Abstract
We previously showed that activation of the A1 adenosine receptor protected the kidney against ischemia–reperfusion injury by induction and phosphorylation of heat shock protein 27 (HSP27). Here, we used mice that overexpress human HSP27 (huHSP27) to determine if kidneys from these mice were protected against injury. Proximal tubule cells cultured from the transgenic mice had increased resistance to peroxide-induced necrosis compared to cells from wild-type mice. However, after renal ischemic injury, HSP27 transgenic mice had decreased renal function compared to wild-type mice, along with increased renal expression of mRNAs of pro-inflammatory cytokines (TNF-α, ICAM-1, MCP-1) and increased plasma and kidney keratinocyte-derived cytokine. Following ischemic injury, neutrophils infiltrated the kidneys earlier in the transgenic mice. Flow cytometric analysis of lymphocyte subsets showed that those isolated from the kidneys of transgenic mice had increased CD3+, CD4+, CD8+, and NK1.1+ cells 3h after injury. When splenocytes or NK1.1+ cells were isolated from transgenic mice and adoptively transferred into wild-type mice there was increased renal injury. Further, depletion of lymphocytes by splenectomy or neutralization of NK1.1+ cells resulted in improved renal function in the transgenic mice following reperfusion. Our study shows that induction of HSP27 in renal tubular cells protects against necrosis in vitro, but its systemic increase counteracts this protection by exacerbating renal and systemic inflammation in vivo.
Keywords: inflammation, keratinocyte-derived cytokine, lymphocyte, natural killer cells, neutrophil
Acute renal failure (ARF) is a disease characterized by high patient morbidity and mortality.1–3 Despite considerable research, there is no effective therapy for ARF.1 Ischemia reperfusion injury (IRI) is the major cause of ARF and occurs frequently due to the obligatory interruption of blood flow and undesirable hemodynamic changes during the peri-operative period.4–6
Our laboratory previously demonstrated that exogenous and endogenous A1 adenosine receptor (AR) activation protected against renal IRI in mice7,8 as well as in rats.9,10 Mechanistically, we have previously shown that A1AR activation phosphorylates heat shock protein 27 (HSP27) in cultured renal proximal tubule cells and chronic activation or overexpression (OE) of A1ARs leads to upregulation of total HSP27.11 This finding was replicated in vivo, as mice treated with a selective A1AR agonist, 2-chloro-N6-cyclopentyladenosine, before renal ischemia showed a reduction in renal corticomedullary necrosis, apoptosis, and inflammation that corresponded with an increase in HSP27 expression.12
HSP27 is a member of family of chaperone proteins that are upregulated in response to increases in temperature, as well as a wide range of cellular stresses including hypoxia, ischemia, and exposure to toxic drugs.13–16 Increased expression of HSP27 serves to defend a cell against injury or death by acting as chaperones facilitating proper polypeptide folding and aberrant protein removal.17–19 Furthermore, HSP27 is a potent antiapoptotic protein and is a key stabilizer of the actin cytoskeleton; both of these cellular effects lead to increased resistance against cell death.20–22 Not surprisingly, OE of HSP27 protected against neuronal and cardiac injury.23–25
In this study, we tested the hypothesis that mice with global OE of HSP27 would show an increased resistance against renal IRI. We determined in this study that although proximal tubules cultured from huHSP27 OE mice were protected against necrosis in vitro, huHSP27 OE mice in vivo had paradoxically worsened renal dysfunction compared to HSP27 wild-type (WT) mice. We subsequently tested the hypothesis that increased renal dysfunction in huHSP27 OE mice is due to the increased leukocyte activation and enhanced renal tubular inflammation after renal ischemia reperfusion (IR).
RESULTS
Human HSP27 mRNA and protein expression in huHSP27 OE mice
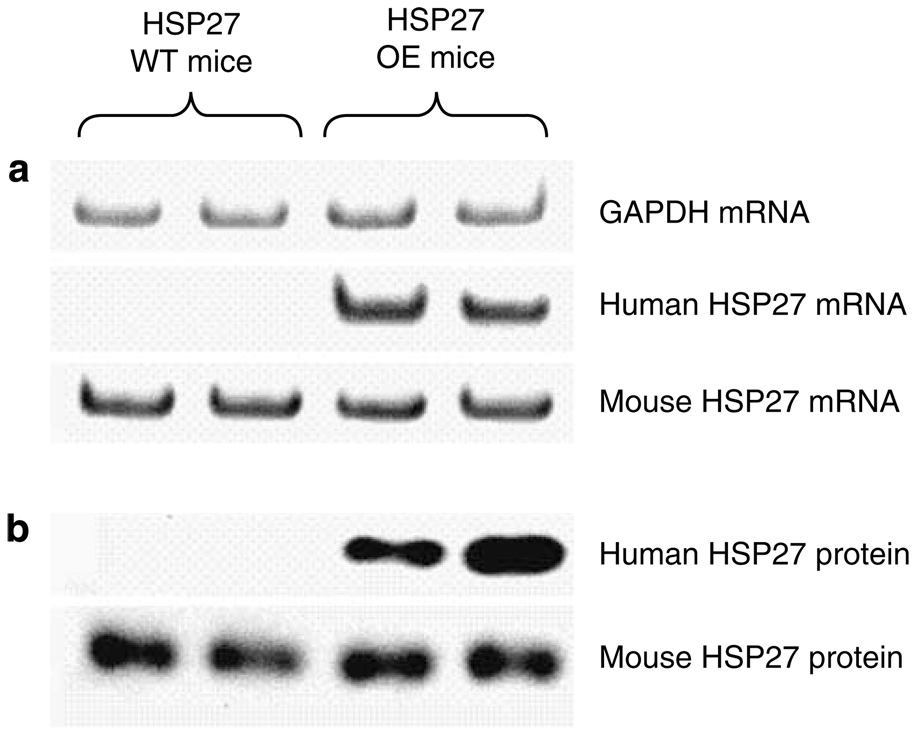
Figure 1 shows a selective human HSP27 transgene expression in huHSP27 OE mice. huHSP27 OE and WT mice expressed equivalent mRNA and protein expression of murine (endogenous) HSP27. However, only the huHSP27 OE mice expressed human HSP27 mRNA and protein distinguishable due to its larger size as a hemagglutininfused protein (Figure 1).
Figure 1. Selective expression of human HSP27 mRNA and protein in HSP27 OE mice.
(a) Representative gel images of semiquantitative RT-PCR results of GAPDH, human HSP27, and mouse HSP27 mRNAs from HSP27 WT and huHSP27 OE mouse renal cortices. (b) Representative immunoblotting images for human HSP27 and mouse HSP27 protein expression in HSP27 WT and huHSP27 OE mouse renal cortices. Representative images from four experiments are shown.
Renal proximal tubules from huHSP27 OE mice show reduced necrosis after H2O2 injury
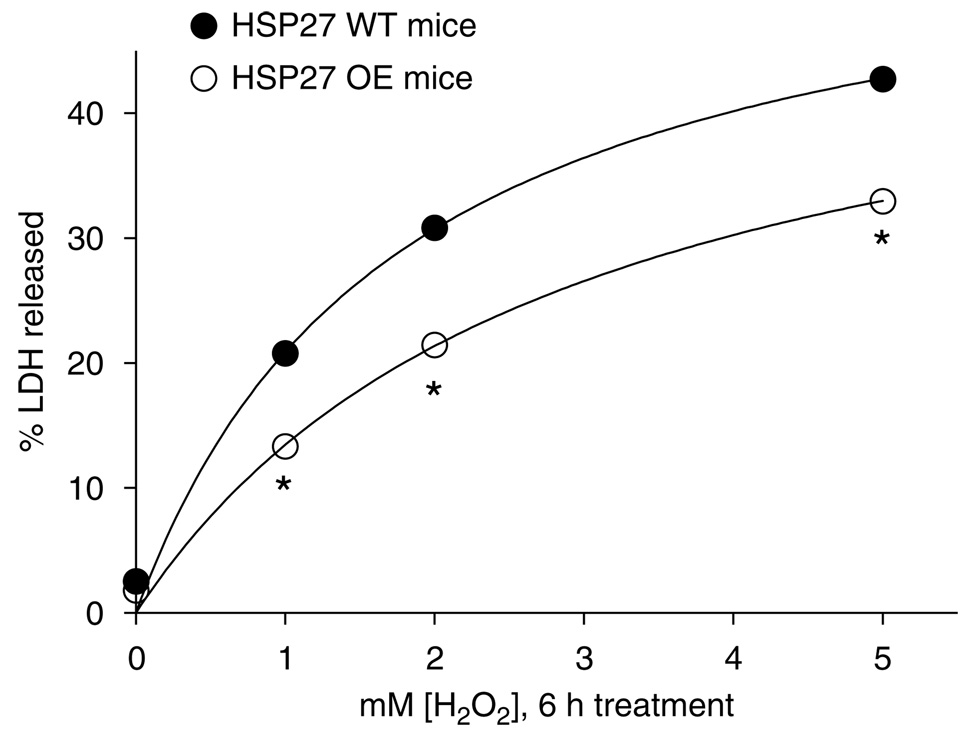
Treatment (6h) with 1–5 mM hydrogen peroxide (H2O2) caused rapid necrosis of proximal tubule cells isolated and cultured from mice (Figure 2; N = 6 for each group). Renal proximal tubules cultured from the huHSP27 OE mice showed increased resistance against H2O2-induced necrosis with reduced lactate dehydrogenase released when compared to the HSP27 WT mice proximal tubules (Figure 2).
Figure 2. LDH release after vehicle or H2O2 treatment (0–5 mM) for 6h in proximal tubule cells cultured from HSP27 WT or huHSP27 OE mice.
