shunts one-carbon groups from DNA synthesis toward synthesis of the primary methyl donor S-Adenosylmethionine (SAM) (6). Two common polymorphisms in the MTHFR gene, 677C>T (rs1801133; Ala222Val) and 1298A>C (rs11801131; Glu429Ala), have been frequently studied. The 677 Val/Val enzyme has been associated with a 65–70% reduction in enzyme activity in vitro (7–9) and an approximately 20% decrease in colorectal cancer (CRC) risk among folate replete individuals in vivo (10–12). However, the published studies suggest that the MTHFR 677 TT genotype does not have the same association with colorectal adenoma risk as it does for colorectal cancer, with a summary odds ratio of approximately 1.0 in meta- analyses (10–12), although some studies have suggested an increased adenoma risk in those with a low folate status (13–17), or among subjects not using multivitamins (18).
The MTHFR 1298A>C polymorphism has been associated with a reduction in enzyme activity relative to the wild type enzyme (AA genotype) in some (8, 19) but not all (9,) in vitro studies. Epidemiologic data regarding associations of this polymorphism with risk of colorectal cancer or adenomas are inconsistent, and do not provide strong support for an independent effect (10, 11).
Although there is now a reasonable body of data assessing how genetic variation in MTHFR is associated with risk of colorectal neoplasia (10, 11, 20), assessment of interactions with folate intake are hampered by the limitations of nutritional epidemiology, most prominently measurement error in the assessment of folate intake. Investigations of associations with adenoma risk are further limited by the fact that most studies have focused on prevalent rather than incidenent adenomas. We recently reported data from a randomized double-blind clinical trial of a 1 mg/day folic acid supplement versus a placebo on the risk of adenoma recurrence (21). In the current analysis, we assess the association of MTHFR genotype with risk of adenoma recurrence in the context of this trial (21, 22) and investigate whether genetic variation in MTHFR modifies the effect of a folic acid supplement on recurrence. Our clinical trial design allowed us largely to avoid the difficulties of nutritional assessment, since we provided a fixed amount of folate as a study treatment. Our study also allows us to assess the effect of genotype on incident rather than prevalent adenomas, making clear the temporal relationships between intake and adenoma occurrence.
Methods
Design
These data were collected as part of a randomized, double blind, placebo-controlled trial of the efficacy of oral aspirin (ASA), folic acid, or both to prevent colorectal adenomas as described in (21, 22). Briefly, this was a three-by-two factorial design in which subjects were randomized to receive 81 mg/day ASA, 325 mg/day ASA or placebo. Within each ASA/placebo group, subjects were additionally randomized to receive supplemental 1 mg folic acid/day or a placebo (22). The study initially focused only on aspirin; 100 subjects who were randomized only to aspirin are not included in this analysis.
Recruitment, Randomization, Treatment and Follow-up
Details of subject eligibility, recruitment, randomization, treatment and follow-up and study outcomes have been described (22, 23). Briefly, subjects were recruited from July 1994 until March 1998 from 9 clinical centers. Eligible subjects were between 21 and 80 years of age, in good health and had received a recommendation for follow-up colonoscopy by their regular medical practitioner. Each eligible subject had at least one or more histologically confirmed colorectal adenomas removed within 3 months of recruitment, or one or more histologically confirmed adenomas removed within 16 months of enrollment as well as a history of two or more confirmed adenomas or an adenoma greater than 1 cm in diameter removed within 16 months of enrollment. Individuals were ineligible if they had a history compatible with a familial colorectal cancer syndrome, invasive colorectal cancer (CRC), any malabsorption syndrome, a medical condition that could be worsened by use of aspirin or folic acid or any medical condition commonly treated with aspirin or folate. A complete colonoscopy with removal of all polyps within three months of enrollment was required for all subjects. Each subject underwent a 3 month run-in period on 325 mg ASA prior to randomization into ASA and folate treatment groups. Only subjects with at least 80% compliance and no other contraindications were randomized. By protocol, all subjects had an anticipated follow-up complete surveillance colonoscopy 34 to 40 months after the qualifying examination.
