Abstract
Two male castrated Whippet littermates were presented at 1 year of age for pallor, tachycardia, systolic heart murmur, dark yellow to orange feces, intermittent lethargy, pigmenturia, and muscle shivering or cramping after exercise. Persistent macrocytic hypochromic anemia with marked reticulocytosis and metarubricytosis was found when CBC results were compared with reference values for Whippets. Increased serum creatine kinase activity and hyperkalemia also were sometimes present over the 4-year period of evaluation. Progressively increasing serum concentrations of N-terminal prohormone brain natriuretic peptide suggested cardiac disease. Erythrocytes from the whippets were less osmotically fragile but more alkaline fragile than those from control dogs. Erythrocyte phosphofructokinase (PFK) activities and 2,3-diphosphoglycerate concentrations were decreased. Restriction enzyme-based DNA test screening and DNA sequencing revealed the same mutation in the muscle-PFK gene of the Whippets as seen in English Springer Spaniel dogs with PFK deficiency. This is the first report of PFK deficiency in Whippet dogs. In addition to causing hemolysis and exertional myopathy, heart disease may be a prominent clinical component of PFK deficiency in this breed and has not been previously recognized in PFK-deficient English Springer Spaniels.
Keywords: Alkaline fragility; cardiomyopathy; 2,3-diphosphoglycerate; hemolytic anemia; hereditary; phosphofructokinase deficiency
The erythrocyte generates energy in the form of ATP during anaerobic glycolysis through the Embden-Meyerhof pathway, and ATP is needed for various erythrocyte functions, including maintaining erythrocyte shape and deformability, transport of ions and other molecules, and synthesis of glutathione. Defects in pyruvate kinase (PK) and phosphofructokinase (PFK), key regulatory enzymes in anaerobic glycolysis, result in impaired ATP production and shortened erythrocyte survival.1 PFK is a homo- or heterotetramer composed of 3 subunits: muscle (M-PFK), liver (L-PFK), and platelet (P-PFK), depending on cell type.2 The M-PFK subunit accounts for ~80% of the PFK present in adult canine erythrocytes, and skeletal muscle contains exclusively M-PFK homotetramers.2 A single nonsense mutation in the M-type PFK gene is responsible for PFK deficiency in English Springer Spaniels, American Cocker Spaniels, and mixed breed dogs. This mutant allele produces an unstable and truncated M-PFK protein.3 Therefore, affected dogs completely lack PFK activity in skeletal muscle4 and have ~20% of normal PFK activity in erythrocytes due to residual P-PFK and L-PFK expression.5
PFK deficiency results in a glycolytic block leading to reduced ATP and 2,3-diphosphoglycerate (DPG) concentrations.5,6 This in turn results in increased hemoglobin (HGB)-oxygen affinity and relative tissue hypoxia, which acts as a persistent stimulus for increased erythropoiesis even when the HCT is normal.7-9 In addition to persistent compensated hemolytic anemia, PFK-deficient dogs (but not humans) may have episodes of intravascular hemolysis following periods of hyperventilation-induced alkalemia due to low DPG concentrations.8,10 Circumstances that can cause hyperventilation in dogs include panting during excitement, excessive barking, high environmental temperatures, and rigorous exercise.8,9
Aside from the hematologic changes, mild metabolic myopathy associated with exertion and a variable degree of muscle wasting can also be present.4,11 However, muscle cramping and severe progressive myopathy occur rarely. Hepatosplenomegaly may be recognized on physical examination.8 PFK-deficient dogs have increased total body iron stores, as indicated by increased serum ferritin concentration and hepatic hemosiderosis.8,12 In this report, we document PFK deficiency in 2 Whippet dogs with anemia, exertional myopathy, and cardiac disease.