*P < 0.05 vs LDH released from proximal tubules isolated from HSP27 WT mice.
huHSP27 OE mice showed increased renal injury after IR
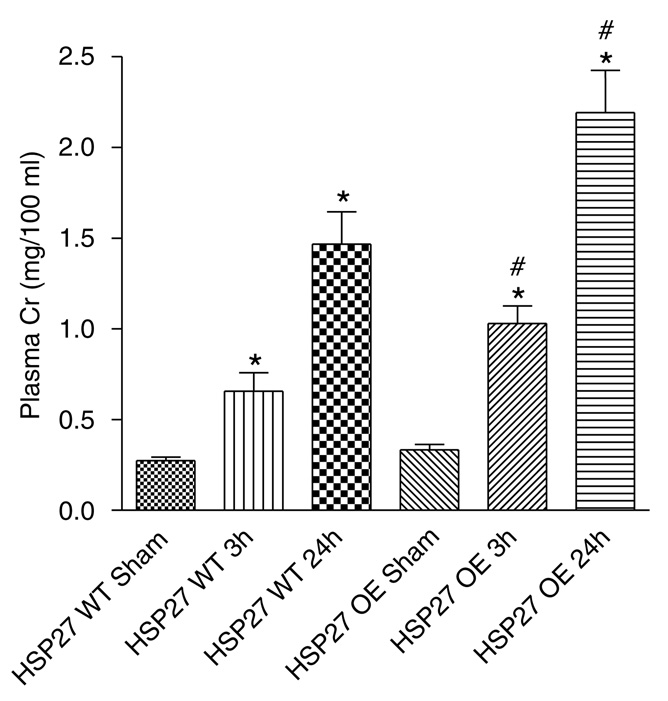
After 30 min of renal ischemia and 3 h reperfusion, plasma creatinine (Cr, mg/100 ml) rose significantly in both HSP27 WT (Cr = 0.6 ± 0.1, N = 6) and huHSP27 OE mice (Cr = 1.0 ± 0.1, N = 6) compared with sham-operated mice at 3h after surgery (Cr = 0.27 ± 0.02, N = 6 for HSP27 WT sham-operated mice and Cr = 0.33 ± 0.03, N = 5 for huHSP27 OE sham-operated mice). Plasma Cr rose even higher at 24 h after renal ischemia in both HSP27 WT (Cr = 1.5 ± 0.2, N = 8) and huHSP27 OE mice (Cr = 2.2 ± 0.2, N = 8). However, huHSP27 OE mice showed significantly higher rise in plasma Cr at both 3 (P < 0.05) and 24 h (Po0.05) after reperfusion (Figure 3).
Figure 3. Plasma creatinine (Cr in mg/100 ml) from shamoperated mice (Sham) or mice subjected to ischemiareperfusion (IR).
For the IR groups, plasma Cr were obtained at 3 (IR 3 h) and 24 h (IR 24h) after reperfusion. *P < 0.05 vs appropriate sham. #P < 0.05 vs HSP27 WT IR at appropriate time. Data are presented as mean ± s.e.m.
Because of the concerns for the potential genetic variability associated with non-congenic strain of mice, we bred our huHSP27 OE and HSP27 WT mice with C57BL/6 mice for three generations. From the resulting male mice, we have performed renal IR experiments and show that the huHSP27 OE mice backcrossed with C57BL/6 mice (Cr = 3.2 ± 0.2 mg/100 ml, N = 7) show increased renal injury after 30 min renal ischemia and 24 h reperfusion compared to the HSP27 WT mice backcrossed with C57BL/6 mice (Cr = 2.2 ± 0.2 mg/100 ml, N = 6). Therefore, OE of huHSP27 in mice backcrossed with C57BL/6 mice continued to result in increased renal injury after IR. We also note that the HSP27 WT mice backcrossed with C57BL/6 now show similar plasma Cr compared to the C57BL/6 mice subjected to renal IR (2.2 ± 0.3 mg/100 ml, N = 6). Moreover, the HSP27 WT and huHSP27 OE mice backcrossed with C57BL/6 mice show higher plasma Cr compared to the plasma Cr values of original HSP27 WT (Cr = 1.5 ± 0.2 mg/100 ml, N = 8) and huHSP27 OE (Cr = 2.2 ± 0.2, N = 8) mice before backcrossing.
Renal tubular necrosis after IR
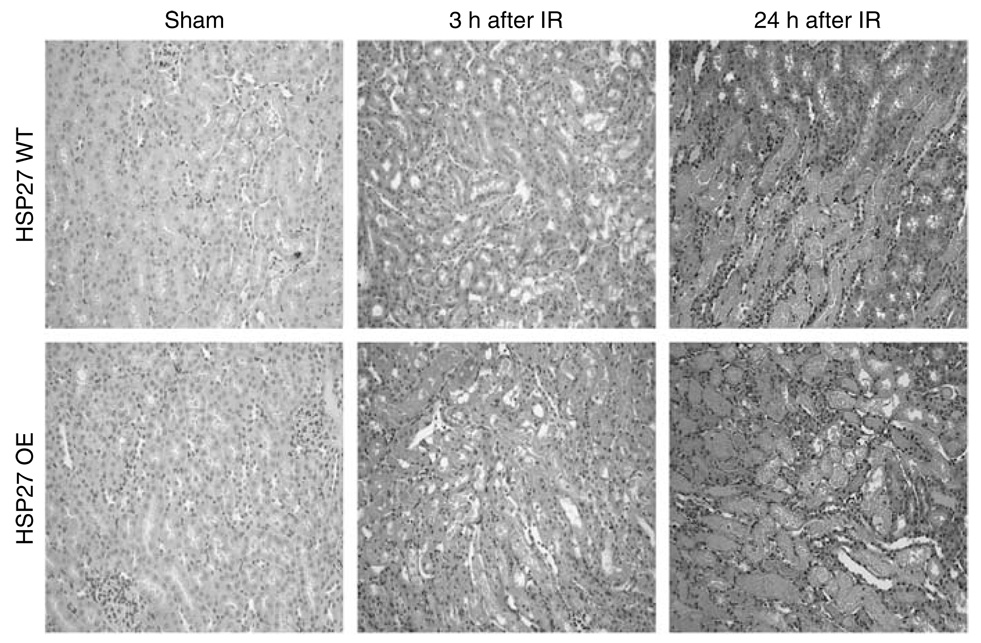
Renal ischemia (30 min) at 37 °C caused significant renal tubular necrosis at 3 and 24 h after IR in both HSP27 WT and OE mice (Figure 4) as evidenced by severe tubular necrosis, medullary congestion and hemorrhage, and development of proteinaceous casts in all mouse kidney sections. Consistent with plasma Cr, huHSP27 OE mice showed increased renal tubular necrosis 3 h after renal IR compared to HSP27 WT mice. However, HSP27 WT and huHSP27 OE mice showed a similar degree of renal tubular necrosis after IR. The Jablonski scale renal injury score histology grading is used to grade renal tubular necrosis after murine renal IRI.26 Thirty min of renal ischemia and 3 h of reperfusion resulted in greater renal injury score in huHSP27 OE mice (renal injury score = 2.0 ± 0.3, N = 4) when compared to HSP27 WT mice (renal injury score = 0.88 ± 0.3, N = 4, P < 0.05). Twenty-four hour of reperfusion resulted in severe acute tubular necrosis in both HSP27 WT mice (renal injury score = 3.0 ± 0.0, N = 4) and huHSP27 OE mice (renal injury score = 2.8 ± 0.4, N = 4).
Figure 4. Representative of photomicrographs of four experiments (hematoxylin and eosin staining, original magnification × 200) of studies with HSP27 WT and huHSP27 OE mice.
Pictures of outer medulla of the kidneys of sham-operated mice and mice subjected to renal ischemia-reperfusion (IR) injury 3 and 24 h prior are shown.
huHSP27 OE mice show increased proinflammatory gene expression in the kidney after renal IRI
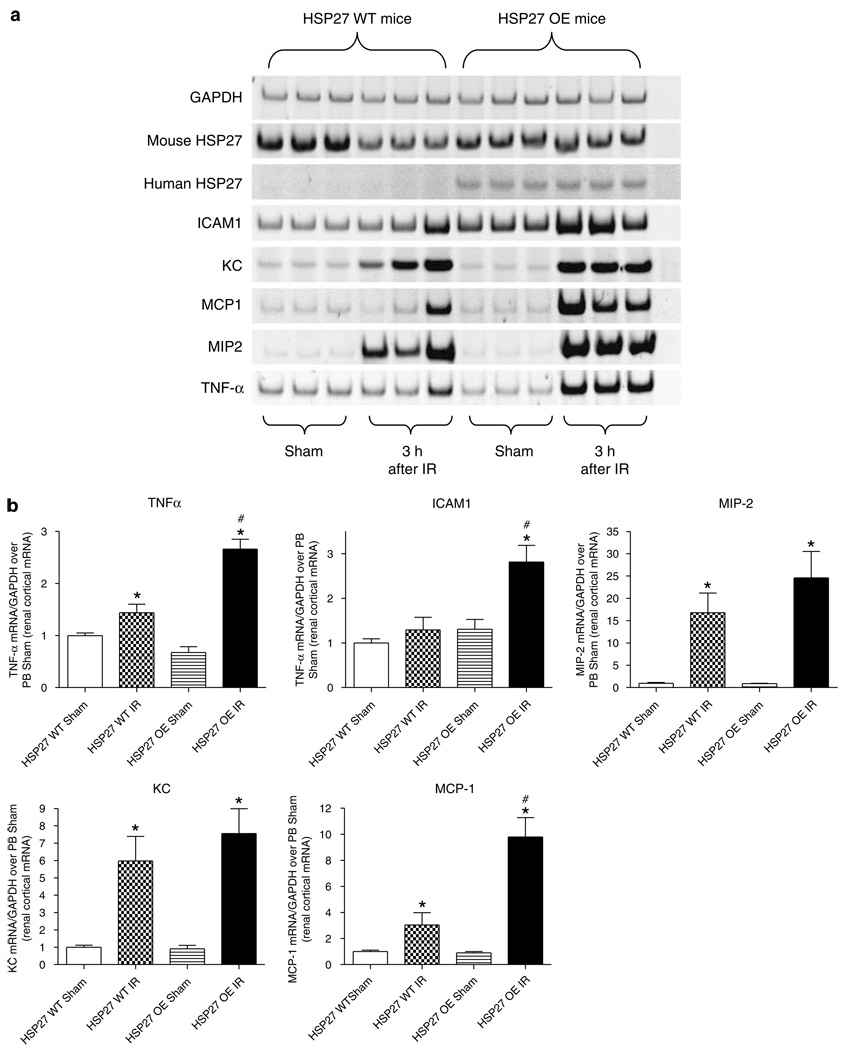
We found significantly increased expression of intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractive protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α) mRNA in huHSP27 OE mice compared to the HSP27 WT mice 3 h after renal ischemia. No significant differences in the expression of keratinocyte-derived cytokine (KC) or macrophage inflammatory protein-2 (MIP-2) mRNA were found between HSP27 WT and OE mice (Figure 5).
