Abstract
Wide variation between hospitals in the quality of critical care lead to many potentially avoidable deaths. Regionalization of critical care is a possible solution; regionalization has been implemented for trauma and neonatal intensive care, and it is under active discussion for medical and cardiac critical care. However, regionalization is only one possible approach to reorganizing critical care services. This commentary introduces the technique of network analysis as a framework for the following: (1) understanding how critically ill patients move between hospitals, (2) defining the roles hospitals play in regional care delivery, and (3) suggesting systematic improvements that may benefit population health.
We examined transfers of critically ill Medicare patients in Connecticut in 2005 as a model system. We found that patients are systematically transferred to more capable hospitals. However, we find the standard distinction of hospitals into either “secondary hospitals” or “tertiary hospitals” poorly explains observed transfer patterns; instead, hospitals show a continuum of roles. We further examine the implications of the network pattern in a simulation of quarantine of a hospital to incoming transfers, as occurred during the severe acute respiratory syndrome epidemic.
Network perspectives offer new ways to study systems to care for critically ill patients and provide additional tools for addressing pragmatic problems in triage and bed management, regionalization, quality improvement, and disaster preparedness.
Key words: critical care, health-care organization, Medicare, network analysis, regionalization
Abbreviations: SARS, severe acute respiratory syndrome
Some hospitals provide better outcomes to their critically ill patients than others. One way to improve critical care is to identify and export elements of care that characterize the best performing ICUs to elevate the practices of other hospitals, through training or telemedicine. A complementary approach is to move critically ill patients from lower quality to higher quality centers, perhaps following a model similar to the regionalization of trauma care. Formal plans to regionalize portions of adult critical care are under active discussion.1, 2 In this commentary, we suggest, first, that variation between hospitals in quality presents an important opportunity to improve outcomes for patients with critical illness; second, that transfer of patients between hospitals is a feasible way to improve care; and third, that the tools of network analysis are revealing and persuasive when applied in this arena.
The Problem: Wide Variation Between Hospitals in the Quality of Critical Care Leads to Large Numbers of Potentially Avoidable Deaths
Critical care quality varies widely across hospitals. Hospital ICUs vary in their compliance with standards of care for the prevention of ventilator-associated pneumonia, catheter-related bloodstream infections, and thromboembolic disease.3, 4 Caring for patients in more experienced hospitals rather than less experienced hospitals is associated with 25 to 50% reductions in the adjusted odds of death for many critical illnesses (Table 1 ).
Table 1.
Examples of Reduction in Mortality of Large-Volume vs Small-Volume Centers
Large relative differences in quality are important given the high absolute mortality seen in critical care settings: 18% of Medicare beneficiaries died within 30 days of hospitalization for myocardial infarction.5, 6 A third of nonpostoperative mechanically ventilated patients died prior to hospital discharge.7 There were 20.7 million critical care patient-days in 2000, up 28.7% vs 1985.8 The combination of large scale and high stakes means that even small improvements in the quality of critical care might save many lives. In eight large states, we estimated that every year 4,000 mechanically ventilated patients die who might have been saved had they been in another hospital.9 Half of those patients died in a low-volume hospital within 5 miles of a high-volume center. Krumholz and colleagues10 have estimated that an additional 10,000 acute myocardial infarction patients might be saved annually if they received the same quality of care as provided by better hospitals. Of note, these lives might be saved using existing technology and knowledge without discovering new therapies.11
Centralization Is a Possible Solution for Potentially Avoidable Deaths
In the 1970s, trauma patients faced similar variations in their quality of care, motivating the creation of formal networks for the care of trauma patients.12 This reorganization of care is associated with remarkable improvements in outcomes.13, 14, 15 Centralization of care in centers of excellence is being considered for other patients, including those with acute myocardial infarction16, 17 and general critical care.1
Three conditions are necessary for centralization to improve public health: (1) transport between centers must be safe18 and timely, (2) some centers must be identifiably better at providing care, and (3) patients must be moved from lower quality centers to higher quality centers. Many quality improvement efforts focus on designating centers of excellence; however, unless patients are directed to these centers, there may be no population health benefits.19, 20 Even in mature trauma systems, getting patients to designated trauma centers is a persistent problem.21, 22
Critical Care Patients Are Transferred Frequently, But We Know Little About These Transfers
Although formal systems transfer trauma and neonatal patients, no such formal system exists for most critically ill patients. Nonetheless, transfers between hospitals are common. In recent data from an all-payer multicenter cohort of critically ill patients, 6.4% of patients in the ICU were admitted directly from another acute care hospital.23 However, little is known about how and why these transfers occur or whether patients systematically move toward sites that provide better care. Patient transfers between two hospitals are known to be generally safe.24, 25, 26
The current system of interhospital critical care transfers is informal, but it is not random. Most hospitals transfer to only a subset of other nearby hospitals. Network analysis provides an approach for examining and improving these patterns and testing whether they achieve the same goals as formal regionalization. Bureaucratic regionalization can be seen as one of several possible approaches to optimizing the flow of patients between hospitals. The remainder of this article introduces these emerging scientific methods and suggests their usefulness for improving the critical care transfer system.
