Abstract
We show that caveolin-1 is a novel binding protein for Mdm2. After oxidative stress, caveolin-1 sequesters Mdm2 away from p53 leading to stabilization of p53 and upregulation of p21Waf1/Cip1 in human fibroblasts. Expression of a peptide corresponding to the Mdm2-binding domain of caveolin-1 is sufficient to upregulate p53 and p21Waf1/Cip1 protein expression and induce premature senescence. Oxidative stress-induced activation of the p53/ p21Waf1/Cip1 pathway and induction of premature senescence are compromised in caveolin-1 null mouse embryonic fibroblasts (MEFs). We also demonstrate that re-introduction of caveolin-1 in oncogenic Ras (RasG12V)-transformed fibroblasts, which express residual levels of caveolin-1, is sufficient to promote cellular senescence. Moreover, caveolin-1 expression in MEFs is required for senescent fibroblast-induced stimulation of cell growth and tumorigenesis of both RasG12V-transformed fibroblasts and MDA-MB-231 breast cancer epithelial cells both in vitro and in vivo. Thus, our results propose caveolin-1 as a key mediator of the antagonistic pleiotropic properties of cellular senescence.
Keywords: Aging, Caveolin, p53, Senescence
Introduction
Antagonistic pleiotropy is an evolutionary theory of aging introduced by G.C. Williams in the 1950s based on the concept that some genes or processes increase the odds of successful reproduction early in life but have harmful effects later in life (1). Because these deleterious effects appear mostly after the organism’s reproduction cycle is over, natural selection cannot eliminate them. Cellular senescence represents an example of antagonistic pleiotropy (reviewed in (2)). By inducing a permanent withdrawal from the cell cycle, cellular senescence acts as a tumor suppressor mechanism and prevents potential cancer cells from proliferating in young individuals (3). However, accumulation of senescent cells overtime may contribute to aging and age-related diseases, including cancer. In fact, senescent fibroblasts secreting metalloproteinases, growth factors, and inflammatory cytokines (4–6) promote the growth and progression of preneoplastic breast cancer cells toward malignancy, and potentiates the transformed phenotype of breast cancer cells (7–10).
Oxidative stress can be considered an example of antagonistic pleiotropy. Reactive oxygen species have been shown to mediate normal biological functions such as smooth muscle cell contraction, regulation of protein tyrosine phosphatase activity, generation of thyroid hormone, cell differentiation, and mitogenic signaling (reviewed in (11)). However, oxidative stress has been shown to induce premature senescence in culture not dependent upon telomere shortening (12, 13). Since oxidative stress induces cellular senescence and free radical levels within cells increase over time, it is possible that oxidative stress may promote the deleterious effects of cellular senescence such as aging and chronic diseases, including cancer.
Caveolae are invaginations of the plasma membrane enriched in cholesterol. Caveolin-1 is the structural protein component of caveolar membranes. Caveolin-1 acts as a scaffolding protein to concentrate and functionally regulate signaling molecules (14, 15). Several independent lines of evidence indicate that caveolin-1 may act as an anti-proliferative protein (16–19). Consistent with this idea, we have previously demonstrated that overexpression of caveolin-1 is sufficient to arrest mouse embryonic fibroblasts (MEFs) in the G0/G1 phase of the cell cycle, reduce their proliferative lifespan, and promote premature cellular senescence (20, 21). In addition, we have shown that oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements (22). However, the signaling pathway that links caveolin-1 to stress-induced premature senescence (SIPS) remains unknown. In addition, the possible role of caveolin-1 as an antagonistic pleiotropic protein is unexplored.
Here, we propose caveolin-1 as a novel antagonistic pleiotropic molecule that contributes to explain, at the molecular and cellular levels, the antagonistic pleiotropic properties of oxidative stress, cellular senescence, and p53.
Materials and Methods
GST fusion proteins pull-down assay
The GST-caveolin-1 (GST-Cav-1) fusion protein constructs were transformed into Escherichia coli (BL21 strain; Novagen, Inc.). After induction of expression through addition of 5 mM isopropyl-β-D-galactoside (Sigma), GST-Cav-1 constructs were affinity purified on glutathione-agarose beads, using the detergent Sarcosyl for initial solubilization. Equal amounts of GST-Cav-1 and GST alone were incubated overnight at 4 °C with cell lysates. After binding, the beads were extensively washed and resuspended in 3X sample buffer and subjected to SDS-PAGE.
Co-culture studies
Three independent clones of either wildtype or caveolin-1 null MEFs were mixed and cultured as one population. Cells were plated into 100mm dishes and subjected to oxidative stress when approximately 50% confluent. Oxidative stress was induced by treating MEFs with 150 µM hydrogen peroxide for 2 hours. After hydrogen peroxide treatment, cells were washed with PBS and cultured in complete medium for 4 days. MEFs were then serum starved for 3 days. Serum starved RasG12V-transformed NIH 3T3 (37,500 cells) or MDA-MB-231 (37,500 cells) cells were layered on top of serum starved MEFs and cultured for 7 days. Ras co-cultures were quantified by counting the number of i) nuclei after DAPI staining, and ii) Ki67 positive cells in 30 random fields per experimental point. MDA-MB-231 co-cultures were quantified by counting the number of colonies after crystal violet staining. Crystal violet staining was performed by incubating the cells with 10% crystal violet in 70% ethanol for 2 minutes followed by extensive washes with PBS. Quantification of crystal violet staining was performed as follows: the image was preprocessed by cropping the central area of each plate, converting to the HSV colorspace, and finding connected regions of pixels with saturation greater than 0.2 on a [0, 1] scale. To reduce noise, only colonies with area greater than 32 pixels were counted (≥0.3mm2).
Extracellular matrix and soluble factors
Wild type and caveolin-1 null MEFs were cultured and treated as listed above for co-culture studies. Conditioned medium was then collected from respective cultures and saved. Plates were washed twice with PBS and cells removed by incubating in Cell Dissociation Buffer Enzyme-Free PBS-based (Gibco). Dishes were washed with PBS three times to remove any residual debris, cells, or dissociation buffer. Conditioned medium was replaced onto the respective dishes. RasG12V-transformed NIH 3T3 cells (37,500 cells) were then added on the dish containing extracellular matrix and soluble factors. Cells were grown for 7 days. Results were quantified by counting the number of either nuclei after DAPI staining or Ki67 positive cells in 30 random fields per experimental point.
Tumorigenesis assays
The animal protocol described in this article were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Nude (nu/nu) mice (5–6 weeks old from Charles River) were injected subcutaneously into the dorsal flap with either 3.25 × 105 RasG12V-transformed NIH 3T3 or MDA-MB-231 cells alone or with 1 × 106 MEFs (100 µl volume). 1 × 106 MEFs alone were injected as a negative control. Four weeks after the injection, tumors were excised and the x and y axes measured to determine tumor volume (size) according to the following formula: volume = 0.5 × width2 × length.
