Abstract
Introduction
We investigated receptor activator of nuclear factor-κB ligand (RANKL) expression by B lymphocytes during early and late aspects of the immune response to Aggregatibacter actinomycetemcomitans, a gram-negative, anaerobic bacterium associated with aggressive periodontal disease.
Methods
Expression of messenger RNA transcripts (tumor necrosis factor-α, Toll-like receptors 4 and 9, interleukins 4 and 10, and RANKL) involved in early (1-day) and late (10-day) responses in cultured rat splenocytes was examined by reverse transcription–polymerase chain reaction (RT-PCR). The immune cell distribution (T, B, and natural killer cells and macrophages) in cultured rat splenocytes and RANKL expression in B cells were determined by flow cytometric analyses. B-cell capacity for induction of osteoclast differentiation was evaluated by coculture with RAW 264.7 cells followed by a tartrate-resistant acid phosphatase (TRAP) activity assay.
Results
The expression levels of interleukins 4 and 10 in cultured cells were not changed in the presence of A. actinomycetemcomitans until cultured for 3 days, and peaked after 7 days. After culture for 10 days, the percentages of B and T cells, the overall RANKL messenger RNA transcripts, and the percentage of RANKL-expressing immunoglobulin G-positive cells were significantly increased in the presence of A. actinomycetemcomitans. These increases were considerably greater in cells isolated from A. actinomycetemcomitans-immunized animals than from non-immunized animals. RAW 264.7 cells demonstrated significantly increased TRAP activity when cocultured with B cells from A. actinomycetemcomitans-immunized animals. The addition of human osteoprotegerin-Fc to the culture significantly diminished such increases.
Conclusion
This study suggests that B-lymphocyte involvement in the immune response to A. actinomycetemcomitans through upregulation of RANKL expression potentially contribute to bone resorption in periodontal disease.
Keywords: Aggregatibacter actinomycetemcomitans, B lymphocytes, immune response, periodontal disease, receptor activator of nuclear factor-κB ligand
Periodontal disease is a specialized infectious disease that involves inflammation of the gums and periodontal bone resorption. During the disease progression, the extensive host immune response may contribute 1to the subsequent tissue destruction (1). It has been suggested that there is a local immunoregulatory imbalance in periodontal disease (2). The progressive lesion has been associated with increased infiltration of T and B lymphocytes (3, 4). The functional potential of lymphocytes in this segment of cellular influx suggests that immune cells could be responsible for the connective tissue destruction and bone resorption in periodontal disease (1, 5–9).
Receptor activator of nuclear factor-κB ligand (RANKL), its receptor RANK, and a decoy receptor osteoprotegerin (OPG) are key molecules in the regulation of osteoclast differentiation, recruitment and function (10–12). Although the interplay between the immune system (innate and adaptive) and inflammatory bone destruction remains unclear, the RANKL–RANK–OPG axis has been recognized as an important signaling system functioning both in bone and immune cell communication (13–15). Recent findings demonstrated that the innate immune response promoted osteoclastogenic activity by activating RANKL via Toll-like receptor (TLR) pathways (16). Others reported that innate immune recognition through TLR signaling is crucial for inflammatory bone loss in response to infection by microorganisms associated with chronic periodontal disease (17). Also, it has been demonstrated that RANKL production by activated T cells directly regulates osteoclastogenesis and bone resorption (18). Our previous studies have demonstrated that regulation of T-lymphocyte function can effect periodontal bone resorption in periodontal disease (19–21). In particular, T lymphocytes specific to Aggregatibacter actinomycetemcomitans, a gram-negative, anaerobic bacterium associated with periodontal disease, have been suggested to modulate periodontal bone resorption in A. actinomycetemcomitans-infected rats through upregulation of RANKL production (21–23). However, the role of B lymphocytes in periodontal bone resorption during the immune response to specific periodontal pathogens has not yet been fully understood. Since B lymphocytes are present in abundance in inflamed periodontal lesions, we hypothesized that B lymphocytes can play a role in bone resorption during the immune response to periodontal pathogens, by the upregulation of RANKL expression. The purpose of this study was to investigate RANKL expression by B lymphocytes during early, late, and primed aspects of the immune response to A. actinomycetemcomitans. Understanding the role of immune B cells in bacterial antigen-induced bone resorption could potentially lead to new treatments for periodontal bone loss (24).
Material and methods
Animals
All animals were inbred heterozygous normal Rowett rats (Rnu/+, male, 3–5 months old) maintained under pathogen-free conditions in laminar flow cabinets. Experiments using these animals were approved by the Forsyth Institute’s Internal Animal Care and Use Committee (IACUC). For some experiments, rats were immunized intraperitoneally with 2 × 108 formalin-killed A. actinomycetemcomitans (ATCC 43718) in phosphate-buffered saline (PBS) as described previously (25). Ten days after immunization rats were boosted intraperitoneally with the same bacteria (2 × 107). Rats injected with PBS only were used as control animals. Rats were sacrificed 4 days after the booster injection.
Tissue extraction and cell culture
Rats were euthanized in a CO2 chamber and the whole spleen was dissected to prepare single-cell suspensions. Single-cell suspensions were applied to Ficoll–Hypaque solution (density 1.088; Sigma Diagnostics, St Louis, MO) and centrifuged (2000 g for 20 min at 20°C) to remove erythrocytes and dead cells. Isolated splenocytes were added to six-well plates (7.5 × 106 per well) in RPMI-1640 complete medium containing 10% fetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mm l-glutamine, and 10 mm HEPES buffer. Cells were cultured at 37°C in a humidified incubator with 5% CO2, in the presence or absence of 2 × 107 formalin-fixed A. actinomycetemcomitans. Cells were cultured for 1 day or for 10 days before collection for analysis.
