Abstract
Antigen/IgE-mediated mast cell activation via FcεRI can be markedly enhanced by the activation of other receptors expressed on mast cells and these receptors may thus contribute to the allergic response in vivo. One such receptor family is the G protein-coupled receptors (GPCRs). Although the signaling cascade linking FcεRI aggregation to mast cell activation has been extensively investigated, the mechanisms by which GPCRs amplify this response are relatively unknown. To investigate this, we utilized prostaglandin (PG)E2 based on initial studies demonstrating its greater ability to augment antigen-mediated degranulation in mouse mast cells than other GPCR agonists examined. This enhancement, and the ability of PGE2 to amplify antigen-induced calcium mobilization, was independent of phosphoinositide 3-kinase but was linked to a pertussis toxin-sensitive synergistic translocation to the membrane of phospholipase (PL)Cγ and PLCβ and to an enhancement of PLCγ phosphorylation. This “trans-synergistic” activation of PLCβ and γ, in turn, enhanced production of inositol 1,4,5-trisphosphate, store operated calcium entry, and activation of protein kinase C (PKC) (α and β). These responses were critical for the promotion of degranulation. This is the first report of synergistic activation between PLCγ and PLCβ that permits reinforcement of signals for degranulation in mast cells.
Keywords: Mast Cell, FcεRI, Gi, signal transduction, phospholipase C, Prostaglandin E2
Introduction
Antigen-dependent mast cell activation, via the aggregation of cell surface high affinity IgE receptors (FcεRI), is essential for the propagation of allergic inflammation [1]. However, the antigen-mediated response may be markedly influenced by co-activation through other cell surface receptors and it has been suggested that these interactions may modulate mast cell-driven reactions in situ [2, 3]. Such receptors, which have been reported to induce mediator release by themselves, enhance antigen-mediated mast cell activation, or inhibit antigen-mediated mast cell activation, include the stem cell factor (SCF) receptor, Kit [4, 5]; toll-like receptors (TLRs) such as TLR2 [6, 7], TLR3 [8], and TLR4 [7, 9]; and G protein-coupled receptors (GPCRs) [10] such as receptors for adenosine (A3 receptor) [11-13], macrophage inflammatory protein-1α (CCR1) [14], the complement component C3a (C3aR) [15], sphingosine-1-phosphate (SIP) (S1P2R) [16] and prostaglandin (PG)E2 (EP1 and 3) [17-19]. It is unclear, however, how the signaling cascades initiated by these various classes of receptors are integrated with FcεRI-mediated signals to modify antigen-mediated mast cell activation.
With regards to the FcεRI, the immediate signaling events elicited upon receptor aggregation follow phosphorylation of the FcεRIβ and γ chains by the Src kinase Lyn and subsequent recruitment and activation of Syk [2, 20]. This latter tyrosine kinase phosphorylates the transmembrane adaptor molecule LAT resulting in the recruitment and activation of phospholipases (PL)Cγ1 and PLCγ2 [21]. The resulting liberation of inositol (1,4,5) tris-phosphate (IP3) and diacylglycerol (DAG), respectively induce a necessary Ca2+ signal [22] and protein kinase C (PKC) activation [23, 24] for degranulation. A parallel pathway, regulated by the Src kinase, Fyn, and leading to activation of phosphoinositide 3-kinase (PI3K), is also critical for optimal degranulation and cytokine production following FcεRI aggregation [25, 26].
Our previous studies investigating potential mechanisms of receptor-mediated signal integration have focused on how the aforementioned signaling events may be modified by those initiated by Kit. Although FcεRI and Kit mediate many signaling events in common, those initiated by Kit alone are insufficient to promote mast cell degranulation [4, 5, 27]. This likely reflects an inability of Kit to induce detectable LAT phosphorylation [4] and PKC activation [5]. In the presence of antigen, however, SCF-dependent Kit activation induces a synergistic enhancement of mast cell degranulation and cytokine production [4, 5, 27]. Previous studies suggested that the LAT-related transmembrane adaptor protein NTAL/LAB/LAT2 [4], PI3K [28], and the tyrosine kinase, Bruton's tyrosine kinase (Btk) [27], which together lead to an enhanced PLCγ1-dependent Ca2+ response [5], participate in the amplification of these responses. As with SCF, ligands for TLR2 and 4 markedly amplify FcεRI-mediated cytokine production in mast cells but, in contrast to SCF, do not potentiate degranulation [7]. This amplification, however, appears to be mediated through MAP kinases rather than the processes described above for Kit [7, 29, 30].
In contrast to these examples, the mechanisms by which GPCRs modify mast cell activation remain largely unknown, although it has been proposed that PI3K [12] and phospholipase D [31] help regulate the A3 receptor-induced potentiation of antigen-mediated degranulation in mast cells. In this study, therefore, we have set out to explore how the signaling cascades initiated by GPCRs and FcεRI are integrated for the synergistic activation of mast cells. We focused these studies on PGE2 as this ligand was found to be a more robust co-activator of mast cells than other GPCR-ligands examined. Here we demonstrate that the enhancement of antigen-mediated mast cell degranulation by PGE2 can proceed independently of PI3K, but is associated with trans-synergy between PLCγ and PLCβ leading to enhanced store operated Ca2+ entry and PKCα and β activation.
Materials and Methods
Bone Marrow Isolation and Mast Cell Differentiation
Mouse bone marrow-derived mast cells (BMMCs) were obtained by flushing bone marrow cells from the femurs of C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), then culturing the cells for 4-6 weeks in RPMI 1640 supplemented with 10% FBS, glutamine (4 mM), sodium pyruvate (1 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), non-essential amino acids (Sigma, St. Louis, MO), HEPES (25 mM), β-mercaptoethanol (50 μM), and mouse recombinant IL-3 (30 ng/ml) (Peprotech, Rocky Hill, NJ). At this point, the BMMC population was greater than 99% pure. Cultures were maintained at 37°C in a humidified incubator of 95% air, 5% CO2.
Cell Activation, Degranulation, and Cytokine Production
For degranulation, cytokine release, and signaling studies, BMMCs were sensitized overnight with anti-mouse monoclonal dinitrophenyl (DNP)-IgE (100 ng/ml) (Sigma) in IL-3-free RPMI medium and then rinsed with HEPES buffer (10 mM HEPES [pH 7.4], 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4•7H2O, 5.6 mM Glucose, 1.8 mM CaCl2•2H2O, 1.3 mM MgSO4•7H2O) containing 0.04% bovine serum albumin (BSA) (Sigma). In the case of preincubation with pertussis toxin (PTX) (Sigma), BMMCs were sensitized overnight and then pre-incubated with PTX (1 μg/ml) for 4 hours before antigen (DNP-human serum albumin) (DNP-HSA) or PGE2 or antigen/PGE2 was added. For degranulation experiments, cells were aliquoted (5×104 cells/well) to individual wells of a 96 well plate and triggered in the same buffer with antigen (0-100 ng/ml) and/or indicated GPCR agonists (Sigma) for 30 minutes. Degranulation was monitored by the release of β-hexosaminidase into the supernatants [32] and calculated as a percentage of the total content (cells and media) after cell activation. For cytokine release studies, cells were sensitized as above, washed with IL-3-free RPMI media, then the cells (5×105 cells/ml) were triggered in this media for 6 hours with antigen (10 ng/ml) and/or PGE2 (1 μM). Cytokines were measured in the cell culture supernatant by mouse TNF-μ, IL-6, and IL-13 Quantikine ELISA kits (R&D Systems, Minneapolis, MN). For signaling studies in which we examined protein phosphorylation by immunoblot analysis, cells were prepared as for degranulation and triggered (1×106 cells/100 μl) in 1.5 ml polyethylene screw cap tubes. The reactions were terminated either as in [33] for whole cell lysates, or as below for cell fractionation experiments.