Results
Oxidative stress promotes the interaction between caveolin-1 and Mdm2
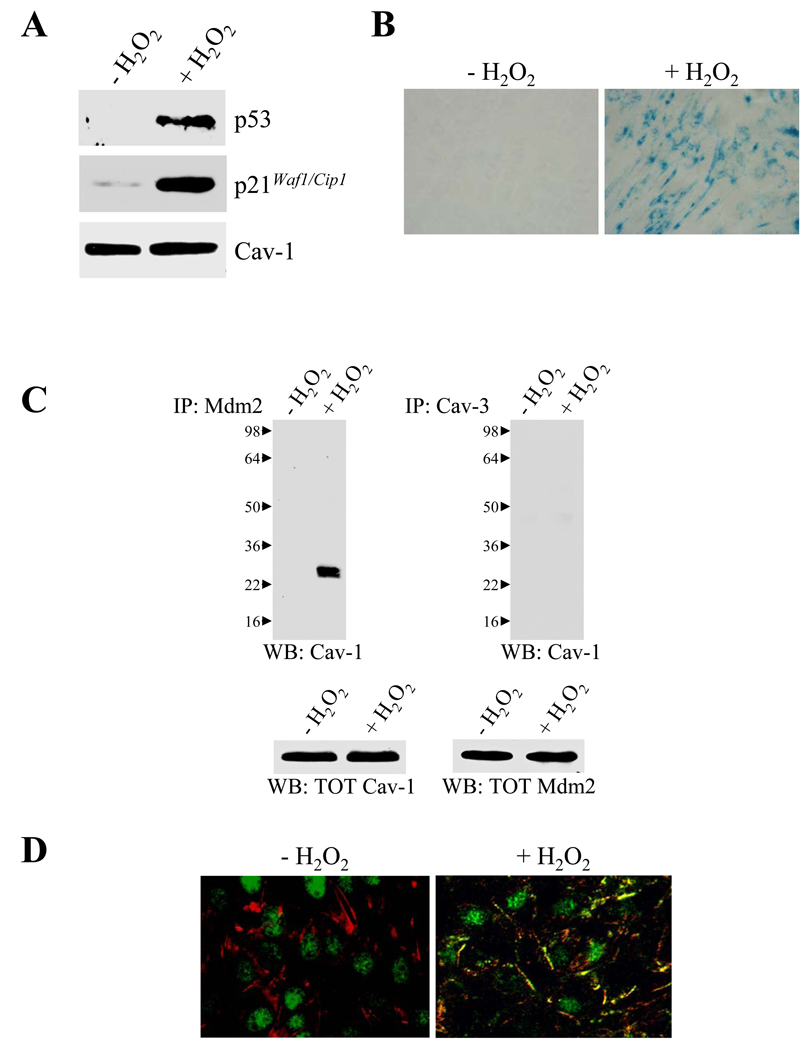
Oxidative stress is a well-established inducer of cellular senescence in culture (12, 13). To begin to investigate the oxidant-promoted and caveolin-1-dependent signaling events that are required for SIPS, we treated WI-38 human diploid fibroblasts with subcytotoxic levels of hydrogen peroxide for 2 hours. Cells were then washed and cultured in normal medium for different periods of time. We found that oxidative stress induced premature senescence of WI-38 cells, as shown by activation of the p53/p21Waf1/Cip1 pathway (Figure 1A) and senescence-associated β-galactosidase activity staining (Figure 1B). To investigate the molecular mechanism underlying activation of p53 after oxidative stress, we focused on the modulation of Mdm2, a well-known negative regulator of p53, by caveolin-1. We found that oxidative stress promoted the interaction between endogenous caveolin-1 and Mdm2, as shown by co-immunoprecipitation studies in WI-38 human diploid fibroblasts (Figure 1C). Consistent with this result, while caveolin-1 was mainly expressed at the plasma membrane and endogenous Mdm2 in the nucleus under resting conditions, Mdm2 was found in caveolin-1-enriched domains at the plasma membrane and in the cytoplasm after oxidative stress in WI-38 cells (Figure 1D, and Supplemental Figures 1A, 1B, and 1C). In support of these data, we show in Supplemental Figures 1D, 1E, and 1F that nuclear p53 levels were low in cells where Mdm2 was expressed in the nucleus before oxidative stress and elevated in cells where Mdm2 left the nucleus upon oxidant stimulation. Thus, by sequestering Mdm2 away from p53, caveolin-1 appears to stabilize p53 after oxidative stress.
Figure 1. Sequestration of Mdm2 by caveolin-1 upon oxidant stimulation.
(A) Immunoblotting. WI-38 cells were treated with subcytotoxic concentrations of hydrogen peroxide for 2 hours. Cells were washed and cultured in normal medium for 24 hours. Cell lysates were matched for total protein content by BCA protein assay and subjected to immunoblotting analysis using antibody probes against p53, p21WAF1/CIP1, and caveolin-1 (Cav-1).
(B) Senescence-Associated (SA) β-galactosidase activity assay. WI-38 cells were treated as in (A). Cells were then subjected to senescence-associated β-galactosidase activity assay after 7 days of recovery following the H2O2 treatment using the Senescence-Associated β-galactosidase Staining Kit (Cell Signaling). A representative field is shown.
(C) Immunoprecipitation assay. WI-38 human diploid fibroblasts were treated with 450 µM H2O2 for 2 hours. After twenty four hours, cell lysates were immunoprecipitated with a monoclonal antibody probe specific for Mdm2 and immunoprecipitates subjected to immunoblotting analysis with anti-caveolin-1 pAb. Total caveolin-1 and Mdm2 expression are shown in the lower panels. Immunoprecipitation with a monoclonal antibody probe specific for caveolin-3, a caveolin-1 homolog that is not expressed in fibroblasts, was used as negative control.
(D) Immunofluorescence analysis. Wi-38 cells were treated as in (A). After twenty four hours of recovery following the H2O2 treatment, cells were double stained with anti-caveolin-1 pAb and anti-Mdm2 mAb. Untreated cells (-H2O2) were used as controls. The expression of these proteins was detected using fluorescent secondary antibodies. Data were collected sequentially in different channels. Representative images were taken using a 20X objective.