RNA isolation and reverse transcription–polymerase chain reaction (RT-PCR)
After the indicated times, cultured splenocytes were lysed in RNA-BEE total RNA isolation solution (Tel-Test, Friendswood, TX) and the total cellular RNAs were isolated according to the manufacturer’s instructions. Isolated RNA (0.1 µg each) was reverse transcribed with the Super-Script synthesis system in the presence of random primers (Invitrogen, Carlsbad, CA). The subsequent complementary DNA was amplified by PCR with Taq DNA polymerase (Invitrogen) as described by the manufacturer. The primer sequences used for the amplification were as follows: RANKL: 5′-TCAGGTGGTCTGCAGCATCGCTCTG-3′ and 5′-AACCCTTAGTTTTCCGTTGCTT-3′ [450 base pairs (bp)]; TLR-4: 5′-GGAATACCTGGACTTTCAGCAC-3′ and 5′-TGTTGCAGTATTCCTTTGGATG-3′ (420 bp); TLR-9: 5′-AACAAGCTGGACCTGTACCATT-3′ and 5′-GATGAATCAGGCTTCTCAGGTC-3′ (300 bp); tumor necrosis factor-α (TNF-α): 5′-GTAGCCCACGTCGTAGCAAA-3′ and 5′-CCCTTCTCCAGCTGGAAGAC-3′ (320 bp); interleukin-4 (IL-4): 5′-AACACCACGGAGAACGAGCTCATC-3′ and 5′-AGTGAGTTCAGACCGCTGACACCT-3′ (150 bp); IL-10: 5′-CACTGCTATGTTGCCTGCTC-3′ and 5′-TTCATGGCCTTGTAGACACC-3′ (460 bp); glyceraldehyde 3-phosphate dehydrogenase (GAPDH): 5′-TCACTGCCACTCAGAAGACTGT-3′ and 5′-GGCCTCTCTCTTGCTCTCAGTA-3′ (520 bp). PCR conditions were 30–35 cycles of 94°C for 30 s; 45–60°C for 30 s; 72°C for 1 min. Amplification of the GAPDH gene was used as an internal control.
Flow cytometry
For cell type distribution, cells were stained with the following monoclonal mouse anti-rat primary antibodies (Serotec, Oxford, UK) to different cell surface markers as follows: OX33 (B cell), R73 (T cell), ED1 (macrophage), NK-RP1 [natural killer (NK) cell]. After washing with PBS, the cells were stained with fluorescein isothiocyanate (FITC) -conjugated rat anti-mouse immunoglobulin (IgG; Jackson Immuno Research, West Grove, PA). At least 20,000 cells were counted for each sample. For the detection of IgG-positive cells, cultured cells were stained with rabbit anti-rat IgG (Sigma, St Louis, MO) followed by phycoerythrin (PE) -conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR). Cells stained only with PE-conjugated goat anti-rabbit immunoglobulin were used as negative controls. For the detection of RANKL-expressing cells, cultured cells were stained with human OPG-Fc (a fusion protein kindly provided by Dr Colin Dunstan from Amgen Inc., Thousand Oaks, CA) or the irrelevant human fusion protein L6-Fc (as negative control), followed by FITC-conjugated goat anti-human IgG (Sigma). At least 20,000 cells were counted for each sample.
Cell viability by acridine orange/ethidium bromide staining
Cell viability was determined morphologically by staining of unfixed, non-permeabilized cells with the DNA-intercalating fluorescent dyes acridine orange and ethidium bromide (26). Briefly, cultured cells were harvested by centrifugation. Cells were then exposed to 40 µg/ml acridine orange (Sigma) and 100 µg/ml ethidium bromide (Bio-Rad, Hercules, CA), and visualized by fluorescence microscopy using an inverted microscope (Leica, Bannockburn, IL). Both the total numbers of cells and the number of live cells were determined on days 0, 1, 3, 7, and 10.
TRAP staining for evaluation of osteoclast differentiation
Splenocytes from immunized animals were cultured in the presence or absence of formalin-fixed A. actinomycetemcomi-tans as described above. B cells were isolated from cultured splenocytes by fluorescence-activated cell sorting (FACS) using mouse anti-rat antibodies to B-cell surface markers (PanB/CD45RA, 1 : 1, Serotec, Oxford, UK) followed by FITC-conjugated rat anti-mouse IgG (Jackson Immuno Research). To evaluate tartrate-resistant acid phosphatase (TRAP) staining for osteoclast differentiation, RAW 264.7 cells (ATCC Cat# TIB-71) were cocultured with purified B cells (1.5 × 105 cells/well) in 96-well plates for 3 days as previously described (8). RAW cells cultured with 200 ng/ml murine soluble RANKL (sRANKL, R&D Systems, Minneapolis, MN) served as a positive control. In some experiments, cocultures were performed in the presence of human OPG-Fc at a concentration of 1 µg/ml, to confirm that the observed osteoclastogenesis of RAW cells was mediated through RANKL–RANK interactions. Addition of an irrelevant fusion protein, L6-Fc, at a concentration of 1 µg/ml was used as a control for OPG-Fc. Cells were stained for TRAP using a leukocyte acid phosphatase kit (Sigma) according to the manufacturer’s instructions. TRAP-positive cells with three or more nuclei were counted microscopically and were considered as osteoclasts.
Results
Expression of immune response markers in cultured splenocytes from naïve rats
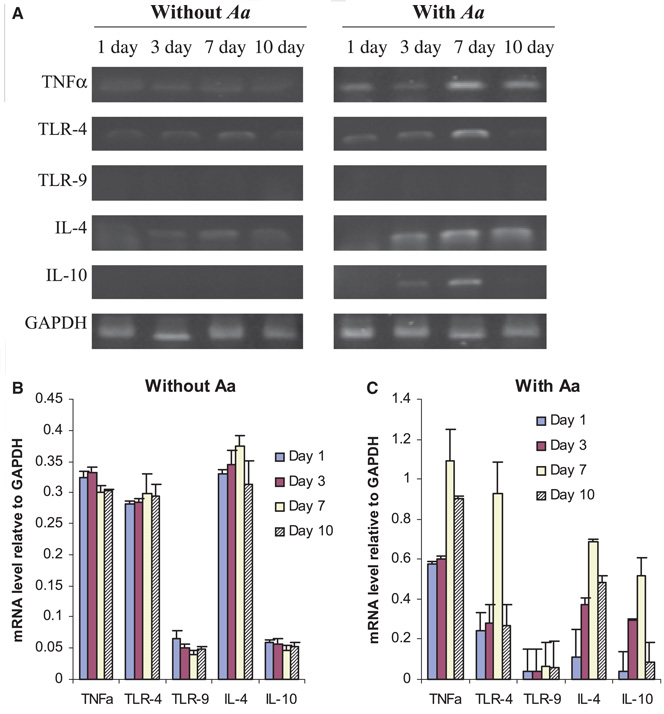
A constitutive level of TNF-α, TLR-4, and IL-4 expression was observed throughout the culture period in the absence of A. actinomycetemcomitans (Fig. 1A,B). Expression of TLR-9 or IL-10 was not detected in the cultured splenocytes in the absence of A. actinomycetemcomitans. However, the messenger RNA (mRNA) transcripts of TNF-α and TLR-4 were immediately increased after cells were cultured with A. actinomycetemcomitans for 1 day. These increases were seen most significantly in cells cultured for 7 days (Fig. 1A,C). Expression of TLR-9 was not detected in splenocytes throughout the culture period in the presence or absence of A. actinomycetemcomitans. The expression levels of IL-4 and IL-10 in the cells were not changed in the presence of A. actinomycetemcomitans until cultured for 3 days or more, and a significant increase of IL-4 and IL-10 expression was observed in cells cultured for 7 days. Furthermore, sustained high levels of both TNF-α and IL-4 expression were observed in cells cultured for 10 days with A. actinomycetemcomitans stimulation. These results suggest that TNF-α and TLR-4 could be associated with the innate aspect of immune response to A. actinomycetemcomitans, while IL-4 and IL-10 are involved in the adaptive immune responses to the bacteria.
