Abstract
A vaccine is likely the most effective strategy for controlling human chlamydial infections. Recent studies have shown immunization with Chlamydia muridarum major outer membrane protein (MOMP) can induce significant protection against infection and disease in mice if its native trimeric structure is preserved (nMOMP). The objective of this study was to investigate the immunogenicity and vaccine efficacy of Chlamydia trachomatis nMOMP in a non-human primate trachoma model. Cynomolgus monkeys (Macaca fascicularis) were immunized systemically with nMOMP and monkeys were challenged ocularly. Immunization induced high serum IgG and IgA ELISA antibody titers, with antibodies displaying high strain-specific neutralizing activity. The PBMC of immunized monkeys produced a broadly cross-reactive, antigen-specific IFN-γ response equivalent to that induced by experimental infection. Immunized monkeys exhibited a highly significant decrease in infectious burden during the early peak shedding periods (days 3-14). However, at later time points they exhibited no difference from control animals in either burden or duration of infection. Immunization had no effect on the progression of ocular disease. These results show that systemically administered nMOMP is highly immunogenic in non-human primates and elicits partially protective immunity against ocular chlamydial challenge. This is the first time a subunit vaccine has shown a marked, significant reduction in ocular shedding in non-human primates. A partially protective vaccine, particularly one that significantly reduces infectious burden following primary infection of children, could interrupt the natural trachoma re-infection cycle. This could have a beneficial effect on the transmission between children and sensitized adults which drives blinding inflammatory disease.
Keywords: Vaccination, Mucosa, Bacterial, Antigens/Peptides/Epitopes, Other Animals
Introduction
Trachoma, a chronic ocular disease caused by Chlamydia trachomatis, is a leading cause of preventable blindness (1). The disease has largely disappeared from Europe and North America, but it continues to be hyperendemic in many of the poorest areas of Africa, Asia, Australia, and the Middle East. Trichiasis is a painful sequela of trachoma where, due to conjunctival scarring caused by chlamydial infection, eyelashes turn inward to touch the cornea. This condition leads to decreased visual acuity, and eventually blindness, through corneal abrasion. The World Health Organization has proposed a four-element approach to eliminate trachoma as a cause of incident blindness, called the SAFE strategy (2). The acronym stands for Surgery to correct trichiasis, Antibiotics to treat infection, and Facial cleanliness and Environmental improvements to reduce chlamydiae transmission. In humans, infection is asymptomatic in many individuals — thus treating only those with clinical symptoms will not control the spread of infection. A vaccination program would have greater impact on decreasing the prevalence of blinding trachoma.
C. trachomatis is also the causative agent of the most common bacterial sexually transmitted disease (STD) and lymphogranuloma venereum (LGV). C. trachomatis strains are divided into 15 serogroups (serovars), of which A-C cause trachoma, D-K cause genital STDs and L1-L3 cause LGV (3-6). Chlamydiae are obligate intracellular parasites exhibiting a unique biphasic life cycle. The infectious, but metabolically inactive, elementary bodies (EBs) attach to cells and enter to form intracellular inclusions. Inside cells, EBs transform into non-infectious, metabolically active reticulate bodies (RB) which divide by binary fission and eventually convert back to EBs (7). During the extracellular EB stage, antibodies present in genital tract or ocular secretions can inhibit infection both in vivo and in tissue culture (8-10). However, the RB, residing within the intracellular inclusion, remains inaccessible to antibodies. Resolution of infection at this stage requires a cell-mediated immune response likely controlled by IFN-γ secreting Th1 cells. Thus, an ideal C. trachomatis vaccine should induce both local neutralizing antibodies to prevent infection by EBs, and a strong Th1 response to limit infection once it is initiated. The bacteria’s intracellular lifestyle, where it resides in a well-protected inclusion, makes the production of either an effective natural or artificial immune response difficult.
Development of a vaccine against C. trachomatis is a high priority. Computer modeling has indicated that even a partially protective vaccine would substantially reduce infections worldwide (11, 12). Efforts to create a vaccine have been unsuccessful to date. In fact, humans vaccinated with killed EBs present more severe disease than non-vaccinated individuals following naturally acquired infection (13-15). This suggests dead intact chlamydiae harbor immunopathogenic components, thus arguing against the use of either inactivated or live-attenuated vaccines. Hence the major effort in the development of a chlamydial vaccine has focused on subunit immunogens capable of evoking protective immunity without sensitization to damaging immunopathogenic antigens.
The major outer membrane protein (MOMP) is regarded as one of the most promising subunit vaccine candidates. Highly immunogenic and immunoaccessible, it elicits both neutralizing antibodies and T cell immunity (10, 16-21). MOMP is the dominant surface protein (contributing to 60% of the total protein mass in the outer membrane) and consists of four variable domains interspersed between five constant domains (22, 23). The four variable domains contain serovar-specific epitopes the five constant domains are highly conserved between the different serovars and contain several conserved CD4 and CD8 T cell epitopes (24-26). MOMP has been used in several vaccine studies, together with various adjuvants and delivery systems. Still, attempts to induce protection using MOMP, MOMP peptides, or plasmids expressing MOMP yielded disappointing results, both in small animal models (27-32) and cynomolgus monkeys (33, 34). These studies demonstrated either no protection or limited protection against C. trachomatis infectious challenge.
An important exception is the recent study by Pal et al. (35) that showed systemic immunizations with MOMP purified in native conformation (nMOMP) induced protection against genital challenge in the murine model. The protective immune response, as measured by post-challenge infectious burden, duration of shedding, and disease (infertility), was equal to that induced by experimental infection. Currently, this remains the most successful attempt of using a chlamydial subunit vaccine to mimic natural immunity. Because of these very encouraging results, we have extended these studies to non-human primates. Here we describe the immunogenicity of nMOMP sub-unit vaccination and the resulting partially protective immunity achieved in the non-human primate ocular trachoma model.