To assess whether a longer follow-up was needed to observe an effect of folate treatment, participants completing their first follow-up colonoscopy were invited to continue their blinded folate treatment for another colonoscopic surveillance cycle (generally 3 to 5 years). In this analysis we defined the second follow-up interval from the time after the year 3 colonoscopy through the subsequent second colonoscopy or October 1, 2004, whichever came first. Of the 1021 participants initially randomized to folate or placebo, 729 (71.4%) continued with their randomized treatment and 607 (59.5%) completed their second follow-up examination with a mean (SD) follow-up time of 41.8 (11.8) months. The current analysis was limited to adenomas that had been found by the time of the protocol year-3 follow-up colonoscopy (36–40 months after the baseline colonoscopy)
Study outcomes
The primary study outcome was the proportion of patients in whom one or more colorectal adenomas were detected in the period starting one year after randomization to the end of the year 3 surveillance follow-up examination. If a year-3 colonoscopy was not performed, we used the last examination at least one year after randomization. Polyps were classified as neoplastic (adenomatous) or nonneoplastic by the study pathologist, who also assessed the degree of dysplasia and the extent of villous component in each adenoma. We defined advanced lesions as invasive carcinoma or adenomas with at least 25% villous component, high grade dysplasia, or an estimated size of 1 centimeter or greater. Patients were considered to have had “multiple adenomas” when there were a total of 3 or more follow-up adenomas by the end of the year 3 exam. Plasma levels of folate were determined by microbiological assays using a colistin sulphate resistant strain of Lactobacillus leichmannii and RBC folate was determined by the ACS:180® folate assay, a competitive immunoassay using direct chemiluminescent technology (Bayer Corporation, Tarrytown NY).
We assessed the MTHFR / recurrence association within strata of post-follow-up RBC and plasma folate levels, age, sex, and subsite of the large bowel (right colon and left colorectum). Median values for folate measurements, taken at baseline and the end of follow-up, and age were defined by reference to the entire population of subjects.
Genotyping
The MTHFR polymorphisms, 677C>T and 1298A>C, were genotyped with the 5'nuclease TaqMan allelic discrimination assay using the ABI7900 (Applied Biosystems, Foster City, CA). Polymerase chain reaction primers and dual-labeled allele discrimination probes were designed using the Primer Express software package (PE Biosystems) as described in (24). Each 384-well assay contained internal quality controls for homozygous wild-type, heterozygous, and homozygous variant alleles for the respective polymorphisms along with no template controls. Genotype calls were determined by SDS 2.1 analysis software.
Statistical Analysis
The statistical analyses were as described in Cole 2007 (21). All analyses of folic acid treatment were performed using an intention to treat strategy, which included all randomized participants who had a follow-up colonoscopy whether or not they had continued using their supplement. To measure the association between folate status and genetic variants and adenoma risk, we estimated genotype-specific risk ratios (and 95% confidence intervals) for one or more adenomas after randomization, calculated with generalized linear regression analyses using a logarithmic linkage and a binomial distribution correcting for over- and under-dispersion. Genotype was coded by the number of variant alleles, 0, 1 or 2. We obtained the relative risks and the p’s for trend using orthogonal linear contrasts. Interactions between genotype and treatment group were evaluated using product interaction terms. We used Wald tests to asses the significance of these terms. All effect estimates were adjusted for age, sex, study center, aspirin treatment group and follow-up time. We assessed Hardy-Weinberg equilibrium by using a contingency table chi-square test to compare observed genotype frequencies to those expected under Hardy-Weinberg equilibrium. All tests of statistical significance are two-sided. We considered the joint genotypes to assess possible effects of compound heterozygosity (8, 25, 26).
Results
A total of 505 patients were randomized to receive placebo and 516 patients were randomized to 1 mg of folic acid per day. A total of 987 patients (96.7 %) underwent a follow-up colonoscopy at least one year following randomization. The two treatment groups were similar with regard to all baseline characteristics (Table 1). Genotypes were distributed randomly across folate treatment groups. Both loci were in Hardy-Weinberg equilibrium, and in strong linkage disequilibrium. There were no subjects with the 677TT/1298AC, 677CT/1298CC or 677TT/1298CC genotypes.