Fig. 1.
Expression of molecular markers of innate and/or adaptive response in cultured splenocytes from naïve rats. Splenocytes from non-immunized rats were cultured for the times indicated and total RNA was extracted from cultured cells. Single-strand complementary DNA (cDNA) was synthesized from 0.1 µg total RNA by reverse transcription, and the specific cDNAs for tumor necrosis factor-α (TNF-α), Toll-like receptors 4 and 9 (TLR-4, TLR-9), interleukins 4 and 10 (IL-4, IL-10), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were amplified by polymerase chain reaction (PCR). (A) PCR fragments were resolved on a 1% agarose gel and stained with ethidium bromide. (B, C). Signal intensity was quantified by densitometry and data are presented as mean ± SE of three independent repeats and the respective transcript levels are expressed as ratios relative to GAPDH; Aggregatibacter actinomycetemcomitans (Aa).
Overall RANKL expression by RT-PCR in cultured splenocytes from naïve rats
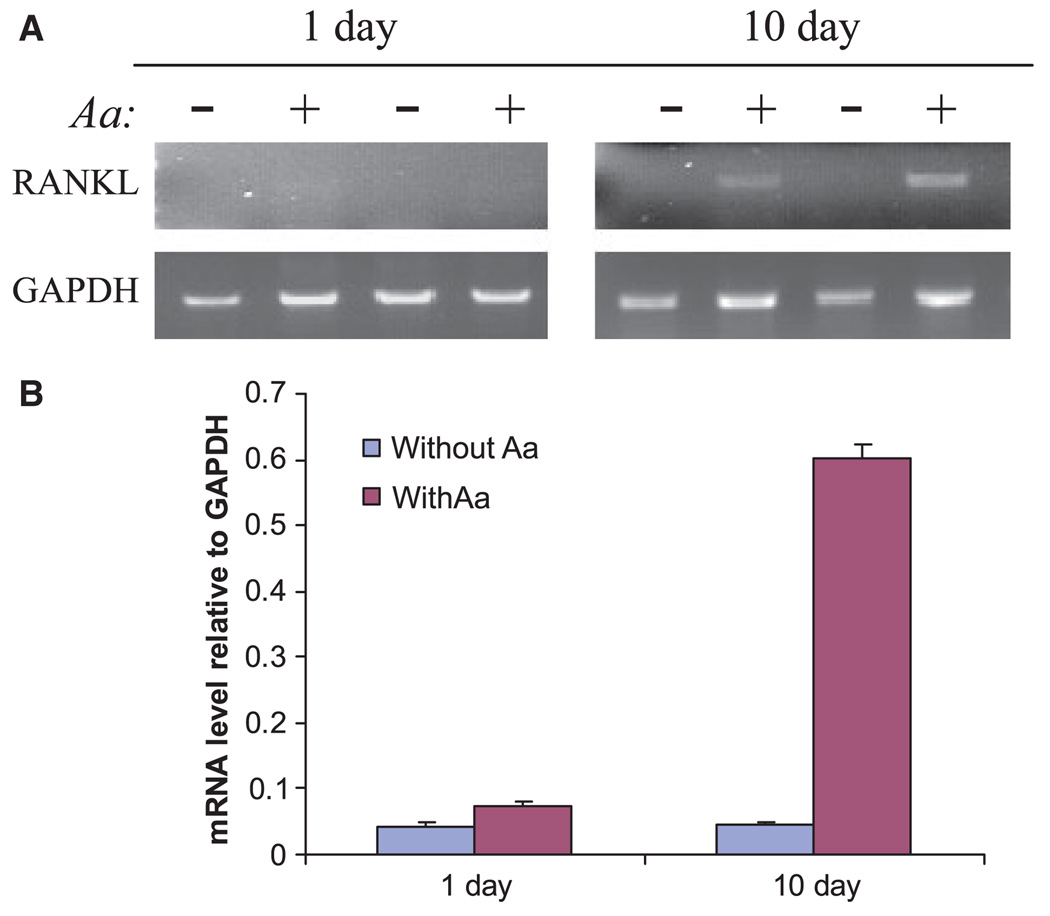
After culture for 1 day, RANKL transcripts were not detectable in the absence of A. actinomycetemcomitans and were barely visible in splenocytes cultured in the presence of A. actinomycetemcomitans for 1 day. However, after culture for 10 days in the presence of A. actinomycetemcomitans, the RANKL transcripts were significantly increased compared with those without A. actinomycetemcomitans stimulation (Fig. 2). These results indicate that RANKL expression is upregulated in B lymphocytes in the late aspect rather than in the earlier aspect of immune responses to A. actinomycetemcomitans bacteria.
Fig. 2.