Figure 5. HSP27 OE mice show reduced proinflammatory mRNA expression after renal IR.
(a) Representative gel images of semiquantitative RT-PCR of mouse and human HSP27 as well as proinflammatory markers ICAM-1, KC, MCP-1, MIP-2, and TNF-α from renal cortices of HSP27 WT and huHSP27 OE mice subjected to sham operation or to renal ischemia and 3 h reperfusion. (b) Densitometric quantifications of relative band intensities normalized to GAPDH from RT-PCR reactions for each indicated mRNA. *P < 0.05 vs appropriate sham. #P < 0.05 vs HSP27 WT IR. Error bars represent 1 s.e.m.
huHSP27 OE mice show increased early renal neutrophil infiltration after IR
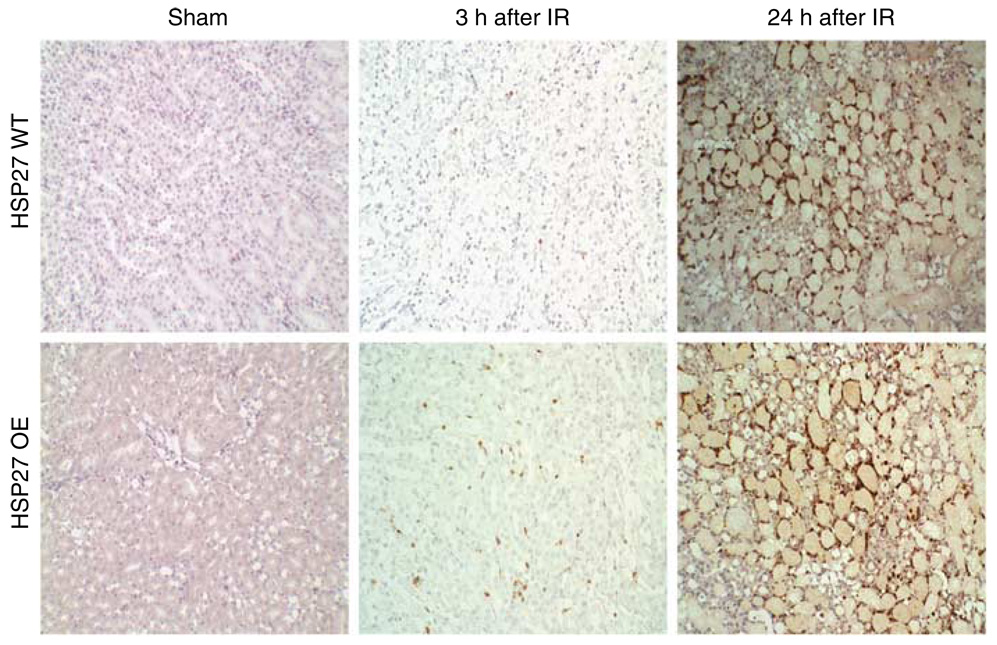
Immunohistochemical assays showed an increase in neutrophil infiltration in the huHSP27 OE mice relative to the HSP27 WT mice at 3 h after renal IRI (Figure 6). At 24 h after renal IR, polymorphonuclear infiltration was similar between the two groups of mice. Manual counting of polymorpho-nuclear neutrophils in hematoxylin and eosin (H&E) slides also showed increased neutrophil infiltration in the huHSP27 OE (0.72 ± 0.20 neutrophils/× 400 field, N = 4) mice relative to the HSP27 WT mice (0.05 ± 0.02 neutrophils/× 400 field, N = 4, P < 0.05) at 3 h after renal IRI.
Figure 6. Representative of photomicrographs of four experiments of immunohistochemistry for neutrophils (original magnification × 200) of the outer medulla of the kidneys of HSP27 WT and huHSP27 OE mice subjected to sham operation or to renal ischemia and 3 or 24 h reperfusion.
Blood KC mRNA and protein expression and renal KC protein expression are increased in huHSP27 OE mice
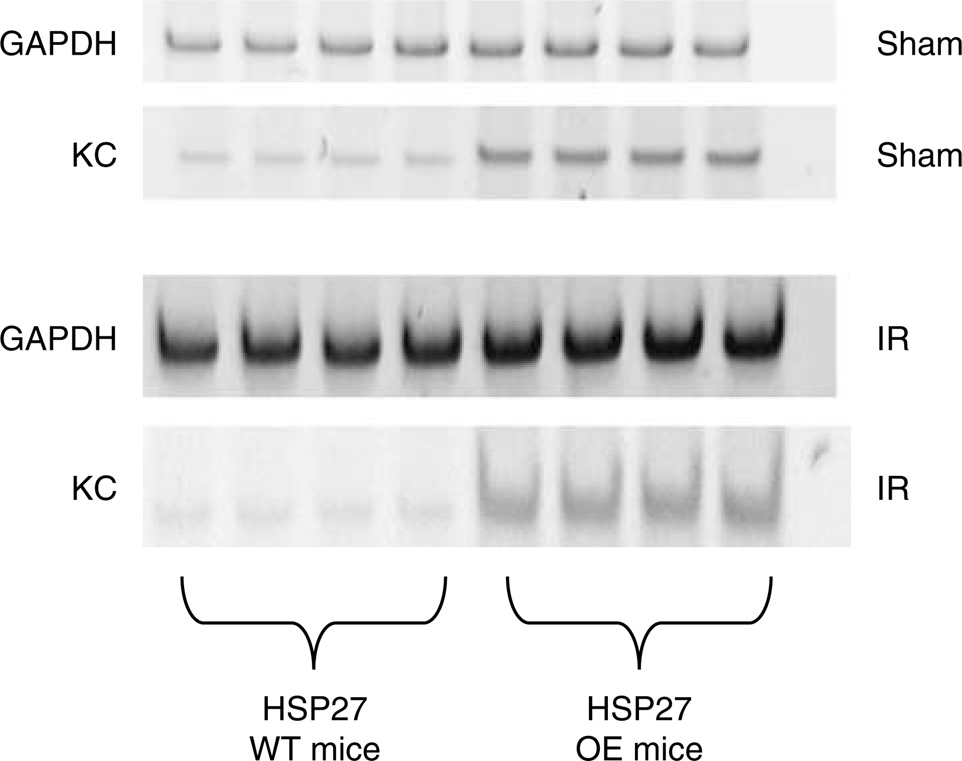
Whole-blood KC mRNA was significantly upregulated in the huHSP27 OE mice compared to the HSP27 WT mice at baseline and at 24 h after renal IR (Figure 7). No other proinflammatory mRNA expression (ICAM-1, TNF-α, MCP-1, MIP-2) was detectable in whole blood. With ELISA, MCP-1 and KC plasma levels were determined 24 h after renal IR. There were no differences in MCP-1 levels (1519 ± 421 pg/ml, N = 8 for HSP27 WT and 1544 ± 465 pg/ml, N = 8 for huHSP27 OE mice), however, plasma KC levels increased significantly higher in the huHSP27 OE mice (36,535 ± 4770 pg/ml, N = 5) compared to the HSP27 WT mice (14,466 ± 6950 pg/ml, N = 5). Moreover, baseline KC levels were higher in the huHSP27 OE mice (538 ± 105 pg/ml, N = 6) compared to the HSP27 WT mice (139 ± 40 pg/ml, N = 6). Finally, baseline kidney KC protein expression was significantly higher in huHSP27 OE mice (18.4±2.4 pg/mg protein, n = 4, P < 0.01) compared to HSP27 WT mice (3.2 ± 0.9 pg/mg protein, n = 4).
Figure 7. Representative gel images of semiquantitative RT-PCR of whole-blood KC of HSP27 WT and huHSP27 OE mice subjected to sham operation or to renal ischemia and 24h reperfusion.