Table 1.
| Characteristic | Placebo (n=461) |
Folic Acid (n=462) |
P-value |
|---|---|---|---|
| Age (y) mean (SD) | 57.5 (9.6) | 57.7 (9.6) | 0.726 |
| Male sex n (%) | 297 (64.4) | 296 (64.1) | 0.910 |
| Race/Ethnicity n (%) | |||
| White | 405 (87.9) | 410 (88.7) | |
| African American | 25 (5.4) | 20 (4.3) | 0.695 |
| Hispanic | 17 (3.7) | 22 (4.8) | |
| Other | 14 (3.0) | 10 (2.2) | |
| Current cigarette smoker n (%) | 60 (13.1) | 70 (15.2) | 0.615 |
| Dietary Intake kcal/d mean (SD) | 1642.3 (649.6) |
1616.2 (656.8) |
0.552 |
| Dietary Folate intake µg/d mean (SD) | 329.0 (165.3) | 317.2 (145.1) | 0.260 |
| Baseline Plasma Folate nmol/L mean (SD) | 23.7 (16.8) | 23.9 (18.1) | 0.873 |
| Year 3 Plasma Folate nmol/L mean (SD) | 30.0 (14.1) | 74.9 (35.9) | <0.0001 |
| Baseline Plasma T-homocysteine µmol/L mean (SD) | 9.8 (2.9) | 9.8 (2.9) | 0.806 |
| Year 3 Plasma T-homocysteine µmol/L mean (SD) | 9.2 (2.5) | 8.9 (2.2) | 0.074 |
| MTHFR 677 C>T genotype | |||
| CC n (%) | 207 (45.0) | 213 (46.2) | |
| CT n (%) | 201 (43.7) | 205 (44.5) | 0.614 |
| TT n (%) | 52 (11.3) | 43 (9.3) | |
| MTHFR 1298 A>C genotype | |||
| AA n (%) | 234 (50.8) | 220 (47.6) | |
| AC n (%) | 193 (41.9) | 195 (42.2) | 0.283 |
| CC n (%) | 34 (7.4) | 47 (10.2) | |
| Joint Genotypes (677/1298) | |||
| CC/AA (baseline) n (%) | 72 (15.7) | 71 (15.4) | |
| CC/AC n (%) | 101 (22.0) | 96 (20.8) | |
| CC/CC n (%) | 34 (7.4) | 46 (10.0) | 0.670 |
| CT/AA n (%) | 110 (23.9) | 106 (23.0) | |
| CT/AC n (%) | 91 (19.8) | 99 (21.5) | |
| CT/CC n (%) | 0 | 0 | |
| TT/AA n (%) | 52 (11.3) | 43 (9.3) | |
| TT/AC n (%) | 0 | 0 | |
| TT/CC n (%) | 0 | 0 |
Of the 1021 subjects randomized to folate treatment or placebo, MTHFR 677C>T and MTHFR 1298A>C genotype data was available for 923 subjects (90%) and of these subjects 23 did not have a year 3 colonoscopy and are not included in further analyses.
Missing data: Smoking status n=4, Dietary folate n=36, Dietary intake n=36,Plasma folate n=67, Baseline HCY n=64, Year 3 Plasma Folate and HCY n=119.
Association between MTHFR genotype and Adenoma Recurrence
The associations of MTHFR genotypes with the risk of one or more recurrent adenomas are shown in Table 2. Relative to those with two wild-type alleles, the adjusted RRs and 95% confidence intervals (CI’s) for any adenoma were 0.89 (0.68–1.16) for the MTHFR 677TT genotype (p for trend over number of variant alleles = 0.42) and 1.14 (0.87–1.50) for the MTHFR 1298CC genotype (p for trend = 0.12). The RR’s and 95% CI’s for any advanced lesion were 0.75 (0.36–1.55) for the 677TT genotype (p for trend = 0.28) and 1.16 (0.58–2.33) for the 1298CC genotype (p for trend = 0.71). Heterozygotes did not have materially different risks than those who were wild type homozygotes. Consideration of the combined 677/1298 genotypes also did not suggest an association (Table 2).
Table 2.