The scaffolding domain of caveolin-1 interacts with the caveolin binding domain of Mdm2 in vitro
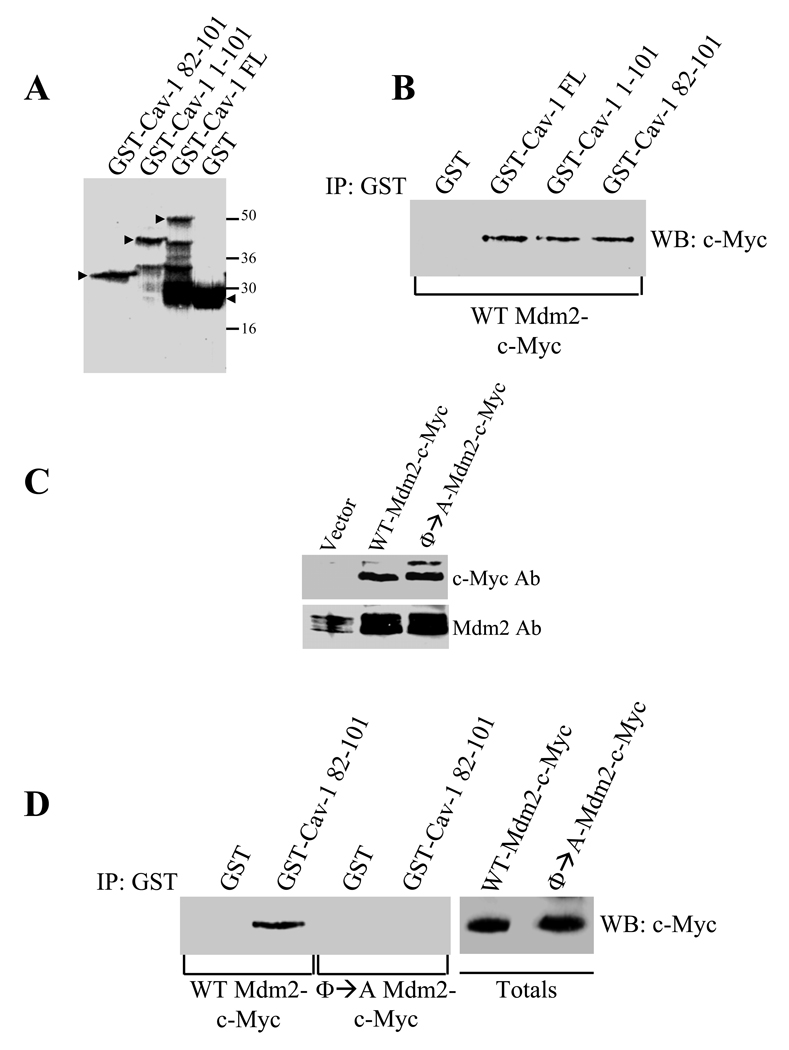
To determine what domain of caveolin-1 is required for the interaction with Mdm2, we performed pulldown assays using a series of caveolin-1 deletion mutants fused to GST. Figures 2A and 2B and Supplemental Figure 2A show that the caveolin scaffolding domain (CSD) of caveolin-1 (residues 82–101) is sufficient for binding to Mdm2. Caveolin-1 directly binds to signaling molecules through a direct interaction between the caveolin scaffolding domain of caveolin-1 and caveolin-binding motifs of signaling molecules. These caveolin-binding motifs include crucial aromatic amino acid residues that are separated by a variable number of X residues (23). A list of peptide ligands previously identified by screening phage-display libraries using a GST fusion protein containing the caveolin-1-scaffolding domain is shown in Supplemental Figure 2B. Interestingly, both mouse and human Mdm2 have a potential caveolin-binding motif (CBM) between amino acids 48 and 60 (Supplemental Figure 2B). To directly assess the importance of residues 48–60 of Mdm2 in the binding to caveolin-1, we generated and expressed an Mdm2 mutant (→A-Mdm2) in which the four aromatic residues of the putative CBM were mutated to alanines (Figure 2C). GST pulldown assays show that WT-Mdm2 but not x02192;A-Mdm2 bound to the caveolin-1 scaffolding domain (Figure 2D and Supplemental Figure 2C), indicating that residues 48–60 of Mdm2 indeed represent a caveolin binding motif.
Figure 2. Identification of the Mdm2-binding domain of caveolin-1 and caveolin-1-binding motif of Mdm2.
(A) and (B) In vitro reconstitution of Mdm2 binding to Caveolin-1. NIH 3T3 cells were transiently transfected with the cDNA encoding Mdm2, containing the c-myc tag at its C-terminus. Cell lysates were incubated with affinity-purified GST alone or GST fused to a series of caveolin-1 deletion mutants (Full-length (FL), residues 1–101, and residues 82–101) immobilized on glutathione-agarose beads (A). The beads were then separated by SDS-PAGE and subjected to Western blot analysis with anti-c-myc monoclonal antibody to detect Mdm2 binding (B).
(C) Generation and expression of Φ→A Mdm2 mutant. NIH 3T3 cells were transiently transfected with wild type Mdm2 or a mutated form of Mdm2 in which the aromatic residues of the putative caveolin-1 binding motif were substituted with alanines. Transfection with the expression vector alone was used as a control. Forty eight hours after transfection, cell lysates were subjected to immunoblotting analysis using either anti-Mdm2 or anti-c-myc mAbs.
(D) In vitro reconstitution of Φ→A Mdm2 mutant binding to the caveolin-1 scaffolding domain. The experiments were performed as described in (B) with the exception that Φ→A Mdm2-c-Myc mutant was transfected in addition to wild type Mdm2-c-Myc and cell lysates were incubated with only affinity-purified GST alone or GST-caveolin-1 82-101.
Caveolin-1 expression activates p53 by preventing binding of Mdm2 to p53
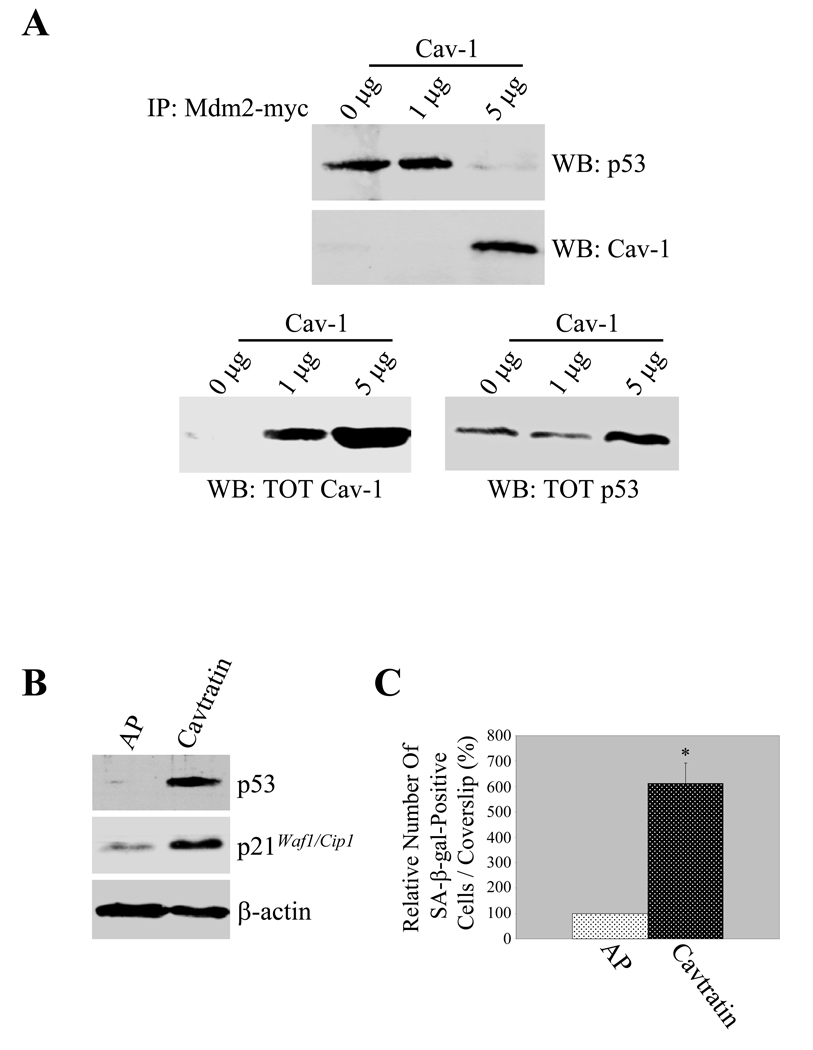
Interestingly, the caveolin binding motif within the Mdm2 sequence overlaps with the p53 binding domain (residues 23–108) (Supplemental Figure 3). Thus, we postulated that by binding to Mdm2, caveolin-1 may prevent binding between Mdm2 and p53. To test this hypothesis, we exogenously expressed Mdm2 in fibroblasts with increasing amount of a caveolin-1-expressing vector. The interaction between Mdm2 and p53 was then assessed by co-immunoprecipitation analysis. Figure 3A illustrates that Mdm2 immunoprecipitated p53 in the absence of overexpression of caveolin-1. In contrast, Mdm2 immunoprecipitated caveolin-1 but not p53 when caveolin-1 was overexpressed (Figure 3A). As a result of less interaction between Mdm2 and p53 after caveolin-1 overexpression, total endogenous p53 expression was increased (Figure 3A).