Materials and Methods
Chlamydia trachomatis Strains
Chlamydia trachomatis serovar A strain A2947 (A2497), serovar A strain A/HAR-13 (A/HAR-13), serovar B strain B/TW-5/OT (B), serovar Ba strain Ba/AP-2/OT (Ba) and serovar C strain C/TW-3/OT (C) were grown in HeLa 229 cells with DMEM (Mediatech, Inc.) containing 10% (v/v) fetal calf serum, 4.5 g/L glucose, 2 mM glutamine, 10 mM HEPES, 1mM sodium pyruvate, 55 M β-mercaptoethanol and 10 μg/ml gentamicin. Density gradient purified EBs were stored in 0.2 M sucrose, 20 mM sodium phosphate and 5 mM glutamic acid buffer (SPG) at -80°C.
Non-human Primates
Six healthy adult male cynomolgus macaques (Macaca fascicularis) maintained in the non-human primate wing at Rocky Mountain Laboratories (Hamilton, MT) and cared for under standard practices implemented by the Rocky Mountain Veterinary Branch, were used for all clinical procedures. Once entered into experiments, animals were housed in single cages. All clinical and handling procedures were reviewed and affirmed by the Animal Care and Use Committee at Rocky Mountain Laboratories and work was conducted in full compliance with the “Guide for Care and Use of Laboratory Animals,” as well as all applicable federal laws and regulations. The facilities are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Vaccination
nMOMP was prepared from A2497 as previously described by Pal et al (35). Three anesthetized macaques (ketamine hydrochloride: 1 mg/kg body weight) received 200 μg of nMOMP divided equally between subcutaneous injections on both sides of the shaved neck and intramuscular injections into the shaved right and left triceps. Injections contained a 3:7 ratio of CpG ODN-2395 (1mg; Coley Pharmaceutical Group) and nMOMP to Montanide ISA 720 (Seppic, Inc.) in a total volume of 1.4 ml per animal (0.35 ml per injection site). Three other macaques received 200 μg of ovalbumin in a similar fashion. Vaccinated and control animals were boosted twice at 71 and 141 days after vaccination (DAV) using the same routes and doses.
Challenge of Vaccinated Monkeys
All six macaques were challenged at 175 DAV with 1×104 A2497 EBs in 20 μl of SPG, placed under the protracted upper and lower eyelids of each eye (2×104 EBs per eye). Following inoculation, upper lids of closed eyes were briefly rotated with sterile forceps to ensure complete coverage of the inoculum, and infected animals were returned to their respective cages.
Culture and Disease Evaluation
Three and 7 days after challenge, and weekly until clearance of disease, swabs from each eye of every animal were cultured on HeLa 229 cells in 96-well microtiter plates. Sterile type 1 calgiswabs (Puritan Medical Products Co.) were pressed onto the inner surface of the upper and lower lid of each eye of anesthetized animals and passed back and forth 8-10 times. Swabs were placed into 2 ml microfuge tubes containing 0.5 ml SPG and 3 sterile glass beads, mixed on an Eppendorf Thermomixer (Brinkmann Instruments, Inc.) for 2 min at 1,400 rpm and 4°C, and titered for recoverable inclusion forming units (IFUs).
Before each swab collection, monkeys were scored for hyperemia and follicle formation on the upper conjunctival surfaces in both eyes, as described by Taylor et al (36). Hyperemia was scored as follows: 0, no hyperemia; 1, mild hyperemia; and 2, severe hyperemia. Follicles were scored as follows: 0, no follicles; 1, one to three follicles; 2, four to ten follicles; 3, more than ten follicles; and 4, follicles too numerous to count. The scores recorded for the upper conjunctival surfaces of both eyes were added for each animal and termed the “clinical response score.” The highest possible score was 12. Statistical analyses of the clinical response and recoverable IFUs were done by the statistical language R and employed the non-parametric Wilcoxon rank sum test. Differences were considered significant at P < 0.05.
Coomassie and Immunoblot Analysis
Purified MOMP was loaded onto 10% polyacrylamide gels to view via Coomassie staining (500 ng/lane) or Western blot analysis (100 ng/lane). To view MOMP trimers, samples were kept below 37°C, while MOMP monomers were viewed by boiling the sample for 5 minutes. GelCode Blue Stain Reagent (Thermo Scientific) was used to stain proteins per manufacturer’s specifications. For immunoblot analysis, proteins were transferred to 0.2 μm nitrocellulose membranes (BioRad) and blocked at room temperature (RT) for 30 minutes. The membranes were rinsed three times for 5 minutes in hybridization buffer (3% BSA; 1X PBS, pH 7.3; 0.05% Tween-20; 0.02% NaN3). Primary antibodies were then added to aliquots of hybridization buffer at a 1:1000 dilution and incubated at RT overnight. The primary antibody solution was removed and the membranes were rinsed two times in wash buffer (3% BSA; 1X PBS, pH 7.3; 0.05% Tween-20) followed by an extended 1 hr wash. Secondary antibody (goat anti-monkey IgG-HRP conjugated or rabbit anti-mouse IgG-HRP conjugated antibody [MP Biomedicals]) was diluted 1:1000 in wash buffer and membranes were incubated at RT for an additional 2 hrs. Membranes were then washed three times in 1X PBS, pH 7.3; 0.05% Tween-20, twice in 1X PBS, and once in H2O. Finally, membranes were treated with Lumi-LightPLUS Western Blotting Substrate (Roche) following manufacturer’s specifications, and antibody-MOMP complexes were visualized via a Typhoon Variable Mode Imager (GE Healthcare).