Association of MTHFR genotype and risk of recurrent adenomas
| Genotype | Any Adenoma | Advanced adenoma† | ||
|---|---|---|---|---|
| 677C>T (n) | No. | RR* (95% CI) | No. | RR* (95% CI) |
| CC (405) | 182 | 1.0 | 45 | 1.0 |
| CT (400) | 177 | 0.97 (0.83–1.14) | 37 | 0.81 (0.36–1.55) |
| TT (93) | 36 | 0.89 (0.68–1.16) | 8 | 0.75 (0.36–1.55) |
| p for trend ‡ | 0.42 | 0.28 | ||
| 1298A>C (n) | ||||
| A (444) | 182 | 1.0 | 43 | 1.0 |
| AC (379) | 178 | 1.14 (0.98–1.33) | 38 | 1.03 (0.68–1.57) |
| CC (77) | 36 | 1.14 (0.87–1.50) | 9 | 1.16 (0.58–2.33) |
| p for trend ‡ | 0.12 | 0.71 | ||
| MTHFR 677/1298 genotype | No. | RR* (95% CI) | No. | RR* (95% CI) |
| CC/AA (137) | 55 | 1.0 | 14 | 1.0 |
| CC/AC (192) | 91 | 1.17 (0.90–1.51) | 22 | 1.08 (0.56–2.07) |
| CC/CC (76) | 36 | 1.17 (0.85–1.60) | 9 | 1.05 (0.47–2.37) |
| CT/AA (214) | 91 | 1.03 (0.80–1.33) | 21 | 0.89 (0.47–1.72) |
| CT/AC (186) | 86 | 1.14 (0.88–1.47) | 16 | 0.79 (0.40–1.58) |
| TT/AA (93) | 36 | 0.98 (0.72–1.35) | 8 | 0.78 (0.34–1.81) |
RR = risk ratio; CI = confidence interval. RRs were adjusted for age, sex, clinical center, treatment assignment, and follow-up time.
Advanced adenoma defined as ≥ 25% tubulovillous or villous adenoma, large adenoma (≥ 1 cm), advanced dysplasia, carcinoma in situ, or invasive cancer.
Wald test used to generate P values
Since the association between MTHFR genotype and adenoma risk is known to interact with folate availability, we also assessed the main effect of genotype after stratifying on folate treatment group (data not shown). Treatment group did not modify the effects of genotype on adenoma risk. For the 677TT genotype, the relative risks for any adenoma were 0.82 (95% CI 0.55, 1.22) and 0.96 (95% CI 0.66, 1.38) among subjects randomized to folate, and placebo respectively (p for interaction =0.85). For the 1298CC genotype, these RR’s were 1.15 (95% CI= 0.79, 1.66) and 1.14 (95% CI = 0.79, 1.71) for the folate and placebo groups respectively (p = 0.83). Genotype RR’s for advanced adenoma risk were also essentially the same in both the supplement and placebo groups. There was some suggestion that the association of MTHFR 677 TT genotype with risk of any adenoma was modified by sex. The adjusted risk ratios for any adenoma were 1.07 (95% CI = 0.79–1.45) in males but 0.48 in females (95% CI 0.25, 0.90; p for interaction=0.05). Control for baseline folate levels did not change either the sex-specific RR’s or the interaction p-value. There was no evidence of modification of genotype associations with all adenomas by age or any baseline or post-treatment folate measure, and findings in bowel subsites were comparable (data not shown). There was no interaction with aspirin treatment (data not shown). The risk ratios for advanced adenomas were not modified by age, sex, baseline or post-treatment folate levels. Risk ratios using 3 or more recurrent adenomas as an endpoint were essentially identical to those for advanced adenomas (data not shown). When we expanded the analysis to consider recurrences occurring within the second surveillance cycle, findings were similar (data not shown).
Modification of the effects of folate supplementation
There was no evidence that the effects of folate supplementation were modified by MTHFR genotypes for any endpoint (Table 3). The folate/placebo risk ratio for all adenomas was 0.93 (0.57, 1.53) among those with the MTHFR 677TT genotype and 1.02 (95% CI 0.62, 1.68) for those carrying the MTHFR 1298CC genotype. Both estimates were essentially the same as the overall result. When we limited the endpoints to adenomas with advanced features, these risk ratios were 1.30 (95% CI 0.34, 4.96) and 1.40 (95% CI 0.36, 5.38) respectively.
Table 3.