Fractionation of Cells and Immunoblotting
Membrane fractions were prepared as previously described [34]. Briefly, BMMCs (2×106 cells/sample) were sensitized and washed as above, then stimulated with antigen (10 ng/ml) and/or PGE2 (1 μM) at 37°C for the indicated times. The reactions were terminated by removing the buffer, washing with ice-cold phosphate-buffered saline, and adding 200 μl of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.4], 2 mM EDTA, 2 mM DTT, 1 mM sodium orthovanadate, 50 mM sodium pyrophosphate, 50 mM sodium fluoride, protease inhibitor cocktail (Roche, Indianapolis, IN) and Sigma phosphatase inhibitor cocktail 1 and 2 (Sigma) then the cells were sonicated. The cell sonicates were then centrifuged at 700×g for 10 minutes to remove intact cells and nuclei. The recovered supernatants were centrifuged at 20,000×g for 30 minutes. Proteins in the pellet fractions were solubilized with 1% Triton X-100, 1% NP40, and 0.1% SDS in lysis buffer for 30 minutes on ice followed by centrifugation at 15,000×g for 15 minutes. The recovered supernatants were saved as membrane fractions. Proteins from the membrane fractions were separated by electrophoresis on 4-12% NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA). Following transfer onto nitrocellulose membranes, the proteins were probed for immunoreactive proteins utilizing the following antibodies: anti-PLCγ1 (1249), anti-PLCγ2 (Q-20), anti-PLCβ2 (Q-15), anti-PLCβ3 (C-20), anti-PKCβI (C-16), and anti-PKCβII (C-18) (Santa Cruz Biotechnology, Santa Cruz, CA); anti-β-actin (clone AC-15) (Sigma); anti-phosphotyrosine, clone 4G10-HRP conjugate, anti-phospho-PLCγ2 (Tyr(P)-759), anti-phospho-AKT (Ser(P)-473), and anti-Kit (Cell Signaling, Beverly, MA); anti-phospho-PLC γ1 (Tyr(P)-783) (BIOSOURCE), and anti-phospho-LAT (Tyr(P)-191), anti-PKCα (clone M4) (Upstate Biotechnology, Inc., Lake Placid, NY). The immunoreactive proteins were visualized by probing with horseradish peroxidase-conjugated anti-mouse (The Jackson Laboratories, West Grove, PA) or anti-rabbit IgG (Amersham Biosciences, Piscataway, NJ), and then by ECL (PerkinElmer Life Sciences, Shelton, CT). Protein loading of the membrane fractions was normalized by stripping and probing for Kit, or alternative by probing identically loaded samples. To quantitate changes in protein phosphorylation, the ECL films were scanned using a Quantity One scanner (Bio-Rad, Hercules, CA).
Intracellular Ca2+ Determination
Ca2+ flux was measured in the BMMCs following loading of the cells with Fura-2 AM ester (Molecular Probes, Eugene, OR) as described [34]. Cells were loaded with Fura-2 AM (2 µM) for 30 minutes at 37 °C, rinsed, and resuspended in HEPES buffer containing 0.04% BSA and sulfinpyrazone (0.3 mM) (Sigma), and then placed in a 96-well black culture plate (2×104 cells/well) (CulturPlat-96 F, PerkinElmer Life Sciences). Fluorescence was measured at two excitation wavelengths (340 and 380 nm) and an emission wavelength of 510 nm. The ratio of the fluorescence readings was calculated following subtraction of the fluorescence of the cells that had not been loaded with Fura-2 AM.
Imaging of Intracellular Ca2+ in Single Cells
For single cell imaging experiments [35], BMMCs were allowed to attach to poly-Lornithine coated cover slips for 1 hour which then were placed in HEPES buffer and loaded with Fura-2 AM (2 µM) for 30 minutes at room temperature. Cells were washed and Fura-2 AM was allowed to de-esterify for a minimum of 15 minutes at room temperature. Measurements of cytosolic Ca2+ levels [Ca2+]i in single BMMC cells were made using an InCyt dual-wavelength fluorescence imaging system (Intracellular Imaging, Cincinnati, OH). The fluorescence emission at 505 nm was monitored with alternating excitation at 340 and 380 nm; [Ca2+]i was monitered as the ratio of fluorescence at 340/380 nm. All measurements shown are means of multiple single-cell Ca2+ traces, and the results are representative of three independent experiments.
IP3 Assay
BMMCs (2×106) were washed with HEPES buffer containing 0.04% BSA and stimulated in same buffer (400 μl) in the absence or presence of antigen (10 ng/ml) and/or PGE2 (1 μM). After 30 seconds, the reaction was terminated by adding 80 μl of ice-cold 100% trichloroacetic acid. IP3 was then extracted from the trichloroacetic acid precipitates using 1,1,2-trichlorofluorethane-trioctylamine. Cellular IP3 concentrations were determined utilizing a commercially available kit (Amersham Biosciences) according to the manufacturer's instructions. The results are expressed as picomoles of IP3 per 2×106 cells.
Statistical analysis
Data were analyzed by a two-tailed Student's t-test. Differences were considered significant when p < 0.05. The n values represent experiments from multiple preparations.
Results
Potentiation of FcεRI-mediated degranulation, cytokine production, and calcium mobilization by PGE2 in a pertussis toxin sensitive manner
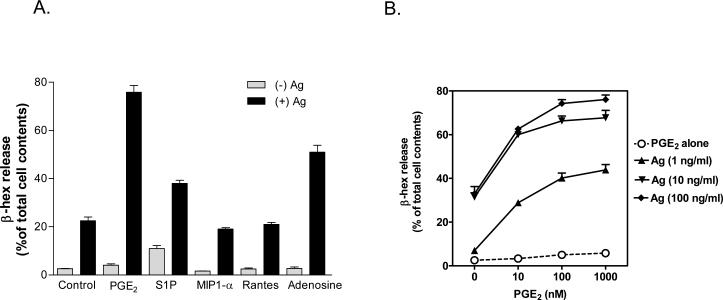
Synergistic responses mediated by FcεRI and GPCRs have been reported in both rodent and human mast cells [10]. However, as studies in human mast cells may be complicated by the additional inhibitory responses with GPCRs such as adenosine and PGE2 [10], we elected to utilize mouse BMMCs for our studies. We screened several GPCR agonists, including S1P, adenosine, MIP1-α, Rantes, and PGE2, known to modify mast cell function [10-12, 14, 16, 18], for their relative abilities to enhance antigen (DNP-human serum albumin (DNP-HSA))-mediated BMMC activation (Fig. 1A). Among these ligands, PGE2 produced the most robust enhancement and we, therefore, selected an optimal concentration (Fig. 1B) of this molecule for further study. As described [17, 18], PGE2 alone failed to stimulate degranulation (Fig 1B) but it significantly potentiated antigen-induced degranulation in a pertussis toxin (PTX)-sensitive manner (Fig. 1C). PTX -pretreatment had no effect on antigen-induced degranulation. Concurrent addition of antigen and PGE2 also resulted in an enhancement of the production of the cytokines IL-6, TNF-α, and IL-13 and this response was completely blocked in the PTX-treated cells (Fig. 1D).