Figure 3. Caveolin-1 expression prevents Mdm2 binding to p53. Activation of the p53/p21Waf1/Cip1/senescence pathway by the caveolin-1 scaffolding domain.
(A) Immunoprecipitation assay. NIH 3T3 fibroblasts were co-transfected with a c-myc-tagged Mdm2-expressing vector and increasing concentrations of a caveolin-1-expressing vector. Twenty four hours after transfection, cells were treated with 150 µM H2O2 for 2 hours. After twenty four hours, cell lysates were immunoprecipitated with a monoclonal antibody probe specific for c-myc and immunoprecipitates subjected to immunoblotting analysis with anti-caveolin-1 and anti-p53 pAbs. Total caveolin-1 and p53 expression are shown in the lower panels.
(B) and (C) Immunoblotting and Senescence-associated (SA) β-galactosidase activity assay. WI-38 cells were incubated with either cavtratin (10µM) or the control peptide antennapedia (AP) (10µM) for either 24 hours (B) or 7 days (C). In (B), cell lysates were subjected to immunoblotting analysis using antibody probes against p53 (pAb) and p21Waf1/Cip1 (mAb). Immunoblotting with anti-β-actin mAb was performed to show equal loading. In (C), cells were subjected to senescence-associated β-galactosidase activity assay. Quantification of SA-β-gal-positive cells is shown. Values represent means ± SEM. *P<0.001.
Expression of the caveolin-1 scaffolding domain is sufficient to activate the p53/p21Waf1/Cip1 pathway and induce premature senescence
Because the interaction between caveolin-1 and Mdm2 is mediated by the scaffolding domain of caveolin-1 (Figure 2B) and overexpression of caveolin-1 is sufficient to prevent the Mdm2/p53 interaction and stabilize p53 (Figure 3A), we then asked whether the expression of the caveolin-1 scaffolding domain is sufficient to activate the p53/p21Waf1/Cip1 pathway. To this end, we took advantage of the cell permeable peptide cavtratin, consisting of the homeodomain of the Drosophila transcription factor antennapedia (AP or penetratin), coupled to the caveolin-1 scaffolding domain (24). Expression of cavtratin, but not the control peptide AP lacking the CSD, upregulated the protein expression of p53 and p21Waf1/Cip1, a downstream target of p53, in WI-38 cells (Figure 3B). Interestingly, expression of cavtratin was sufficient to induce premature senescence in WI-38 cells (Figure 3C).
Lack of caveolin-1 prevents the oxidant-induced activation of the p53/p21Waf1/Cip1 pathway and induction of premature senescence
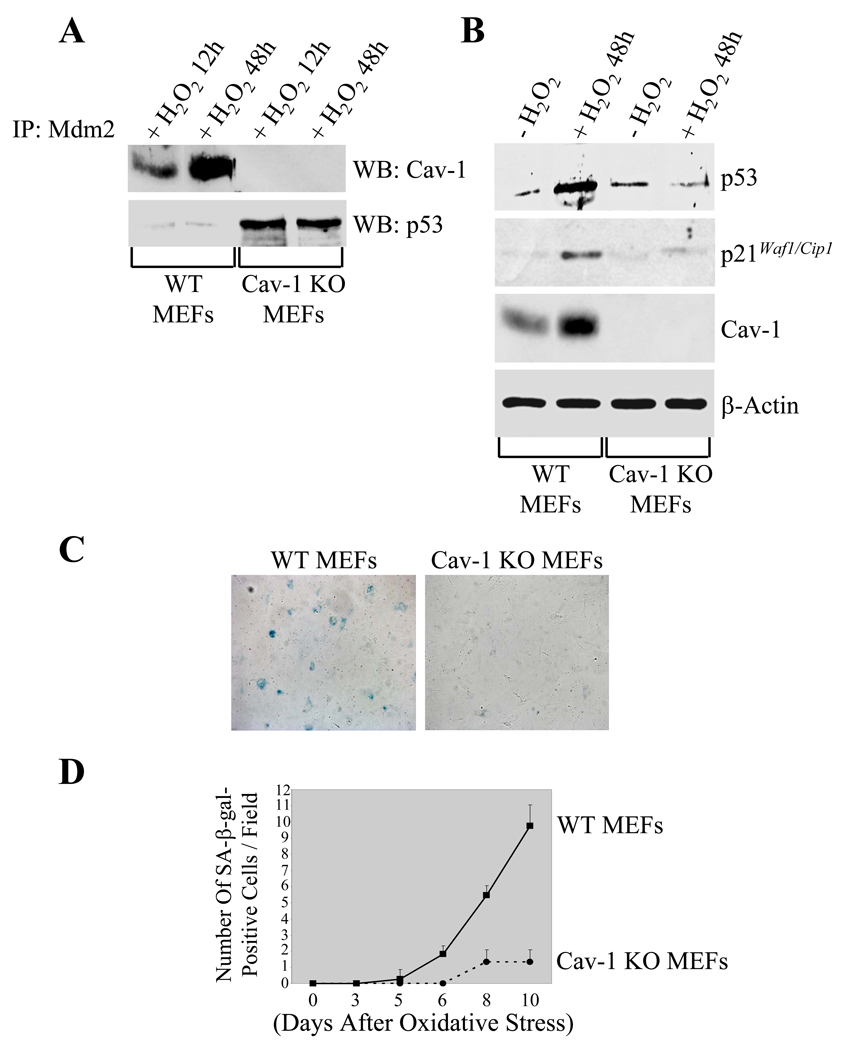
Based on the above data, one would not expect a full activation of the p53/p21Waf1/Cip1 pathway after oxidative stress in cells which do not express caveolin-1. To address this point, mouse embryonic fibroblasts (MEFs) were derived from wild type and caveolin-1 null mice, which do not express caveolin-1, and subjected to oxidative stress. Endogenous Mdm2 was immunoprecipitated with a monoclonal specific antibody probe and immunoprecipitates subjected to immunoblotting analysis using polyclonal antibodies against either caveolin-1 or p53. Figure 4A shows that oxidative stress promoted the interaction between caveolin-1 and Mdm2 in wild type but not caveolin-1 null MEFs. Interestingly, the interaction between Mdm2 and p53, as assessed by coimmunoprecipitation studies, was significantly higher in caveolin-1 null MEFs, as compared to wild type MEFs (Figure 4A). Consistent with the notion that Mdm2 is a negative regulator of p53, we show in Figure 4B that the oxidant-induced stabilization of p53 is dramatically compromised in the absence of caveolin-1. Thus, more interaction between Mdm2 and p53 in caveolin-1 null cells results in reduced total p53 expression. Reduced activation of p53 in caveolin-1 null cells was confirmed by reduced upregulation of p21Waf1/Cip1 after oxidative stress (Figure 4B). Since activation of the p53/p21Waf1/Cip1 pathway plays a key role in SIPS, we subjected wild type and caveolin-1 null MEFs to senescent-associated β-galactosidase staining at different time points after oxidative stress. Figures 4C and 4D demonstrate that the number of senescent-associated β-galactosidase-positive cells was significantly reduced in caveolin-1 null MEFs, as compared to wild type MEFs.