Effect of folate treatment on the risk of adenomas, by MTHFR genotype
| Genotype | Any Adenoma | Advanced Adenoma† | ||
|---|---|---|---|---|
| Ns § | Folate/Placebo RR* (95% CI) | Ns § | Folate/Placebo RR (95% CI) | |
| 677C>T (n) Folate/Placebo§ | Folate /Placebo§ | Folate/Placebo | ||
| CC (204/201) | 96/86 | 1.09 (0.87–1.36) | 21/22 | 0.96 (0.54–1.68) |
| CT (203/197) | 93/84 | 1.06 (0.85–1.33) | 22/15 | 1.48 (0.78–2.79) |
| TT (43/50) | 16/20 | 0.93 (0.57–1.53) | 4/4 | 1.30 (0.34–4.96) |
| C.1298A>C (n) | ||||
| AA (218/226) | 91/91 | 1.01 (0.81–1.26) | 22/21 | 1.11 (0.62–1.98) |
| AC (189/190) | 94/84 | 1.11 (0.89–1.39) | 21/17 | 1.22 (0.66–2.27) |
| CC (44/33) | 20/16 | 1.02 (0.62–1.68) | 6/3 | 1.40 (0.36–5.38) |
| MTHFR 677/1298 Genotype§ | ||||
| CC/AA (69/68) | 27/28 | 0.90 (0.60–1.35) | 4/10 | 0.39 (0.13–1.19) |
| CC/AC (92/100) | 49/42 | 1.25 (0.92–1.72) | 13/9 | 1.41 (0.62–3.22) |
| CC/CC (43/33) | 20/16 | 1.04 (0.63–1.72) | 6/3 | 1.44 (0.37–5.54) |
| CT/AA (106/108) | 48/43 | 1.12 (0.82–1.53) | 14/7 | 2.05 (0.85–4.95) |
| CT/AC (97/89) | 45/41 | 0.99 (0.72–1.37) | 8/8 | 0.99 (0.38–2.55) |
| TT/CC (43/50) | 16/20 | 0.93 (0.56–1.53) | 4/4 | 1.30 (0.34–4.95) |
RR = risk ratio for effect of 1 mg/day folate supplement versus placebo on adenoma recurrence risk; CI = confidence interval. RRs were adjusted for age, sex, clinical center, and aspirin treatment assignment.
Number of subjects in folate/placebo treatment groups.
Advanced adenoma defined as tubulovillous or villous adenoma, large adenoma (≥ 1 cm), carcinoma in situ, or invasive cancer.
Discussion
We studied the association between MTHFR 677C>T and 1298A>C genotypes and the risk of recurrent adenomas in subjects participating in a randomized clinical trial of aspirin and folic acid supplementation. Overall, MTHFR genotype was not associated with recurrence risk for all adenomas or for advanced adenomas, regardless of folate treatment group. Neither MTFHR genotype appeared to alter the effects of folate supplementation on adenoma risk.
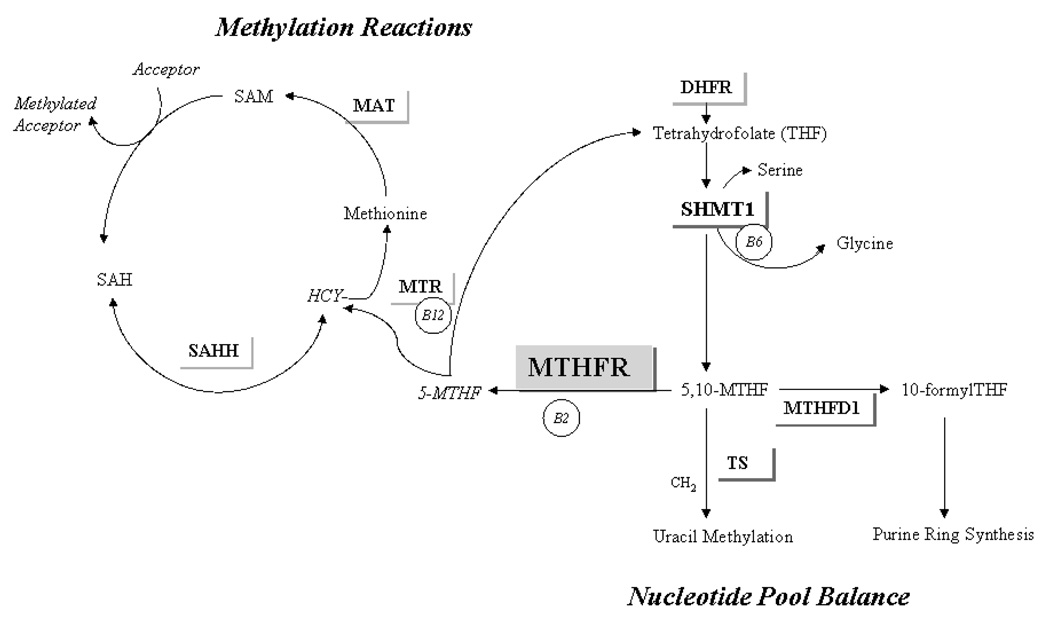
The MTHFR enzyme plays a key role in intra-cellular folate distribution, irreversibly moving one-carbon groups away from nucleotide synthesis and toward maintenance of the universal methyl-donor SAM (see Figure 1). When one-carbon groups are not limiting, individuals with the 677 TT genotype appear to retain more folates for nucleotide synthesis at the expense of DNA and protein methylation reactions (6, 27). In the context of these well-described functional effects, the decreased CRC risk observed in 677T homozygotes in epidemiologic studies (10, 11, 20) emphasizes the importance of imbalanced nucleotide pools over disturbed methylation reactions in folate-associated colorectal carcinogenesis (3).