Fig 1. The effect of PGE2 on antigen-induced mast cell activation.
A-B. BMMCs were sensitized overnight and then challenged with the indicated GPCR agonists (PGE2, 1 μM; S1P, 40 μM; MIP1-α, 100 ng/ml; Rantes, 50 ng/ml; Adenosine, 10 μM) or antigen (Ag) (DNP-HSA) or Ag/GPCR agonist added concurrently for 30 min for β-hexosaminidase (β-hex) release. C-D. BMMCs were sensitized overnight and then pre-incubated with or without PTX (1 μg/ml) for 4 hours before antigen (Ag) (DNP-HSA) or PGE2 or Ag/PGE2 was added. After incubation for 30 minutes (C) or 6 hours (D), β-hexosaminidase (β-hex) release (C) or cytokine production (D) was measured according to “Materials and Methods”. E. BMMCs were loaded with Fura-2 AM and changes in [Ca2+] were monitored after treatment Ag (10 ng/ml) or PGE2 (1 μM) or Ag together with PGE2. The Ca2+ data are representative of n=3 experiments conducted in duplicate. The data in A and B are presented as means ± S.E. of (n=3-4) separate experiments conducted in duplicate. ###, p < 0.001 for comparison with Ag/PGE2 (A). ***, p < 0.001 by Student's t-test (B).
Antigen alone produced a sustained increase in intracellular calcium concentrations ([Ca2+]i), whereas PGE2 alone induced a rapid but transient increase in [Ca2+]i (Fig. 1E). When added together, however, PGE2 and antigen produced a markedly enhanced increase in [Ca2+]i. As was the case for degranulation and cytokine production, the increase in [Ca2+]i in response to PGE2 and the enhancement of this response by antigen was completely attenuated by PTX treatment (Fig. 1E). These data confirm that PGE2 enhances antigen-mediated responses in mast cells and support the conclusion that the PGE2 receptor is linked to the Gi subfamily of GPCRs [17, 18].
Lack of enhancement of early protein phosphorylation events by PGE2
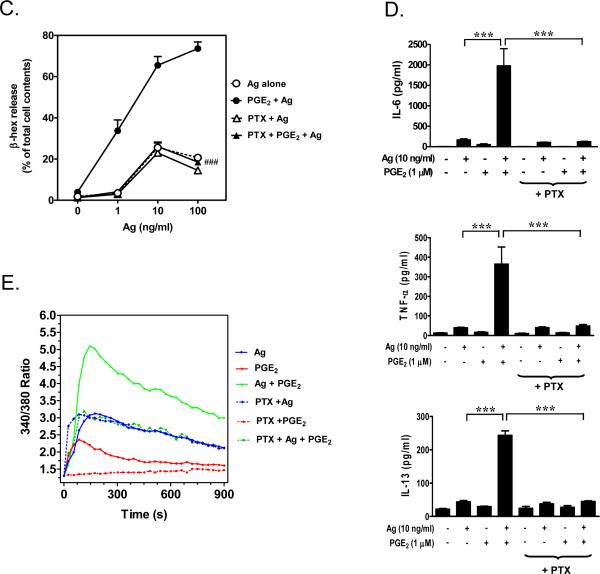
Having confirmed that PGE2 produced a robust enhancement of antigen-mediated degranulation and cytokine production which was associated with a synergistic calcium signal, we utilized this molecule to elucidate the mechanism of potentiation, focusing on the degranulation response. We first examined the initial signaling events that lead to the calcium signal required for degranulation [2]. In contrast to GPCRs which initiate their signaling via Gα and Gβγ subunits, FcεRI-mediated signaling is propagated by the initial activation of tyrosine kinases leading to protein tyrosine phosphorylation and activation of downstream enzymes. As expected, phosphorylation of numerous proteins was increased in response to antigen (Fig. 2A). However, PGE2 had no obvious effect on total tyrosine protein phosphorylation either in the presence or absence of antigen (Fig. 2A). We next examined the Lyn and Syk [2]-dependent phosphorylation of the transmembrane adaptor molecules NTAL (LAB/LAT2) and LAT with the use of an antibody (anti-(pY191)-LAT) that recognizes a common tyrosine-phosphorylated epitope in both LAT and NTAL [4]. We have previously demonstrated that the synergistic phosphorylation of NTAL is required for the ability of Kit to potentiate FcεRI-induced degranulation [4]. As shown in Figure 2B and C, although an increase in NTAL and LAT phosphorylation was observed in response to antigen at all time points, PGE2 had no effect on the phosphorylation of these molecules and, furthermore, PGE2 failed to enhance the antigen responses. Therefore, the synergy occurred downstream of the activation of the early tyrosine phosphorylation events including the phosphorylation of NTAL and LAT.
Fig 2. Protein tyrosine phosphorylation and phosphorylation of LAT/NTAL in response to antigen and/or PGE2.
A, B. BMMCs were sensitized overnight and treated with antigen (Ag), PGE2, or Ag/PGE2 concurrently for the indicated times, and protein samples were prepared as described in “Materials and Methods”. Following gel electrophoresis, the whole-cell extracts were immunoblotted with anti-phosphotyrosine (4G10-HRP) (A) or anti-phospho-LAT (Y191) antibodies (B). The blots are representative of three to five independent experiments. Protein loading of the samples was normalized by stripping and then probing for β-actin. C. The data were generated by the scanning the blots in B in three independent experiments, then normalizing to the response at 120 seconds obtained with Ag/PGE2. The data in C are presented as means ± S.E. of (n=3) separate experiments.
Enhancement of antigen-induced degranulation by PGE2 is PI3K independent
We previously demonstrate that the FcεRI-dependent increase in [Ca2+]i is regulated by both a PLCγ-dependent/PI3K-independent and a PI3K-dependent mechanism [34]. Therefore, we next investigated whether a synergistic activation of PI3K and PLCγ may account for the ability of PGE2 to enhance the antigen-mediated elevation of [Ca2+]i and the degranulation response.
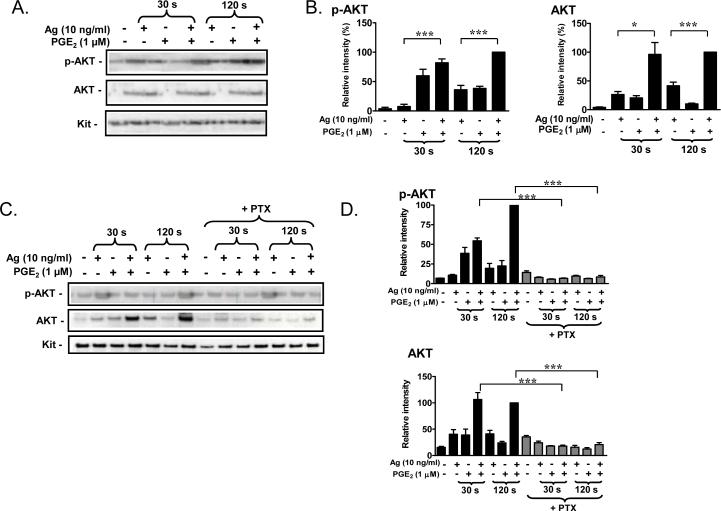
In contrast to the FcεRI which primarily utilizes the PI3K-p110δ isoform, Gβγ subunits, released from Gαi proteins transiently activate the p110γ PI3K isoform [36] although activation of either form leads to the phosphorylation and activation of AKT, which is thus used as a surrogate marker for PI3K activation [36, 37]. As membrane-associated AKT may be more reflective of its PI3K-dependent activation state [38], we examined the association and phosphorylation of AKT in membrane fractions. When antigen and PGE2 were added concurrently, there was an additive phosphorylation of AKT which was accompanied by a substantial enhancement in the translocation of total AKT to the membrane (Fig. 3A, B). Thus, the observed additive effect on phosphorylation of membrane-associated AKT likely reflects enhanced translocation of AKT rather than a net increase in phosphorylation. As was the case with degranulation, the ability of PGE2 to enhance AKT translocation and phosphorylation was completely inhibited in PTX-treated cells (Fig. 3C, D) again implying that this response was mediated via Gi.