Theoretical Perspective and Testable Hypotheses
Critical care transfers reflect relationships between hospitals. This conception is informed by a significant social scientific tradition demonstrating that organizational performance can be understood in terms of the specific connections a given organization has with others.27 The selection of relationships is a key strategic decision.28, 29, 30 An organization's history of relationships places constraints on its formation of new ties, making some ties easy and some more difficult.30 These relationships make some resources readily accessible; they may even change awareness that resources exist, such as novel therapies in the ICU. For example, it may be important not only where hospitals send their critically ill patients, but also from what other hospitals those referral centers receive patients. This interdependence between second-order (and more distant) relationships is captured using tools known collectively as network analytics.31
A common heuristic view of health-care transfers is that patients move from secondary care hospitals to tertiary care hospitals. The “network perspective” allows us to test hypotheses that can formalize and evaluate this dominant view:
H1 (Secondary or Tertiary)
Hospitals can be divided into two mutually exclusive categories: secondary hospitals, which send out transfers, and tertiary hospitals, which accept transfers. A competing hypothesis is that some hospitals both send and receive critically ill patients.
H2 (Satellites of a Single Center)
Each secondary hospital is a satellite of a single population center; patients are transferred to one of a few tertiary hospitals in that center. A competing hypothesis is that hospitals send patients to several different other hospitals.
H3 (Informal Regionalization)
Transfers systematically move patients toward hospitals with more extensive facilities. A competing hypothesis is that patients are as likely to move to lower resourced hospitals as higher resourced hospitals, as might be the case if transfers were designed merely to distribute patient load, such as in electrical power grids.
Beyond these specific testable hypothesis about network structure, the network perspective offers a planning perspective. The experience of severe acute respiratory syndrome (SARS) in Toronto offers one motivation. An exploratory hypothesis is as follows.
H4 (Simulation)
The effects of closure of a single hospital on other hospitals can be simulated using network data. A competing hypothesis is that network data tell us little about the importance of single hospitals.
In the case of SARS, quarantine due to the detection of an unknown highly transmissible airborne pathogen led to the closure of specific central hospitals. We use our data to simulate the impact of one such closure on the transfer options and patient loads for other hospitals in a system.
A central concern of network science is to link a given structure to outcomes. A review of that literature exceeds the scope of this commentary, but in Table 2 we provide examples of additional policy-relevant scientific questions that may benefit from a network perspective.
Table 2.
Application of Network Concepts to Public Health Problems in Critical Care
| Public Health Problem | Network Concept | Testable Hypothesis |
|---|---|---|
| Will regionalization of adult critical care services improve outcomes? | Centralization: networks quantitatively differ in the extent to which relationships are concentrated in a few nodes. | Regions of the country with more centralized referral networks have improved risk-adjusted population outcomes from selected critical illnesses. |
| Which patients should be transferred? | The benefits of network position may be a function of characteristics of the individual who occupies that position. | The benefits of a transfer will result from an interaction between characteristics of the patient, the sending hospital and the receiving hospital. |
| Does health insurance limit a patient's treatment options for critical illness? | Network regression allows statistical comparison of different networks. | Transfer networks for different insurers will be statistically indistinguishable. |
| Do for-profit hospitals “cream skim” patients? | Individual hospital characteristics can be statistically correlated with quantitative measures of network position. | Hospital for-profit status will not be associated with position in the network. |
Illustrative Example: Data and Methods
Variations in quality between hospitals might provide a public health opportunity if transfers are being used effectively, but are they? As a starting point, we examine transfers of critically ill patients in the state of Connecticut.