Figure 1. MTHFR and one carbon metabolism.
MTHFR, methylenetetrahydrofolate reductase; DHFR, dihydrofolate reductase; THF, tetrahydrofolate; SHMT1, cytosolic serine hydroxymethyltransferase; 5,10-MTHF, 5,10-methylentetrahydrofolate, MTHFD1, methyltetrahydrofolate hydrogenase; 5-MTHF, 5-methyltetrahydrofolate; HCY, homocysteine; MTR, 5- Methyltetrahydrofolate-homocysteine s-methyltransferase; MAT, Methionine adenosyltransferase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; SAHH, S-adenosylhomocysteine hydrolase; B2, B6, B12, B-vitamin cofactors.
Regarding the 677 T allele, our clinical trial data are generally consistent with those from adenoma case control and cohort studies, none of which have reported a significant association between the MTHFR 677TT genotype and adenoma prevalence (11, 13, 14, 16, 28–32), even after stratifying on adenoma type (13, 28, 31, 32). Our findings are also consistent with an analysis of incident adenomas in a similar clinical trial of 500 μg folic acid / day with or without 300 mg/day aspirin (33). However, the results of both these trials differ from those of the observational analysis of incident adenomas in another trial (18), which reported an increased risk for any recurrent adenoma and an approximate doubling in the risk of advanced adenomas in subjects with the 677 TT genotype, but only in those not taking a multivitamin supplement.
The aggregate data from case control, cohort studies and clinical trials suggest that overall, the MTHFR 677C>T genotype has at most a weak association with adenoma risk. However, some (13, 16, 30, 31, 34) though not all (17, 29) studies have reported lower MTHFR 677TT relative risks among those with higher folate status, and increased relative risks have been reported in subjects who use alcohol, a known folate antagonist (13, 17, 28–30, 32). All these findings suggest that folate availability may modify adenoma risk in 677 TT homozygotes. To reduce the incidence of neural tube defects, fortification of the North American food supply with 140 μg folic acid per 100 g cereal-grain product, began on a voluntary basis in 1996 and became mandatory on January 1, 1998. Recruitment in this study overlapped a time period of increasing supplementation of the US and Canadian food supply, so our study population was relatively folate replete at the start of the study (21). We also observed a small, non-significant decrease in recurrence risk for those with the TT genotype. Thus our finding of a small, non-significant decrease in recurrence risk for subjects with the TT genotype are consistent with those from some prior studies indicating that this genotype may decrease adenoma risk modestly in those with adequate folate availability.
It is possible that longer term folate supplementation may modify the association between the risk of incident adenomas in those with the MTHFR 677 TT genotype. We considered this, in part, by extending our analysis to adenomas that occurred in all subjects who were followed for an additional colonoscopic surveillance cycle. However, this analysis did not lead to any change in our conclusions.
In general, our findings regarding the 1298A>C variant are similar to those seen in the other aspirin/folate trial (33) and the literature (10). There is significant heterogeneity in the biological (9, 35) and epidemiological data for the MTHFR 1298A>C CC genotype (10) and CRC risk. The lack of consistency in the association with the 1298 A>C polymorphism may reflect a lack of true functional effect or it may imply the presence of an important modifier of such an effect.
Our results and those of Hubner et al (33), showing that the MTHFR C677T TT genotype did not significantly modify the effect of folic acid supplementation in a similar trial, may be a reflection of the interaction between genotype and folate availability. Yamada et al demonstrated experimentally that the enzyme coded for by the 677 T allele becomes indistinguishable from the wild type enzyme in the presence of folic acid (9). It is possible that 677TT subjects in the supplement group (1700 μg folate equivalents/day in our study) had a functionally wild type MTHFR enzyme during the follow-up period, suggesting that our ability to assess a genotype effect may have been limited to subjects in the placebo group. The fact that we did not observe an association even here, suggests additional support for the conclusion that MTHFR genotype does not substantially affect adenoma recurrence risk. Nonetheless, all our subjects were relatively folate-replete at the beginning of the trial, and so it is possible that these associations may be different in populations with lower folate intake.