Receptor activator of nuclear factor-κB ligand (RANKL) messenger RNA expression by reverse transcription–polymerase chain reaction (RT-PCR) in cultured splenocytes from naïve rats. Splenocytes from non-immunized rats were cultured for 1 day or 10 days in the presence or absence of Aggregatibacter actinomycetemcomitans (Aa). After indicated times cultured splenocytes were collected and total RNA was extracted. Single-strand complementary DNA (cDNA) was synthesized from 0.1 µg total RNA by reverse transcription, and the specific cDNAs for RANKL and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were amplified by PCR. (A) PCR fragments were resolved on a 1% agarose gel and stained with ethidium bromide. (B) Signal intensity was quantified by densitometry and data are presented as mean ± SE of three independent duplicates and the respective transcript levels are expressed as ratios relative to GAPDH.
Cell type distribution of cultured splenocytes
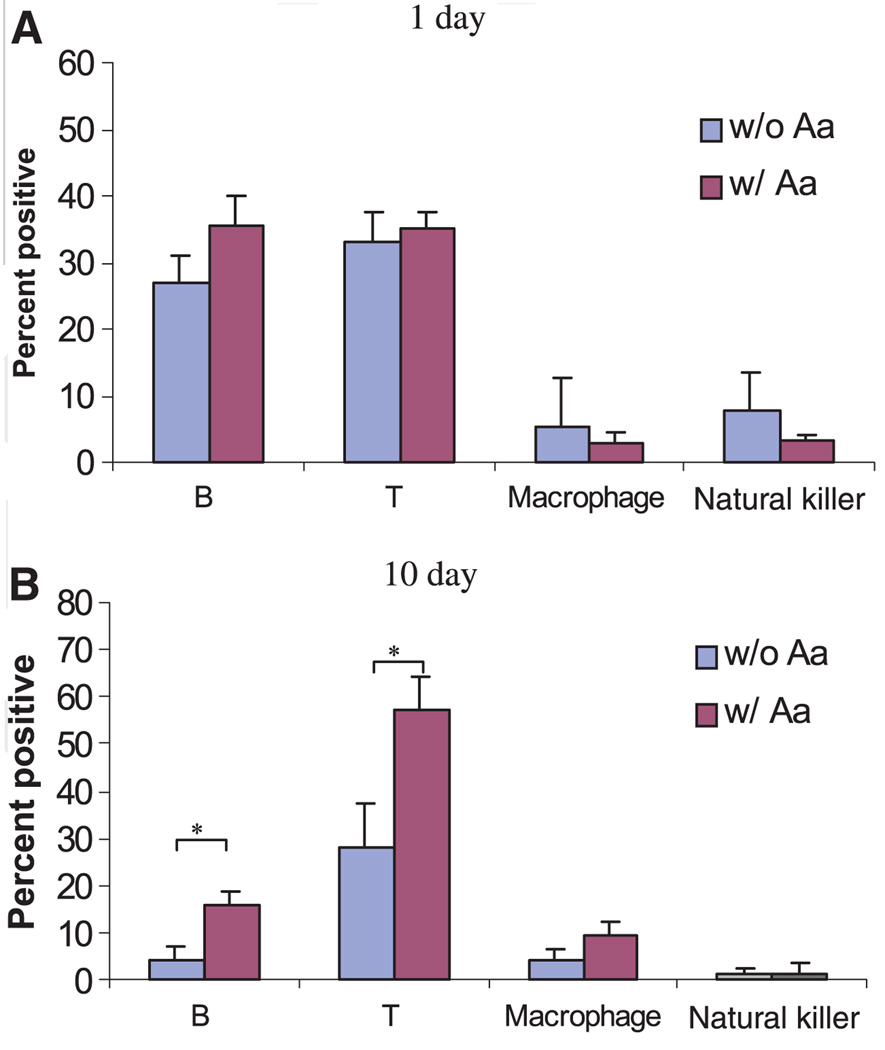
After culture for 1 day, the percentage of B cells, T cells, macrophages, and NK cells did not change in the presence or absence of A. actinomycetemcomitans (Fig. 3A). However, after culture for 10 days, the percentages of B cells and T cells were both significantly increased in the presence of A. actinomycetemcomitans compared with those without A. actinomycetemcomitans stimulation (15.9% vs. 4.2% for B cells; 57.5% vs. 28.2% for T cells, respectively) (Fig. 3B). These data suggest that B and T cells could be major cell populations that respond to A. actinomycetemcomitans antigen challenge in vitro.
Fig. 3.
Cell type distribution of cultured splenocytes from non-immunized rats. Non-immune splenocytes were cultured for (A) 1 day or (B) 10 days in the presence or absence of Aggregatibacter actinomycetemcomitans (Aa). Cells were stained with the mouse anti-rat antibodies against different surface makers: OX33 for B cells, R73 for T cells, ED1 for macrophages, and NK-RP1 for natural killer cells. The cells were then stained with fluorescein isothiocyanate-conjugated rat anti-mouse immunoglobulin G. The percentage of each cell type in cultured splenocytes was determined by flow cytometry. At least 20,000 cells were counted for each sample (n = 4, mean ± SD, *P < 0.05, t-test).