Cytokine Analysis
Levels of IL-4, IL-10, IL-12p70, IFN-γ and TNF-α were determined from culture supernatants of non-human primate PBMC stimulated with UV-killed EBs of A2497, B and C. Blood was collected from each anesthetized animal into a sterile heparinized cell preparation tube (Becton, Dickinson and Co.) and processed according to the manufacturer’s instructions. Following hypotonic lysis of remaining red blood cells, collected PBMC were washed twice in PBS, counted, and adjusted to 5×106 cells per ml in DMEM culture medium. Two hundred microliters of each cell suspension was then placed into flat-bottom microtiter plate wells in triplicate, and either 50 μl of killed EBs at a total protein concentration of 50 μg/ml or 50 μl of SPG, was added to test or control wells, respectively. Plates were rocked at 37°C for 1 hr, then further incubated at 37°C for 72 hr. Following incubation, 130 μl of culture supernatant was collected from each well, placed into polypropylene microtiter plates, sealed, frozen at -20°C, and sent for multiplex cytokine analysis at LINCO Diagnostic Services, Inc. (St. Charles, MO 63304).
Serum and Tear Collection
Serum was obtained from venous blood and tears were collected by absorbing onto autoclaved and dried 5 × 20 mm filter strips (Whatman No. 41). Each strip was placed under the lower eyelid and allowed to saturate with tears. Saturated strips were then placed into microfuge tubes containing 0.2 ml of PBS, refrigerated overnight, and frozen until assayed.
ELISA
Serum and tear IgG and IgA antibody titers for each animal were determined by ELISA. Two-fold dilutions of serum or tears (100 μl/well) were added to the wells of Immulon 2 HB flat-bottom 96-well plates (ThermoLabsystems) coated with formalin-fixed A2497, A/HAR-13, Ba or C EBs (1 μg of total protein per well). Following 37°C incubation for 2 hr, plates were washed 3 times and 100 μl of biotin-conjugated goat anti-monkey IgA or IgG (Rockland Immunochemicals) (1:60,000 and 1:5,000, respectively) was added to appropriate wells for another 1 hr incubation at 37°C. Plates were then washed, incubated with 100 μl/well of streptavidin (1:5,000), washed, and exposed to 100 μl of p-Nitrophenyl Phosphate (Invitrogen) for 30 min in the dark at RT. Reactions were stopped with 50 μl of 1N NaOH per well and analyzed on a plate reader at 405 nm.
In Vitro Serum Neutralization Assays
C. trachomatis neutralization assays were performed in HAK cells as previously described (37). Briefly, 1×106 EBs per ml was added to two-fold dilutions of pre-immune and immune sera from vaccinated macaques and incubated for 1 hr at 37°C in microfuge tubes placed on a rotator. Two hundred microliters of each dilution was then inoculated (in triplicate) onto 4×105 HAK cells per well in a 24-well plate and incubated with rocking for 2 hr at 37°C. Inocula were then removed, and monolayers were fed with DMEM containing 1 μg/ml of cycloheximide and incubated at 37°C for 36-42 hr. Following methanol fixation, monolayers were stained with a chlamydial-specific mAb (A-20) and counted for IFUs. Percent specific neutralization for each dilution was calculated as (preimmune IFUs — immune IFUs)/preimmune IFUs × 100.
Results
Immunized Monkey Sera Recognizes both Trimeric and Monomeric MOMP Under Western Blot Analysis
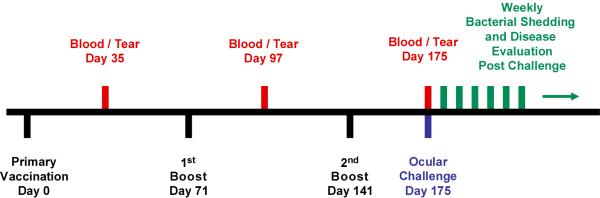
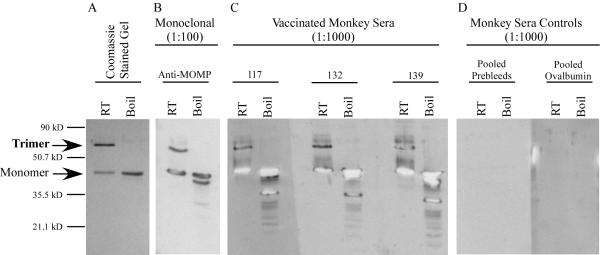
A schematic diagram showing the immunization and challenge schedule of cynomolgus monkeys is shown in Figure 1. Initially we analyzed the serum antibody response by Western blotting under conditions that detect both trimeric nMOMP (65 kDa) and monomeric MOMP (42 kDa). Coomassie staining revealed a strong trimeric nMOMP band and a less abundant 42 kDa monomeric MOMP band. No other protein bands were detected. After boiling, only the monomeric form of MOMP was found (Fig. 2A). Western blots were then performed using sera from immunized and control animals. Sera from all three nMOMP immunized monkeys reacted with both trimeric and monomeric MOMP (Fig. 2C), while sera from both the OVA immunized animals and pre-bleed controls were negative (Fig. 2D). A serovar A-specific mAb (A-20) was used as a positive control. The A-20 antibody recognized both trimeric and monomeric MOMP (Fig. 2B).
FIGURE 1. Immunization and infectious challenge schedule for nMOMP vaccinated monkeys.
Three cynomolgus monkeys were immunized with nMOMP three times. The immunizations were a combination of intramuscular and subcutaneous injections. Three other monkeys were immunized with OVA in a similar fashion. Approximately one month after each immunization, blood and tear samples were collected to monitor the immune response. Thirty-four days after the second boost, all animals were challenged with serovar A strain A2497. Chlamydial shedding and gross clinical response were monitored weekly after challenge.