Renal apoptosis after IRI
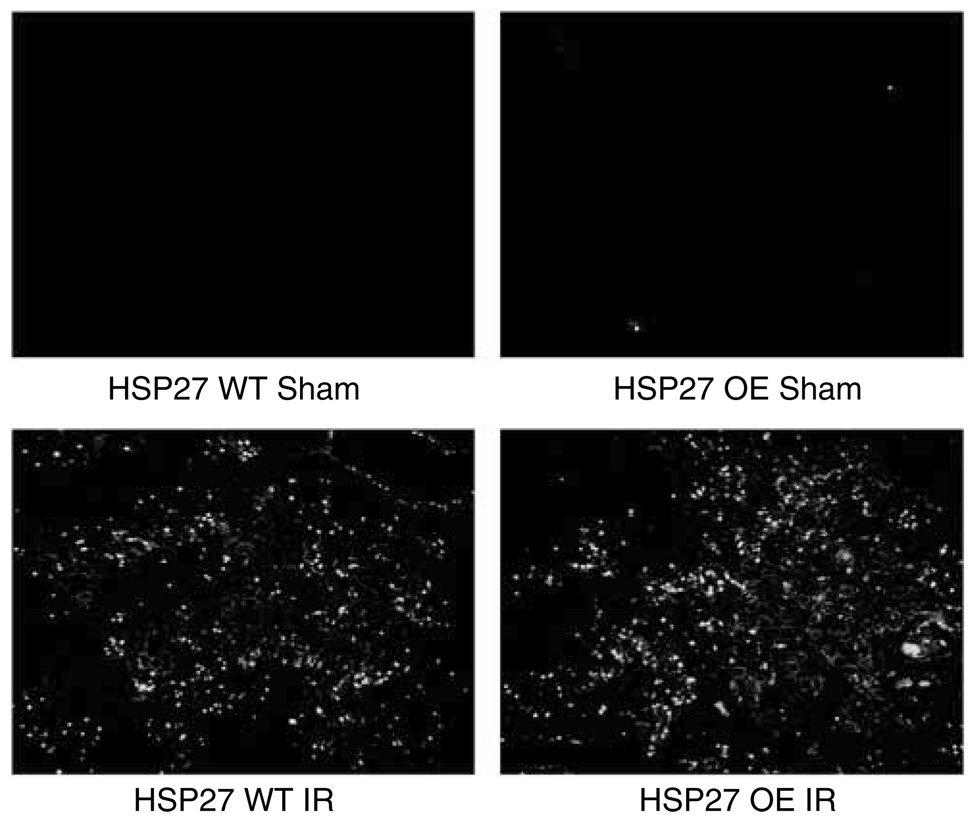
We failed to detect significant terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL)-positive cells in kidney sections (corticomedullary junction) from sham-operated mice (Figure 8). Mice subjected to 30 min of renal ischemia and 24 h of reperfusion demonstrated TUNEL-positive cells in the corticomedullary junction. However, there were no differences in TUNEL-positive cells between huHSP27 OE mice and HSP27 WT mice (Figure 8). Finally, the degree of renal tubular apoptosis was also quantified by counting the number of apoptotic bodies in proximal tubules in the corticomedullary area of the kidney (expressed as apoptotic bodies per tubule) on the H&E-stained sections. A total of 25–50 tubules/field were counted for each treatment group, and kidneys from four experiments were examined. Renal IRI equivalently increased the number of apoptotic bodies within the proximal tubules in both HSP27 WT (0.22 ± 0.1 apoptotic bodies/tubule, N = 4) and huHSP27 OE mice (0.21 ± 0.1 apoptotic bodies/tubule, N = 4).
Figure 8. Representative fluorescent photomicrographs of kidney sections from identical fields illustrate apoptotic nuclei (TUNEL fluorescent stain).
HSP27 WT and huHSP27 OE mice were subjected to sham operation or to renal ischemia and 24 h reperfusion. Representative of six independent experiments.
FACS analysis of lymphocyte subtype
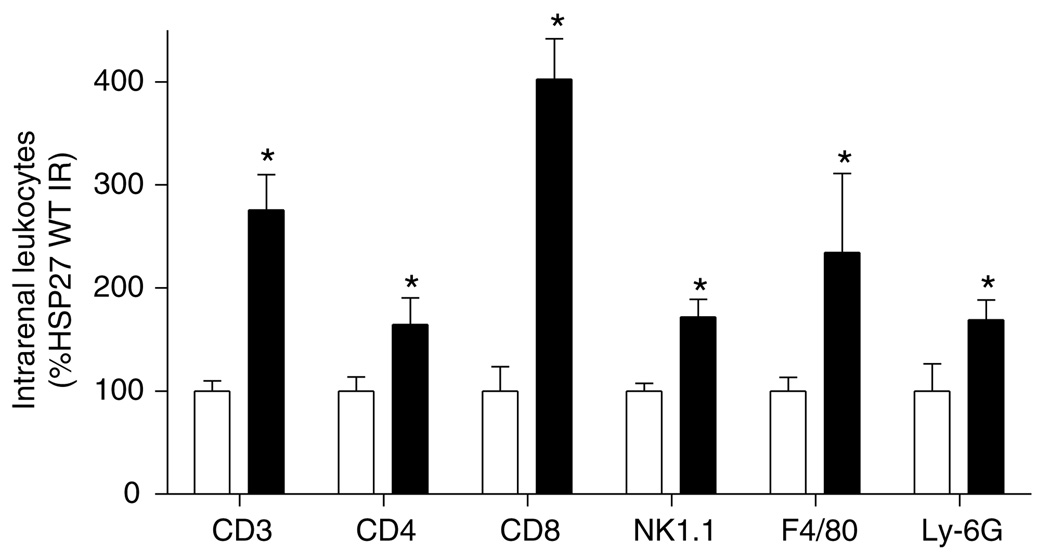
Utilizing flow cytometry, we identified subpopulations of leukocytes that infiltrated the kidney following renal IR in HSP27 WT and OE mice. Flow cytometry staining of kidney leukocytes showed increased renal trafficking of CD3, CD4, CD8, F4/80, Ly-6G, and NK1.1+ cells in both HSP27 WT and huHSP27 OE mice 3h after renal IRI. However, significantly higher number of leukocytes infiltrated the kidneys in huHSP27 OE mice (Figure 9).
Figure 9. Kidney lymphocyte subtype (CD3, CD4, CD8, and NK1.1), macrophage (F4/80), and neutrophil (LY-6G) content after renal IR injury in HSP27 WT (white bars) and huHSP27 OE mice (black bars).
Kidney leukocyte subtype content in mice 3 h after reperfusion was quantified using flow cytometry. Values are expressed as percent HSP27 WT mice subjected to IR. *P < 0.05 vs HSP27 WT IR. Error bars represent 1 s.e.m.
Transfer of splenocytes or NK1.1 + cells from huHSP27 OE mice increases renal IRI in HSP27 WT mice
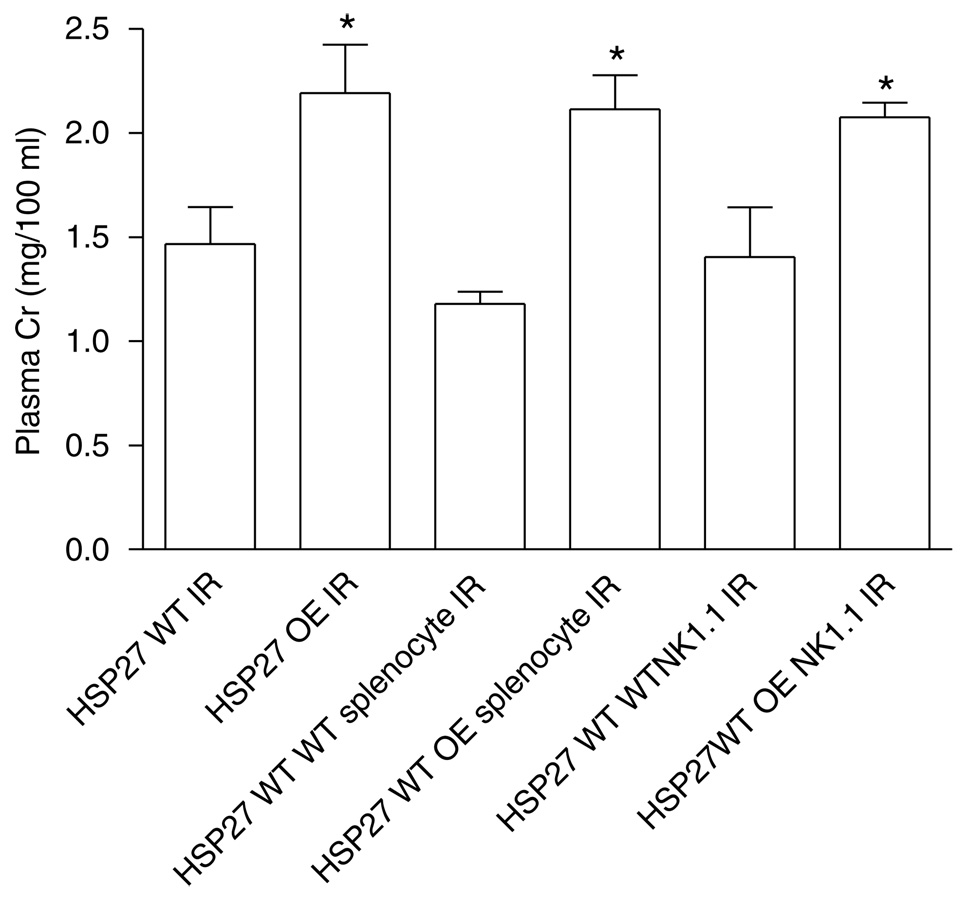
The adoptive transfer of splenocytes (Cr = 2.1 ± 0.2, N = 6) or NK1.1 ± cells (Cr = 2.1 ± 0.1, N = 6) isolated from huHSP27 OE mice increased renal injury at 24 h in HSP27 WT mice (Figure 10). When the splenocytes (Cr = 1.2 ± 0.2, N = 5) or the NK1.1 + cells (Cr = 1.6 ± 0.3, N = 6) from HSP27 WT mice were given to the HSP27 WT mice, there were no increases in renal injury (Figure 10).
Figure 10. Plasma creatinine (Cr in mg/100 ml) from HSP27 WT and huHSP27 OE mice subjected to 30min renal ischemia and 24h reperfusion.
Some HSP27 WT mice were injected with huHSP27 OE splenocytes or NK1.1 + cells before renal ischemia. *P < 0.05 vs HSP27 WT 24h IR. Data are presented as mean ± s.e.m.