Fig 3. AKT phosphorylation and the effects of the PI3K inhibitor, wortmannin.
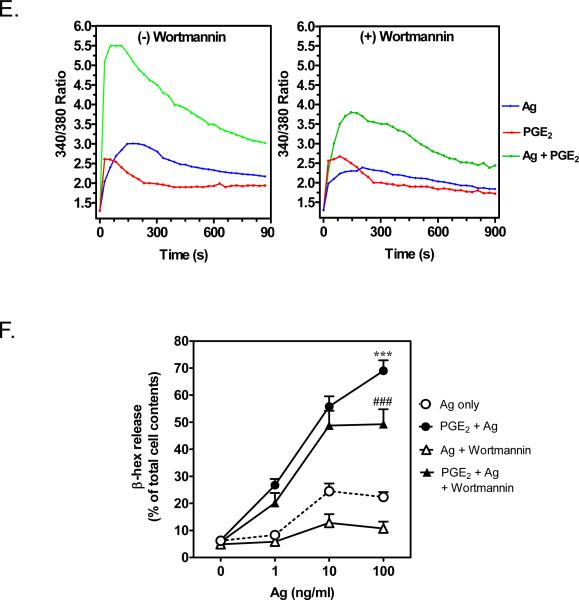
A, C. BMMCs were sensitized and pretreated with or without PTX (1 μg/ml) for 4 h, followed by treatment with Ag or PGE2, or Ag/PGE2 for the indicated times, then membrane fractions were prepared. Following gel electrophoresis, the proteins were probed with antibodies recognizing phosphorylated-AKT (S473) or AKT. Blots are representative of three independent experiments. Protein loading of the samples was normalized by stripping and then probing for c-Kit. B, D. The data were generated by the scanning the blots A and C respectively in three independent experiments, and then normalizing to response at 120 seconds obtained with Ag/PGE2. The data in B and D are presented as means ± S.E. of (n=3) separate experiments. E. BMMCs were loaded with Fura-2 AM and preincubated with or without wortmannin (100 nM) for 10 minutes. And then changes in [Ca2+]i were monitored after treatment Ag (10 ng/ml) or PGE2 (1 μM) or Ag together with PGE2. The Ca2+ data are representative of n=3 experiments conducted in duplicate. F. After preincubation with wortmannin (100 nM) for 10 minutes, cells were treated with Ag, PGE2, or Ag/PGE2 concurrently for 30 minutes for β-hex release. The data in F are presented as means ± S.E. of (n=4) separate experiments conducted in duplicate. *, p < 0.05 and ***, p < 0.001 by Student's t-test (B, D). ***, p < 0.001 and ###, p<0.001 for comparison with Ag alone (100 ng/ml) and wortmannin / Ag (100 ng/ml), respectively (F).
Based on the above observations, we investigated whether PI3K was responsible for the synergistic enhancement of the antigen-dependent increase in [Ca2+]i and degranulation by PGE2. For these studies, we utilized the PI3K inhibitor wortmannin (100 nM) which completely suppressed antigen- and PGE2-induced AKT phosphorylation (data not shown). As shown in Figure 3E, the increase of [Ca2+]i by PGE2 was unaffected by wortmannin pretreatment. However, as previously described [34] the antigen-mediated increase in [Ca2+]i, particularly the sustained phase, was partially reduced by wortmannin pretreatment. Nevertheless, the enhancement of [Ca2+]i by PGE2 and antigen together was not inhibited when the wortmannin-sensitive component of the antigen-induced response was taken into account (Fig. 3E). Similarly, antigen-induced degranulation was substantially inhibited by wortmannin. However, PGE2 still markedly potentiated the residual antigen-mediated degranulation in the wortmannin-treated cells (Fig. 3F). Similar results were obtained with the PI3K inhibitor LY294002 (10 μM) (data not shown). Thus, although PGE2 enhanced antigen's ability to induce PI3K mediated responses in the membrane fraction, this response was not necessary for the ability of PGE2 to augment degranulation.
Enhancement of antigen-mediated degranulation by PGE2 is associated with enhanced PLC activity
FcεRI regulates activation of PLCγ1 and PLCγ2, whereas the Gi subfamily of GPCR activates PLCβ isozymes though Gβγ [39, 40]. IP3 directly induces the release of Ca2+ from intracellular stores, indirectly leading to the influx of extracellular Ca2+ via store operated calcium entry (SOCE) following depletion of intracellular stores [41, 42]. We therefore hypothesized that activation of PLCβ by PGE2 concurrently with the activation of PLCγ through FcεRI may allow additive or synergistic enhancement of signals generated through Ca2+ and PKC and, thus, degranulation.
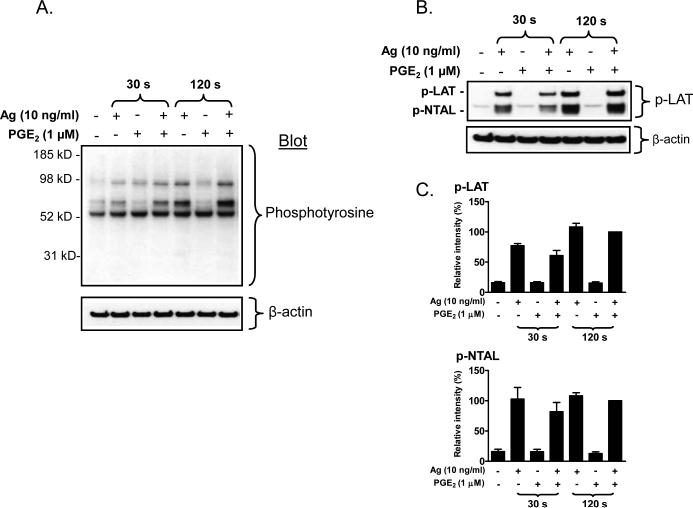
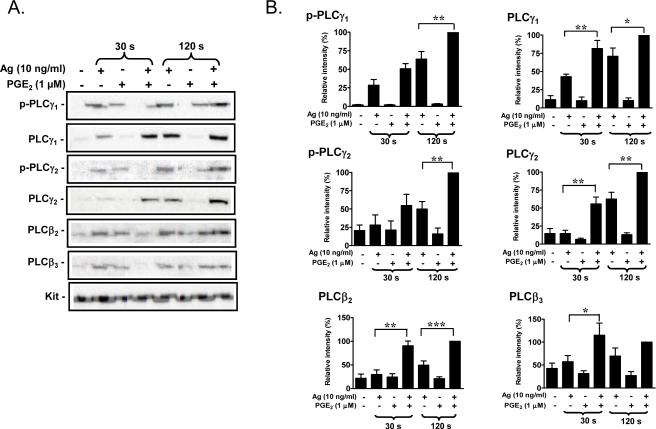
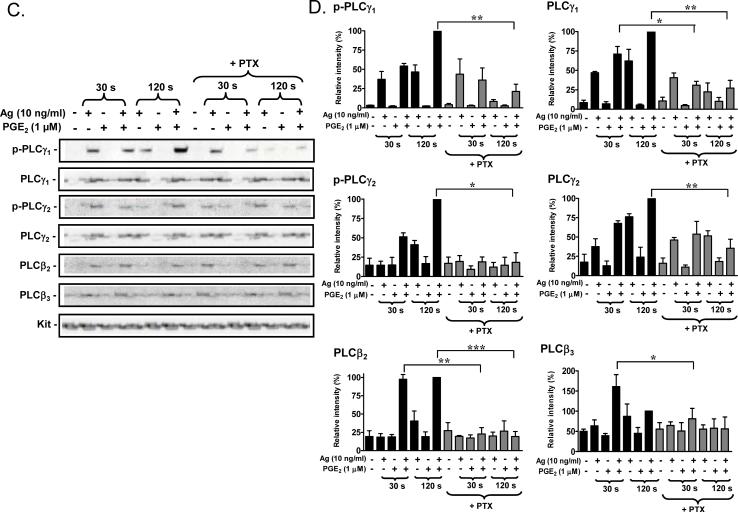