FIGURE 2. Immunized monkeys recognize both trimeric and monomeric MOMP by western blot analysis.
nMOMP was purified from C. trachomatis serovar A strain A2497 infected cells. The purity of the immunogen and its heat-labile trimeric state were evaluated by SDS-PAGE followed by Coomassie staining or immunoblotting with or without boiling. (A) Coomassie staining of nMOMP. The trimeric and the monomeric forms are indicated by arrows on the left. (B) Control immunoblot of a Serovar A specific monoclonal antibody (A-20). (C) Immunoblot using the three immunized monkey sera. (D) Immunoblot using pools of OVA and pre-bleed controls.
Kinetics and Specificity of the Serum ELISA Antibody Responses Against Different Trachoma Serovars
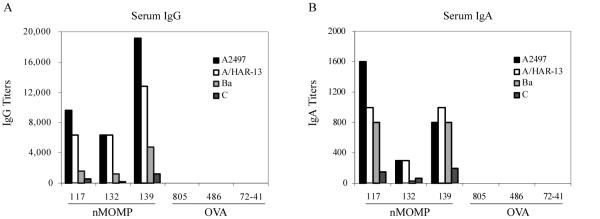
The serum and tear antibody responses of the immunized monkeys were measured by ELISA using formalin-fixed A2497 EBs. Low serum IgG and IgA titers were detected post-primary immunization, but reasonably high titers were measured after the first and second boosts (Fig. 3A and B). Tear IgG antibodies were of very low titer and tear IgA antibodies were not detectable (data not shown).
FIGURE 3. Kinetics of the serum ELISA IgG and IgA titers against serovar A EB (A2497).
The serum antibody responses of the immunized monkeys were measured by ELISA using formalin-fixed EBs (A2497). (A) Serum IgG and (B) Serum IgA. ELISA titers were measured approximately 30 days after the primary vaccination and the first and second boosts.
The trachoma strain specificity of the serum antibody response was also evaluated by ELISA using EBs of four different trachoma strains as coating antigens. For both IgG and IgA, the highest titers were measured against the serovar A strains (A2497 and A/HAR-13), followed by the serovar Ba and C strains (Fig. 4A and B).
FIGURE 4. Titer and specificity of the serum ELISA antibody responses for different trachoma serovars.
The trachoma strain specificity of the serum antibody response was evaluated by ELISA using EBs of four different strains. (A) Serum IgG and (B) Serum IgA. ELISA titers were measured 34 days after the second boost.
Titer and Specificity of the Serum Neutralizing Antibody Responses Against Different Trachoma Serovars
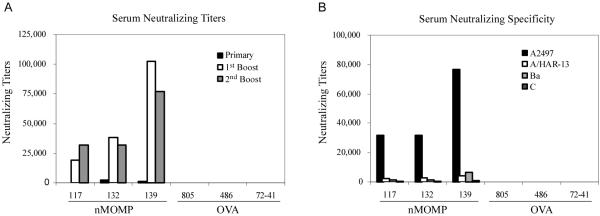
We next assayed the serum of immunized monkeys for neutralizing antibodies. Interestingly, the sera of all three immunized monkeys revealed high neutralizing titers following immunization (1:20,000 -1:100,000) (Fig. 5A). The trachoma strain specificity of these neutralizing titers was also evaluated. Surprisingly, and unlike the ELISA antibody titers, these experiments showed highly specific neutralizing activity against strain A2497 (Fig. 5B), the strain from which nMOMP was prepared. Neutralizing titers against the other three trachoma strains, including the other serovar A strain (A/HAR-13), were relatively low (1:2,400 – 1:4,000) compared to the homotypic titers.
FIGURE 5. Titer and specificity of the serum neutralizing antibody responses for different trachoma serovars.
The infection neutralization capability of the serum antibodies was measured in an in vitro neutralization assay in HAK cells. (A) The kinetics of the neutralization titers were measured approximately 30 days after the primary vaccination and the first and second boosts using A2497. (B) The trachoma strain specificity of these neutralizing titers was measured 34 days after the second boost.
Cytokine Secretion Profiles of PBMC from nMOMP Immunized Monkeys Pulsed with Different Trachoma Serovars
The antigen-specific cytokine-mediated immune response and its serovar specificity were evaluated by profiling the Th1/Th2 cytokine response of PBMCs. The cytokine profile showed that IFN-γ, a Th1 cytokine, was consistently induced after being pulsed with antigen (Table I). The data also revealed that, unlike the serum ELISA and neutralizing titers, IFN-γ induction was heterotypic, not dependent on the serovar used for stimulating the PBMCs.
Table I.