Case Presentations
Two male castrated Whippet littermates around 1 year of age were initially examined at 2 different veterinary practices in Oxon, Oxfordshire, UK, in 2003 and had follow-up examinations over the next 4 years. On presentation, Whippet 1 had tachycardia (160 bpm), a grade I/VI left-sided systolic heart murmur, and pale mucous membranes. Whippet 2 was presented for lethargy and pallor after hydrotherapy as postoperative management for a fractured front limb. This dog developed frequent episodes of pyrexia (40.2°C) and later cardiac abnormalities characterized by tachycardia (170 bpm) and a left-sided heart murmur, which progressed from grade III to V. Nocturnal coughing and delayed capillary refill time were noted. Both clients reported dark pigmenturia, mild lethargy, shivering, and occasionally anorexia after exercise. The clients also recalled occasional muscle flinching or cramping in the large muscle groups of the thighs and shoulders. Muscle atrophy of the gluteal muscle of the right hind limb of Whippet 2 was later noted. Persistent dark yellow to orange feces had been observed in both dogs, which was interpreted to indicate the presence of increased bile pigments. There was no evidence of hepatosplenomegaly in either dog. Deteriorating cardiac function became a major clinical problem in Whippet 2, apparently resulting in his death. A necropsy was not performed.
Laboratory findings
Pertinent hematology findings from both dogs gathered over the 4 years documented the persistence of macrocytic hypochromic erythrocytes associated with normal to decreased HCT and HGB concentrations based on reference intervals for nonsighthound breeds; based on reference values for healthy Whippets the dogs were persistently anemic (Table 1).13 Serial blood smear examinations revealed moderate anisocytosis and marked polychromasia, consistent with marked reticulocytosis. Persistent reticulocytosis and metarubricytosis along with macrocytic hypochromic erythrocytes were interpreted as partially compensated hemolytic anemia. No evidence of hemoparasites, hematotoxicity, immune-mediated disease (negative direct Coombs' test result) or blood loss was found. Total WBC counts were generally in the reference interval, but mild lymphocytosis and reactive lymphocytes were occasionally seen. Whippet 1 frequently had apparent thrombocytopenia; however, breed-specific reference values for platelets were not available.
Table 1.
Hematology results in 2 Whippets with phosphofructokinase (PFK)-deficiency.*
| Analyte | Whippet 1 | Whippet 2 | PFK-deficient Spaniels† | Reference Values ‡ (Whippets) |
|---|---|---|---|---|
| RBC ( × 1012/L) | 4.1 (3.5-4.7) | 4.4 (4.1-4.9) | 4.1±0.3 | 5.0-8.5 |
| HGB (g/L) | 110 (95-126) | 127 (117-137) | 110±10 | 120-180 (181-234) |
| HCT (L/L) | 0.40 (0.30-0.47) | 0.44 (0.38-0.47) | 0.35±0.02 | 0.37-0.55 (0.53-0.68) |
| MCV (fL) | 100 (78-106) | 97 (92-100) | 85±2 | 60-80 |
| MCHC (g/L) | 270 (250-340) | 300 (290-310) | 318±0.7 | 310-340 |
| Nucleated | 19.2 | 4.6 | 1.3±0.9 | 0 |
| RBCs ( × 109/L) | (6.7-39.1) | (2.8-7.9) | ||
| Reticulocytes* ( × 109/L) | 1000, 1184 | 857 (848-1320) | 859±320 | < 60 |
| Platelets ( × 109/L) | 104 (84-126) | Adequate on smear | 257±62 | 200-500 |
Values for Whippets are median (minimum-maximum) of 5-11 data points. Only 2 reticulocyte counts were determined for Whippet 1.
Values are mean ± SD for 9 English Springer Spaniels.12
Reference intervals for nonsighthound breeds of dogs at Axiom Veterinary Laboratory UK. For HGB and HCT, minimum-maximum values from 15 healthy Whippets are given in parentheses.13
Hyperkalemia was occasionally noted (Whippet 1, 6.0-7.2mmol/L; Whippet 2, 4.4-7.6mmol/L). Increases in serum creatine kinase activity were generally mild (Whippet 1, 161-2140 U/L; Whippet 2, 225-350 U/L) except on 1 occasion (Whippet 1, 2140 U/L). Slightly increased serum amylase, alkaline phosphatase, and alanine aminotransferase activities were noted in both Whippets. Prominent hyperbilirubinuria (3+; grading scale, 0 to 3+) was present in most urine samples that were analyzed. On 1 occasion, presumptive hemoglobinuria (positive blood on the dipstick, but no erythrocytes in the sediment) was found in Whippet 1.