PLCγ requires phosphorylation of tyrosine residues for activation, and both PLCβ and PLCγ isozymes require membrane localization for biological function [43]. As expected, antigen induced the translocation of PLCγ1 and PLCγ2 and their respective phosphorylated forms (phospho-PLCγ1 (Y783) and phospho-PLCγ2 (Y759)) to the cell membrane (Fig 4A, B). Unexpectedly, however, this response was potentiated by PGE2, although the enhancement of phosphorylation appeared to be delayed compared to the translocation response. The combination of antigen and PGE2 also resulted in a marked enhancement of the translocation of PLCβ2 and PLCβ3 to the membrane (Fig. 4A, B). Interestingly, although PGE2 on its own failed to induce PLCβ translocation as shown in Figure 4A and B, antigen alone appeared to induce some translocation of both PLCβ2 and PLCβ3 at the 120 seconds time point. The synergistic enhancement of translocation of PLCγ1, PLCγ2, PLCβ2 and PLCβ3 to the membrane fraction was completely reversed by PTX treatment (Fig. 4C, D), again supporting the role of Gi in these synergistic responses. Taken together, the above data suggest that the ability of PGE2 to potentiate antigenmediated degranulation may be a consequence of trans-synergy in the phosphorylation and membrane translocation of PLCβ and PLCγ isozymes.
Fig 4. Translocation of PLCγ and PLCβ to the membrane in response to antigen and/or PGE2.
A. BMMCs were sensitized overnight and treated with antigen (Ag), PGE2, or Ag/PGE2 concurrently for the indicated times, and membrane fractions were prepared as described in “Experimental procedures”. Following gel eletrophoresis, the proteins were probed with antibodies recognizing phosphorylated PLCγ1 (Y783), phosphorylated PLCγ2 (Y759), PLCγ1, PLCγ2, PLCγ2, or PLCγ3. Blots are representative of four to five independent experiments. Protein loading of the membrane fraction was normalized by stripping and then probing for c-Kit. C. BMMCs were pretreated with or without PTX (1 μg/ml) for 4 hours, followed by treatment with Ag, PGE2, or Ag/PGE2 for the indicated times, and membrane fractions were prepared. B, D. The data were generated by the scanning the blots in four to five independent experiments of A and C respectively, and then normalizing to the response at 120 seconds obtained with Ag/PGE2. The data in B (n=5) and D (n=4) were presented as means ± S.E. of separate experiments. *, p < 0.05, **, p < 0.01 and ***, p < 0.001 by Student's t-test.
Enhanced antigen-induced IP3 production and store-operated calcium entry by PGE2
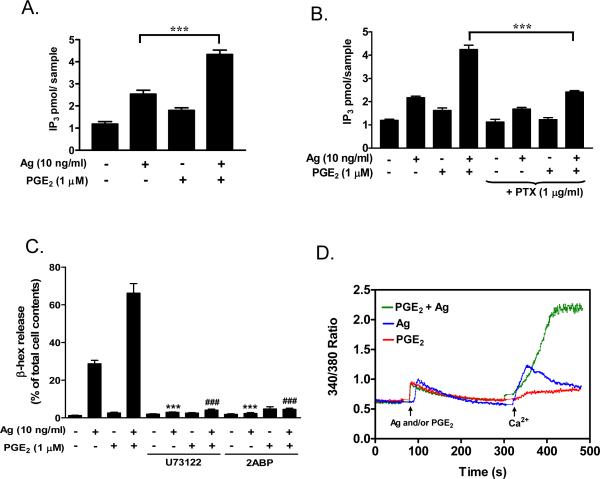
We thus next investigated whether the enhanced phosphorylation and/or translocation of PLCβ and PLCγ resulted in increases in IP3 production and Ca2+ mobilization. Based on kinetic studies (data not shown), we measured IP3 levels after 30 seconds of cell activation as this represented the time of maximal PGE2 response. From Figure 5A it can be seen that antigen and PGE2 individually increased levels of IP3 by 2.1 and 1.5 fold respectively compared to basal levels, but together, they strongly stimulated the generation of IP3 up to 3.7 fold. PTX-treatment blocked the IP3 response to PGE2 and the augmented response to both stimulants (Fig. 5B). In contrast the results obtained with wortmannin, the ability of PGE2 to enhance antigen-mediated degranulation, was attenuated by both the PLC inhibitor U73122 (1 μM) and the IP3 receptor antagonist 2ABP (2-amino-ethoxydiphenylborate) (50 μM) (Fig. 5C). These data provide support for the conclusion that the enhanced PLC-dependent IP3 production is critical for the potentiation effect of PGE2 on antigen-induced degranulation..
Fig 5. IP3 generation and Ca2+ mobilization.
A, B. BMMCs (A) or PTX (1 μg/ml, 4 hours)-preincubated BMMCs (B) were washed with HEPES buffer containing 0.04% BSA and stimulated with antigen (Ag) (10 ng/ml), PGE2 (1 μM, or Ag/PGE2. After 30 seconds, the samples were processed and the IP3 measurement assays conducted as described under “Materials and Methods”. The data in A (n=4) and B (n=2) are presented as means ± S.E. of separate experiments conducted in duplicate. C. After preincubation with U73122 (1 μM) or 2ABP (50 μM) for 10 minutes, cells were treated with Ag, PGE2, or Ag/PGE2 concurrently for 30 minutes for β-hex release. The data in C are presented as means ± S.E. of (n=3) separate experiments conducted in duplicate. ***, p < 0.001 by Student's t-test (A and B). ***, p < 0.001 and ###, p<0.001 for comparison with Ag alone and Ag/PGE2, respectively (C). D. BMMCs were stimulated with Ag or PGE2, or Ag/PGE2 in the absence of external Ca2+. After 5 min Ca2+ was added and Ca2+ influx was monitored using an InCyt dual-wavelength fluorescence imaging system. The data are representative of three independent experiments.