Cytokine secretion profiles of PBMC from nMOMP immunized monkeys pulsed with different trachoma serovars
| Monkey # | Immunogen | Serovar | TNF-α | IL-4 | IL-10 | IL-12 | IFN-γ |
|---|---|---|---|---|---|---|---|
| 117 | nMOMP | A | —* | — | — | — | 56 |
| 117 | nMOMP | B | — | — | — | 8 | 79 |
| 117 | nMOMP | C | — | — | — | 4 | 55 |
| 132 | nMOMP | A | — | — | — | — | 14 |
| 132 | nMOMP | B | — | — | — | 4 | 26 |
| 132 | nMOMP | C | — | — | — | — | 12 |
| 139 | nMOMP | A | — | 4 | — | 4 | 223 |
| 139 | nMOMP | B | — | — | — | 8 | 213 |
| 139 | nMOMP | C | — | — | — | 7 | 118 |
| 805 | OVA | A | — | — | — | — | — |
| 805 | OVA | B | — | — | — | — | — |
| 805 | OVA | C | — | — | — | — | — |
| 486 | OVA | A | — | — | — | — | — |
| 486 | OVA | B | — | — | — | — | — |
| 486 | OVA | C | — | — | — | — | — |
| 72-41 | OVA | A | — | — | — | — | — |
| 72-41 | OVA | B | — | — | — | 11 | — |
| 72-41 | OVA | C | 7 | — | — | 4 | — |
pg/ml
minus sign is equal to <3 pg/ml
Ocular Challenge of Non-Human Primates Immunized with nMOMP
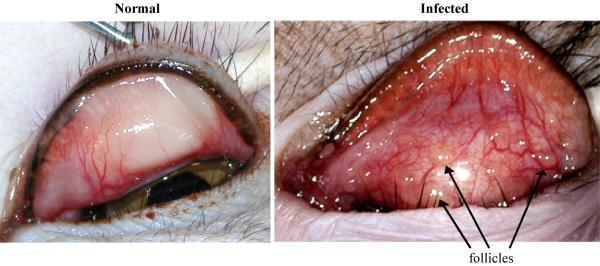
At 34 days following the third immunization, the nMOMP and OVA immunized non-human primates were ocularly challenged with strain A2497. Chlamydial shedding and gross clinical pathology were evaluated at regular intervals to monitor the course of infection. Gross clinical pathology was scored based on hyperemia and follicle formation on the upper conjunctiva. Figure 6 shows examples of an uninfected naïve upper conjunctiva before challenge and an infected conjunctiva four weeks post-challenge. The infected conjunctiva exhibits the maximum clinical score of 12, presenting with severe hyperemia, edema, and multiple large follicles.
FIGURE 6. Follicular conjunctivitis in non-human primates.
These images are examples of both an uninfected naïve and an infected upper conjunctiva four weeks post-challenge. The infected conjunctiva exhibits severe hyperemia, edema and large follicles.
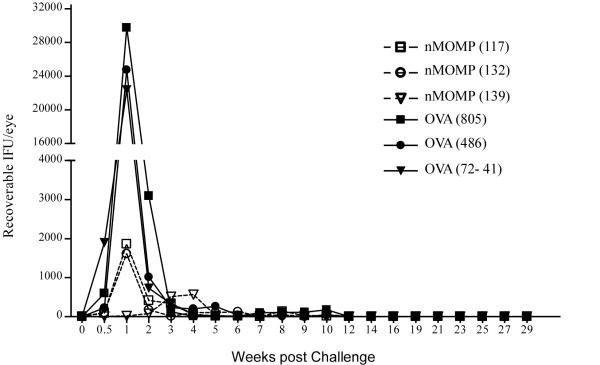
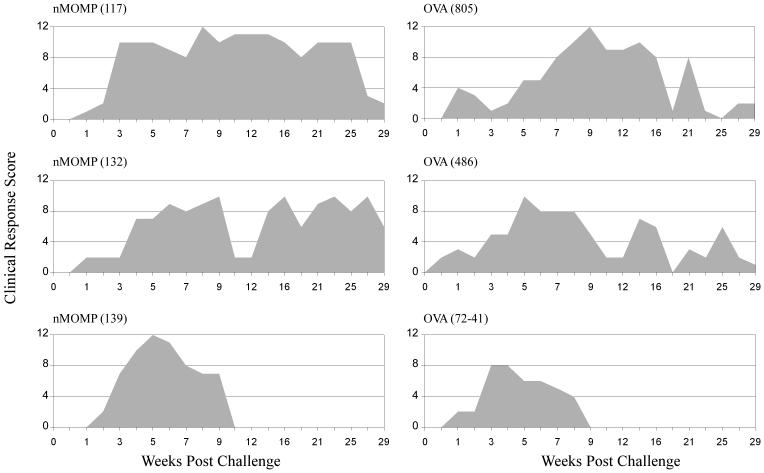
The nMOMP immunized animals shed significantly less organisms during the first two weeks post-challenge (p = 0.002-0.026), with 98% (70-fold) reduction at the peak shedding period observed on Day 7 (Fig. 7). The total infectious burden (total bacterial shedding during the entire experiment) for these nMOMP immunized animals was reduced by 94% (18-fold). However, the duration of bacterial shedding did not differ significantly from the control animals. Interestingly, monkey #139 had a delayed peak shedding period (week 4 instead of week 1) that corresponded to the highest serum ELISA IgG and neutralizing titers and the highest IFN-γ response. Furthermore and surprisingly, the high nMOMP neutralizing titers, strong IFN-γ response, and reduced chlamydial shedding in the immunized animals did not manifest in a significant difference in gross clinical pathology between the two groups (Fig. 8).
FIGURE 7. Chlamydial shedding after ocular challenge of non-human primates.
The nMOMP and OVA immunized non-human primates were ocularly challenged with strain A2497. Chlamydial shedding was evaluated at weekly intervals to monitor the course of infection. The nMOMP immunized animals shed significantly fewer organisms during the first two weeks post-challenge (p =0.002-0.026, Wilcoxon rank sum test).
FIGURE 8. Gross clinical pathology after ocular challenge of non-human primates.
The nMOMP and OVA immunized non-human primates were ocularly challenged with strain A2497. Gross clinical pathology was evaluated at weekly intervals to monitor the course of infection. The clinical response score was determined based on the hyperemia and follicular formation of the upper conjunctiva of both eyes, with 0 indicating no disease and 12 indicating maximum disease. There was no significant difference in the gross clinical response between the two groups.