Depletion of NK1.1 + cells or splenectomy unmasks the protective phenotype of HSP27 after renal IR in mice
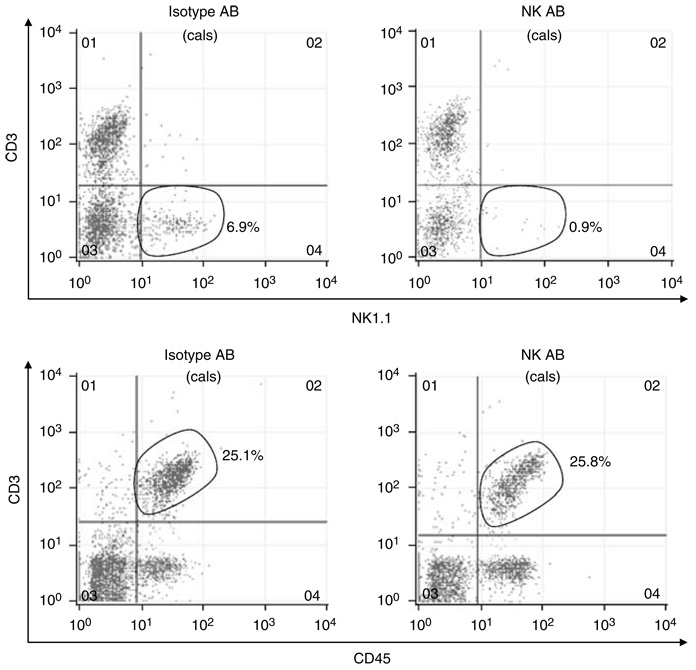
Figure 11 shows our ability to deplete NK1.1 + cells selectively in mice. Mice were injected with either NK1.1 antibody (PK136, 500 µg/mouse) or isotype control (mouse IgG2a). After 24 h, spleen and blood leukocytes were isolated and subjected to fluorescence-activated cell sorting (FACS). Our PK136 antibody selectively depleted NK1.1 + cells in blood (Figure 11, representative of three independent experiments) and spleen (not shown) without effecting CD3 + T cells.
Figure 11. Representative FACS analysis of CD3 + lymphocytes and NK1.1+ lymphocytes in blood of HSP27 WT mice 24h after treatment with either NK1.1+ neutralizing antibody (PK136) or with isotype control.
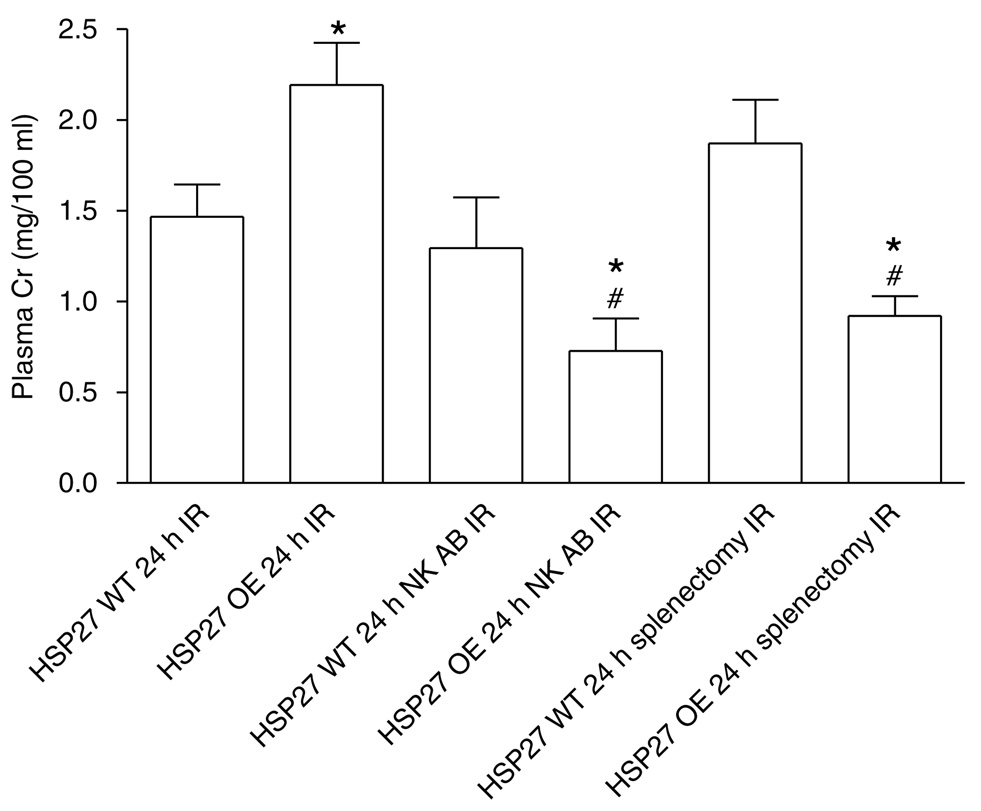
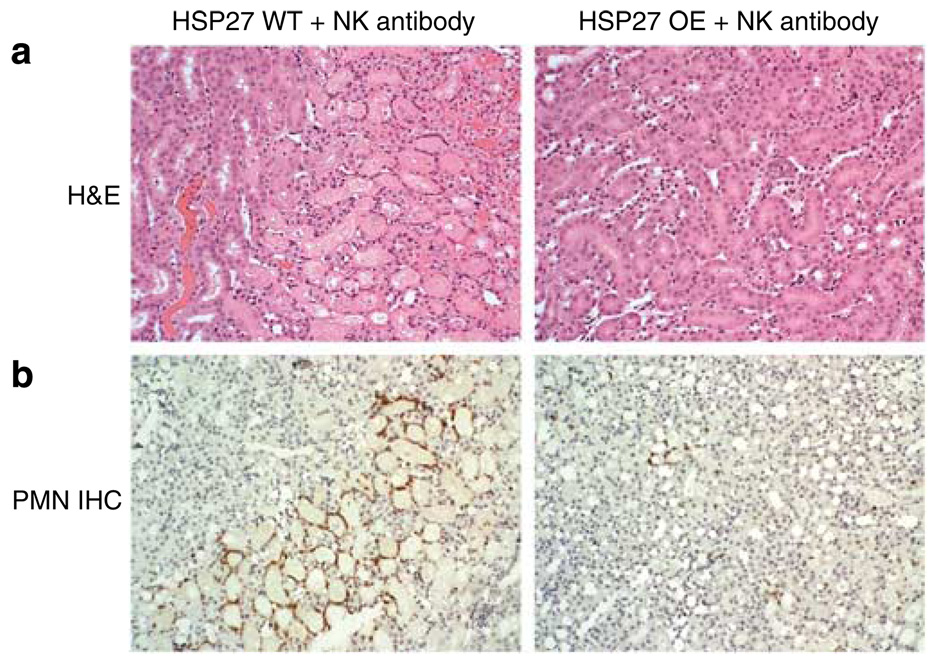
huHSP27 OE mice subjected to renal IRI after splenectomy (Cr = 0.9 ± 0.1, N = 8) or NK1.1+ cell depletion (Cr = 0.7 ± 0.2, N = 6) were significantly protected against renal IRI compared to the HSP27 WT (Cr = 1.5 ± 0.2, N = 8) or non-depleted huHSP27 OE mice at 24h (Cr = 2.2 ± 0.2, N = 8; Figure 12). However, HSP27 WT mice failed to improve their renal function after splenectomy (Cr = 1.8 ± 0.2, N = 7) or NK1.1+ cell depletion (Cr = 1.3 ± 0.3, N = 6). Moreover, NK1.1+ neutralizing antibody treatment led to significant protection against renal tubular necrosis in huHSP27 OE mice (renal injury score = 1.3 ± 0.4, N = 4; Figure 13a) compared to HSP27 WT mice treated with NK1.1 + neutralizing antibody (renal injury score = 2.3 ± 0.4, N = 4). huHSP27 OE mice pretreated with NK1.1+ neutralizing antibody also showed reduced neutrophil infiltration compared to the HSP27 WT mice pretreated with NK1.1+ antibody (Figure 13b). Manual counting of polymorphonuclear cells in H&E slides also showed reduced neutrophil infiltration in the huHSP27 OE mice treated with NK1.1+ antibody (0.87 ± 1 neutrophils/× 400 field, N = 4) relative to the HSP27 WT mice treated with NK1.1 + antibody (33 ± 9 neutrophils/× 400 field, N = 4, P < 0.001) at 24h after renal IRI. Similar protection against renal tubular damage and neutrophil infiltration was observed in splenectomized huHSP27 OE mice (data not shown).
Figure 12. Plasma creatinine (Cr in mg/100 ml) from HSP27 WT and huHSP27 OE mice subjected to 30min renal ischemia and 24h reperfusion.
Some HSP27 WT and OE mice were injected with NK1.1 + neutralizing antibody (NK AB) or were splenectomized before renal ischemia. *P < 0.05 vs appropriate HSP27 WT 24h IR. #P < 0.05 vs huHSP27 OE 24h IR. Data are presented as mean ± s.e.m.
Figure 13. NK1.1 + neutralizing antibody decreases renal necrosis and PMN infiltration.
(a) Representative photomicrographs (hematoxylin and eosin staining, original magnification × 200) from studies with HSP27 WT and huHSP27 OE mice injected with NK1.1 + neutralizing antibody. Pictures of outer medulla of the kidneys of sham-operated mice and mice subjected to renal ischemia-reperfusion (IR) injury 24 h prior are shown. (b) Representative photomicrographs of immunohistochemistry for neutrophils (original magnification × 200) of the outer medulla of the kidneys of HSP27 WT and huHSP27 OE mice injected with NK1.1 + neutralizing antibody and subjected to renal ischemia and 24 h reperfusion.
DISCUSSION
The major findings of this study are that (1) isolated proximal tubules from huHSP27 OE mice were protected against H2O2-induced necrosis compared to the proximal tubules isolated from HSP27 WT mice; (2) in contrast, huHSP27 OE mice showed worse renal function, increased renal tubular inflammation (early leukocyte influx and renal proinflammatory mRNA expression), and exacerbated early renal tubular necrosis after renal IRI in vivo; (3) huHSP27 OE mice showed increased plasma KC levels at baseline and 24 h after renal IRI; (4) huHSP27 OE mice had increased renal KC protein expression; (5) adoptive transfer of NK1. 1 + cells or splenocytes isolated from huHSP27 OE mice exacerbated renal injury in HSP27 WT mice; and (6) depletion of NK1.1 + cells or splenectomy unmasked the protective effects of HSP27 OE against renal IRI in mice.
