Abstract
Atmospheric deposition of anthropogenic Hg has led to increased Hg concentrations in many ecosystems. Modeling is an effective method for predicting the complex dynamics of Hg fate and transport in watersheds; such models require accurate concentrations for water column methylmercury, CH3Hg+, as input parameters, yet these concentrations are very difficult to measure precisely as they are so low. We developed a method for aqueous CH3Hg+ quantification in Lake Champlain VT, where ambient CH3Hg+ concentrations are < 0.04 ng l-1. The analysis utilized species specific isotope dilution, purge and trap, gas chromatography ICP-MS and provided instrument detection limits of ca 0.2 fM (0.04 pg l-1) and method detection limits of 15 fM (0.003 ng l-1) for CH3Hg+ which are amongst the lowest reported. Artifactual methylation of inorganic Hg2+ was shown to be minor and the precision of the isotope dilution method was generally < 5% relative standard deviation; much lower than would have been the case for an external calibration approach. The method is accurate even at low concentrations of ca. 0.025 ng l-1. This combination of precision, accuracy and low detection allow for quantification of significant differences in CH3Hg+ concentration between bays and over time within bays of Lake Champlain where mean CH3Hg+ concentrations differ by only 0.006 ng l-1 at concentrations as low as 0.014 ng l-1.
Introduction
Mercury is a ubiquitous contaminant that is widely dispersed through the environment mostly via atmospheric deposition. Mercury, as the more toxic CH3Hg+ species, is also bioaccumulated by organisms, which can result in very high concentrations in top level predators. This has obvious ecosystem and human health implications. The latter point is exemplified by the Hg fish advisories that exist in most US states. Such bioaccumulation occurs in freshwater systems despite the fact that the aqueous CH3Hg+ concentrations of many water bodies are low, in many cases below the detection limit of current analytical methodologies. In order to successfully model Hg dynamics in freshwater lakes it is essential to be able to quantify the ambient CH3Hg+ concentrations in the water column, both dissolved and in the particulate load.
Sensitive analytical methods for CH3Hg+ quantification do exist, in fact the most common low level Hg speciation method, purge and trap gas chromatography coupled to atomic fluorescence spectroscopy, was developed over 15 years ago and remains the most popular method for determining sub ng l-1 concentrations of CH3Hg+ in environmental samples(1). The procedure involves reacting CH3Hg+ in a water sample or, indeed, an extract of biological tissue or sediment, with sodium tetraethylborate to form the volatile methylethylmercury. Other very similar methods involve propylation(2) or butylation(3) to form a volatile species. The volatile species can be purged from solution to the gas phase and trapped on a solid phase such as Tenax. The Hg species are thermally desorbed from the trap onto a packed column GC, the eluting mercury species are then pyrolytically reduced to Hg0 which is then quantified by AFS. This method can provide detection limits on the order of 0.01 ng l-1 for aqueous samples. However, when applied to the analysis of water samples, recovery of CH3Hg+ is be incomplete, with only 5-60% recovery reported depending on the water sample matrix and specifically the dissolved organic carbon (DOC) and sulfide concentrations(4).
A number of methods have been advanced to improve CH3Hg+ recoveries from natural waters and distillation is the most accepted method. The method involves distilling the CH3Hg+ from a water sample as the chloride complex. Horvat et al.(4) have shown this method to be more effective than solvent extraction in terms of CH3Hg+ recovery and the method has been adopted by the EPA as a standard method (EPA 6030). However, the method itself is not without its drawbacks; the sample is distilled relatively slowly such that the total distillation time is 5-6 hrs. More importantly the sample is exposed to new vessels and apparatus, and reagents are added to the sample prior to distillation, which increases the possibility for contamination or increased blank levels. Recently, a liquid chromatographic technique for determining aqueous Hg speciation has been reported which relies on the strong and pH dependent complexation of CH3Hg+ with thiourea(5). This method can reportedly achieve detection limits for CH3Hg+ of 0.007 ng l-1 based on a 40 ml sample and is the first reported liquid chromatographic method with equivalent detection limits to the GC methodologies.