RANKL expression by naïve B cells after A. actinomycetemcomitans challenge
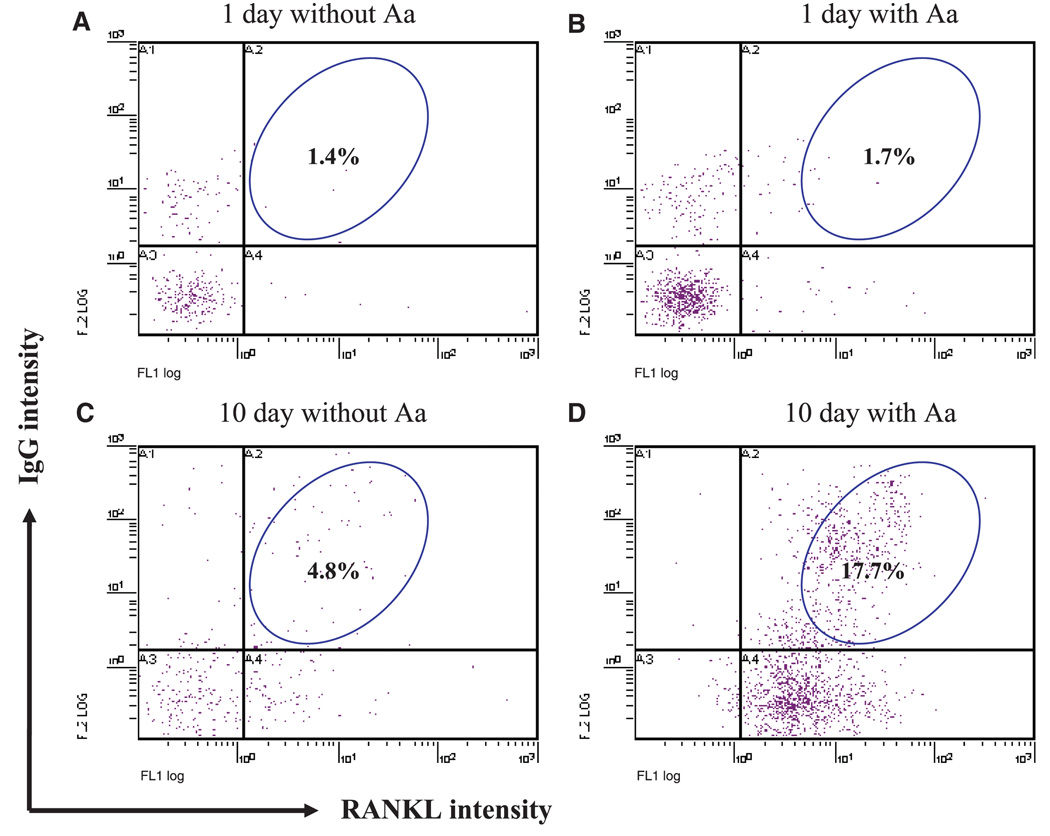
To study the level of RANKL expression in B-cell populations, rat splenocytes were cultured in the presence or absence of A. actinomycetemcomitans for 1 day or 10 days, and RANKL-expressing IgG-positive cells were detected by double staining. Since our preliminary data indicated that the majority of B cells in spleen are IgG-positive (data not shown), we use this subtype to represent the potentially active B-cell population. As shown in Fig. 4, after culture for 1 day the percentage of RANKL-expressing IgG-positive cells demonstrated little change in the absence (1.4%) or presence (1.7%) of A. actinomycetemcomitans (Figs 4A,B). However, the percentage of RANKL-expressing IgG-positive cells were greatly increased after culture for 10 days in the presence of A. actinomycetemcomitans (17.7%), compared with those without A. actinomycetemcomitans stimulation (4.8%) (Figs 4C,D). This suggests that immune B cells (significant increase in IgG intensity) express increased levels of RANKL during late aspects of the response in vitro in the presence of A. actinomycetemcomitans, compared with the early aspect of response. This might be attributed to the B-cell immune response in vitro after stimulation by inflammatory cytokines secreted by antigen-specific T cells.
Fig. 4.
Receptor activator of nuclear factor-κB ligand (RANKL) expression in immunoglobulin G-positive (IgG+) cells. Splenocytes from non-immunized rats were cultured for 1 day or 10 days in the presence or absence of Aggregatibacter actinomycetemcomitans (Aa). Cells were stained with rabbit anti-rat IgG followed by phycoerythrin-conjugated goat anti-rabbit IgG for the detection of IgG+ cells. Cells were also stained with human osteoprotegerin (OPG) -Fc (a fusion protein kindly provided by Dr Colin Dunstan from Amgen Inc., Thousand Oaks, CA) or the irrelavant human peptide L6-Fc (as controls), followed by fluorescein isothiocyanate-conjugated goat anti-human IgG for the detection of RANKL-expressing cells. At least 20,000 cells were counted for each sample.
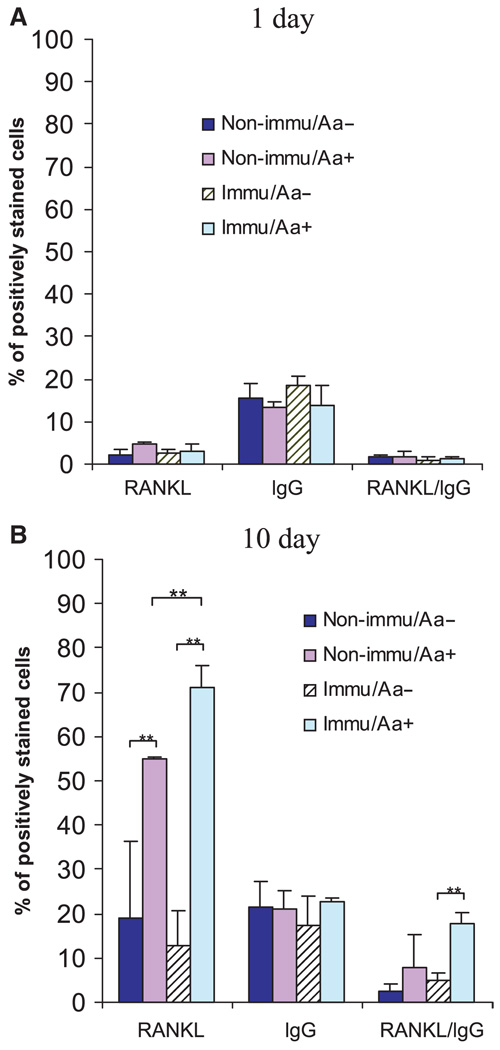
RANKL expression by primed B cells after A. actinomycetemcomitans challenge
To further test this hypothesis, we evaluated the number of RANKL-expressing, IgG-positive cells in the A. actinomycetemcomitans-immunized vs. non-immunized splenocytes (Fig. 5A). At day 1, the overall RANKL expression was low in all tested groups despite the immunization and the presence or absence of A. actinomycetemcomitans in the culture. There were no differences in RANKL-expressing IgG-positive cells in the presence or absence of A. actinomycetemcomitans, and between the non-immunized and immunized groups (Fig. 5A). However, at day 10, the overall RANKL expression in cultured cells was significantly increased in the presence of A. actinomycetemcomitans, as compared with cultured cells in the absence of A. actinomycetemcomitans (Fig. 5B). This increase was observed both in the immunized group (RANKL+ cells: 71% vs. 12%, P < 0.01), and in the non-immunized group (RANKL+ cells: 54% vs. 19%, P < 0.01). More importantly, cells from the immunized group showed significantly increased levels of RANKL expression compared with cells from the non-immunized group (71% vs. 54%, P < 0.01) when they were cultured in the presence of A. actinomycetemcomitans (Fig. 5B). Therefore, the adaptive response to immunization and boost are involved in major upregulation of RANKL expression. To focus on B cells, we evaluated RANKL-expressing IgG-positive cells. RANKL-expressing IgG-positive cells were significantly increased, more than fourfold, in the immunized group (from 4% to 17%, P < 0.01). Interestingly, the percentage of IgG-positive cells was unchanged through-out the different groups tested on either day 1 or day 10, suggesting that the significant increase of RANKL-expressing B cells was a result of specific antigen-driven RANKL expression by B cells, not a relative increase of the B-cell percentage.