Both dogs had progressively increasing serum concentrations of the N-terminal fragment of the prohormone brain natriuretic peptide (NT-proBNP; VETSIGN Canine CardioSCREEN, Guildhay Limited, Guildford, UK; reference interval <300 pmol/L), from 631 to 1032 pmol/L in Whippet 1 and 1138 to 5337 pmol/L in Whippet 2. The owners opted not to pursue further cardiac diagnostics such as echocardiography, knowing the effect of stress on their PFK-deficient pets.
Persistent hemolytic anemia in 2 littermates suggested the possibility of an inherited erythrocyte defect. In vitro studies were used to compare erythrocytes from the Whippets with those of healthy non-Whippet control dogs that were bled and assayed under identical conditions. Erythrocyte osmotic and alkaline fragility tests were performed as previously described.9,14 Erythrocytes from the affected dogs were less osmotically fragile than those from control dogs (Figure 1A). Additionally, erythrocytes from the affected Whippets were more alkaline fragile than erythrocytes from control dogs, exhibiting increasing hemolysis when the pH was 7.4 (Figure 1B). Erythrocyte 2,3-DPG content was decreased in Whippet 1 and 2 to 73% and 51% of the mean control value, respectively (2,3DPG UV-test, Roche Diagnostics, Burgess Hill, UK; Table 2). When measured spectrophotometrically,9 erythrocyte PFK activity was decreased in Whippets 1 and 2 to 16.5% and 12.4% of the mean control value, respectively (Table 2). Results of the PCR-based restriction enzyme test (PennGen, University of Pennsylvania) used to diagnose PFK deficiency in English Springer Spaniels revealed a mutant allele pattern in both Whippets. Additional sequencing of the PCR product showed the same nonsense G2228A point mutation as seen in English Springer Spaniels, American Cocker Spaniels, and mixed breed dogs.3,6 No family information was available to trace the gene defect.
Figure 1.
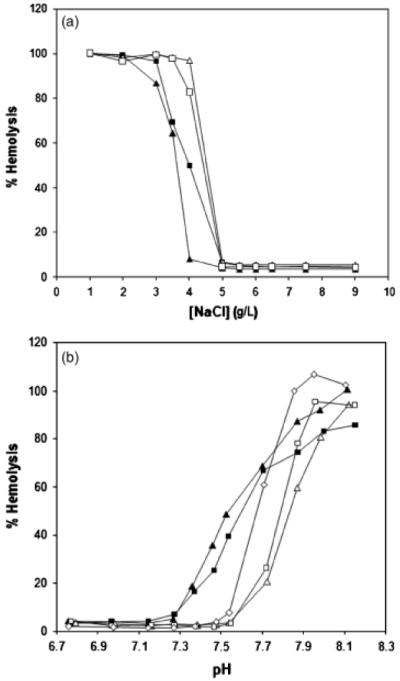
(A) Osmotic fragility. Erythrocytes from PFK-deficient Whippets are more osmotically stable than erythrocytes from control dogs. (B) Alkaline fragility. Normal canine erythrocytes hemolyze at ~pH 7.6, whereas erythrocytes from PFK-deficient whippets begin to hemolyze at a lower pH, near 7.4. Whippet 1 ■, Whippet 2 ▲, Control 3 △, Control 4 □, Control 5 ◇.
Table 2.
Erythrocyte phosphofructokinase (PFK) activities and 2,3-di-phosphoglycerate (DPG) concentrations in dogs.*
| Parameter | Whippet 1 | Whippet 2 | Controls (n = 3) | Reference Interval† |
|---|---|---|---|---|
| PFK (U/g HGB) | 1.6 | 1.2 | 6.4-12.3 | 7.4-11.9 |
| DPG (mmol/L RBC) | 4.8 | 3.3 | 6.9-7.0 | 4.7-7.3 |
PFK assays for Whippets and controls were performed on 2-day-old samples shipped (transit time about 15 hours) from the UK to the University of Florida. DPG assays were done on fresh Whippet and control samples.
Established at the University of Florida. Reference intervals for 2,3-DPG were determined using Kit 35-UV, Sigma Chemical Co, St. Louis, MO, USA.