The methods reported in this study were developed for a modeling study of Hg dynamics in Lake Champlain, VT, USA that build on previous efforts to establish a mass balance for Hg in the lake(6). Lake Champlain has low aqueous phase Hg concentrations (≪ 1 ng l-1) with CH3Hg+ less than 0.04 ng l-1, yet consumption advisories have been issued for top trophic-level game fish in the lake. Prior efforts to elucidate trophic transfers of mercury in the ecosystem were frustrated by the inability to quantify CH3Hg+ concentrations in the water column. In order to have meaningful input parameters for the model it was necessary to provide accurate detectable CH3Hg+ determinations in the low pg L-1 range. We used the increased sensitivity of high resolution ICP-MS coupled to the purge and trap, thermal desorption GC methodology commonly used with AFS detection, Other studies have shown that coupling high resolution ICP-MS, in low resolution mode, with cold vapor generation can achieve lower Hg detection limits than available with AFS(7). Additionally, we used species specific isotope dilution for quantification. Isotope dilution involves adding a known mass and concentration of an enriched stable isotope to a known mass of sample(8,9). In addition to excellent precision and accuracy, a further advantage of isotope dilution is that the isotope spike should equilibrate with all the particular species in the sample given sufficient equilibration time. Hence the use of isotope dilution with ICP-MS detection should not require a distillation step. We assume that all the ‘dissolved’ CH3Hg+ and Hg2+ in a water sample is labile, and therefore isotopically exchangeable. Distillation and extraction procedures for fresh water samples implicitly make the same assumption as they also rely on the reversible desorption of complexed CH3Hg+. A further benefit of isotope dilution for Hg speciation is that loss of Hg2+ during the analysis is compensated for by an equivalent loss of enriched isotope spike, and the isotope dilution calculation based on the inorganic peak area should provide accurate quantification of the Hg2+ in the sample(10).
The objectives of this study were to establish detection limits for CH3Hg+ and Hg2+ in freshwater samples employing the increased sensitivity of sector field ICP-MS in low resolution mode and to investigate whether isotopic exchange using enriched stable isotopes can provide quantitative results for CH3Hg+ in the presence of DOC without the requirement of sample distillation. The accuracy and precision of the method and its applicability to replicate ultra-trace determination of CH3Hg+ in Lake Champlain is briefly illustrated.
Materials and Methods
Instrumentation
The purge and trap apparatus including a Hg gold trap for scrubbing the He purging gas, gas flowmeter, glass reaction vessels and spargers and Tenax traps and the trap desorption module were purchased from Brooks Rand (Seattle, WA). The GC column was made in-house from a 60 cm quartz tube (6 mm OD) that was heated and shaped to fit in a GC laboratory oven (Model 20, Quincy Lab. Inc., Chicago, IL); the column was silanized and packed with 15% OV3 on Chromosorb. The voltage output from trap desorption module was set to heat the Tenax trap to 200°C within 30 sec, confirmed by thermocouple readout. The GC oven was set at 85-90°C. Either He or He with 100 ppm Xe was used as the carrier gas for the GC at a flow rate of 70 ml min-1. This set-up is essentially the same as that described initially by Liang et al.(1). The outflow from the GC was mixed with an Ar stream (using the nebulizer gas supply of the ICP-MS) and then introduced directly into the ICP-MS. The use of a mixed gas (Xe in He) allows certain ICP-MS conditions (XYZ stage position and ‘nebulizer’ gas flow) to be optimized daily with the GC on-line. The ICP-MS was an Element 2 sector field instrument (Thermo Electron, Bremen, Germany) operated in low resolution mode. Instrument operating conditions are given in Table 1. Deadtime correction was performed according to Nelms et al.(11) Element specific chromatograms were exported in ASCII format and data processed in Microsoft XL.
Table 1.