Fig. 5.
Receptor activator of nuclear factor-κB ligand (RANKL) -expressing immunoglobulin G-positive (IgG+) cells in immunized vs. non-immunized animals. The same experiments as described in Fig. 3 were repeated on animals immunized intraperitoneally with Aggregatibacter actinomycetemcomitans (Aa) or with phosphate-buffered saline (as non-immunized controls). Splenocytes from these rats were cultured for (A) 1 day or (B) 10 days in the presence or absence of A. actinomycetemcomitans. Cells were then double-stained for the detection of RANKL-expressing IgG+ cells. At least 20,000 cells were counted for each sample (n = 4, mean ± SD, **P < 0.01, t-test).
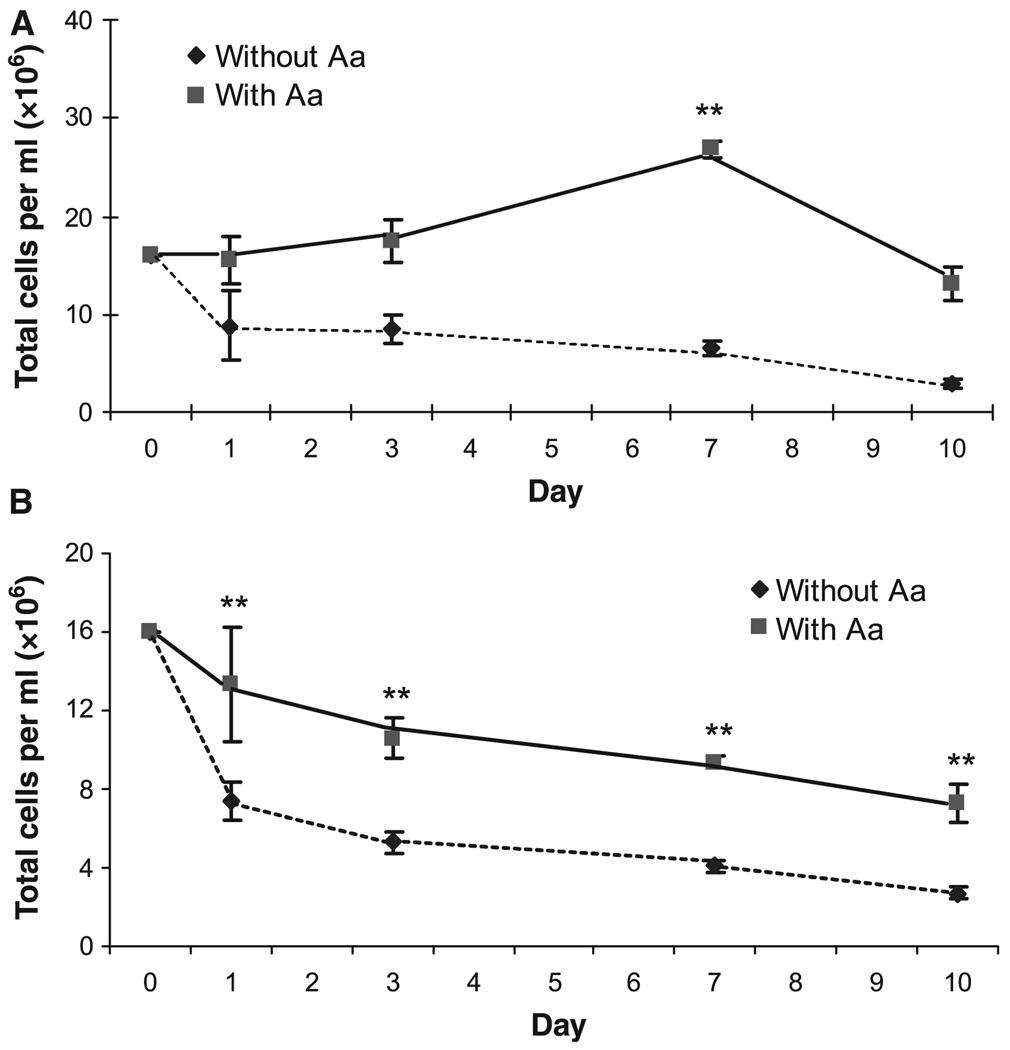
Cell viability of cultured splenocytes in the presence or absence of A. actinomycetemcomitans
To determine the effect of A. actinomycetemcomitans on cell proliferation and survival, cultured cells were enumerated after staining with the DNA-intercalating fluorescent dyes acridine orange and ethidium bromide (26). Total cell concentration (live and dead) was gradually decreased in the cultures in the absence of A. actinomycetemcomitans stimulation, indicating a higher rate of cell death than cell proliferation (Fig. 6A). In the presence of A. actinomycetemcomitans, the cell concentration remained unchanged during the first 3 days of culture, but was greatly increased at day 7 (P < 0.01, compared with day 0), indicating, transiently, an augmented rate of cell proliferation as opposed to cell death (Fig. 6A). Live cell concentrations were also determined to ascertain viability conditions in the presence or absence of A. actinomycetemcomitans. Although both conditions showed a gradual decrease in concentration of live cells, the number of cells cultured with A. actinomycetemcomitans was significantly higher compared with the number of cells cultured without A. actinomycetemcomitans at each time-point, probably because of the relatively greater level of proliferation (Fig. 6B). Taken together with the significant increase in RANKL expression on A. actinomycetemcomitans-cultured cells (Fig. 5B), these results suggested that there is a greatly increased rate of survival of B cells that are activated and express more RANKL. This enhanced viability/survival may lead to increased RANKL+ cells that can participate in bone resorption related to bacterial infection.