The advantages of our study include the high follow-up rate for subjects and the randomization to folate treatment, a design which largely avoids the measurement error inherent in nutritional epidemiology and minimizes the potential for confounding in the assessment of the main effects of folate. Additionally, almost all studies of MTHFR and adenomas to date have been studies of prevalent adenomas, complicating inferences regarding the timing of the neoplasm and the folate intake. Since all subjects in this clinical trial were cleared of polyps at baseline, the adenomas we detected at follow-up were incident lesions (missed polyps aside). This design thus allows us to estimate the association of genotype with incidence rather than prevalence of adenomas further strengthening the impression that the MTHFR 677 TT genotype does not modify the risk of adenoma development. Disadvantages include the requirement that all subjects in the study cohort had at least one adenoma before randomization, a design feature that limits the generalizability of our results. Also, the study population had high baseline folate levels and it is unclear how this might have modified the functional effects of the MTHFR 677 T allele. Finally despite the substantial size of the study, the small genotype-specific N’s, especially for stratified estimates, limits the inferences that are possible from the data.
In summary, we assessed the association between MTHFR genotype and the risk of recurrent colorectal adenomas and did not observe any significant association with recurrence risk for either genotype. These findings were the same for any adenomas and adenomas with advanced features and were not modified by baseline folate, post-follow-up folate or folate supplementation. Our results are consistent with numerous previous studies and extend to incident recurrent adenomas the finding that MTHFR genotype is not substantially associated with adenoma risk.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400 and grants 5 RO1 CA059005 and U54 CA 100971 from the National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
We thank all the individuals who participated in this clinical trial.
References
- 1.Hoffman RM. Altered methionine metabolism and transmethylation in cancer. Anticancer Res. 1985;5(1):1–30. [PubMed] [Google Scholar]
- 2.Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71(1–2):121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- 3.Eto I, L KC. Essential Nutrients in Carcinogenesis. New York: Academic Press; 1996. [Google Scholar]
- 4.Giovannucci E, Stampfer MJ, Colditz GA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85(11):875–884. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132(8 Suppl):2350S–2355S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Stampfer MJ, Giovannucci E, et al. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997;57(6):1098–1102. [PubMed] [Google Scholar]
- 7.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg I, Tran P, Christensen B, Sibani S, Rozen RA. second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64(3):169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci U S A. 2001;98(26):14853–14858. doi: 10.1073/pnas.261469998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kono S, Chen K. Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci. 2005;96(9):535–542. doi: 10.1111/j.1349-7006.2005.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little J, Sharp L, Duthie S, Narayanan S. Colon cancer and genetic variation in folate metabolism: the clinical bottom line. J Nutr. 2003;133(11 Suppl 1):3758S–3766S. doi: 10.1093/jn/133.11.3758S. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Han S, Li Y, Mao Y, Xie Y. Different roles of MTHFR C677T and A1298C polymorphisms in colorectal adenoma and colorectal cancer: a meta-analysis. J Hum Genet. 2007;52(1):73–85. doi: 10.1007/s10038-006-0082-5. [DOI] [PubMed] [Google Scholar]
- 13.Levine AJ, Siegmund KD, Ervin CM, et al. The methylenetetrahydrofolate reductase 677C-->T polymorphism and distal colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2000;9(7):657–663. [PubMed] [Google Scholar]