GC-ICP-MS operating conditions
| Plasma gas flows (Argon) | |
| Cool | 16 L min-1 |
| Auxiliary | 0.9 L min-1 |
| Sample | 1.1-1.3 L min-1 (optimized daily) |
| GC gas flow (He, He/Xe) | |
| 0.07 L min-1 | |
| ICP-MS method parameters | |
| Isotopes measured: | 199Hg, 200Hg, 201Hg, 202Hg. |
| Mass window | 20% |
| Search window | 0 |
| Integration window | 20% |
| Samples per peak | 50 |
| Sampling time | 0.025 s |
| Runs and passes | 300, 1 |
| Total run time | 300 sec |
Mass bias and consideration of method uncertainty
Isotope ratio mass bias for high mass elements measured by high resolution ICP-MS is <1% per amu. For the ICP-MS used for these studies average mass bias for Hg201:Hg202 for presumed natural abundance samples and certified concentration standards analysed by cold vapor samples introduction was 0.6% (n= 6). Mass bias for 207Pb:206Pb analysis of the NIST certified reference material was 0.38% (n=38). We consider the main sources of uncertainty of the overall isotope dilution method to be the value of the spike concentration and measured isotope ratio in the sample. The latter is affected by carryover from the purging vessels and/or trap, methylation and demethylation reactions, mass bias and errors in processing peak area ratios within XL. The pooled standard deviation of sample measurement is influenced by all these factors. The pooled standard deviation for CH3Hg+ (expressed as a concentration) of triplicate sampling of 18 lake water samples over a period of 5 months was 0.002 ng L-1. the standard deviation of 26 blank readings over the same time period is 0.001 ng L-1. A mass bias of 0.6% on the Hg201:Hg202 would lead to an error in concentration on the order of 0.0002 ng L-1. The individual uncertainty factors have a greater effect on Hg2+ quantification where reagent blank contamination and carryover effects are greater such the pooled standard deviation for Hg2+ is 0.035 ng L-1 compared with a mass bias correction of 0.6% resulting in a concentration correction of 0.003 ng L-1. Hence we consider that effect of mass bias on overall uncertainty is small in relation to the other factors. Given the difficulties in effectively determining mass bias ‘on-line’ for this GC-ICP-MS method and its relatively small overall contribution, mass bias corrections were not applied.
Reagents
Sodium tetraethylborate (>98%) was purchased from Strem Chemicals (Newburyport, MA) as individual 1g solid samples sealed under Ar. A 1% NaBEt4 solution was made up in 100 ml 2% KOH in an Ar atmosphere. The solution was then aliquoted into 1ml vials which were immediately frozen. Individual vials of the 1% NaBEt4 were thawed immediately prior to use and kept at 4°C between usages. Once thawed, the solution was kept in use for a maximum of four hours before being discarded. The 2M acetate buffer was made from sodium acetate and pH adjusted with acetic acid. Samples were not acidified but kept dark at 4 °C and analysed after 24 hrs. Enriched stable Hg isotopes of 199Hg and 201Hg were obtained from Oak Ridge laboratories (Oak Ridge, TN) and Cambridge Isotope laboratories (Andover, MA) as HgO. An enriched 201CH3Hg+ solution was prepared by reaction of an inorganic 201Hg solution with methylcobalamin as previously described(12,13). The relative isotopic abundance and the integrity of the CH3Hg+ species were confirmed frequently by purge and trap GC-ICP-MS analysis. The concentration of total dissolved Hg in either the 199Hg2+ spike or the CH3201Hg+ and subsequent dilutions of these stock solutions were calculated by reverse isotope dilution using ICP-MS certified Hg standards (Spex CertiPrep, Metuchen, NJ) following BrCl oxidation of the CH3Hg+ solutions and quantification by cold vapor ICP-MS. A reagent grade CH3Hg+ solution (Strem Chemicals, Newburyport, MA) was serially diluted to give working solutions of natural abundance CH3Hg+. These solutions were quantified for total dissolved Hg by CV-ICP-MS following BrCl treatment by isotope dilution using the 199Hg2+.
Ethylation of water samples
To determine absolute detection limits, about 60–70 ml of deionized water was weighed into the reaction vessels and 10 μl of a 100 ng l-1 natural CH3Hg2+ and Hg2+ was added, i.e. 1 pg of each Hg species as Hg. 200 μl of acetate buffer was added and 50ul of ethylating reagent. Twenty minutes were allowed for the ethylation reaction and were followed by 20 minutes of purging. After purging, He gas was passed directly through the Tenax trap for five minutes to remove any residual moisture. After drying, the trap was analysed by thermal desorption GC-ICP-MS.
Enriched isotope equilibration experiment
To assess the kinetics of equilibration between the enriched isotope spikes and the ambient Hg species in the presence of natural organic matter (NOM) kinetic studies were conducted with three different well characterized NOM solutions. Suwannee River aquatic NOM (SR), Nordic Lake aquatic NOM (NL) and Pony Lake (Antarctica) fulvic acid (PL) were obtained from the International Humic Substances Society (IHSS, St Paul, MN) as freeze dried solids. Solutions of each NOM were made at a concentration of 10 mg l-1 i.e. approximately 5 mg l-1 DOC. Water quality data for Mallets Bay, Lake Champlain in 2007 record a mean DOC concentration of 4 mg l-1 (http://www.vtwaterquality.org/cfm/champlain/). The NOM water samples were spiked at approximately 0.11 ng l-1 CH3Hg+ and 2 ng l-1 Hg2+ and placed in the dark for seven days to equilibrate. After equilibration, 50 ml of each spiked NOM solution was pipetted into the reaction vessels. 200 ul of acetate buffer was added to these samples and an appropriate amount of the CH3201Hg2+ and 199Hg2+ for isotope dilution quantification was added. The samples were ethylated, reacted and purged as above (time 0 in the kinetics experiment). The remaining 900 ml of NOM solution were then spiked with equivalent amounts of the enriched isotope spikes per ml of sample as the 50 ml samples. The samples were not acidified but placed in the dark and analysed by purge and trap GC-ICP-MS at selected time intervals with multiple time points for the first 24 hours and continued analysis to 48 hrs or longer. Duplicate experiments were conducted for each NOM sample.