Fig. 6.
Cell viability after exposure to Aggregatibacter actinomycetemcomitans. Splenocytes were cultured for the indicated time in the presence or absence of A. actinomycetemcomitans (Aa). Cultured cells were then harvested and stained with 40 µg/ml acridine orange and 100 µg/ml ethidium bromide, and visualized by fluorescence microscopy using an inverted microscope. Each experiment was performed in triplicates and the results were presented as mean ± SD (**P < 0.01, t-test).
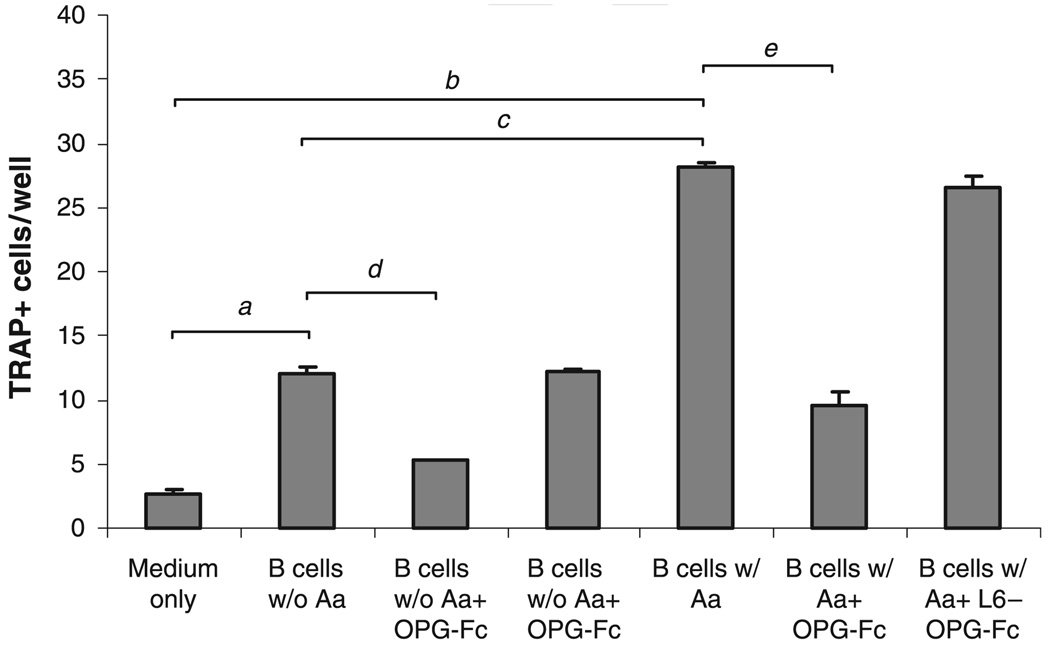
Induction of osteoclast differentiation by B cells
The TRAP activity assay was performed to test the FACS-purified B cells for the ability to induce osteoclast differentiation. Osteoclast precursor cells (RAW 264.7 cells) demonstrated significantly increased TRAP+ multinucleated cells when cocultured with purified B cells as compared with medium-only controls (a,bP < 0.01, Fig. 7). Cocultures of RAW cells with purified B cells from splenocytes cultured with A. actinomycetemcomitans exhibited a significantly larger number of multinucleated TRAP+ cell formation compared with B cells not previously cultured with A. actinomycetemcomitans (cP < 0.001, Fig. 7). Addition of human OPG-Fc into the culture significantly diminished such increases (d,eP < 0.01, Fig. 7), suggesting that the observed induction of osteoclastogenesis of RAW cells is RANKL-dependent. No such inhibitory effect was observed in RAW cells cocultured with purified B cells in the presence of L6-Fc.
Fig. 7.
Induction of osteoclast differentiation by purified B cells. Splenocytes from immunized animals (n = 3) were cultured in the presence or absence of formalin-fixed Aggregatibacter actinomycetemcomitans as described in the Materials and methods. B cells were purified from cultured splenocytes by fluorescence-activated cell sorting (FACS). RAW 264.7 cells were cocultured with purified B cells for 3 days. For some experiments, cocultures were performed in the presence or absence of human osteoprotegerin (OPG)-Fc at a concentration of 1 µg/ml. An irrelevant fusion protein, L6-Fc, was used as control. For tartrate-resistant acid phosphatase (TRAP) staining, cells were stained using a Leukocyte Acid Phosphatase kit (Sigma) and multinucleated TRAP-positive cells were counted. Each experiment was performed in triplicate and the results were presented as mean ± SE (a,b,d,eP < 0.01, cP < 0.001, t-test).
Discussion
It has been suggested that alveolar bone resorption in periodontal disease is mediated by enhanced immune-cell-induced osteoclastogenesis (27). RANKL is involved in both physiological osteoclastogenesis and pathological bone loss (28, 29). Recent findings indicated that B cells are key participants in RANKL-mediated bone resorption (8, 30). Our current results show that the percentage of B cells and T cells in cultured naïve splenocytes are significantly increased in the presence of the antigen A. actinomycetemcomitans (Fig. 3B). Meanwhile, the level of RANKL mRNA was also upregulated in the cultured splenocytes after A. actinomycetemcomitans antigenic stimulation. These data suggest that B and T cells are major cell populations that respond to A. actinomycetemcomitans antigen challenge in vitro and that these cells have an increased level of RANKL expression after stimulation/restimulation with antigen. Our previous studies have demonstrated that T lymphocytes specific to A. actinomycetemcomitans can modulate periodontal bone resorption in A. actinomycetemcomitans-infected rats through upregulation of RANKL production (21, 22). The present study and our more recent data (8) support the previous observation and further suggest that B cells can be another abundant source of RANKL with marked potential to induce bone resorption during periodontal infection.