Discussion
PFK deficiency has been diagnosed in English Springer Spaniels, American Cocker Spaniels, and mixed breed dogs,6,8,9 but this is the first report in the Whippet breed. Classic signs of persistent hemolysis with hemolytic crises and the less common exertional myopathy were observed in addition to marked cardiac signs not previously reported with PFK deficiency. Laboratory findings associated with PFK deficiency can be mistaken for other causes of hemolytic anemia. However, the continual presence of macrocytic hypochromic erythrocytes and marked reticulocytosis in these mildly anemic Whippets was a key indicator of PFK deficiency.8 HCT and HGB concentration were generally higher than those in PFK-deficient English Springer Spaniels, but are higher normally in healthy Whippets compared with English Springer Spaniels.13 Platelet counts are generally lower in Greyhounds than in nonsighthound breeds, and 5% of healthy Greyhounds have platelet counts of 50-100 × 109/L.15 This may have been a contributing factor to the apparent thrombocytopenia observed occasionally in Whippet 1.
Previously reported laboratory findings in PFK-deficient dogs, such as persistent hyperbilirubinuria, mildly to moderately increased serum creatine kinase activity, and occasionally, hyperkalemia8 were also observed in these Whippets. Intermittent hyperkalemia cannot be explained by lysis of mature erythrocytes because dog erythrocytes have low K+ concentrations. In contrast, dog reticulocytes have high K+ concentrations because of the presence of a Na+, K+-activated ATPase pump that is lost as reticulocytes mature into erythrocytes.11,16 Hyperkalemia might result from release of K+ not only from reticulocytes but also from muscle cells in PFK-deficient dogs.9 However, it should be noted that PFK-deficient reticulocytes are not alkaline fragile, because the DPG concentration is higher in these cells.10
The absence of increased erythrocyte osmotic fragility in the Whippets made a membrane or ion transport defect an unlikely cause of hemolysis. Erythrocytes in dogs with immune-mediated hemolytic anemia, a frequent misdiagnosis in PFK-deficient dogs, tend to have increased osmotic fragility, providing further evidence, in addition to the negative Coombs' test results and lack of spherocytosis, to exclude this diagnosis. The osmotic fragility test is a rough index of RBC surface-to-volume ratio.17 Increased resistance to hemolysis in these PFK-deficient Whippets may have been due to the high number of reticulocytes present, which have greater surface-to-volume ratios than mature erythrocytes.17
Normal canine erythrocytes are uniquely more alkaline fragile than those of other species and start to hemolyze at pH 7.6 in vitro.18,19 The mechanism of the alkaline fragility of canine erythrocytes is unknown, but might be related to facilitated calcium entry under conditions of increased pH.16 PFK-deficient erythrocytes begin to lyse at a lower pH, near pH 7.4.7,8 This is due to decreased erythrocyte DPG content, the major impermeant anion in canine erythrocytes, which leads to increased pH within affected erythrocytes.8-10 Consequently, mild systemic alkalemia can induce intravascular hemolytic crises in dogs with PFK deficiency. The low erythrocyte DPG concentration in these Whippets helped eliminate PK deficiency from the differential diagnosis, because PK deficiency is associated with increased DPG concentration.20,21
As was done in this study, a definitive diagnosis of PFK deficiency can be made in adult dogs by measuring total erythrocyte PFK activity. This enzyme assay is limited in that affected dogs cannot be accurately diagnosed until 3 months of age because neonatal dogs express large amounts of L-PFK isoform in erythrocytes.22 Furthermore this test requires considerable technical expertise that is limited to a few specialized laboratories and requires freshly collected and properly shipped EDTA blood samples. Fortunately, a mutation-specific DNA test has been developed that can clearly differentiate normal, carrier, and affected dogs regardless of age or sample condition; this test uses a cheek brush or EDTA blood sample and can be sent by regular mail.3 The assumption that both Whippets had the same PFK mutation, based on the positive DNA restriction enzyme screening tests, was confirmed by sequencing analysis of the M-PFK mutation. Unfortunately, the pedigree could not be further investigated in these cases. While the presence of the same mutation can be readily explained in English Springer and American Cocker Spaniels—2 related breeds—as well as in mixed breed dogs with English Springer Spaniels in their ancestry, the relationship of these Whippets to other PFK-deficient dogs remains elusive. Based on pedigree analyses the PFK mutation occurred before the 1960s in the English Springer Spaniel breed, and an ancient mutation common to both breeds appears more likely than the separate spontaneous occurrence of exactly the same mutation in 2 different breeds.