Renal IRI is the leading cause of ARF in hospitalized patients.1,27 Moreover, the mortality and morbidity of perioperative ARF are very high and remain virtually unchanged for the past 40 years.3,28 We have demonstrated in our earlier studies that activation of renal A1ARs produced renal protection in vivo as well as in vitro.7,8,11,29 The mechanisms of renal protection with A1AR activation are mediated at least in part by phosphorylation as well as upregulation of HSP27 protein expression.11,12 Transient activation of renal tubular A1ARs led to phosphorylation of HSP27 whereas OE or chronic stimulation of A1ARs led to upregulation of HSP27. To the best of our knowledge, modulation of HSP27 expression on renal function after IRI in vivo has never been studied previously.
HSP27 is generally regarded as a cytoprotective protein and is well known to attenuate apoptosis, stabilize cytoskeletal architecture, and decrease necrotic cell death.19,22,30 It is well established that HSP27 phosphorylation or upregulation improves cell survival against stress or injury.14,30 These principles as well as our previous findings led us to hypothesize that huHSP27 OE mice would show improved renal tubular survival against necrotic injury in vitro and better renal function after IRI in vivo. As we expected, the proximal tubule cells cultured from huHSP27 OE mice showed increased resistance against H2O2-induced necrosis compared to the cells cultured from HSP27 WT mice. However, we unexpectedly demonstrated that the huHSP27 OE mice showed enhanced renal IRI with a heightened inflammatory response (earlier intrarenal leukocyte (for example, neutrophils, lymphocytes) infiltration, increased renal tubular proinflammatory mRNA expression, and higher plasma and kidney KC levels) after renal IRI. We also show enhanced renal necrosis early (3 h after reperfusion) after IR. Therefore, although HSP27 expression in renal proximal tubules is associated with increased resistance against necrosis in vitro, systemic HSP27 OE led to increased systemic and renal tubular inflammation in these mice, leading to worsened renal injury after IR in vivo.
Although HSP27 expression is usually associated with increased tolerance against cell death and injury, HSP27 may also orchestrate physiological events that increase immune cell-mediated cytotoxicity. For instance, HSP27 phosphorylation enhances neutrophil chemotaxis and function.31 Furthermore, a cytoplasmic OE of HSP25, a murine form of HSP27, increases natural killer (NK) cell-mediated lysis in a murine melanoma model.32 In addition, OE of HSP27 in a breast cancer cell line increases T-cell-mediated cytolysis.33 In HeLa cells, HSP27 is required for proinflammatory cell signaling and the expression of proinflammatory genes.34 In this study, we also showed increased inflammatory changes in huHSP27 OE mice leading to worse renal dysfunction after in vivo renal IRI. Our study also suggests that exogenous proinflammatory signaling distant to the organ or site of injury is important in regulating injury as increased inflammatory response overrides the protective effects of renal tubule HSP27 overexpression. We propose that although the antiapoptotic and anti-necrotic effects of HSP27 on the renal tubules promote direct cellular protection, the enhanced systemic inflammation of huHSP27 OE mice promotes the exacerbation of immune-mediated renal tubule injury after IR.
With regard to the previous studies involving huHSP27 OE mice, Hollander et al.23 demonstrated that OE of HSP27 protected against cardiac IRI evidenced by reduced release of Cr kinase, lipid peroxidation, and myocyte protein oxidation. Similar results were obtained by Efthymiou et al.35 utilizing the same strain of huHSP27 OE transgenic mice utilized in this study. In these previous studies, an isolated Langendorff-perfused heart IR model was utilized. Therefore, the effects of circulating inflammatory cells in cardiac IRI could not be tested. It remains to be determined whether the cardiac protection persists in intact, whole animal models of cardiac IRI.
Neutrophils, macrophages, and lymphocytes have important functions in initiating and propagating inflammation after renal IR.4,36,37 In this study, we showed that huHSP27 OE mice had increased early (3 h) neutrophil infiltration after renal IR. The appearance of neutrophils as well as macrophages mediates antigen-independent mechanisms of tissue damage due to innate immunity activation.38–41 Neutrophil infiltration begins early, within hours after the initiation of reperfusion and peaks at ~24 h after reperfusion. However, although renal neutrophil infiltration after IRI is a well-known phenomenon, the direct role of neutrophils in promoting the pathogenesis of renal IRI has been controversial. Some studies report a beneficial effect of neutrophil neutralization/blockade whereas other studies showed no effects.36,38,40,42,43 Our study showed that the huHSP27 OE mice had an early increase in neutrophil infiltration (3 h) as well as worsened renal injury after IR show. Therefore, we conclude that neutrophil infiltration after renal IR does directly contribute to increased renal injury after IR and that neutrophil-mediated renal injury may outweigh the cyto-protective effects of renal tubular HSP27 OE.
We detected increased T lymphocyte as well as NK1.1 + cell infiltration in huHSP27 OE mice compared to HSP27 WT mice 3 h after renal IR. The pathophysiological role of NK1.1 + cells and T lymphocytes in initiating liver and cardiac IRI has been described.37,44,45 In the kidney, NK1.1 + cells as well as T cells have important functions in the pathogenesis of IRI.44,46,47 When the mice were splenectomized (to deplete circulating lymphocytes) or treated with a neutralizing NK1.1 + antibody, less renal injury occurred in the huHSP27 OE mice. In fact, after splenectomy and NK1.1 + cell depletion, huHSP27 OE mice had significantly better renal function compared to the splenectomized or NK1.1+ cell-depleted HSP27 WT mice. These findings further support the hypothesis that huHSP27 OE mice have exacerbated inflammatory response after renal IR and depletion of key cellular components of the inflammatory response uncovers the protective phenotype of systemic HSP27 OE. Surprisingly, depletion of NK1.1 + cells or splenectomy failed to protect HSP27 WT mice against renal IRI. This finding contrasts with the study by Li et al.48 where NK1.1 cell depletion using the same antibody as in this study provided profound protection against renal IR. Our study suggests that the NK1.1 cells are important in generating renal injury only in huHSP27 OE mice. The reason for this discrepancy is not clear. Strain differences may explain this discrepancy as Li et al. used a C57BL/6 strain whereas we used mice with a C57BL/10 and CBA/Ca background.
The role of increased inflammation after renal IR causing worsened renal dysfunction in huHSP27 OE mice is further corroborated by our adoptive cellular transfer studies. HSP27 WT mice infused with splenocytes or NK1.1 + cells isolated from huHSP27 OE (but not from HSP27 WT) mice showed increased renal injury. These data further support our conclusion that the increased renal dysfunction in huHSP27 OE mice after IR is due to the enhanced inflammatory and immune-mediated renal injury.
One of the limitations of this study is that we do not conclusively identify the specific leukocyte subtype(s) responsible for the exacerbated renal injury in huHSP27 mice after renal IR. We implicate one of the cell types involved as NK1.1 cells as we show increased renal injury in HSP27 WT infused with huHSP27 OE NK1.1 cells. However, we cannot rule out the heightened proinflammatory role of other leukocytes such as macrophages and T cells in huHSP27 OE mice as these cell types have also been shown to be important in renal IRI. Identifying the specific role of T lymphocytes and/or macrophages in creating the enhanced proinflammatory phenotype in huHSP27 remains to be investigated.
We also demonstrated that huHSP27 OE mice had increased expression of whole-blood KC mRNA and protein expression at baseline and after renal IR. Moreover, we demonstrate increased renal KC protein expression in huHSP27 mice. It is unclear as to why overexpression of systemic HSP27 would lead to upregulation of kidney and blood KC expression in mice. Nevertheless, we propose that increased renal KC production may account for the differences in renal neutrophil infiltration after IR. Although the rodents lack a homologue of human IL-8, KC is one of the two murine IL-8-like CXC cytokines that acts through CXCR2 and functions as granulocyte chemoattractant similar to the function of IL-8.41,49 KC is a well known and major chemoattractant for neutrophils that subsequently cause tissue and cellular damage after injury and inflammation.41 In a rat model of cold ischemia followed by kidney transplantation, inhibition of the chemokine receptor CXCR2 reduced kidney graft failure due to ischemia/reperfusion.50 KC is also important in initiating injury in other diseases associated with increased inflammation. Frink et al.51 demonstrated that administration of the anti-KC antibody before trauma and hemorrhage in mice prevented increases in KC plasma levels, which was accompanied by amelioration of neutrophil infiltration and edema formation in lung and liver after trauma and hemorrhage.
We have previously demonstrated that A1AR agonists or OE of A1ARs produced renal protection in vivo as well as protection against renal tubule cell death in vitro through increases in renal tubular HSP27 expression.7,8,11,29 Therefore, unlike HSP27, systemic A1AR activation produces both in vivo and in vitro renal protection. We have performed pilot studies and determined that A1AR activation failed to upregulate HSP27 in two leukocyte cell lines (Jurkat lymphocyte cell line, RAW 263.7 macrophage cell line, data not shown). Therefore, we speculate that a selective upregulation of renal HSP27 with A1AR activation leads to renal protection whereas global HSP27 OE leads to increased renal injury after IR due to increased leukocyte activation and KC overproduction.
Although significant efforts had been made to maintain a constant hybrid background, because of the concerns for the potential genetic variability associated with non-congenic strain of mice, we bred our huHSP27 OE and HSP27 WT mice with C57BL/6 mice for three generations. We showed OE of huHSP27 in mice backcrossed with C57BL/6 mice continued to result in increased renal injury after IR. Therefore, although strain differences do have an important function in renal IR injury, global OE of human HSP27 protein led to increased renal injury after IR before and after backcrossing with the C57BL/6 strain.
In summary, we demonstrate in this study that global OE of HSP27 does not confer protection after renal IRI. In contrast to what we hypothesized and what others have reported in cardiac and neuronal injury studies, OE of HSP27 in vivo led to increased renal injury after IR. HSP27 does serve to protect the renal proximal tubules from necrosis in vitro but its protective effects are counteracted by increased immune-mediated renal tubular injury after IR. Future studies will compare the direct cytotoxicity profiles of NK1.1+ cells and lymphocytes between HSP27 WT and huHSP27 OE mice.