From Figure 1E, it was evident that PGE2 synergistically enhanced the antigen-mediated increase in [Ca2+]i. To examine which component of the antigen-mediated Ca2+ signal was affected by PGE2, we determined the release of Ca2+ from intracellular pools, and Ca2+ influx using single cell imaging techniques. The addition of PGE2 to BMMCs in Ca2+-free buffer induced an increase in [Ca2+]i (Fig. 5D), however, the subsequent addition of extracellular Ca2+, failed to produce substantial influx of Ca2+, indicating that the Ca2+ signal produced by PGE2 was primarily due to release from intracellular stores without a substantial depletion of these stores. In contrast, antigen produced not only a release of Ca2+ from intracellular stores but influx of Ca2+ when extracellular Ca2+ was re-introduced indicating that depletion of intracellular stores was sufficient to activate influx presumably via SOCE. The magnitude of the Ca2+ signal in response to the simultaneous addition of PGE2 and antigen in the absence of extracellular Ca2+ was not different to that elicited by either antigen or PGE2 alone. However, when extracellular Ca2+ was added, the secondary influx of Ca2+ was synergistically enhanced to give increases in [Ca2+]i comparable to those observed after maximal depletion of intracellular stores with thapsigargin (data not shown), These data suggest that the ability of PGE2 to enhance the Ca2+ signal was primarily due to potentiation of Ca2+ influx possibly as a consequence of more complete depletion of intracellular stores.
The effect of PGE2 on antigen-induced translocation of PKC isozymes
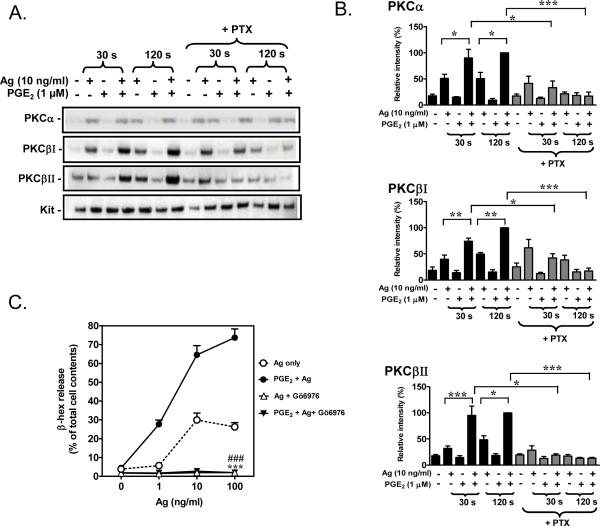
The augmented activation of PLC and increase in [Ca2+]i would likely extend to enhanced activation of conventional PKCs (α, βI, βII, and γ) [44]. Based on previous studies on the role of representative PKC isozymes on mast cell degranulation [22] and the fact that PKC activation follows translocation to the plasma membrane, we examined the effect of PGE2 and antigen on membrane translocation of PKCα, βI and βII. Antigen but not PGE2 alone, induced membrane translocation of PKCα, βI and βII (Fig. 6A, B). However, when added together with antigen, PGE2-potentiated membrane translocation of all three isozymes. This potentiation was effectively attenuated by PTX pre-treatment (Fig. 6 A, B). Activation of PKC is critical for degranulation [22, 24] and cytokine production [24]. Accordingly, both antigen-mediated degranulation and the ability of PGE2 to enhance this response (Fig. 6C), in addition to their effects on cytokine production (data not shown), were effectively blocked by pre-incubation with Gö6976, an inhibitor of Ca2+ dependent isozymes of PKC.
Fig 6. PKC translocation.
A. BMMCs were sensitized overnight and pre-incubation with or without PTX (1 μg/ml) for 4 hours. Cells were treated with antigen (Ag) or PGE2, or Ag/PGE2 concurrently for the indicated times, and membrane fractions were prepared as described in “Materials and Methods”. These fractions were analyzed by immunoblotting with anti-PKCα, PKCβI and PKCβII antibodies. Blots are representative of three independent experiments. Protein loading of the membrane fraction was normalized by probing for c-Kit. B. The data were generated by the scanning the blots (A) in three independents experiments, and then normalizing to response at 120 seconds obtained with Ag/PGE2. C. BMMCs were sensitized overnight and then pre-incubated with Gö6976 (1 μM) for 30 minutes before Ag, PGE2, or Ag/PGE2 was added concurrently for 30 minutes for β-hex release. The data in B (n=3) and C (n=3) are presented as means ± S.E. of separate experiments. *, p < 0.05 and **, p < 0.01 and ***, p < 0.001 by Student's t-test (B). ***, p < 0.001 and ###, p<0.001 for comparison with Ag (100 ng/ml) alone and Ag (100 ng/ml)/PGE2, respectively (C).
Discussion
In this study, we have examined how the signals induced by Gi-linked GPCRs are integrated with those elicited by the FcεRI for the synergistic enhancement of mast cell degranulation. Of the various GPCR agonists that influence antigen-mediated mast cell activation, we selected PGE2 for detailed study as it enhanced antigen-mediated responses (Fig. 1) to a greater extent than other GPCR agonists examined such as S1P, and adenosine.
Four subtypes of PGE2 GPCRs, have been described: EP1, EP2, EP3, and EP4 [45]. EP1 is coupled to Gq and can induce Ca2+ mobilization, whereas, both EP2 and EP4 are coupled to Gs and activate adenylate cyclase. EP3 is reported to have four isozymes (EP3α, β, γ, and δ ) which are coupled to different G proteins (Gi, Gs and Gq) and, as such, mediate their effects via different signaling mechanisms [46]. Human mast cells express EP2, 3, and 4 [47] and it has been reported that PGE2 both stimulates and inhibits antigen-mediated degranulation in these cells [19, 48]. Mouse BMMCs express EP1, 3 and 4 receptors [18] and PGE2 appears to enhance antigen-mediated responses in these cells in the absence of an inhibitory response [10]. Recent pharmacological studies have indicated that the observed ability of PGE2 to enhance mast cell degranulation and IL-6 production are mediated through the EP1 or EP3 receptors [17]. Studies using EP3 receptor-deficient BMMCs, however, have indicated that this is the primary EP receptor subtype responsible for the actions of PGE2 on mast cells [18]. In our study, all responses mediated by PGE2, including its ability to potentiate antigen-mediated degranulation and cytokine production (Fig. 1) were inhibited by pre-treatment with PTX, supporting the conclusion that these responses are mediated by Gi-linked EP3 receptors.
Unlike, the FcεRI, GPCRs do not require activation of tyrosine kinases and subsequent protein phosphorylation for propagation of their responses but rely instead on coupling to a hetero-trimetric protein complex of Gα and Gβγ subunits [49]. This would explain why PGE2 had little effect on the total tyrosine phosphorylation of proteins including NTAL and LAT (Fig. 2). The ability of antigen and PGE2 to synergistically enhance the membrane translocation and phosphorylation of AKT in a PTX and wortmannin-sensitive manner (Fig. 3) in BMMCs, is likely attributable to activation of both PI3Kγ which is linked to GPCR signaling [12, 50], and PI3Kδ which contributes to FcεRI signaling [51]. Nevertheless, our results suggest that the potentiation of antigen-mediated degranulation by PGE2 was independent of PI3K. Although wortmannin completely blocked AKT phosphorylation (data not shown), and markedly reduced antigen-mediated degranulation, there was still a substantial amplification of residual antigen-mediated Ca2+ mobilization (Fig. 3) and degranulation (Fig. 3) by PGE2 in the wortmannin-treated cells. This is in contrast to the report that adenosine potentiates antigen-mediated degranulation in a PI3K-dependent manner [12]. This difference suggests that GPCRs may vary in their requirements for PI3K in their ability to modify antigen-mediated responses.