Data Sources
Data on hospitals come from the American Hospital Association Annual Survey. Critical care transfers are identified in the fee-for-service Medicare claims, using the 2005 MedPAR file. We included medical, surgical, cardiac, and burn units but excluded stepdown units.32 We defined a critical care transfer as occurring between two hospitals (A and B) when a patient was observed to be in hospital A until a certain day, and then in hospital B beginning on the same day or the next day, and the patient used critical care in both hospitals.
Network Visualization
Hospitals are represented as nodes, and transfers form connections between the nodes that may be analyzed.33 These transfers can be plotted, with nodes at their relative latitude and longitude. The network can also be visualized using algorithms that move hospitals close together if they exchange more patients.34 Finally, the networks can also be represented in a sociomatrix, a numeric table that facilitates statistical analyses (Fig 1 , bottom right, F, and supplementary table).
Figure 1.
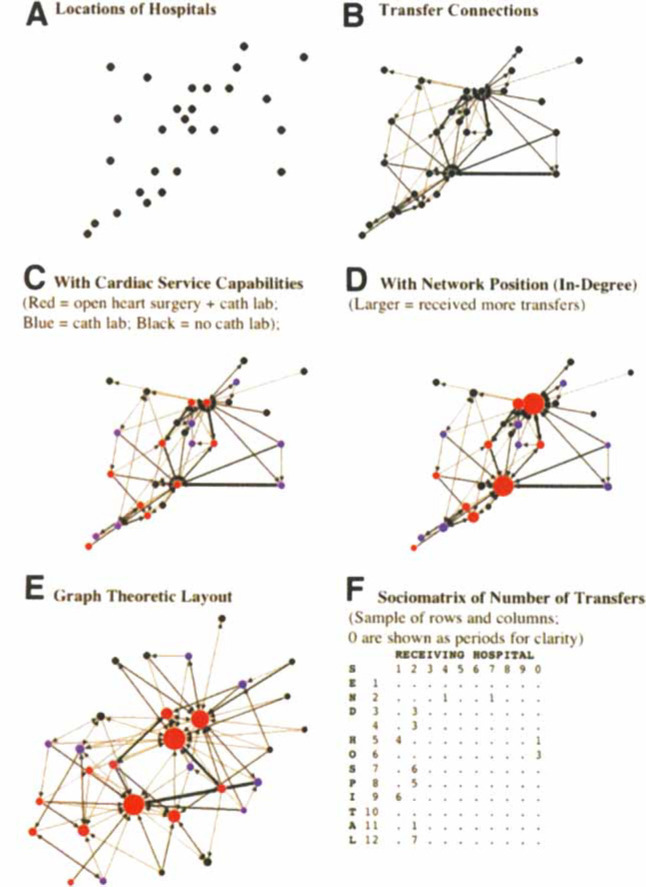
Critical Care Transfers in Connecticut, 2005. Cath lab = cardiac catheterization lab (interventional or diagnostic).
Simulation of the Closure of a Hospital
The network representation facilitates simulation of closure of a hospital to outside transfers. We perform a first-order simulation, holding all other relationships constant. We assume that patients are redistributed to other hospitals in the state proportionately to their previous acceptance. Thus, hospital No. 1 transferred out three fee-for-service Medicare patients, one of whom went to No. 20. In simulating the quarantine of hospital No. 20, this implies that the two other hospitals to which No. 1 transferred would each receive 0.5 additional patients.
Illustrative Example: Results and Discussion
We can visualize the critical care transfer network and assess how well the network achieves public health goals.