MATERIALS AND METHODS
Mice
Heterozygous breeder pairs of mice overexpressing (OE) human HSP27 were generated as described previously.52 huHSP27 OE transgenic mice overexpress human HSP27 tagged with hemagglutinin to track the expression of the transgene. The generation and initial characterization of the huHSP27 OE and WT mice with a C57BL/10 and CBA/Ca background has been described previously.52 Heterozygous HSP27 transgenic mice were bred and resulting male littermates (huHSP27 OE or HSP27 WT mice) were used in the subsequent studies. We performed tail DNA PCR genotyping as well as human HSP27 RT–PCR from the RNAs extracted on every mouse studied using primers that distinguish human from mouse HSP27. In preliminary studies, we also confirmed human HSP27 protein overexpression with immunoblotting for mouse and human form of HSP27 (Santa Cruz Biotechnologies, CA) as described previously.11,53 We also purchased C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) to breed with huHSP27 OE and HSP27 WT mice.
Primary culture of renal proximal tubules
Proximal tubules from male HSP27 WT and huHSP27 OE mice were enriched using the methods of Vinay et al.54 and cultured in 50:50 mix of Dulbecco’s modified Eagle’s medium high glucose and F12 plus 5% fetal bovine serum (FBS).
Induction of necrosis in cultured mouse renal proximal tubules
After 24 h deprivation of FBS, primary cultures of renal proximal tubules isolated from HSP27 WT and huHSP27 OE mice were treated with 1–5 mM H2O2 for 0–6 h to induce necrosis. We determined in preliminary studies that the ability of H2O2 to kill renal tubule cells is dose and time related. Our preliminary studies also showed that high-dose (1–5mM) treatment with H2O2 for 1–6 h causes negligible apoptosis (data not shown).
Renal ischemia-reperfusion injury
After Columbia University IACUC approval, adult (25–30 g) male huHSP27 OE mice and their littermate control HSP27 WT mice were anesthetized with an intraperitoneal injection of pentobarbital (50mg/kg or to effect), and placed supine on a heating pad under a warming light to maintain body temperature at 37 °C. Additional pentobarbital was given as needed based on the mouse’s response to a tail pinch. Bilateral flank incisions were made and following right nephrectomy, a microaneurysm clip occluded the left renal pedicle (artery and vein) for 30 min. Ischemia (30 min) was chosen as previous studies have shown that this length of ischemic injury minimizes mortality whereas maximizing reproducible IRI mice.7,8 After 30 min, the microaneurysm clip was removed, 0.5 cc of saline was given intraperitoneally, the wounds closed in two layers and animals were allowed to awaken from anesthesia. The sham mice were subjected to anesthesia and right nephrectomy only.
Adoptive transfer of splenocytes or NK1.1 + cells
huHSP27 OE or WT spleens were crushed, splenocytes were passed through a nylon cell strainer (BD Biosciences, San Jose, CA) and collected in phosphate-buffered saline. Red blood cells were lyzed and single-cell splenocyte suspensions from huHSP27 OE or WT mice were transferred intravenously (6×106 to 1×107 splenocytes per transfer, 200 µl) to the HSP27 WT mice. NK1.1 + lymphocytes were isolated utilizing SpinSep murine NK cell enrichment kit (StemCell Technologies, Vancouver, Canada, BC), resulting in >90% pure NK1.1 + cells. To confirm NK1.1 + phenotype and purity of isolated cells, column-purified cells were stained for 30 min with fluorescein isothiocyanate-conjugated anti-mouse NK1.1 (PK136; eBioscience, San Diego, CA) and analyzed with Quanta FACS (Beckman Coulter, Fullerton, CA). Purified NK1.1 + cells isolated from HSP27 WT or huHSP27 OE mice were injected intravenously (1–1.2 × 106 NK1.1 + cells per transfer, 200 µl) to HSP27 WT mice. The recipient HSP27 WT mice were subjected to renal IR 24 h later.
NK1.1 + cell depletion and splenectomy
NK cells were depleted by injecting each mouse with 500 mg rabbit anti-mouse NK1.1 + antibody (eBioscience) intravenously 24 h before renal ischemia. Splenectomized mice had their spleens removed 15 min before renal ischemia.
Assessment of renal function after ischemia-reperfusion injury
Renal function was assessed by measurement of plasma Cr 3 or 24 h after renal ischemia by a colorimetric method based on the Jaffe reaction.55
Histological examinations to detect necrosis and apoptosis
Histological examinations to detect necrosis and apoptosis were performed as described previously.7,8 Morphological assessment was performed by an experienced renal pathologist (VDD), who was unaware of the treatment each animal had received. An established grading scale (renal injury scores of 0–4) for assessment of necrotic injury to the proximal tubules was used for the histopathological assessment of IR-induced damage (3 and 24 h after IR), as outlined by Jablonski et al.26 Renal tubular apoptosis was assessed by counting the number of apoptotic bodies in proximal tubules in the outer stripe of the corticomedullary junction (expressed as mean number of apoptotic bodies per tubule). This area is the most severely injured area after renal IRI. At least 25–30 tubules were counted per field and six fields were examined per slide.
Assessment of renal apoptosis with TUNEL Staining
We used TUNEL staining to detect DNA fragmentation in apoptosis 24 h after renal injury. In situ labeling of fragmented DNA was performed with TUNEL staining (green fluorescence) with a commercially available in situ cell death detection kit (Roche, Indianapolis, IN), according to the manufacturer’s instructions.
Assessment of renal inflammation
Renal inflammation after IRI was determined with (1) detection of neutrophil infiltration with immunohistochemistry 3 and 24 h after renal IR; (2) measurements of mRNA encoding markers of inflammation (Table 1),8 including KC, ICAM-1, MCP-1, MIP-2, and TNF-α; and (3) immunophenotyping of T lymphocyte (CD3+, CD4+, CD8+), NK1.1 + lymphocyte (PK136), macrophage (F4/80), and neutrophil (LY-6G) influx into the kidneys after IR with flow cytometry.
Table 1.
Primers used to amplify cDNAs encoding mouse or human HSP27, mouse GAPDH, and proinflammatory cytokines based on published GenBank sequences for mice
| Primer | Accession number | Sequence (sense, antisense) | Product size (bp) | Cycle number | Annealing T (°C) |
|---|---|---|---|---|---|
| Mouse HSP27 | NM_024441 | 5′-CCTAAGGTCTGGCATGGTA-3′ | 373 | 26 | 66 |
| 5′-AGGAAGCTCGTTGTTGAAGC-3′ | |||||
| Human HSP27 | NM_001540 | 5′-GTCCCTGGATGTCAACCAC-3′ | 259 | 21 | 66 |
| 5′-GACTGGGATGGTGATCTCG-3′ | |||||
| Mouse KC | J04596 | 5′-CAATGAGCTGCGCTGTCAGTG-3′ | 203 | 23 | 60 |
| 5′-CTTGGGGACACCTTTTAGCATC-3′ | |||||
| Mouse MIP-2 | X53798 | 5′-CCAAGGGTTGACTTCAAGAAC-3′ | 282 | 22 | 60 |
| 5′-AGCGAGGCACATCAGGTACG-3′ | |||||
| Mouse ICAM-1 | X52264 | 5′-TGTTTCCTGCCTCTGAAGC-3′ | 409 | 21 | 60 |
| 5′-CTTCGTTTGTGATCCTCCG-3′ | |||||
| Mouse TNF-α | X02611 | 5′-CCTCAGCCTCTTCTCCTTCCT-3′ | 290 | 24 | 65 |
| 5′-GGTGTGGGTGAGGAGCA-3′ | |||||
| Mouse MCP-1 | NM_011333 | 5′-ACCACAGTCCATGCCATCAC-3′ | 312 | 22 | 60 |
| 5′-CACCACCCTGTTGCTGTAGCC-3′ | |||||
| Mouse GAPDH | M32599 | 5′-ACCACAGTCCATGCCATCAC-3′ | 450 | 15 | 65 |
| 5′-CACCACCCTGTTGCTGTAGCC-3′ |
ICAM-1, intercellular adhesion molecule-1; KC, keratinocyte-derived chemokine; MCP-1, monocyte chemoattractant protein 1; MIP-2, macrophage inflammatory protein 2; TNF-α, tumor necrosis factor-α.
Respective anticipated RT-PCR product size, PCR cycle number for linear amplification, and annealing temperatures used for each primer are also provided.
Neutrophil immunohistochemistry
Neutrophil immunohistochemistry was performed as described previously7,8 with a monoclonal antibody raised against Ly-6G (551459; BD Biosciences). A primary antibody that recognized IgG2b (MCA691XZ; Serotec, Raleigh, NC) was used as a negative isotype control in all experiments. Intrarenal neutrophil infiltration was also quantified by manual counting of polymorphonuclear cells in H&E slides by a renal pathologist who was blinded to the treatment groups. Neutrophils were quantified in 10 randomly chosen microscope fields (magnification × 400) in the corticomedullary junction, and results were expressed as neutrophils counted per × 400 HPF.