Discussion
We have shown that nMOMP is highly immunogenic in non-human primates. Systemic immunization elicited high levels of serovar-specific serum IgG and IgA antibodies, but very low levels of tear IgG and undetectable levels of tear IgA antibodies, as measured by ELISA. Notably, serum from vaccinated monkeys was shown to contain high strain-specific neutralizing antibodies. Conversely, immunization induced a broad trachoma strain cross-reactive IFN-γ response. This immune response resulted in highly significant protection against homotypic ocular challenge, reducing the infectious burden greater than 70-fold over the first two weeks post-challenge. However, protection was restricted to early time periods post-challenge, with minimal differences observed between vaccinated and control monkeys in either infectious burden or duration at later time points. Surprisingly, these marked early differences in organism burden in the conjunctival epithelia did not reduce the severity of ocular disease. Nevertheless, this is the first time a subunit vaccine has shown a significant reduction in ocular shedding in non-human primates. Although two previous studies described partial protection in non-human primates after subunit vaccination (33, 38), this protection was limited to a transient decrease in clinical response with no significant reduction observed in shedding.
A major and perhaps important finding of this work was the high strain-specific neutralizing titers generated following immunization with nMOMP. We believe the native trimeric structure of MOMP could be the reason for achieving such high strain-specific neutralizing titers. Interestingly, neither this high titer nor strain specificity was found by ELISA when using purified formalin-fixed EBs as antigen. Serum ELISA antibody titers showed virtually identical titers against the two serovar A strains (A2497 and AHAR-13), with lower but measurable titers against the heterologous Ba and C trachoma serovars. The significance of this finding is unclear, but it is consistent with previous findings that protective immunity against C. trachomatis ocular infection is serovar-specific, with little to no cross protection against different serovars (39-41). Indirectly, these findings implicate serovar-specific neutralizing antibodies in ocular immunity. The exquisite degree of strain specificity found in serum neutralizing antibodies of nMOMP immunized monkeys was unexpected and unpredicted, as the MOMPs of strains A2497 and AHAR-13 differ by only four amino acids (42). Two of these differences are located in MOMP variable domains (amino acid #80 and #153 in VDs I and II, respectively), and the other two in constant regions. According to the 2-D model of MOMP (43), VD I is the latching loop for the trimers and that loop should be critical for trimer formation. Also, recombinant phage clones expressing MOMP antigenic determinants revealed that protective serotype-specific mAbs recognized epitopes in VD I and II (26). Although speculative, these findings argue the four amino acid substitutions, either independently or collectively, may change the structural properties of trimeric nMOMP. These substitutions could thus generate immunodominant determinants recognized by highly efficient infection blocking neutralizing antibodies.
Immunization with nMOMP resulted in an antigen-specific production of IFN-γ by PBMC. The response was broadly cross-reactive, as different trachoma strains were equally effective in its induction. IFN-γ is thought to play an important role in resolving C. trachomatis infection; however our findings indicate it is not sufficient to significantly alter the course of ocular infection. Possible explanations for this finding are that systemic cellular immunity was ineffective mucosally or perhaps the levels of IFN-γ generated by systemic immunization were simply insufficient. A more accurate role for IFN-γ in ocular immunity against chlamydial infection may require, like neutralizing antibodies, strategies capable of targeting local ocular immune responses. Typically, C. trachomatis infections generate primarily homotypic immunity, providing less protection against heterotypic challenges (39-41). Our findings suggest protective immunity is not solely reliant on the cytokine-mediated immune response — homotypic neutralizing antibodies could also be important factors. Due to the limited number of primates available for experiments, we were unable to challenge nMOMP vaccinated animals with strains other than A2497. Heterotypic challenge using other serovars could better define the role of IFN-γ in achieving the protection observed in our experiments. Similarly, heterotypic challenge with A/HAR-13 would help characterize the in vivo function of the strain specificity of the neutralizing serum antibodies.
The immunity induced by systemic immunization with nMOMP was equivalent or even better than the immunity induced by experimental infection in non-human primates. In a previous experiment, monkeys were infected with A2497 twice and immunity was evaluated 3 weeks later (unpublished data). The serum ELISA IgG and IgA titers and the IFN-γ response of PBMCs were comparable to that induced by nMOMP immunization. However, serum neutralizing titers were about 10 fold lower (4000-8000 versus 32000-76800) than those measured after nMOMP immunization. Because the nMOMP immunizations induced chlamydial-specific IFN-γ and serum neutralizing antibody responses that correlated with a significant reduction in the level of early bacterial shedding, the lack of impact on gross pathology post-challenge was puzzling. In mice, systemic immunization with nMOMP induced protective immunity comparable to that induced by infection with live bacteria. Discrepancies between the results of the murine and non-human primate experiments underscore the importance of using the non-human primate trachoma model in pre-clinical studies. Aside from the inherent differences between host and chlamydial species, these discrepancies likely result from differences in infection sites. Both the ocular conjunctiva and the upper female genital tract are mucosal sites; however, the ocular mucosa is probably more regionally isolated. Lacking a practical genital model in non-human primates, we were unable to investigate this further. Another interesting aspect of the partial protection induced by nMOMP immunization in non-human primates is that it does not differ much from the protection induced by experimental infection. A single experimental infection in primates also induces only partial protection, with a 1-2 log decrease in shedding and limited reduction in pathology (41, 44). This suggests an ideal vaccine of non-human primates and humans will need to produce immunity that actually exceeds that of natural infection. At present the underlying mechanisms for this disparity remain unclear, but it is certain that a better understanding of human immunity and C. trachomatis virulence factors capable of altering natural and vaccine-mediated immunity are needed. Vaccines that can provide effective protection of the eye will probably require immunization strategies that target regional ocular immune induction sites.