Visualization of the Network
Figure 1, top left, A, maps the 30 Connecticut hospitals engaging in a critical care transfer of a Medicare fee-for-service patient during 2005. Figure 1, top right, B, adds transfer information to the map, with arrowheads reflecting the direction of the transfer and line thickness proportional to the number of transfers. Because cardiovascular disease is a common indication for transfer, Figure 1, middle left, C, adds information about hospital capabilities: catheterization laboratory, cardiac surgery, or neither. In Figure 1, middle right, D, hospitals that receive more transfers are shown with larger markers, embedding network characteristics in the graphical representation. Figure 1, bottom left, E, a network visualization algorithm, demonstrates the transfer networks in Connecticut as having two distinct centers, one in New Haven, one in Hartford. Hartford has two major hospitals of nearly equal importance in this network. The other hospitals in the state are more peripheral, and many are connected to both core cities. This initial test provides little graphical support for hypothesis H2 (satellites of a single center).
Connectivity
About 77% (23 of 30) hospitals both send and receive critically ill transfers, showing little support for hypothesis H1 (secondary or tertiary). The median hospital sends critically ill transfer patients to three other hospitals. Only 10% of hospitals (3 of 30 hospitals) transfer to just one hospital; 23% of hospitals (7 of 30 hospitals) transfer critically ill patients to ≥ 5 other hospitals within Connecticut. Again, there is little support for hypothesis H2 (satellites of a single center). In the Connecticut data, the density is 11% (95% binomial confidence interval, 9.0 to 13.3), meaning that 11% of all hospital-to-hospital pairings are reflected in an observed patient transfer.
Centralization or Load Sharing
Graphical evidence indicates that transfers are funneled toward central hospitals with a cardiac surgery capacity (Fig 1, bottom left, E). This is consistent with the goals of regionalization (supporting hypothesis H3) and counter to a “load-sharing” alternative hypothesis of the function of transfers.
Impact of Loss of a Hospital
In the event that the ICU in a central hospital was to close to outside transfers as in quarantine for SARS, we can simulate the results of the increased burden that would fall on other hospitals in the state if no new transfer relationships are formed. In Table 3 , we quarantine hospital No. 20, which had received 126 Medicare ICU transfers from within the state during 2005. In order to accept the patients that hospital No. 20 would have taken, 17 other hospitals accept patients, ranging from 0.2 to 41.8 additional patients, or an 11 to 300% increase in their usual receipt of transfers. One hospital would become isolated and need a new transfer recipient. Hospitals with additional ICU beds at baseline receive more patients, but the correlation is imperfect (Pearson product moment correlation = 0.595, p = 0.007) [Table 3].
Table 3.
Simulated Impact of Closure of a Hospital to Transfers*
| No. 20 Not Accepting Patients |
||||
|---|---|---|---|---|
| Hospital | Observed Transfers Received | Reported ICU Beds | Marginal Transfers Received | Additional Workload, % |
| 1 | 43 | 75 | 9.0 | 21 |
| 2 | 74 | 42 | 41.8 | 56 |
| 3 | 0 | 6 | 0.0 | 0 |
| 4 | 1 | 0 | 0.2 | 20 |
| 5 | 1 | 20 | 0.6 | 56 |
| 6 | 1 | 14 | 3.0 | 300 |
| 7 | 3 | 20 | 3.0 | 100 |
| 8 | 1 | 7 | 0.0 | 0 |
| 9 | 0 | 6 | 0.0 | 0 |
| 10 | 28 | 32 | 8.5 | 31 |
| 11 | 3 | 10 | 1.0 | 33 |
| 12 | 2 | 9 | 0.4 | 20 |
| 13 | 1 | 8 | 0.2 | 17 |
| 14 | 4 | 24 | 0.0 | 0 |
| 15 | 1 | 9 | 0.4 | 44 |
| 16 | 0 | 10 | 0.0 | 0 |
| 17 | 0 | 10 | 0.0 | 0 |
| 18 | 3 | 21 | 0.0 | 0 |
| 19 | 0 | 12 | 0.0 | 0 |
| 20 | 126 | 78 | ||
| 21 | 0 | 12 | 0.0 | 0 |
| 22 | 75 | 50 | 30.6 | 41 |
| 23 | 1 | 12 | 0.0 | 0 |
| 24 | 23 | 32 | 2.6 | 11 |
| 25 | 0 | 14 | 0.0 | 0 |
| 26 | 3 | 14 | 0.9 | 31 |
| 27 | 1 | 22 | 0.8 | 83 |
| 28 | 4 | 16 | 2.4 | 59 |
| 29 | 6 | 24 | 0.0 | 0 |
| 30 | 45 | 9 | 10.5 | 23 |
Values are given as No., unless otherwise indicated.