Interestingly, both Whippets had marked clinical signs of exertional myopathy similar to those seen in PFK-deficient humans. In contrast, PFK-deficient English Springer and Cocker Spaniel dogs generally have relatively mild muscle signs. Despite a complete lack of PFK activity, canine skeletal muscle can function well because it is less dependent on anaerobic glycolysis than human skeletal muscle. Skeletal muscle in dogs has a higher oxidative rate than that of humans because dogs lack the classical fast twitch, glycolytic (type IIB) muscle fibers.23
The increased severity of myopathic signs in these Whippets may be associated with their muscle conformation, athletic nature, and genetic makeup. A mutation in the gene responsible for production of myostatin, a negative regulator of skeletal muscle growth affecting fast-twitch muscles (linked to sprinting ability) has recently been identified in Whippets.24 Whippets that were heterozygous for the mutant allele were of intermediate musculature, and elite racing dogs. However, Whippets homozygous for the deletion were heavily muscled, similar to Belgian blue cattle with the same defect, and poor performers.24,25 Whippet 2 was larger than the average Whippet. Therefore, it is possible that this mutation may also be present and have contributed to poor exercise tolerance. Furthermore, owners of Whippets with homozygous myostatin deletion (referred to as “bully” Whippets) reported muscle cramping in the shoulder and thigh similar to these PFK-deficient Whippets.24 Molecular studies were not performed to further define the myostatin defect in the 2 Whippets of this report.
Cardiac disease was part of the initial presenting concerns in both Whippets, and deteriorating cardiac function apparently resulted in the death of Whippet 2. Persistent tachycardia, cardiac murmurs, and rising serum NT-proBNP concentrations in both Whippets supported a diagnosis of cardiac disease. Cardiac disease has not been described as a component of PFK deficiency in dogs, even though PFK activity in heart muscle was found to be 29% of normal in a deficient dog.11 Cases of the infantile variant of PFK deficiency have been reported in humans in whom cardiomyopathy led to early death. Excessive extralysosomal glycogen storage and reduced PFK activity were seen in biopsies of heart, skeletal muscle, and liver from these patients.26 Progressive cardiac and neurologic disease was also identified in an elderly woman with PFK deficiency.27 In both Whippets, it is possible the heart disease was related to PFK deficiency rather than occurring as an independent, concomitant cardiomyopathy. Histopathologic evaluation of cardiac muscle would have been required to better define this relationship and the severity of the cardiomyopathy.
Biochemical variables used to detect cardiac disease in dogs include atrial natriuretic peptide (ANP),28 NT-proANP,29 BNP,28,30,31 NT-proBNP,32 and cardiac troponin-I.30 Application of these assays has allowed earlier detection of (occult) heart disease and provides a tool to monitor cardiac disease, as was done in these Whippets.28,30 NT-proBNP is a B-type natriuretic peptide synthesized and released by myocardial tissue in response to increased stress on the myocardial wall; high concentrations are associated with congestive heart failure.30,33,34
There is no practical curative therapy available for PFK deficiency in dogs, although experimental bone marrow transplantation from compatible donors (littermates) has successfully corrected the hematologic, and to some extent, skeletal muscle abnormalities.35 However, restriction of excessive exercise, heat exposure, barking, and other activities associated with hyperventilation can reduce the number and severity of intravascular hemolytic crises and exertional myopathies in dogs and thereby extend longevity.6,8,9
Acknowledgments
The corresponding author would like to thank Jakob Hayes for his support, the veterinary clinicians Jane Bailey, Libby Morten, Michelle Darboe, and Karen Coggles, and generous cooperation of the owners. Thank you to Malcolm Silk-stone, Sophie Cunnington, Melanie Pate, and Christopher D'Agorne for their scientific, technical, and statistical contributions, respectively, and Mrs. Gerber, Anne Tiley, and Sara Still for their clerical assistance. This work was supported in part by NIH RR02512.