Figure 4. Activation of the p53/p21Waf1/Cip1 pathway and induction of premature senescence by oxidative stress are inhibited in caveolin-1 null MEFs.
(A) Immunoprecipitation assay. Mouse embryonic fibroblasts were derived from wild type (WT MEFs) and caveolin-1 null (Cav-1 KO MEFs) mice. Cells were treated with 150 µM H2O2 for 2 hours, washed, and cultured in normal medium for 12 and 48 hours. Cell lysates were then immunoprecipitated with a monoclonal antibody probe specific for Mdm2 bound to agarose beads and immunoprecipitates subjected to immunoblotting analysis with anti-caveolin-1 and anti-p53 pAbs.
(B) Immunoblotting. Wild type (WT MEFs) and caveolin-1 null (Cav-1 MEFs) were treated with 150 µM H2O2 for 2 hours, washed, and cultured in normal medium for 48 hours. Untreated cells were used as controls. Cell lysates were then subjected to immunoblotting analysis using antibody probes against p53 (pAb), p21Waf1/Cip1 (mAb), and caveolin-1 (pAb). Immunoblotting with anti-β-actin mAb was performed to show equal loading.
(C) and (D) Senescence-Associated (SA) β-galactosidase activity assay. Wild type (WT MEFs) and caveolin-1 null (Cav-1 KO) MEFs were treated with 150 µM hydrogen peroxide for 2 hours. Cells were washed and cultured in normal medium for different periods of time (3, 5, 6, 8, and 10 days). Cells were then subjected to senescence-associated β-galactosidase activity assay. A representative field of the staining 10 days after oxidative stress is shown in (C). Quantitation of the SA-β-galactosidase activity assay is shown in (D).
We conclude that caveolin-1 expression is required for activation of the p53 pathway and induction of premature senescence after oxidative stress (Supplemental Figure 4).
Caveolin-1 mediates the antagonistic pleiotropic properties of cellular senescence
To investigate whether caveolin-1 represents a key mediator of the antagonistic pleiotropic properties of cellular senescence, we asked whether i) caveolin-1 expression in cancer cells induces senescence and thus inhibits cancer cell growth and ii) caveolin-1-dependent senescence of fibroblasts promotes proliferation of cancer cells and tumorigenesis.
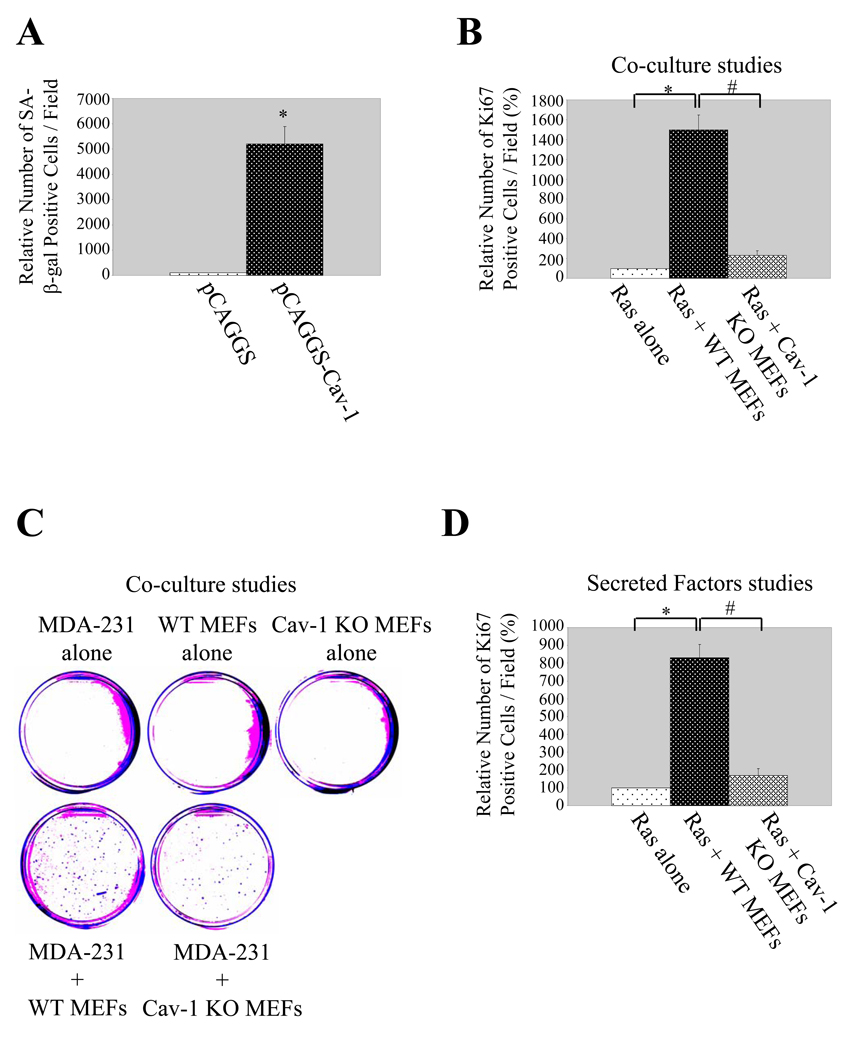
To this end, caveolin-1 was overexpressed in oncogenic Ras (RasG12V)-transformed NIH 3T3 cells, a well-established model system to study cell transformation, where caveolin-1 mRNA and protein levels are dramatically reduced (17). We show in Supplemental Figure 5A and Figure 5A that overexpression of caveolin-1 in these cells was sufficient to induce cellular senescence, as compared to RasG12V-transformed NIH 3T3 cells expressing vector alone.