Discussion
These results suggest that, in the state of Connecticut in 2005, critically ill patients are being systematically transferred to more capable hospitals. There is little evidence of a clean separation of hospitals into either secondary or tertiary care roles; instead, hospitals display a continuum of roles. The simulation data suggest that closure of any individual prominent hospital will cause fewer hospitals to be isolated than might be implied by the secondary/tertiary model, but will also cause wider statewide ramifications even without new transfer relationships being formed.
Practical Value of Network Analysis
Viewing critical care delivery as a network offers several distinct approaches to improve it.
Triage and Bed Management
If hospital A accepts a patient from hospital B, then A has less availability if hospital C wishes to transfer a patient. More generally, the network structure of critical care transfers induces interdependencies between ICUs that are likely invisible to the ICUs themselves. Hospital B is dependent on the census and capacity of hospital C in a way that is not readily transparent. In some areas patients are frequently refused admission to an ICU because of a lack of available beds.35 This, in part, may be due to poor understanding of the existing transfer system, further hampered by the lack of data distinguishing transfer for capacity from transfers for additional expertise or technology. A management perspective that considers the informally integrated network may allow better prevention of congestion and bed lock.
Regionalization
Trauma networks were able to improve outcomes by combining improved care at central sites with increased flow of patients to those sites. The goals of regionalization in other areas of critical care are the same. The data from Connecticut suggest that cardiac patients are already being directed to sites that provide improved care. If this finding is true more generally, then the goals of regionalization might be met without a large reorganization of the current system. Instead, research and policy can focus on increasing the flow of patients over the current network.
Quality Improvement
The decision to transfer, and the decision where to transfer, are processes of care. Like any other process of care, they can be improved. The network perspective suggests an emphasis on choice of transfer hospital. Transfers to lower quality hospitals could be identified and targeted for improvement; transfers to high-quality hospitals could be an important goal when patients' needs exceed a hospital's capabilities. Network analysis can make visible untapped nearby opportunities for new transfer relationships.
Disaster Preparedness
Recent experience with natural and manmade disasters has made it clear that local resources are the first line of response. As local hospitals are overwhelmed, they will rely on regional referral sites to accept patients. Network science allows a better understanding of these existing lines of transfer and suggests flexibility if the traditional transfer lines are disrupted. A large hospital might not only be disabled from accepting patients (as we modeled) but need to send patients out when incapacitated. Planners could simulate the consequences of removing a central node from the system, anticipating any number of disaster scenarios; integration of network analysis with agent-based models may be of great value here.36 In cases of actual emergency, hospitals could quickly triage patients along existing transfer lines and identify new transfer lines when existing ones are broken. In the case of a flu epidemic or another outbreak of SARS, visualization of the network can identify at-risk areas and help contain an outbreak by minimizing transfers to unaffected regions.
Conclusion
One in five Americans will die in an ICU,37 and critical care expenditures account for 0.5% of the gross domestic product.8 As the population ages and demand for critical care rises, it will be essential to use existing resources effectively. Wide variation in the processes and outcomes of critical care between hospitals means that thousands of lives and millions of dollars may be saved by getting critically ill patients to more effective hospitals along existing transfer lines. Network analytic techniques offer the potential to improve the efficiency of the current transfer network, informing hospitals on the optimal distribution of patients within regions, and providing a direct way to improve the quality of care for the critically ill. Network analysis can also provide novel information about hospital quality and aid policy makers in understanding the effects of network stressors such as epidemics and disasters.
Network analysis is already widely applied outside of health care. To date, there have been comparatively few applications in health care. Interhospital transfers represent a natural extension of this emerging field, offering new ways to improve the way we care for critically ill hospitalized patients. As we debate the value of formal regionalization for critical care or other conditions, the network analyses can reveal how much we have achieved already and how much more we might achieve.
Footnotes
There are no conflicts of interest for any of the authors of this manuscript.