References
- 1.Harvey JW. The erythrocyte: physiology, metabolism, and biochemical disorders. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical Biochemistry of Domestic Animals. 6th ed. Elsevier Inc.; San Diego: 2008. pp. 173–240. [Google Scholar]
- 2.Vora S, Giger U, Turchen S, Harvey JW. Characterization of the enzymatic lesion in inherited phosphofructokinase deficiency in the dog: an animal analogue of human glycogen storage disease type VII. Proc Natl Acad Sci USA. 1985;82:8109–8113. doi: 10.1073/pnas.82.23.8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith BF, Stedman H, Rajpurohit Y, et al. The molecular basis of canine muscle-type phosphofructokinase deficiency. J Biol Chem. 1996;271:20070–20074. doi: 10.1074/jbc.271.33.20070. [DOI] [PubMed] [Google Scholar]
- 4.Giger U, Kelly AM, Teno PS. Biochemical studies of canine muscle phosphofructokinase deficiency. Enzyme. 1988;40:25–29. doi: 10.1159/000469137. [DOI] [PubMed] [Google Scholar]
- 5.Harvey JW, Pate MG, Mhaskar Y, Dunaway GA. Characterization of phosphofructokinase-deficient canine erythrocytes. J Inherit Metab Dis. 1992;15:747–759. doi: 10.1007/BF01800017. [DOI] [PubMed] [Google Scholar]
- 6.Giger U, Smith BF, Woods CB, Patterson DF, Stedman H. Inherited phosphofructokinase deficiency in the American Cocker spaniel. J Am Vet Med Assoc. 1992;201:1569–1571. [PubMed] [Google Scholar]
- 7.Giger U, Reilly MP, Asakura T, Baldwin CJ, Harvey JW. Autosomal recessive inherited phosphofructokinase deficiency in English Springer spaniels. Anim Genet. 1986;17:15–23. doi: 10.1111/j.1365-2052.1986.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 8.Giger U, Harvey JW. Hemolysis caused by phosphofructokinase deficient English Springer spaniels: seven cases (1983-86) J Am Vet Med Assoc. 1987;191:453–459. [PubMed] [Google Scholar]
- 9.Giger U, Harvey JW, Yamaguchi RA, et al. Inherited phosphofructokinase deficiency in dogs with hyperventilation induced hemolysis: increased in vitro and in vivo alkaline fragility of erythrocytes. Blood. 1985;65:345–351. [PubMed] [Google Scholar]
- 10.Harvey JW, Sussman WA, Pate MG. Effect of 2,3-diphosphoglycerate concentration on the alkaline fragility of phosphofructokinase-deficient canine erythrocytes. Comp Biochem Physiol B. 1988;89B:105–109. doi: 10.1016/0305-0491(88)90269-6. [DOI] [PubMed] [Google Scholar]
- 11.Harvey JW, Calderwood Mays MB, Gropp KE, Denaro FJ. Polysaccharide storage myopathy in canine phosphofructokinase deficiency (type VII glycogen storage disease) Vet Pathol. 1990;27:1–8. doi: 10.1177/030098589002700101. [DOI] [PubMed] [Google Scholar]
- 12.Harvey JW, Smith JE. Haematology and clinical chemistry of English Springer spaniel dogs with phosphofructokinase deficiency. Comp Haematol Intl. 1994;4:70–74. [Google Scholar]
- 13.Hilppo M. Some haematological and clinical-chemical parameters of sight hounds (Afghan hound, saluki and whippet) Nord Vet Med. 1986;38:148–155. [PubMed] [Google Scholar]
- 14.Jain NC. Schalm's Veterinary Hematology. 4th ed. Lea & Febiger; Philadelphia: 1986. pp. 69–71.pp. 551–554. [Google Scholar]
- 15.Santoro SK, Garrett LD, Wilkerson M. Platelet concentrations and platelet-associated IgG in greyhounds. J Vet Intern Med. 2007;21:107–112. doi: 10.1892/0891-6640(2007)21[107:pcapii]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Parker JC. Solute and water transport in dog and cat red blood cells. In: Ellory JC, Lew VL, editors. Membrane Transport in Red Cells. Academic Press Inc.; New York: 1977. pp. 427–465. [Google Scholar]
- 17.Dacie JV, Lewis SM, Gordon-Smith EC. Investigations of the hereditary haemolytic anaemias. In: Dacie JV, Lewis SM, editors. Practical Haematology. 6th ed Churchill Livingstone; London: 1984. pp. 152–172. [Google Scholar]