Figure 5. Caveolin-1 induces senescence of cancer cells and mediates senescent fibroblast-induced proliferation of cancer cells in vitro and in vivo.
(A) Senescence-Associated (SA) β-galactosidase activity assay. Caveolin-1-and pCAGGS-expressing cells were derived as described in Supplementary Figure 5A. Cells were then subjected to senescence-associated β-galactosidase activity assay. Quantitation of the SA-β-galactosidase activity assay is shown. Values represent means ±SEM. *P<0.001.
(B). Co-culture studies using RasG12V-transformed NIH 3T3 cells. RasG12V-transformed NIH 3T3 cells were cultured alone or with either wild type or caveolin-1 null MEFs as described in Materials and Methods. Cells were then stained with anti-Ki67 mAb. Ki67 staining was detected using fluorescent secondary antibodies. Quantification of Ki67 staining is shown. Values represent means ± SEM. *, #P<0.001.
(C) Co-culture studies using MDA-MB-231 cells. MDA-MB-231 cells were cultured alone or with either wild type or caveolin-1 null MEFs as described in Materials and Methods. Colony formation was then detected by staining the cells with crystal violet (A representative image is shown in (C); quantification of the staining is represented in Supplementary Figure 5F).
(D) Secreted factors studies. RasG12V-transformed NIH 3T3 cells were grown with secreted factors from hydrogen peroxide-treated wild type and caveolin-1 null MEFs as described in Materials and Methods. Cells were then stained with anti-Ki67 mAb. Ki67 staining was detected using fluorescent secondary antibodies. Quantification of Ki67 staining is shown. Values represent means ± SEM. *, #P<0.001.
We then subjected wild type and caveolin-1 null MEFs to oxidative stress to induce cellular senescence (150µM H2O2 for 2 hours followed by culturing the cells for 7 days in normal medium) and examined the ability of these cells to promote cell proliferation of RasG12V-transformed NIH 3T3 cells in co-culture studies. RasG12V-transformed NIH 3T3 cells were seeded onto an equal number of hydrogen peroxide-treated wild type and caveolin-1 null cells (50–70% confluent lawns). Both RasG12V-transformed NIH 3T3 cells and MEFs were serum-starved for 72 hours before co-culture experiments to i) avoid any influence of growth factors contained in culture medium; ii) arrest caveolin-1 null MEFs, which otherwise would have proliferated given the fact that oxidative stress failed to induce senescence in the absence of caveolin-1 (Figure 4); and iii) arrest the proliferation of RasG12V-transformed NIH 3T3 cells.
We first examined cell proliferation by staining the co-cultures with DAPI and quantified the number of smaller and more intensely stained RasG12V-transformed NIH 3T3 nuclei (Supplemental Figure 5B and Supplemental Figure 5C). Alternatively, actively proliferating RasG12V-transformed NIH 3T3 cells were quantified by markers of cellular proliferation: Ki67 staining and BrdU incorporation (Supplemental Figures 5B, Figure 5B, and Supplemental Figure 5D, respectively). Growth-arrested MEFs resulted negative for Ki67 staining (data not shown). We demonstrate that cellular proliferation of RasG12V-transformed NIH 3T3 was dramatically enhanced when co-cultured with wild type MEFs subjected to oxidative stress, as compared to RasG12V-transformed NIH 3T3 cells alone (Supplemental Figure 5B, Supplemental Figures 5C, 5D, and Figure 5B). Importantly, the ability of hydrogen peroxide-treated caveolin-1 null MEFs to potentiate the growth of RasG12V-transformed NIH 3T3 cells was dramatically inhibited (Supplemental Figure 5B, Supplemental Figures 5C, 5D, and Figure 5B).
The ability of senescent MEFs to stimulate proliferation of cancer cells in a caveolin-1-dependent manner was not limited to RasG12V-transformed fibroblasts. We demonstrate in Supplemental Figure 5E that H2O2-treated/serum-starved WT MEFs promoted a 3.5-fold-increase of cell growth of serum-starved MDA-MB-231, a breast cancer epithelial cell line, as assessed by counting the number of colonies formed in a Petri dish after DAPI staining (colonies were defined as groups of cells containing at least 20 cells). In contrast, the capacity of caveolin-1-lacking MEFs subjected to oxidative stress to enhance colony formation of MDA-MB-231 cells was reduced by ~30%, as compared to H2O2-treated caveolin-1-expressing MEFs (Supplemental Figure 5E). A more striking difference was noted when only larger colonies (≥0.3mm2) were detected by crystal violet staining. In fact, no colonies were formed by serum-starved MDA-MB-231 cells, WT MEFs, and caveolin-1 null MEFs alone (Figure 5C and Supplemental Figure 5F). Colonies formed when MDA-MB-231 cells were co-cultured with senescent wild type MEFs (Figure 5C and Supplemental Figure 5F). The number of colonies was dramatically reduced (250% reduction) when MDA-MB-231 cells were co-cultured with caveolin-1 null MEFs subjected to oxidative stress (Figure 5C and Supplemental Figure 5F), as compared to H2O2-treated wild type MEFs.
Secreted factors mediate the ability of caveolin-1-expressing senescent MEFs to stimulate the proliferation of RasG12V-transformed NIH 3T3 cells
How do caveolin-1-expressing senescent MEFs stimulate the proliferation of RasG12V-transformed NIH 3T3 cells? Stimulation of cell proliferation may occur by either direct cell-cell interaction and/or secreted insoluble extracellular matrix factor / secreted soluble factor contained in the medium. To directly address these possibilities, we evaluated the proliferation properties of serum-starved RasG12V-transformed NIH 3T3 cells after culturing the cells with conditioned medium from hydrogen-peroxide treated wild type and caveolin-1 null MEFs (cells were treated as described above for co-culture studies) onto the same dishes used to grow MEFs after the cells were removed by calcium chelation without altering the extracellular matrix layer. We show in Supplemental Figure 5G and Figure 5D that the combination of conditioned medium / extracellular matrix factors from H2O2-treated wild type but not caveolin-1 null MEFs stimulated the proliferation of RasG12V-transformed NIH 3T3 cells. These results indicate that secreted factors released by senescent MEFs in a caveolin-1-dependent fashion are sufficient to stimulate RasG12V-transformed NIH 3T3 cell proliferation.