This analysis was supported in part by a Fellows Career Development Award from the American Thoracic Society, National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Cardiopulmonary Epidemiology Training Grant No. 5T32HL007891 and NIH/NHLBI No. 1K08HL091249-01.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Barnato AE, Kahn JM, Rubenfeld GD. Prioritizing the Organization and Management of Intensive Care Services in the United States: the PrOMIS Conference. Crit Care Med. 2007;35:1003–1006. doi: 10.1097/01.CCM.0000259535.06205.B4. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL. Evaluating and improving the quality of care for acute myocardial infarction: can regionalization help? JAMA. 2006;295:2177–2179. doi: 10.1001/jama.295.18.2177. [DOI] [PubMed] [Google Scholar]
- 3.University Healthsystem Consortium . Adult ICU Benchmarking Project: findings and conclusions. University Healthsystem Consortium; Oak Brook, IL: 2003. [Google Scholar]
- 4.Pronovost PJ, Needham DM, Berenholtz SM. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 5.Krumholz HM, Wang Y, Mattera JA. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–1692. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 6.Skinner JS, Chandra A, Staiger DO. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112:2634–2641. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn JM, Goss CH, Heagerty PJ. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 8.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985–2000: an analysis of bed numbers, use, and costs. Crit Care Med. 2004;32:1254–1259. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]
- 9.Kahn JM, Linde-Zwirble WT, Wunsch H. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med. 2008;177:285–291. doi: 10.1164/rccm.200708-1214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krumholz HM, Normand S-LT, Spertus JA. Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement. Health Affairs. 2007;26:75–85. doi: 10.1377/hlthaff.26.1.75. [DOI] [PubMed] [Google Scholar]
- 11.Cabana MD, Rand CS, Powe NR. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 12.Nathens AB, Brunet FP, Maler RV. Development of trauma systems and effect on outcomes after injury. Lancet. 2004;363:1794–1801. doi: 10.1016/S0140-6736(04)16307-1. [DOI] [PubMed] [Google Scholar]
- 13.Mullins RJ, Veum-Stone J, Helfand M. Outcomes of hospitalized injured patients after institution of a trauma system in an urban area. JAMA. 1994;271:1919–1924. doi: 10.1001/jama.1994.03510480043032. [DOI] [PubMed] [Google Scholar]
- 14.Durham R, Pracht E, Orban B. Evaluation of a mature trauma system. Ann Surg. 2006;243:775–785. doi: 10.1097/01.sla.0000219644.52926.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKenzie EJ, Rivara FP, Jurkovich GJ. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 16.Hannan EL, Wu C, Walford G. Volume-outcome relationships for percutaneous coronary interventions in the stent era. Circulation. 2005;112:1171–1179. doi: 10.1161/CIRCULATIONAHA.104.528455. [DOI] [PubMed] [Google Scholar]
- 17.Jollis JG, Roettig ML, Aluko AO. Implementation of a statewide system for coronary reperfusion for ST-segment elevation myocardial infarction. JAMA. 2007;298:2371–2380. doi: 10.1001/jama.298.20.joc70124. [DOI] [PubMed] [Google Scholar]
- 18.Fan E, McDonald RD, Adhikari NKJ. Outcomes of interfacility critical care adult patient transport: a systematic review. Crit Care. 2006;10:R6. doi: 10.1186/cc3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington DT, Connolly M, Biffl WL. Transfer times to definitive care facilities are too long: a consequence of an immature trauma system. Ann Surg. 2005;241:961–968. doi: 10.1097/01.sla.0000164178.62726.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tallon JM, Fell DB, Ackroyd-Stolarz S. Influence of a new province-wide trauma system on motor vehicle trauma care and mortality. J Trauma. 2006;60:548–552. doi: 10.1097/01.ta.0000209336.66283.ea. [DOI] [PubMed] [Google Scholar]
- 21.Vassar MJ, Holcroft JJ, Knudson MM. Fractures in access to and assessment of trauma systems. J Am Coll Surg. 2003;197:717–725. doi: 10.1016/S1072-7515(03)00749-X. [DOI] [PubMed] [Google Scholar]