- 14.Marugame T, Tsuji E, Kiyohara C, et al. Relation of plasma folate and methylenetetrahydrofolate reductase C677T polymorphism to colorectal adenomas. Int J Epidemiol. 2003;32(1):64–66. doi: 10.1093/ije/dyg004. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Giovannucci EL, Hunter DJ. MTHFR polymorphism, methyl-replete diets and the risk of colorectal carcinoma and adenoma among U.S. men and women: an example of gene-environment interactions in colorectal tumorigenesis. J Nutr. 1999;129(2S Suppl):560S–564S. doi: 10.1093/jn/129.2.560S. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich CM, Kampman E, Bigler J, et al. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev. 1999;8(8):659–668. [PubMed] [Google Scholar]
- 17.Chen J, Giovannucci E, Hankinson SE, et al. A prospective study of methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms, and risk of colorectal adenoma. Carcinogenesis. 1998;19(12):2129–2132. doi: 10.1093/carcin/19.12.2129. [DOI] [PubMed] [Google Scholar]
- 18.Martinez ME, Thompson P, Jacobs ET, et al. Dietary factors and biomarkers involved in the methylenetetrahydrofolate reductase genotype-colorectal adenoma pathway. Gastroenterology. 2006;131(6):1706–1716. doi: 10.1053/j.gastro.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Weisberg IS, Jacques PF, Selhub J, et al. The 1298A-->C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156(2):409–415. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 20.Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol. 2004;159(5):423–443. doi: 10.1093/aje/kwh066. [DOI] [PubMed] [Google Scholar]
- 21.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 22.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 23.Cole BF, Baron JA, Sandler RS, et al. A randomised trial of folic acid to prevent colorecta adenomas. AACR 96th Annual Meeting; 2005. abstract. [Google Scholar]
- 24.Gibson CS, MacLennan AH, Dekker GA, et al. Genetic polymorphisms and spontaneous preterm birth. Obstet Gynecol. 2007;109(2 Pt 1):384–391. doi: 10.1097/01.AOG.0000252712.62241.1a. [DOI] [PubMed] [Google Scholar]
- 25.Chango A, Boisson F, Barbe F, et al. The effect of 677C-->T and 1298A-->C mutations on plasma homocysteine and 5,10-methylenetetrahydrofolate reductase activity in healthy subjects. Br J Nutr. 2000;83(6):593–596. doi: 10.1017/s0007114500000751. [DOI] [PubMed] [Google Scholar]
- 26.van der Put NM, Gabreels F, Stevens EM, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62(5):1044–1051. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Giovannucci E, Kelsey K, et al. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996;56(21):4862–4864. [PubMed] [Google Scholar]
- 28.Hirose M, Kono S, Tabata S, et al. Genetic polymorphisms of methylenetetrahydrofolate reductase and aldehyde dehydrogenase 2, alcohol use and risk of colorectal adenomas: Self-Defense Forces Health Study. Cancer Sci. 2005;96(8):513–518. doi: 10.1111/j.1349-7006.2005.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannucci E, Chen J, Smith-Warner SA, et al. Methylenetetrahydrofolate reductase, alcohol dehydrogenase, diet, and risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2003;12(10):970–979. [PubMed] [Google Scholar]
- 30.Boyapati SM, Bostick RM, McGlynn KA, et al. Folate intake, MTHFR C677T polymorphism, alcohol consumption, and risk for sporadic colorectal adenoma (United States) Cancer Causes Control. 2004;15(5):493–501. doi: 10.1023/B:CACO.0000036447.45446.2c. [DOI] [PubMed] [Google Scholar]
- 31.Ulvik A, Evensen ET, Lien EA, et al. Smoking, folate and methylenetetrahydrofolate reductase status as interactive determinants of adenomatous and hyperplastic polyps of colorectum. Am J Med Genet. 2001;101(3):246–254. doi: 10.1002/ajmg.1370. [DOI] [PubMed] [Google Scholar]
- 32.Marugame T, Tsuji E, Inoue H, et al. Methylenetetrahydrofolate reductase polymorphism and risk of colorectal adenomas. Cancer Lett. 2000;151(2):181–186. doi: 10.1016/s0304-3835(99)00412-7. [DOI] [PubMed] [Google Scholar]
- 33.Hubner RA, Muir KR, Liu JF, et al. Folate metabolism polymorphisms influence risk of colorectal adenoma recurrence. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1607–1613. doi: 10.1158/1055-9965.EPI-06-0274. [DOI] [PubMed] [Google Scholar]
- 34.van den Donk M, Buijsse B, van den Berg SW, et al. Dietary intake of folate and riboflavin, MTHFR C677T genotype, and colorectal adenoma risk: a Dutch case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1562–1566. doi: 10.1158/1055-9965.EPI-04-0419. [DOI] [PubMed] [Google Scholar]
- 35.Wilson A, Platt R, Wu Q, et al. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab. 1999;67(4):317–323. doi: 10.1006/mgme.1999.2879. [DOI] [PubMed] [Google Scholar]