Results and Discussion
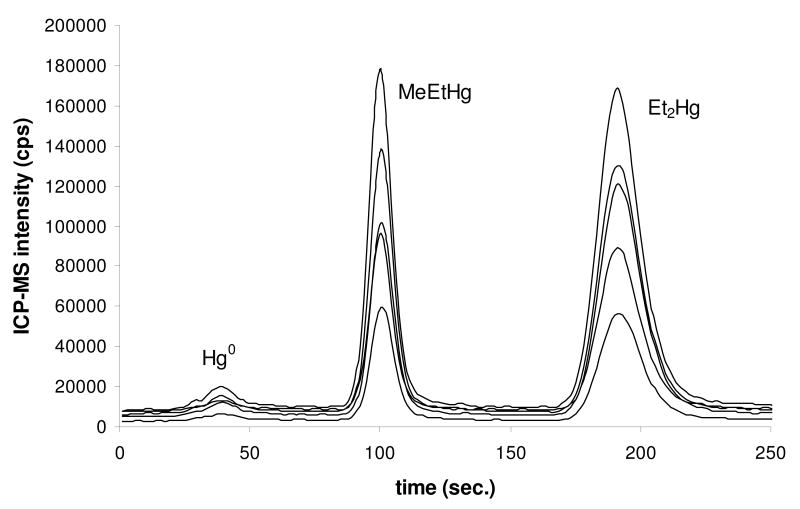
To establish instrument sensitivity and absolute detection limits a solution containing 1 picogram of natural abundance CH3Hg+ and Hg2+ was run in quadruplicate. An example GC-ICP-MS chromatogram is shown in Figure 1. Clearly even for a total Hg content of 1 pg for each species the instrumental technique has great sensitivity. For surface water samples, normal procedure uses a sample volume of about 70 ml. Using this as an example volume, Figure 1 corresponds to an aqueous concentration of 14 pg l-1 for CH3Hg+ and Hg2+. Absolute detection limits were calculated as 3σ of the chromatographic baseline noise and the 202Hg peak height value for 1 pg was used to calculate the sensitivity factor. Using these parameters the average absolute detection limit was 3 fg or, assuming a sample volume of 70 ml, 0.04 pg l-1 for each species. Instrument detection limits are a best case scenario, representing the lowest possible attainable detection limits based solely on the signal-to-noise ratio of the ICP-MS. Method detection limits, determined on multiple blank samples processed through the entire procedure (Table 2, see later discussion) are invariably higher than IDLs. Notwithstanding that fact, the use of the high sensitivity Element2 ICP-MS clearly provides excellent sensitivity for Hg speciation measurements. Instrumental sensitivity is further enhanced through use of gas chromatography (or gas phase sample introduction in general) as sample introduction for ICP-MS. This is because gas phase analysis is 100% efficient in terms of analyte transport to the ICP, which also contributes to low detection limits.
Figure 1.
purge and trap GC-ICP-MS chromatogram of 1 pg MeHg (peak @ 100 sec) and Hg2+ (peak @ 190 sec). Peaks, in order of increasing signal intensity, are Hg isotopes 198, 199, 200, 201, 202. The concentrations correspond to an aqueous concentration of 14 pg l-1 based on a sample volume of 70 mls.
Table 2.