- 18.Waddell WJ. Lysis of dog erythrocytes in mildly alkaline isotonic media. Am J Physiol. 1956;186:339–342. doi: 10.1152/ajplegacy.1956.186.2.339. [DOI] [PubMed] [Google Scholar]
- 19.Iampietro PF, Burr MJ, Fiorica V, McKenzie JM, Higgins EA. PH-dependent lysis of canine erythrocytes. J Appl Physiol. 1967;23:505–510. doi: 10.1152/jappl.1967.23.4.505. [DOI] [PubMed] [Google Scholar]
- 20.Searcy GP, Tasker JB, Miller DR. Animal model: pyruvate kinase deficiency in dogs. Am J Pathol. 1979;94:689–692. [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman BL, Giger U. Inherited erythrocyte pyruvate kinase deficiency in the West Highland white terrier. J Small Anim Pract. 1990;31:610–616. [Google Scholar]
- 22.Harvey JW, Reddy GR. Postnatal hematological development in phosphofructokinase-deficient dogs. Blood. 1989;74:2556–2561. [PubMed] [Google Scholar]
- 23.Snow DH, Billeter R, Mascarello F, et al. No classical type IIB fibers in dog skeletal muscle. Histochemistry. 1982;75:53–65. doi: 10.1007/BF00492533. [DOI] [PubMed] [Google Scholar]
- 24.Mosher DS, Quignon P, Bustamante CD, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PloS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amit R, Bashan N, Abarbanel JM, et al. Fatal familial infantile glycogen storage disease: multisystem phosphofructokinase deficiency. Muscle Nerve. 1992;15:455–458. doi: 10.1002/mus.880150406. [DOI] [PubMed] [Google Scholar]
- 27.Finsterer J, Strollberger C, Kopsa W. Neurological and cardiac progression of glycogenosis type VII over an eight-year period. South Med J. 2002;95:1436–1440. [PubMed] [Google Scholar]
- 28.Asano K, Masuda K, Okumura M, Kadosawa T, Fujinaga T. Plasma atrial and brain natriuretic peptide levels in dogs with congestive heart failure. J Vet Med Sci. 1999;61:523–529. doi: 10.1292/jvms.61.523. [DOI] [PubMed] [Google Scholar]
- 29.Prosek R, Sisson DD, Oyama MA, Solter PF. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B-type natriuretic factor, endothelin, and cardiac troponin-I. J Vet Intern Med. 2007;21:238–242. doi: 10.1892/0891-6640(2007)21[238:dcandi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Oyama MA, Sisson DD, Solter PF. Prospective screening for occult cardiomyopathy in dogs by measurement of plasma atrial natriuretic peptide and B-type natriuretic peptide and cardiac troponin-I concentrations. Am J Vet Res. 2007;68:42–47. doi: 10.2460/ajvr.68.1.42. [DOI] [PubMed] [Google Scholar]
- 31.DeFrancesco TC, Rush JE, Rozanski EA, et al. Prospective clinical evaluation of an ELISA B-type natriuretic peptide assay in the diagnosis of congestive heart failure in dogs presenting with a cough or dyspnea. J Vet Intern Med. 2007;21:243–250. doi: 10.1892/0891-6640(2007)21[243:pceoae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Fine DM, DeClue AE, Reinero CR. Evaluation of circulating amino terminal-pro-B-type natriuretic peptide concentration in dogs with respiratory distress attributable to congestive heart failure or primary pulmonary disease. J Am Vet Med Assoc. 2008;XX:1674–1679. doi: 10.2460/javma.232.11.1674. [DOI] [PubMed] [Google Scholar]
- 33.Hall C. NT-ProBNP: the mechanism behind the marker. J Cardiac Fail. 2005;11:S81–S83. doi: 10.1016/j.cardfail.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Hall C. Essential biochemistry and physiology of (NT-pro) BNP. Eur J Heart Fail. 2004;17:257–260. doi: 10.1016/j.ejheart.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 35.McCully K, Giger U. Using near-infrared spectroscopy coupled to magnetic resonance spectroscopy to evaluate canine muscle oxygen saturation: evaluation and treatment of M-type phosphofructokinase deficiency. In: Tavitian B, Leroy-Willig A, Ntziachristos V, editors. International Textbook of In Vivo Imaging in Vertebrates. John Wiley & Sons, Ltd.; London: 2007. pp. 265–269. [Google Scholar]