Computer modeling studies have predicted even a partially efficacious vaccine would have a significant effect on decreasing chlamydial transmission (11, 12). Considerably reducing chlamydial shedding throughout communities could successfully interrupt the trachoma re-infection transmission cycle. This approach would in fact be similar to that of mass antibiotic treatment, which reduces transmission by temporarily reducing the total infectious burden in a community. However, the effect of mass antibiotic treatments is unsustainable and treatment must be repeated as infections return over time (45-48). In contrast, a vaccine providing long-lasting partial protection need be administered only once, or at least less frequently, to achieve a sustainable effect on transmission. In our study, systemic immunization with nMOMP reduced the total infectious burden by 94% (18-fold). Thus, a community-wide nMOMP immunization could significantly impact the fight against blinding trachoma by interrupting the re-infection cycle. Admittedly, this vaccination campaign could face significant logistical difficulties, such as the high production cost of the immunogen and the delivery and storage of the vaccine in rural African villages. This might be overcome by expressing nMOMP in a surrogate system and combining it with other childhood vaccines. Nevertheless, it is foreseeable that nMOMP vaccination in combination with antibiotic treatment could be the most effective way to eliminate trachoma.
Acknowledgments
We would like to thank the Rocky Mountain Veterinary Branch of RML/NIAID, Stephen Porcella, Daniel Bozinov and Craig Martens for help with statistical analysis, Anita Mora for assistance in graphic art, Kelly Matteson for manuscript formatting, and Kena Swanson for critical review of the manuscript.
1 This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health and by Public Health Service grant AI-32248 (to L. M. de la Maza) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
4 Abbreviations used in this paper
- MOMP
major outer membrane protein
- nMOMP
native-MOMP
- STD
sexually transmitted disease
- LGV
lymphogranuloma venereum
- EB
elementary bodies
- RB
reticulate bodies
- SPG
sucrose phosphate glutamate
- DAV
days after vaccination
- IFU
inclusion forming units
Footnotes
Disclosures The authors have no financial conflict of interest.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Global elimination of blinding trachoma. May 16, 1998. 106Resolution WHA 51.11 adopted by the World Health Assembly. 1998.
- 3.Grayston JT, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Schachter J. Chlamydial infections (First of three parts) N. Engl. J. Med. 1978;298:428–434. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J. Chlamydial infections (Third of three parts) N. Engl. J. Med. 1978;298:540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- 6.Schachter J. Chlamydial infections (Second of three parts) N.Engl.J.Med. 1978;298:490–495. doi: 10.1056/NEJM197803022980905. [DOI] [PubMed] [Google Scholar]
- 7.Moulder JW. The relation of basic biology to pathogenic potential in the genus Chlamydia. Infection. 1982;10(Suppl 1):S10–18. doi: 10.1007/BF01640709. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell HD, Perry LJ. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982;38:745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peeling RW, Brunham RC. Neutralization of Chlamydia trachomatis: kinetics and stoichiometry. Infect Immun. 1991;59:2624–2630. doi: 10.1128/iai.59.8.2624-2630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YX, Stewart S, Joseph T, Taylor HR, Caldwell HD. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]
- 11.de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–127. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- 12.Ward ME, Webber GM, Shahani AK. Computer modelling of trachoma control strategies. In: Oriel D, Ridgway G, Schachter J, Taylor-Robinson D, Ward M, editors. Chlamydial infections. Cambridge University Press; Cambridge: 1986. pp. 154–157. [Google Scholar]
- 13.Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 14.Nichols RL, Bell SD, Jr., Haddad NA, Bobb AA. Studies on trachoma. VI. Microbiological observations in a field trial in Saudi Arabia of bivalent rachoma vaccine at three dosage levels. Am J Trop Med Hyg. 1969;18:723–730. [PubMed] [Google Scholar]
- 15.Woolridge RL, Grayston JT, Chang IH, Cheng KH, Yang CY, Neave C. Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma vaccine in Taiwan school children. Am J Ophthalmol. 1967;63(Suppl):1645–1650. doi: 10.1016/0002-9394(67)94158-x. [DOI] [PubMed] [Google Scholar]
- 16.Murdin AD, Su H, Manning DS, Klein MH, Parnell MJ, Caldwell HD. A poliovirus hybrid expressing a neutralization epitope from the major outer membrane protein of Chlamydia trachomatis is highly immunogenic. Infect Immun. 1993;61:4406–4414. doi: 10.1128/iai.61.10.4406-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson EM, Cheng X, Markoff BA, Fielder TJ, de la Maza LM. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect Immun. 1991;59:4147–4153. doi: 10.1128/iai.59.11.4147-4153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson EM, Cheng X, Pal S, de la Maza LM. Effects of antibody isotype and host cell type on in vitro neutralization of Chlamydia trachomatis. Infect Immun. 1993;61:498–503. doi: 10.1128/iai.61.2.498-503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su H, Morrison RP, Watkins NG, Caldwell HD. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990;172:203–21. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su H, Caldwell HD. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992;175:227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toye B, Zhong GM, Peeling R, Brunham RC. Immunologic characterization of a cloned fragment containing the species-specific epitope from the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1990;58:3909–3913. doi: 10.1128/iai.58.12.3909-3913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens RS, Mullenbach G, Sanchez-Pescador R, Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986;168:1277–128. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens RS, Sanchez-Pescador R, Wagar EA, Inouye C, Urdea MS. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SK, DeMars R. Epitope clusters in the major outer membrane protein of Chlamydia trachomatis. Curr Opin Immunol. 2001;13:429–426. doi: 10.1016/s0952-7915(00)00237-5. [DOI] [PubMed] [Google Scholar]