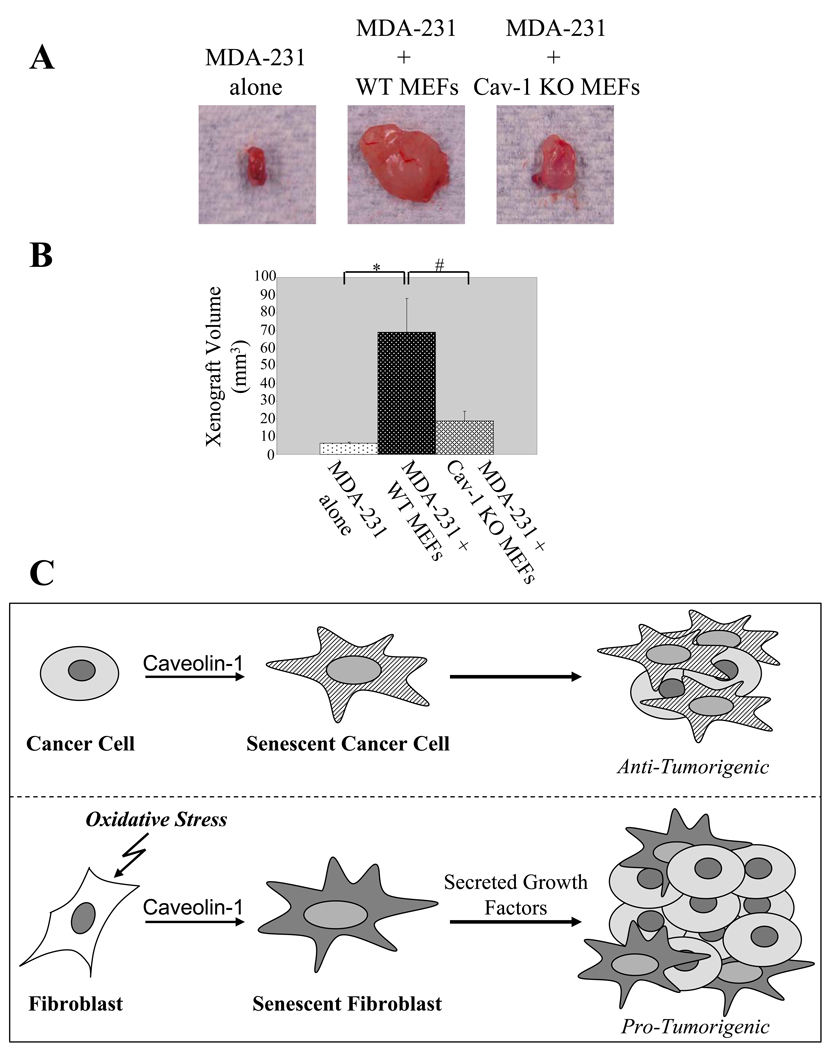
Caveolin-1 expression is required for senescent fibroblast-induced tumorigenesis in vivo
To assess whether caveolin-1 plays an important role in tumorigenesis promoted by senescent fibroblasts in vivo, we injected either RasG12V-transformed NIH 3T3 or MDA-MB-231 cells, alone or with hydrogen peroxide-treated wild type and caveolin-1 null MEFs, into immuno-compromised (nu/nu) mice. Mice were sacrificed 4 weeks later and tumor size determined. Injection of either wild type or caveolin-1 null MEFs alone were done as controls and did not form tumors (data not shown). As expected, both RasG12V-transformed NIH 3T3 (Supplemental Figures 6A and 6B) and MDA-MB-231 (Figures 6A and 6B) cells alone formed tumors 4 weeks after injection. Interestingly, wild type but not caveolin-1 null MEFs subjected to oxidative stress potentiated tumorigenesis of both RasG12V-transformed NIH 3T3 (Supplemental Figures 6A and 6B) and MDA-MB-231 (Figures 6A and 6B) cells. The small increase of tumor size that caveolin-1 null MEFs induced when co-cultured with MDA-MB-231, but not RasG12V-transformed NIH 3T3 cells, may either reflect a variation due to the different cancer cell line used in the experiment or indicate that, in the case of MDA-MB-231 cells, there may be a caveolin-1-independent pathway in fibroblasts that contributes, although minimally, to potentiation of tumorigenesis. Together, these data indicate that senescent fibroblasts create a microenvironment that stimulates the growth of neoplastic cells in vivo in a caveolin-1-dependent manner.
Figure 6. Caveolin-1 mediates senescent fibroblast-induced tumorigenesis in vivo.
(A) and (B) Tumor xenografts studies in nude mice. MDA-231 cells were subcutaneously injected alone or with either hydrogen peroxide-treated wild type or caveolin-1 null MEFs into the dorsal flap of nude (nu / nu) mice. Mice were sacrificed four weeks later and tumor size measured. Representative images are shown in (A); quantification of tumor size is shown in (B). Values in (B) represent means ± SEM. *, #P<0.001.
(C) Schematic diagram summarizing the role of caveolin-1 as a key mediator of the antagonistic pleiotropic properties of cellular senescence. Anti-tumorigenic effect of caveolin-1-mediated cellular senescence: by inducing cellular senescence of cancer cells, including breast cancer epithelial cells, caveolin-1 expression inhibits cancer cell growth. Pro-tumorigenic effect of caveolin-1-mediated cellular senescence: caveolin-1 indirectly promotes tumor cell growth by inducing senescence of fibroblasts, which release secreted factors that stimulate proliferation of cancer cells.
Discussion
The p53 pathway responds to a variety of intrinsic and extrinsic stress signals that can disrupt the fidelity of DNA replication (25). Activation of p53 initiates a program that may induce cellular senescence (26). Thus, stresses that would compromise genome stability can be accommodated by preventing cell cycle progression through p53 activation. This is a tumor suppressor action of p53 that is beneficial to young organisms, as demonstrated by the fact that the p53 gene is mutated in approximately 50% of all human cancers (27). However, p53 action has been linked to the ageing process (28). Mouse models of gain of p53 function show increased tumor suppression but decreased longevity and early aging phenotypes (29, 30). Thus, the p53 tumor suppressor gene is believed to act in an antagonistically pleiotropic manner (31). Interestingly, Scrable and colleagues have analyzed data from mouse models of accelerated aging and determined that the harmful effects of p53 on aging are not attributable only to the p53 gene but involve also the NAD+-dependent deacetylase SIRT1 (28), suggesting that genes that have modifying effects on p53 activity but evolve independently may contribute to the deleterious actions of p53 late in life.
What are the signals that activate p53? These include gamma or UV irradiation, alkylation of bases, depurination of DNA, and reaction with oxidative free radicals (32). Activation of p53 occurs through different mechanisms, including dissociation of Mdm2 from p53. In fact, p53 is a target of the E3 ubiquitin ligase activity of Mdm2, resulting in proteosomal degradation of p53 (33). Mdm2 also suppresses p53 transcriptional activity and shuttles p53 out of the nucleus (34).
Here, we demonstrated that caveolin-1 is a novel Mdm2 binding protein. We found that oxidative stress promotes the interaction between caveolin-1 and Mdm2, which is sequestered in caveolar membranes away from p53. We identified amino acids 48–60 of Mdm2 as the caveolin-1 binding motif. Interestingly, the caveolin-1 binding motif of Mdm2 overlaps with the p53 binding domain of Mdm2 (aa 23–108). We showed that by binding to Mdm2, caveolin-1 prevents the interaction between p53 and Mdm2. As a result, p53 gets stabilized and p21 up-regulated. Activation of this pathway is compromised in caveolin-1 null MEFs, which do not express caveolin-1, and do not undergo cellular senescence after oxidative stress. These data are consistent with several independent lines of evidence suggesting that caveolin-1 may function as a "tumor suppressor protein" in mammalian cells (35, 36).