Semiquantitative reverse transcription-PCR assay
Semiquantitative reverse transcription-PCR assay for proinflammatory mRNAs was performed as described previously.11,56,57
Isolation of lymphocytes from mouse kidney and flow cytometry analyses of leukocyte subtypes
Flow cytometric analysis was performed with a Quanta SC flow cytometer (Beckman Coulter, Miami, FL) to analyze the whole-kidney leukocyte content as described previously.58 These studies assessed kidney content of T cell (CD3, CD4, and CD8), NK cells (PK136), macrophages (F4/80), and neutrophils (Ly-6G) in huHSP27 OE or WT mice subjected to 30 min renal ischemia and 3 h reperfusion. In brief, kidney was extracted, minced, thoroughly crushed, and then passed through a 50-µm mesh prewetted with phosphate-buffered saline containing 10% FBS. The kidney cell suspension was diluted with phosphate-buffered saline containing 10% FBS and carefully layered on an equal volume of Histopaque-1083 (Sigma, St Louis, MO) to isolate mononuclear cells. Samples were centrifuged at 400 g for 30 min at room temperature. The pellet from this spin was processed to detect neutrophils. The mononuclear cell interface containing lymphocytes and macrophages was collected, washed twice with phosphate-buffered saline with 10% FBS, and pelleted. The mononuclear cells recovered from the mouse kidney (105 cells in a volume of 100 µl) were preincubated with an anti-mouse CD16/32 (2.4G2) antibody for 10min to block nonspecific antibody binding. Samples were then incubated with different combinations of antibodies (for example, CD45 + CD3 + 7AAD, CD3 + CD4 + 7AAD) for 20 min at room temperature. Flow cytometry was conducted under conditions to eliminate the interference of nonspecific staining by dead and nonleukocyte (for example, renal) cells. For discrimination of viable and dead cells, samples were stained with 7-AAD for 5min at room temperature. Anti-mouse CD45 was used to determine the total leukocyte cell number. Kidney leukocyte infiltrations were expressed as percent of HSP27 WT mice subjected to renal IR.
Antibodies for FACS
Purified rat IgG2a (clone YTH71.3) was purchased from Serotec (Oxford, UK). Anti-CD3e (clone 145–2C11), anti-F4/80 (clone BM8), anti-Ly-6G (Gr-1), anti-CD45 (clone 30-F11) were purchased from eBioscience. Anti-NK1.1 (LY-55; clone PK136), anti-CD4/L3T4 (clone GK1.5), and anti-CD8α/Lyt-2 (clone 53-6.7) were obtained from Beckman Coulter.
Measurement of plasma cytokines by ELISA
Murine plasma MCP-1 (eBioscience) and KC (R&D Systems, Minneapolis, MN) concentrations were measured using commercially available ELISA kits according to the manufacturer’s instructions from sham-operated mice and mice subjected to renal IR 24 h before.
Statistical analysis
The data were analyzed with t-test when means between two groups were compared or with one-way (for example, plasma Cr) ANOVA plus Tukey’s post hoc multiple comparison test to compare mean values across multiple treatment groups. The ordinal values of the Jablonski scale were analyzed by the Kruskal–Wallis nonparametric test with Dunn’s post-test comparison among groups. In all cases, P < 0.05 was taken to indicate significance. All data are expressed as mean ± s.e.m.
Reagents
Unless otherwise specified, all chemicals were obtained from Sigma.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant RO1 DK-58547.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Jones dr, Lee HT. Protecting the kidney during critical illness. Curr Opin Anaesthesiol. 2007;20:106–112. doi: 10.1097/ACO.0b013e328013f83c. [DOI] [PubMed] [Google Scholar]
- 2.Tang IY, Murray PT. Prevention of perioperative acute renal failure: what works? Best Pract Res Clin Anaesthesiol. 2004;18:91–111. doi: 10.1016/j.bpa.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabb H, O'Meara YM, Maderna P, et al. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- 5.Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol. 1998;18:490–497. [PubMed] [Google Scholar]
- 6.Molitoris BA. Actin cytoskeleton in ischemic acute renal failure. Kidney Int. 2004;66:871–883. doi: 10.1111/j.1523-1755.2004.00818.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee HT, Gallos G, Nasr SH, et al. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 8.Lee HT, Xu H, Nasr SH, et al. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 9.Lee HT, Emala CW. Protein kinase C and G(i/o) proteins are involved in adenosine- and ischemic preconditioning-mediated renal protection. J Am Soc Nephrol. 2001;12:233–240. doi: 10.1681/ASN.V122233. [DOI] [PubMed] [Google Scholar]
- 10.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am J Physiol Renal Physiol. 2000;278:F380–F387. doi: 10.1152/ajprenal.2000.278.3.F380. [DOI] [PubMed] [Google Scholar]
- 11.Lee HT, Kim M, Jan M, et al. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int. 2007;71:1249–1261. doi: 10.1038/sj.ki.5002227. [DOI] [PubMed] [Google Scholar]
- 12.Joo JD, Kim M, Horst P, et al. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–F1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- 13.Garrido C, Bruey JM, Fromentin A, et al. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- 14.Bruey JM, Ducasse C, Bonniaud P, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 15.Arrigo AP, Firdaus WJ, Mellier G, et al. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods. 2005;35:126–138. doi: 10.1016/j.ymeth.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- 17.Wyttenbach A, Sauvageot O, Carmichael J, et al. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 18.Samali A, Robertson JD, Peterson E, et al. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:hpmotc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul C, Manero F, Gonin S, et al. Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber NC, Toma O, Wolter JI, et al. Mechanisms of xenon- and isoflurane-induced preconditioning—a potential link to the cytoskeleton via the MAPKAPK-2/HSP27 pathway. Br J Pharmacol. 2005;146:445–455. doi: 10.1038/sj.bjp.0706324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27) Biochem Soc Symp. 1999;64:79–89. [PubMed] [Google Scholar]
- 22.Huot J, Houle F, Spitz DR, et al. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 23.Hollander JM, Martin JL, Belke DD, et al. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 24.Martin JL, Mestril R, Hilal-Dandan R, et al. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96:4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- 25.Wagstaff MJ, Collaco-Moraes Y, Smith J, et al. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- 26.Jablonski P, Howden BO, Rae DA, et al. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 28.Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–1162. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 29.Lee HT, Emala CW. Preconditioning and adenosine protect human proximal tubule cells in an in vitro model of ischemic injury. J Am Soc Nephrol. 2002;13:2753–2761. doi: 10.1097/01.asn.0000032421.79225.6e. [DOI] [PubMed] [Google Scholar]
- 30.Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096. [DOI] [PubMed] [Google Scholar]
- 31.Jog NR, Jala VR, Ward RA, et al. Heat shock protein 27 regulates neutrophil chemotaxis and exocytosis through two independent mechanisms. J Immunol. 2007;178:2421–2428. doi: 10.4049/jimmunol.178.4.2421. [DOI] [PubMed] [Google Scholar]
- 32.Jantschitsch C, Trautinger F, Klosner G, et al. Overexpression of Hsp25 in K1735 murine melanoma cells enhances susceptibility to natural killer cytotoxicity. Cell Stress Chaperones. 2002;7:107–117. doi: 10.1379/1466-1268(2002)007<0107:oohikm>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahvi DM, Carper SW, Storm FK, et al. Overexpression of 27-kDa heat-shock protein in MCF-7 breast cancer cells: effects on lymphocyte-mediated killing by natural killer and gamma delta T cells. Cancer Immunol Immunother. 1993;37:181–186. doi: 10.1007/BF01525433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alford KA, Glennie S, Turrell BR, et al. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta- activated kinase-1 (TAK1)-mediated signaling. J Biol Chem. 2007;282:6232–6241. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 35.Efthymiou CA, Mocanu MM, de Belleroche J, et al. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 2004;99:392–394. doi: 10.1007/s00395-004-0483-6. [DOI] [PubMed] [Google Scholar]
- 36.Thornton MA, Winn R, Alpers CE, et al. An evaluation of the neutrophil as a mediator of in vivo renal ischemic-reperfusion injury. Am J Pathol. 1989;135:509–515. [PMC free article] [PubMed] [Google Scholar]
- 37.Rabb H, Daniels F, O'Donnell M, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 38.Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002;90:133–138. doi: 10.1159/000049032. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 40.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384–399. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 41.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747. [PubMed] [Google Scholar]
- 42.Klausner JM, Paterson IS, Goldman G, et al. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256:F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 43.De Greef KE, Ysebaert DK, Ghielli M, et al. Neutrophils and acute ischemia-reperfusion injury. J Nephrol. 1998;11:110–122. [PubMed] [Google Scholar]
- 44.Rabb H. Immune modulation of acute kidney injury. J Am Soc Nephrol. 2006;17:604–606. doi: 10.1681/ASN.2006010060. [DOI] [PubMed] [Google Scholar]
- 45.Rabb H. The T cell as a bridge between innate and adaptive immune systems: implications for the kidney. Kidney Int. 2002;61:1935–1946. doi: 10.1046/j.1523-1755.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 46.Wright FS, Okusa MD. Functional role of tubuloglomerular feedback control of glomerular filtration. Adv Nephrol Necker Hosp. 1990;19:119–133. [PubMed] [Google Scholar]
- 47.Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Huang L, Sung SS, et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 49.Molls RR, Savransky V, Liu M, et al. Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2006;290:F1187–F1193. doi: 10.1152/ajprenal.00342.2005. [DOI] [PubMed] [Google Scholar]
- 50.Cugini D, Azzollini N, Gagliardini E, et al. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int. 2005;67:1753–1761. doi: 10.1111/j.1523-1755.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 51.Frink M, Hsieh YC, Hsieh CH, et al. Keratinocyte-derived chemokine plays a critical role in the induction of systemic inflammation and tissue damage after trauma-hemorrhage. Shock. 2007;28:576–581. doi: 10.1097/shk.0b013e31814b8e0d. [DOI] [PubMed] [Google Scholar]
- 52.Akbar MT, Lundberg AM, Liu K, et al. The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem. 2003;278:19956–19965. doi: 10.1074/jbc.M207073200. [DOI] [PubMed] [Google Scholar]
- 53.Lee HT, Kim M, Jan M, et al. Anti-inflammatory and anti-necrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–F78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 54.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981;241:F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- 55.SLOT C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 56.Lee HT, Emala CW, Joo JD, et al. Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis. Shock. 2007;27:373–379. doi: 10.1097/01.shk.0000248595.17130.24. [DOI] [PubMed] [Google Scholar]
- 57.Lee HT, Kim M, Kim J, et al. TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol. 2007;27:416–424. doi: 10.1159/000105124. [DOI] [PubMed] [Google Scholar]
- 58.Lee HT, Kim M, Kim M, et al. Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–F722. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]