Our results indicated that the transcript levels of TNF-α and TLR-4 (indicative of an innate response) were significantly upregulated within 1 day of the cells being exposed to A. actinomycetemcomitans, whereas the expression levels of IL-4 and IL-10 in the cells were not increased in the presence of A. actinomycetemcomitans until after 3–7 days of culture (Fig. 1). These observations confirmed that TNF-α and TLR-4 could be actively involved in the innate immune response to A. actinomycetemcomitans, while IL-4 and IL-10 cytokines can be associated with adaptive immunity. However, after culture for 1 day, there were no changes in the percentage of RANKL-expressing IgG-positive cells in cultured splenocytes. Furthermore, the percentage of RANKL-expressing IgG-positive cells in cultured splenocytes was significantly increased after culture for 10 days in the presence of A. actinomycetemcomitans, and the increase was much greater in the primed, immunized animals than in the non-immunized animals. These data suggest that RANKL expression is upregulated in B lymphocytes in the adaptive immune response rather than in the innate immune response to A. actinomycetemcomitans bacteria, and preimmunization of animals with A. actinomycetemcomitans leads to an enhanced B-cell response including increased RANKL expression. Previous findings indicated that the in vitro innate immune response itself is not sufficient to markedly stimulate RANKL expression by immune cells (31). However, it is very clear that adaptive immune response give rise to abundant RANKL expression and potential for induction of bone resorption (21–23). This suggests that B cells primed by immunization could undergo apoptosis during culture in the absence of antigen, or be stimulated by specific antigen (A. actinomycetemcomitans) whereupon they manifest greatly enhanced RANKL expression. We conclude that B lymphocytes have considerable potential to contribute to increased periodontal bone resorption during the adaptive immune response to A. actinomycetemcomitans in periodontal disease and that this effect could be associated with the upregulation of RANKL expression. This is particularly significant in light of our recent findings that more than 90% of B cells recovered from human periodontal diseased tissues express RANKL, as opposed to about 54% of T cells (7). The combined effects of A. actinomycetemcomitans activation to enhance RANKL expression and cell turnover therefore combine to increase the potential for periodontal bone resorption. Further studies are warranted to determine the nature of T-cell and B-cell immune responses to oral bacteria and the contributions of RANKL-expressing, antigen-specific B lymphocytes to periodontal bone resorption.
Acknowledgments
Human osteoprotegerin-Fc fusion protein was kindly provided by Dr Colin Dunstan from Amgen Inc., Thousand Oaks, CA. This work was supported by National Institutes of Health grant DE-03420 from the National Institute of Dental and Craniofacial Research. Dr Xiaoping Lin was partially supported by Shengjing Hospital, China Medical University.
References
- 1.Taubman MA, Yoshie H, Ebersole JL, Smith DJ, Olson CL. Host response in experimental periodontal disease. J Dent Res. 1984;63:455–460. doi: 10.1177/00220345840630031801. [DOI] [PubMed] [Google Scholar]
- 2.Stoufi ED, Taubman MA, Ebersole JL, Smith DJ, Stashenko PP. Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. J Clin Immunol. 1987;7:235–245. doi: 10.1007/BF00915729. [DOI] [PubMed] [Google Scholar]
- 3.Seymour GJ, Powell RN, Cole KL, et al. Experimental gingivitis in humans. A histochemical and immunological characterization of the lymphoid cell subpopulations. J Periodontal Res. 1983;18:375–385. doi: 10.1111/j.1600-0765.1983.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 4.Malberg K, Molle A, Streuer D, Gangler P. Determination of lymphocyte populations and subpopulations extracted from chronically inflamed human periodontal tissues. J Clin Periodontol. 1992;19:155–158. doi: 10.1111/j.1600-051x.1992.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 5.Tew J, Engel D, Mangan D. Polyclonal B-cell activation in periodontitis. J Periodontal Res. 1989;24:225–241. doi: 10.1111/j.1600-0765.1989.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 6.Katz J, Michalek SM. Effect of immune T cells derived from mucosal or systemic tissue on host responses to Porphyromonas gingivalis. Oral Microbiol Immunol. 1998;13:73–80. doi: 10.1111/j.1399-302x.1998.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Matsuyama T, Hosokawa Y, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006;176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 9.Han X, Kawai T, Taubman MA. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol 2000. 2007;45:76–94. doi: 10.1111/j.1600-0757.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 10.Motoyoshi K. Biological activities and clinical application of M-CSF. Int J Hematol. 1998;67:109–122. doi: 10.1016/s0925-5710(98)00010-3. [DOI] [PubMed] [Google Scholar]
- 11.Udagawa N, Takahashi N, Jimi E, et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone. 1999;25:517–523. doi: 10.1016/s8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 12.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 13.Lorenzo J, Choi Y. Osteoimmunology. Immunol Rev. 2005;208:5–6. doi: 10.1111/j.0105-2896.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 14.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 15.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 16.Kim KW, Cho ML, Lee SH, et al. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol Lett. 2007;110:54–64. doi: 10.1016/j.imlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Ukai T, Yumoto H, Gibson FC, III, Genco CA. Macrophage-elicited osteoclastogenesis in response to bacterial stimulation requires Toll-like receptor 2-dependent tumor necrosis factor-alpha production. Infect Immun. 2008;76:812–819. doi: 10.1128/IAI.01241-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Shimauchi H, Eastcott JW, Smith DJ, Taubman MA. Antigen direction of specific T-cell clones into gingival tissues. Immunology. 1998;93:11–19. doi: 10.1046/j.1365-2567.1998.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–2109. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 21.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001;12:125–135. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 22.Valverde P, Kawai T, Taubman MA. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Miner Res. 2004;19:155–164. doi: 10.1359/JBMR.0301213. [DOI] [PubMed] [Google Scholar]
- 23.Teng YT, Nguyen H, Gao X, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–R67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taubman MA, Kawai T, Han X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J Clin Periodontol. 2007;34:367–369. doi: 10.1111/j.1600-051X.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita K, Eastcott JW, Taubman MA, Smith DJ, Cox DS. Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect Immun. 1991;59:1529–1534. doi: 10.1128/iai.59.4.1529-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay D, Sundereshan S, Rao C, Karande AA. Placental protein 14 induces apoptosis in T cells but not in monocytes. J Biol Chem. 2001;276:28268–28273. doi: 10.1074/jbc.M010487200. [DOI] [PubMed] [Google Scholar]
- 27.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 29.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 30.Choi Y, Woo KM, Ko SH, et al. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8(+) T cells. Eur J Immunol. 2001;31:2179–2188. doi: 10.1002/1521-4141(200107)31:7<2179::aid-immu2179>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 31.Alnaeeli M, Penninger JM, Teng YT. Immune interactions with CD4 + T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006;177:3314–3326. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]