These findings pointed to the possibility that PGE2 acts through PLC in a PI3Kindependent manner to enhance mast cell activation (Fig. 4, 5). We have previously demonstrated in human mast cells that the PLCγ-dependent increase in calcium mobilization produced by antigen challenge is also independent of PI3K [34]. Both FcεRI and GPCRs can activate PLC, however, these receptors are linked to different PLC isozymes which have different modes of activation: The FcεRI is linked to PLCγ1 and PLCγ2 whereas GPCRs are linked to PLCβ isozymes [20, 52]. As there is little evidence that PLCβ1 is expressed in BMMCs [53], we focused on PLCβ2 and PLCβ3. Both PLCβ and PLCγ isozymes require membrane localization for activation, however, whereas activation of PLCγ isozymes also requires phosphorylation of critical tyrosine within their catalytic domains [39], the PLCβ isozymes are activated by Gβγ subunits [54]. Membrane localization of PLCγ1 is mediated via binding of its SH2 domain to phosphorylated tyrosine residues within the transmembrane adaptor molecule LAT [52].
Given the different modes of activation of PLCβ and PLCγ, it was therefore surprising to observe that PGE2 enhanced the antigen-mediated phosphorylation and translocation of PLCγ1 and PLCγ2 to the cell membrane and that antigen and PGE2 induced the synergistic membrane translocation of the PLCβ isozymes (Fig. 4). Nevertheless, these data do demonstrate that there is "trans-synergy" between the pathways leading to the activation of both PLC isozymes accounting for the enhancement of IP3 production and increased [Ca2+]i (Fig. 5). It is of interest, however, that as with antigen, PGE2 alone induced sufficient IP3 generation to increase [Ca2+]i even though PGE2 induced no detectable PLCβ translocation (Fig. 4, 5). This may be explained by Gβγ-dependent activation of PLCβ constitutively associated with the membrane.
As far as we are aware, this is the first report of trans-synergistic interactions between PLCβ and PLCγ which provides a means for integrating signaling responses for the enhanced activation of mast cells. Therefore, the mechanism(s) leading to this response need to be clarified although several possibilities exist. The apparent activation of PI3K by PGE2 (Fig. 3) is unlikely to account for this response because of the responses in the presence of wortmannin noted here. A more likely possibility is the activation of PLCγ by Src kinases which have been noted to be activated through GPCRs [52, 55, 56]. Preliminary studies (Kuehn and Gilfillan, unpublished observations) indeed indicate that the Src family kinase inhibitor PP2 (30 μM) markedly attenuates the ability of PGE2 to enhance antigen-mediated degranulation. The enhanced activation of PLCβ in antigenstimulated cells could conceivably occur through an autocrine/paracrine pathway as a result of release of adenosine [12] or S1P [57] both of which act through GPCRs in mast cells. A more speculative possibility is inherent in the common pleckstrin homology (PH) domain, C2 domain, and EF-hand domains in PLCβ and PLCγ. The increase in [Ca2+]i by one or the other receptor might, for example, promote activation of either PLC through the EF-hand domain or C2 domain.
The synergistic increase in [Ca2+]i following the increases in PLC activation and IP3 generation produced by co-stimulation with antigen and PGE2 was primarily due to a marked enhancement of SOCE (Fig. 5). PGE2 alone produced a transient production of IP3 and a release of intracellular Ca2+ which was insufficiently sustained to initiate SOCE upon addition of extracellular Ca2+, possibly a consequence of down-regulation of GPCR activation [53]. This is in contrast to antigen, which can sustain depletion of intracellular Ca2+ pools to allow activation of SOCE after delayed addition of Ca2+ [58]. The enhanced SOCE that was observed with the combination of antigen and PGE2 could be due to the more complete depletion of intracellular stores as a consequence of enhanced and prolonged IP3 production.
The synergistic increase in PLC activation produced by PGE2 and antigen also provides an explanation for the observed enhancement of membrane localization of the PKCα and β isozymes (Fig. 6A, B). By elevating intracellular [Ca2+]i levels, PLC regulates the activation of the Ca2+-dependent PKC isozymes in stimulated mast cells. Essential requirements for PKC activation in FcεRI-induced mast cells function have been defined from studies conducted in RBL-2H3 cells [22, 23] and from studies conducted in PKC isozyme-deficient mice and mouse BMMCs [24, 59, 60]. Among these PKC isozymes, PKCβ has been demonstrated as being important for mast cell degranulation and IL-6 generation [24]. Thus, the synergistic enhancement of the translocation of the PKCβ isozymes which we observed would be expected to contribute to the amplification of the antigen-mediated degranulation response by PGE2. This conclusion was supported by the ability of the PKCα and β inhibitor Gö6976 to not only block the antigen-mediated degranulation but also the ability of PGE2 to potentiate this response (Fig. 6C).
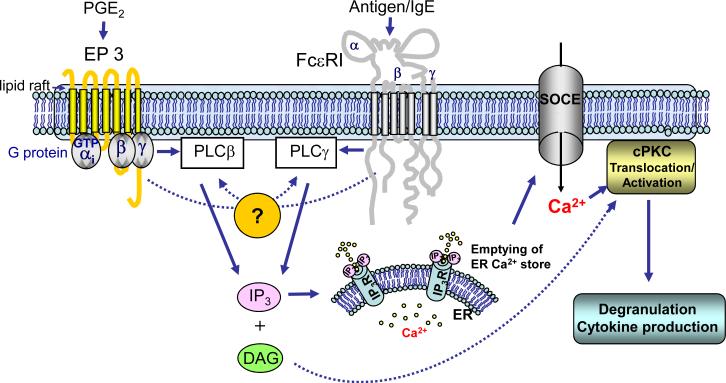
In summary, in this study we have demonstrated that the Gi-linked receptor for PGE2 potentiates FcεRI-mediated mast cell degranulation by utilizing a novel PI3Kindependent integration pathway involving trans-synergy in the activation of PLCβ and PLCγ (Fig. 7). This, in turn, induces an enhanced increase in [Ca2+]i via SOCE, and enhanced PKC translocation, critical signals for degranulation to occur. The novel finding that PLCβ and PLCγ trans-synergizes raises a question of mechanism for which several possibilities exist. These studies potentially provide a paradigm for the mechanism by which other GPCRs linked to Gi also enhance antigen-dependent mast cell activation. However, these data also illustrate the point that the different classes of receptors that are known to enhance antigen-mediated responses in mast cells likely do so through entirely different mechanisms. For this reason, it would be of interest to examine whether co-activation of multiple receptors would further enhance mast cell degranulation and cytokine production, whether such interaction occur in vivo, and how these interactions could be inhibited. A comprehensive understanding of these interactions and their roles in disease states would be important considerations in the treatment of allergic disorders.
Fig 7.
Proposed mechanism of integration of GPCR and FcεRI signaling mechanisms for the potentiation of mast cell degranulation and cytokine production. Binding of PGE2 to EP3, which is coupled to Gi, enhances antigen-mediated mast cell activation following FcεRI aggregation. The initial receptor-proximal signaling events regulate trans-synergy in the activation of membrane-associated PLCγ and PLCβ concurrently with enhanced PI3K-dependent AKT phosphorylation. This latter response, however, is not required for the ability of PGE2 to enhance antigen-mediated degranulation and cytokine production and, thus, has not been included in this diagram. The synergistic activation of PLCγ and PLCβ hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to similarly synergistically enhance the production of inositol 1,4,5-trisphosphate (IP3) and, by inference, diacylglycerol (DAG). IP3 then binds to the IP3 receptor to mobilize ER stored Ca2+ and following the enhanced depletion of intracellular stores this promotes enhanced influx of extracellular Ca2+ via SOCE. The enhanced levels of intracellular Ca2+ and DAG then enhance the activation of conventional PKC (cPKC) which, together with the calcium signal, is critical for degranulation and cytokine production.