Method parameters: Instrument detection limits, method detection limits (based on a 60 ml sample), and sample spike recoveries (n=4, spike concentrations of 0.025 ng l-1 CH3Hg and 0.25 ng l-1 inorganic Hg.
| CH3Hg | inorganic Hg | |
|---|---|---|
| IDL (ng l-1) | 0.00007 | 0.00007 |
| Blank mean (ng l-1, n=27) | 0.0023 | 0.0243 |
| Blank σ (ng l-1, n=27) | 0.0010 | 0.0180 |
| MDL (ng l-1) | 0.005 | 0.074 |
| Spike recovery (%) (RSD) | 106 (3.4) | 98 (10) |
One of the complicating factors in the analysis of Hg species in natural waters is the fact that complexation with DOC or sulfide may mean CH3Hg+ and Hg2+ are not reactive to ethylation. The incomplete recovery of CH3Hg+ from natural waters was the initial reason for the development of distillation and solvent extraction methods(14). Similarly, inorganic Hg bound by DOC has been shown to be non-reactive to reduction by SnCl2, an effect which has been used to investigate the complexation characteristics of Hg with DOC(15). For low level CH3Hg+ determination, distillation has been shown to be effective in isolating dissolved CH3Hg+ from the DOC matrix. However, species specific isotope dilution should also lead to complete quantification if the enriched stable isotope species fully equilibrates with the ambient Hg species. We conducted a kinetics experiment to establish the time for the isotope spike to equilibrate with natural Hg species complexed to three natural DOM samples. The isolated NOM samples were prepared at 5 mg l-1 DOC, which is the approximate mean concentration in Lake Champlain. Many studies have shown that in oxic surface waters both Hg2+ and CH3Hg+ are predominantly bound to thiol functional groups on NOM. Even under reducing conditions, where complexation with S2- would be predicted thermodynamically, the interaction between DOM and Hg2+ persists(16). The binding of Hg species with thiol functional groups on NOM is strong and conditional stability constants of ca. 12-17 for CH3Hg+(17-19) and 22 -32 for Hg2+(20,21) are reported for soils and natural water NOM. Synchrotron X-ray spectroscopy has shown that binding of Hg2+ to humic acid occurs with reduced S functional groups and is bidentate(22).
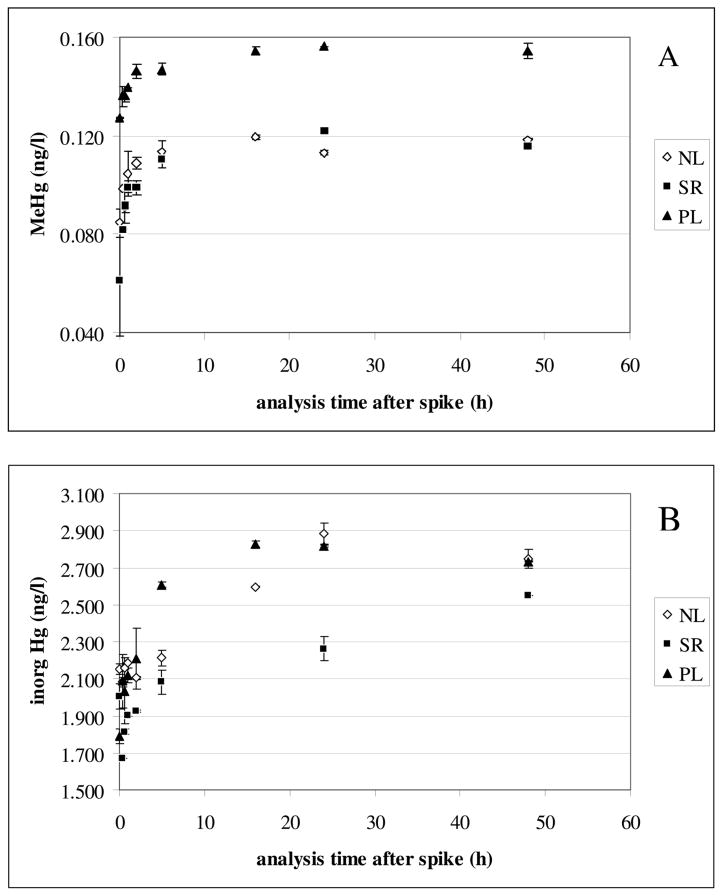
In terms of using isotope dilution for quantification of Hg species, if equilibrium of the enriched isotope spikes with the ambient Hg species is not instantaneous, the added spike will be more reactive to ethylation than the natural Hg species leading to a higher isotope ratio and a lower calculated concentration for the natural Hg species than the initial added amount. As the species spike equilibrates with the natural Hg species, the isotope ratio should decrease and the predicted concentration should approach the expected concentration. The results of this kinetic experiment are displayed in Figure 2A,B. Initially the apparent calculated CH3Hg+ concentration of both for the NL and SR solutions was 65% of the nominal added concentration. The enriched spike continued to equilibrate over the time frame of the kinetic experiment and after 10 hrs. equilibration the apparent concentration for CH3Hg+ plateau at 0.12 ng l-1, which is 109% recovery of the added concentration. This > 100% recovery may be due to uncertainties in the concentrations of the original natural abundance spikes and the enriched isotope spikes, or may simply indicate that both NL and SR contain low concentrations of CH3Hg+. The PL sample exhibited the same concentration vs. time response as NL and SR, but in the case of PL the apparent CH3Hg+ was initially 0.125 ng l-1 and approximately 0.16 ng l-1 ng l-1 from 20 hrs through the duration of the kinetic study. In this case it appears that the PL sample contains CH3Hg+, in addition to the added spike. The PL sample has the higher proportion of S (3%), therefore increased binding of CH3Hg+ could be expected. The Hg2+ data was more complex than the CH3Hg+; the time to reach a stable apparent