- 25.Stephens RS, Wagar EA, Schoolnik GK. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988;167:817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baehr W, Zhang YX, Joseph T, Su H, Nano FE, Everett KD, Caldwell HD. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988;85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berry LJ, Hickey DK, Skelding KA, Bao S, Rendina AM, Hansbro PM, Gockel CM, Beagley KW. Transcutaneous immunization with combined cholera toxin and CpG adjuvant protects against Chlamydia muridarum genital tract infection. Infect Immun. 2004;72:1019–1028. doi: 10.1128/IAI.72.2.1019-1028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong-Ji Z, Yang X, Shen C, Lu H, Murdin A, Brunham RC. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect Immun. 2000;68:3074–3078. doi: 10.1128/iai.68.6.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pal S, Barnhart KM, Wei Q, Abai AM, Peterson EM, de la Maza LM. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine. 1999;17:459–465. doi: 10.1016/s0264-410x(98)00219-9. [DOI] [PubMed] [Google Scholar]
- 31.Shaw AC, Gevaert K, Demol H, Hoorelbeke B, Vandekerckhove J, Larsen MR, Roepstorff P, Holm A, Christiansen G, Birkelund S. Comparative proteome analysis of Chlamydia trachomatis serovar A, D and L2. Proteomics. 2002;2:164–186. doi: 10.1002/1615-9861(200202)2:2<164::aid-prot164>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Zhang DJ, Yang X, Shen C, Brunham RC. Characterization of immune responses following intramuscular DNA immunization with the MOMP gene of Chlamydia trachomatis mouse pneumonitis strain. Immunology. 1999;96:314–321. doi: 10.1046/j.1365-2567.1999.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campos M, Pal S, O’Brien TP, Taylor HR, Prendergast RA, Whittum-Hudson JA. A chlamydial major outer membrane protein extract as a trachoma vaccine candidate. Invest Ophthalmol Vis Sci. 1995;36:1477–1491. [PubMed] [Google Scholar]
- 34.Taylor HR, Whittum-Hudson J, Schachter J, Caldwell HD, Prendergast RA. Oral immunization with chlamydial major outer membrane protein (MOMP) Invest Ophthalmol Vis Sci. 1988;29:1847–1853. [PubMed] [Google Scholar]
- 35.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis Major Outer Membrane Protein Can Elicit an Immune Reponse as Protective as That Resulting from Inoculation with Live Bacteria. Infect Immun. 2005;73:8153–8160. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor HR, Prendergast RA, Dawson CR, Schachter J, Silverstein AM. An animal model for cicatrizing trachoma. Invest Ophthalmol Vis Sci. 1981;21:422–433. [PubMed] [Google Scholar]
- 37.Su H, Watkins NG, Zhang YX, Caldwell HD. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor GR, Hyde K, Wensley RT, Delamore IW. Polymerase chain-reaction amplification and detection of HIV DNA-sequences in the peripheral-blood. Br.J.Haematol. 1988;69:127. [Google Scholar]
- 39.Dawson C, Wood TR, Rose L, Hanna L. Experimental inclusion conjunctivitis in man. 3. Keratitis and other complications. Arch Ophthalmol. 1967;78:341–349. doi: 10.1001/archopht.1967.00980030343015. [DOI] [PubMed] [Google Scholar]
- 40.Tarizzo ML, Nataf R, Nabli B. Experimental inoculation of thirteen volunteers with agent isolated from inclusion conjunctivitis. Am J Ophthalmol. 1967;63:1120–1128. doi: 10.1016/0002-9394(67)94093-7. [DOI] [PubMed] [Google Scholar]
- 41.Taylor HR. Development of immunity to ocular chlamydial infection. Am J Trop Med Hyg. 1990;42:358–364. doi: 10.4269/ajtmh.1990.42.358. [DOI] [PubMed] [Google Scholar]
- 42.Kari L, Whitmire WM, Carlson JH, Crane DD, Reveneau N, Nelson DE, Mabey DC, Bailey RL, Holland MJ, McClarty G, Caldwell HD. Pathogenic diversity among Chlamydia trachomatis ocular strains in non-human primates is affected by subtle genomic variations. J Infect Dis. 2008;197:449–456. doi: 10.1086/525285. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Marañón MJ, Bush RM, Peterson EM, Schirmer T, de la Maza LM. Prediction of the membrane-spanning beta-strands of the major outer membrane protein of Chlamydia. Protein Sci. 2002;11:1854–1861. doi: 10.1110/ps.3650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor HR, Johnson SL, Prendergast RA, Schachter J, Dawson CR, Silverstein AM. An animal model of trachoma II. The importance of repeated reinfection. Invest Ophthalmol Vis Sci. 1982;23:507–515. [PubMed] [Google Scholar]
- 45.Broman AT, Shum K, Munoz B, Duncan DD, West SK. Spatial clustering of ocular chlamydial infection over time following treatment among households in a village in Tanzania. Invest Opthalmol Vis Sci. 2006;47:99–104. doi: 10.1167/iovs.05-0326. [DOI] [PubMed] [Google Scholar]
- 46.Chidambaram JD, Alemayehu W, Melese M, Lakew T, Yi E, House J, Cevallos V, Zhou Z, Maxey K, Lee DC, Shapiro BL, Srinivasan M, Porco T, Whitcher JP, Gaynor BD, Lietman TM. Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. JAMA. 2006;295:1142–1146. doi: 10.1001/jama.295.10.1142. [DOI] [PubMed] [Google Scholar]
- 47.Leitman T, Porco T, Dawson C, Blower S. Global elimination of trachoma: how frequently should we administer mass chemotherapy? Nat Med. 1999;5:572–576. doi: 10.1038/8451. [DOI] [PubMed] [Google Scholar]
- 48.West SK, Munoz B, Mkocha H, Holland MJ, Aguirre A, Solomon AW, Foster A, Bailey RL, Mabey DC. Infection with Chlamydia trachomatis after mass treatment of a trachoma hyperendemic community in Tanzania: a longitudinal study. Lancet. 2005;366:1296–1300. doi: 10.1016/S0140-6736(05)67529-0. [DOI] [PubMed] [Google Scholar]