- 22.Nathens AB, Jurkovich GJ, MacKenzie EJ. A resource-based assessment of trauma care in the United States. J Trauma. 2004;56:173–178. doi: 10.1097/01.TA.0000056159.65396.7C. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman JE, Kramer AA, McNair DS. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 24.American College of Emergency Physicians Appropriate interhospital patient transfer. Ann Emerg Med. 1993;22:766–767. [PubMed] [Google Scholar]
- 25.American College of Emergency Physicians Interfacility transportation of the critical care patient and its medical direction. Ann Emerg Med. 2006;47:305. doi: 10.1016/j.annemergmed.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Guidelines Committee of the Society for Critical Care Medicine Guidelines for the transfer of critically ill patients. Crit Care Med. 1993;21:931–937. [PubMed] [Google Scholar]
- 27.Granovetter MS. Economic action and social structure: the problem of embeddedness. Am J Sociol. 1985;91:481–510. [Google Scholar]
- 28.Burt RS. Structural holes: the social structure of competition. Harvard University Press; Cambridge, MA: 1992. [Google Scholar]
- 29.Podolny JM, Baron JN. Resources and relationships: social networks and mobility in the workplace. Am Sociol Rev. 1997;62:673–693. [Google Scholar]
- 30.Gulati R. Managing network resources: alliances, affiliations, and other relational assets. Oxford University Press; New York, NY: 2007. [Google Scholar]
- 31.Watts DJ. Networks, dynamics, and the small-world phenomenon. Am J Sociol. 1999;105:493–527. [Google Scholar]
- 32.Halpern NA, Pastores SM, Thaler HT. Critical care medicine use and cost among Medicare beneficiaries 1995–2000: major discrepancies between two United States federal Medicare databases. Crit Care Med. 2007;35:692–699. doi: 10.1097/01.CCM.0000257255.57899.5D. [DOI] [PubMed] [Google Scholar]
- 33.Wasserman S, Faust K. Social network analysis: methods and applications. Cambridge University; Cambridge, UK: 1994. [Google Scholar]
- 34.Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Software Pract Ex. 1991;21:1129–1164. [Google Scholar]
- 35.Joynt G, Gomersall C, Tan P. Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome. Intensive Care Med. 2001;27:1459–1465. doi: 10.1007/s001340101041. [DOI] [PubMed] [Google Scholar]
- 36.Miller JH, Page SE. Complex adaptive systems: an introduction to computational models of social life. Princeton University Press; Princeton, NJ: 2007. [Google Scholar]
- 37.Angus DC, Barnato AE, Linde-Zwirble WT. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 38.Chen EW, Canto JG, Parsons LS. Relation between hospital intra-aortic balloon counterpulsation volume and mortality in acute myocardial infarction complicated by cardiogenic shock. Circulation. 2003;108:951–957. doi: 10.1161/01.CIR.0000085068.59734.E4. [DOI] [PubMed] [Google Scholar]
- 39.Canto JG, Every NR, Magid DJ. The volume of primary angioplasty procedures and survival after acute myocardial infarction. N Engl J Med. 2000;342:1573–1580. doi: 10.1056/NEJM200005253422106. [DOI] [PubMed] [Google Scholar]
- 40.Magid DJ, Calonge BN, Rumsfeld JS. Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. JAMA. 2000;284:3131–3138. doi: 10.1001/jama.284.24.3131. [DOI] [PubMed] [Google Scholar]
- 41.McGrath PD, Wennberg DE, Dickens JD., Jr Relation between operator and hospital volume and outcomes following percutaneous coronary interventions in the era of the coronary stent. JAMA. 2000;284:3139–3144. doi: 10.1001/jama.284.24.3139. [DOI] [PubMed] [Google Scholar]
- 42.Nathens AB, Jurkovich GJ, Maier RV. Relationship between trauma center volume and outcomes. JAMA. 2001;285:1164–1171. doi: 10.1001/jama.285.9.1164. [DOI] [PubMed] [Google Scholar]
- 43.Needham DM, Bronskill SE, Rothwell DM. Hospital volume and mortality for mechanical ventilation of medical and surgical patients: a population-based analysis using administrative data. Crit Care Med. 2006;34:2349–2354. doi: 10.1097/01.CCM.0000233858.85802.5C. [DOI] [PubMed] [Google Scholar]