Acknowledgements
This work was supported by the Intramural Programs of NIAID and NHLBI within the National Institutes of Health.
References
- [1].Metcalfe DD, Baram D, Mekori YA. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- [2].Gilfillan AM, Tkaczyk C. Nat Rev Immunol. 2006;6(3):218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- [3].Jensen BM, Metcalfe DD, Gilfillan AM. Inflamm Allergy Drug Targets. 2007;6(1):57–62. doi: 10.2174/187152807780077255. [DOI] [PubMed] [Google Scholar]
- [4].Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. Blood. 2004;104(1):207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- [5].Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Blood. 2004;104(8):2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- [6].McCurdy JD, Olynych TJ, Maher LH, Marshall JS. J Immunol. 2003;170(4):1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- [7].Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. Blood. 2006;107(2):610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. J Allergy Clin Immunol. 2004;114(1):174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- [9].Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. J Immunol. 2001;167(4):2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- [10].Kuehn HS, Gilfillan AM. Immunol Lett. 2007;113(2):59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. J Immunol. 2003;171(1):338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- [12].Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Immunity. 2002;16(3):441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- [13].Ramkumar V, Stiles GL, Beaven MA, Ali H. J Biol Chem. 1993;268(23):16887–16890. [PubMed] [Google Scholar]
- [14].Miyazaki D, Nakamura T, Toda M, Cheung-Chau KW, Richardson RM, Ono SJ. J Clin Invest. 2005;115(2):434–442. doi: 10.1172/JCI18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ali H, Panettieri RA., Jr. Respir Res. 2005;6:19. doi: 10.1186/1465-9921-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. J Exp Med. 2004;199(7):959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gomi K, Zhu FG, Marshall JS. J Immunol. 2000;165(11):6545–6552. doi: 10.4049/jimmunol.165.11.6545. [DOI] [PubMed] [Google Scholar]
- [18].Nguyen M, Solle M, Audoly LP, Tilley SL, Stock JL, McNeish JD, Coffman TM, Dombrowicz D, Koller BH. J Immunol. 2002;169(8):4586–4593. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- [19].Wang XS, Lau HY. Allergy. 2006;61(4):503–506. doi: 10.1111/j.1398-9995.2006.01043.x. [DOI] [PubMed] [Google Scholar]
- [20].Rivera J, Gilfillan AM. J Allergy Clin Immunol. 2006;117(6):1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- [21].Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. Immunity. 2000;12(5):525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- [22].Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. J Biol Chem. 1993;268(3):1749–1756. [PubMed] [Google Scholar]
- [23].Chang EY, Szallasi Z, Acs P, Raizada V, Wolfe PC, Fewtrell C, Blumberg PM, Rivera J. J Immunol. 1997;159(6):2624–2632. [PubMed] [Google Scholar]
- [24].Nechushtan H, Leitges M, Cohen C, Kay G, Razin E. Blood. 2000;95(5):1752–1757. [PubMed] [Google Scholar]
- [25].Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J. Nat Immunol. 2002;3(8):741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- [26].Gu H, Saito K, Klaman LD, Shen J, Fleming T, Wang Y, Pratt JC, Lin G, Lim B, Kinet JP, Neel BG. Nature. 2001;412(6843):186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- [27].Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. J Biol Chem. 2005;280(48):40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- [28].Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Nature. 2004;431(7011):1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- [29].Zaidi AK, Thangam ER, Ali H. Immunology. 2006;119(3):412–420. doi: 10.1111/j.1365-2567.2006.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kikawada E, Bonventre JV, Arm JP. Blood. 2007 doi: 10.1182/blood-2006-10-052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ali H, Choi OH, Fraundorfer PF, Yamada K, Gonzaga HM, Beaven MA. J Pharmacol Exp Ther. 1996;276(2):837–845. [PubMed] [Google Scholar]
- [32].Chaves-Dias C, Hundley TR, Gilfillan AM, Kirshenbaum AS, Cunha-Melo JR, Metcalfe DD, Beaven MA. J Immunol. 2001;166(11):6647–6656. doi: 10.4049/jimmunol.166.11.6647. [DOI] [PubMed] [Google Scholar]
- [33].Tkaczyk C, Metcalfe DD, Gilfillan AM. J Immunol Methods. 2002;268(2):239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- [34].Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. J Biol Chem. 2003;278(48):48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- [35].Venkatachalam K, Ma HT, Ford DL, Gill DL. J Biol Chem. 2001;276(36):33980–33985. doi: 10.1074/jbc.C100321200. [DOI] [PubMed] [Google Scholar]
- [36].Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. J Biol Chem. 1998;273(30):19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- [37].Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. Cell. 1994;77(1):83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- [38].Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. J Biol Chem. 1997;272(50):31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- [39].Rhee SG, Bae YS. J Biol Chem. 1997;272(24):15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- [40].Singer WD, Brown HA, Sternweis PC. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- [41].Bolotina VM. Sci STKE. 2004;2004(243):pe34. doi: 10.1126/stke.2432004pe34. [DOI] [PubMed] [Google Scholar]
- [42].Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. Nat Cell Biol. 2004;6(2):113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- [43].Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mol Cell Biol. 2004;24(22):9986–9999. doi: 10.1128/MCB.24.22.9986-9999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Parker PJ, Murray-Rust J. J Cell Sci. 2004;117(Pt 2):131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- [45].Chung KF. Sci STKE. 2005;2005(303):pe47. doi: 10.1126/stke.3032005pe47. [DOI] [PubMed] [Google Scholar]
- [46].Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, Ito S, Ichikawa A, Narumiya S. Nature. 1993;365(6442):166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- [47].Feng C, Beller EM, Bagga S, Boyce JA. Blood. 2006;107(8):3243–3250. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kay LJ, Yeo WW, Peachell PT. Br J Pharmacol. 2006;147(7):707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Deshpande DA, Penn RB. Cell Signal. 2006;18(12):2105–2120. doi: 10.1016/j.cellsig.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [50].Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Science. 1997;275(5298):394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- [51].Okkenhaug K, Ali K, Vanhaesebroeck B. Trends Immunol. 2007;28(2):80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rhee SG. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ali H, Fisher I, Haribabu B, Richardson RM, Snyderman R. J Biol Chem. 1997;272(18):11706–11709. doi: 10.1074/jbc.272.18.11706. [DOI] [PubMed] [Google Scholar]
- [54].Clapham DE, Neer EJ. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- [55].Luttrell LM, Daaka Y, Lefkowitz RJ. Curr Opin Cell Biol. 1999;11(2):177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- [56].Ma YC, Huang J, Ali S, Lowry W, Huang XY. Cell. 2000;102(5):635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- [57].Rosen H, Goetzl EJ. Nat Rev Immunol. 2005;5(7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- [58].Choi OH, Lee JH, Kassessinoff T, Cunha-Melo JR, Jones SV, Beaven MA. J Immunol. 1993;151(10):5586–5595. [PubMed] [Google Scholar]
- [59].Leitges M, Gimborn K, Elis W, Kalesnikoff J, Hughes MR, Krystal G, Huber M. Mol Cell Biol. 2002;22(12):3970–3980. doi: 10.1128/MCB.22.12.3970-3980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lessmann E, Leitges M, Huber M. Int Immunol. 2006;18(5):767–773. doi: 10.1093/intimm/dxl012. [DOI] [PubMed] [Google Scholar]