H-ras G12V is a mutation that accumulates in normal adult tissue and represents one of the most common somatic mutations which lead to cancer development (37). We show here that over-expression of caveolin-1 in caveolin-1-negative RasG12V-transformed NIH 3T3 cells is sufficient to induce cellular senescence. This data is consistent with previously published data where we show that an adenoviral-mediated over-expression of caveolin-1 in caveolin-1 negative MCF7 breast cancer epithelial cells was sufficient to restore stress-induced cellular senescence (22). Since cellular senescence is a natural tumor suppressor mechanism (38–42), it may represent one of the molecular mechanisms through which caveolin-1 acts as a tumor suppressor protein. Because most malignant tumors eventually acquire cells that can proliferate indefinitely and, in many cases, are resistant to senescence-inducing signals (43), our data indicate that caveolin-1 replacement therapy in caveolin-1-negative cancer cells may represent a way to revert these cells to a “senescence-prone” phenotype making them good targets of therapeutic drugs.
We also found that caveolin-1 expression in senescent fibroblasts is required for their ability to secrete insoluble extracellular matrix and/or soluble growth factors and accelerate the proliferation and tumorigenesis of neoplastic cells in vitro and in vivo, respectively. These data are consistent with the known secretory properties of senescent fibroblasts and support the theory that the senescent microenvironment may contribute together with multiple factors, including somatic mutations, to cancer development in older individuals. Based on these data, we propose caveolin-1 as a major regulator of the antagonistic pleiotropic properties of cellular senescence (Figure 6C). We can speculate that interventions aimed at targeting caveolin-1 expression in senescent fibroblasts may represent a novel therapeutic approach, in combination with chemotherapy and radiation, for the treatment of late-life cancer.
Supplementary Material
Acknowledgments
This work was supported by grant number R01AG022548 from the National Institutes of Health (NIH) (to F.G.). JNB was supported by National Institute of Health Predoctoral Training Grant in Pharmacological Sciences (T32GM008424) and NRSA F31 AG032182. We thank Dave Bradley for quantification of crystal violet staining.
References
- 1.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 2.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 4.Linskens MH, Feng J, Andrews WH, et al. Cataloging altered gene expression in young and senescent cells using enhanced differential display. Nucleic Acids Res. 1995;23:3244–3251. doi: 10.1093/nar/23.16.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millis AJ, Hoyle M, McCue HM, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- 6.West MD, Pereira-Smith OM, Smith JR. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989;184:138–147. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- 7.Dilley TK, Bowden GT, Chen QM. Novel mechanisms of sublethal oxidant toxicity: induction of premature senescence in human fibroblasts confers tumor promoter activity. Exp Cell Res. 2003;290:38–48. doi: 10.1016/s0014-4827(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 8.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 10.Roninson IB. Oncogenic functions of tumour suppressor p21(Waf1/Cip1/Sdi1): association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002;179:1–14. doi: 10.1016/s0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- 11.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frippiat C, Chen QM, Zdanov S, et al. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- 13.Chen QM, Bartholomew JC, Campisi J, et al. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332(Pt 1):43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couet J, Li S, Okamoto T, Scherer PS, Lisanti MP. Molecular and cellular biology of caveolae: Paradoxes and Plasticities. Trends in Cardiovascular Medicine. 1997;7:103–110. doi: 10.1016/S1050-1738(97)00001-7. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, A family of scaffolding proteins for organizing "pre-assembled signaling complexes" at the plasma membrane. J. Biol. Chem., (Mini-review) 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 16.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc. Natl. Acad. Sci., USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelman JA, Wycoff CC, Yasuhara S, et al. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J. Biol. Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 18.Engelman JA, Lee RJ, Karnezis A, et al. Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for mammary tumorigenesis. J. Biol. Chem. 1998;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- 19.Sargiacomo M, Scherer PE, Tang Z-L, Casanova JE, Lisanti MP. In vitro phosphorylation of caveolin-rich membrane domains: Identification of an associated serine kinase activity as a casein kinase II-like enzyme. Oncogene. 1994;9:2589–2595. [PubMed] [Google Scholar]
- 20.Volonte D, Zhang K, Lisanti MP, Galbiati F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13:2502–2517. doi: 10.1091/mbc.01-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galbiati F, Volonte D, Liu J, et al. Caveolin-1 Expression Negatively Regulates Cell Cycle Progression by Inducing G(0)/G(1) Arrest via a p53/p21(WAF1/Cip1)-dependent Mechanism. Mol Biol Cell. 2001;12:2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 24.Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849–2856. doi: 10.1158/0008-5472.CAN-06-4082. [DOI] [PubMed] [Google Scholar]
- 25.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 26.Jin S, Levine AJ. The p53 functional circuit. J Cell Sci. 2001;114:4139–4140. doi: 10.1242/jcs.114.23.4139. [DOI] [PubMed] [Google Scholar]
- 27.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 28.Ungewitter E, Scrable H. Antagonistic pleiotropy and p53. Mech Ageing Dev. 2008 doi: 10.1016/j.mad.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier B, Gluba W, Bernier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyner SD, Venkatachalam S, Choi J, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 31.Zafon C. Jekyll and Hyde, the p53 protein, pleiotropics antagonisms and the thrifty aged hypothesis of senescence. Med Hypotheses. 2007;68:1371–1377. doi: 10.1016/j.mehy.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 33.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 34.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 35.Sager R, Sheng S, Anisowicz A, et al. RNA Genetics of Breast Cancer: Maspin as a Paradigm. Cold Spring Harbor Sym. Quant. Biol. 1994;LIX:537–546. doi: 10.1101/sqb.1994.059.01.060. [DOI] [PubMed] [Google Scholar]
- 36.Engelman JA, Zhang XL, Lisanti MP. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998;436:403–410. doi: 10.1016/s0014-5793(98)01134-x. [DOI] [PubMed] [Google Scholar]
- 37.Cha RS, Thilly WG, Zarbl H. N-nitroso-N-methylurea-induced rat mammary tumors arise from cells with preexisting oncogenic Hras1 gene mutations. Proc Natl Acad Sci U S A. 1994;91:3749–3753. doi: 10.1073/pnas.91.9.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. doi: 10.1016/s0955-0674(00)00155-1. [DOI] [PubMed] [Google Scholar]
- 39.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black EJ, Clark W, Gillespie DA. Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr Biol. 2000;10:1119–1122. doi: 10.1016/s0960-9822(00)00699-0. [DOI] [PubMed] [Google Scholar]
- 41.Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 42.Wynford-Thomas D. Cellular senescence and cancer. J Pathol. 1999;187:100–111. doi: 10.1002/(SICI)1096-9896(199901)187:1<100::AID-PATH236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 43.Yeager TR, DeVries S, Jarrard DF, et al. Overcoming cellular senescence in human cancer pathogenesis. Genes Dev. 1998;12:163–174. doi: 10.1101/gad.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.