Hg2+ concentration was longer, all three NOM samples equilibrated at a higher Hg2+ concentration than originally spiked (2 ng l-1) and there was greater variability for the time series data. Given the very high affinity of Hg2+ for NOM it is not surprising that all three NOM samples appear to contain low Hg2+ concentrations. The known stronger binding of Hg2+ with NOM explains the longer time required for equilibrium of the spike. The greater variability of the Hg2+ data is a consequence of the high uncertainty in quantification of low level Hg2+ by this method. The analysis was not conducted in a clean room and contamination and background Hg2+ are a much bigger concern than for CH3Hg+ determination. As a result of our kinetic study we established a 24hr equilibration time for the enriched isotope spikes prior to speciation analysis.
Figure 2.
Hg species concentration determined by ID-GC-ICP-MS as a function of equilibration time after the isotope spike. NL = Nordic Lake aquatic NOM, SR =Suwannee River aquatic NOM, PL = Pony Lake reference fulvic acid.
The binding of the Hg species, and hence the isotopic exchange with the enriched spikes will also be a function of pH; not only because it effects the reactivity of the thiol groups on NOM but also the overall structure of fulvic and humic acids change with pH. However, we did not pursue investigation of pH effects as it was outside the original aims of the study. In our methodology, field samples were not acidified for preservation or storage, rather they were transported back to the laboratory at the end of the collection day, and filtered and spiked within 24 hours. Acidified samples require pH adjustment to pH 5 prior to the ethylation procedure, in effect this introduces two additional sample handling stages and two reagents to be added to the sample which could have a detrimental effect on detection limits as each reagent addition has some risk of sample contamination. It has been shown that CH3Hg+ in water samples is stable without acidification for short periods (48hrs)(23). The main goal of this study was to detect low levels of CH3Hg+, therefore samples were not acidified but were processed and analysed within 48 hrs and kept dark and at 4°C in the interim.
A benefit of the ID method is excellent precision. Because the quantification calculation is based only on the isotope ratio in the sample, instrument drift and matrix suppression are accounted for. The purge and trap method for Hg speciation involves multiple steps where quantitative reaction or recovery of Hg species may not fully take place; i.e quantitative ethylation of Hg species, quantitative purging to the gas phase or trapping on Tenax. An additional source of variability is a change in ICP-MS sensitivity over the course of a run. Indeed, we have observed quite large relative standard deviations (r.s.d.) for peak areas in the Hg202 chromatograms of replicates of the same filtered water sample analysed consecutively. However, precision of the isotope ratio measurements, and hence the final calculated concentration, is excellent because the isotope dilution method can account for incomplete reaction/species recovery and changing instrumental conditions. For example, peak areas for natural CH3Hg202 and inorganic Hg202 for five replicate analysis (65 ml) of a surface water gave an ‘external’ precision based on the Hg202 peak areas for CH3Hg+ and Hg2+ of 39% and 21% r.s.d. respectively. However, the precision (r.s.d.) based on the isotope ratios Hg201:Hg202 for CH3Hg+ was 0.5% and Hg199:Hg202 for Hg2+ was 1%. Nevertheless, it is still desirable to achieve the maximum Hg signal intensity as the uncertainty of the ratio calculation will be less in this case.
Assessing the accuracy of this method for these ultra-low level concentrations is difficult because no certified reference materials are available in this concentration range (i.e. < 0.5 ng l-1) and there do not appear to be any aqueous CH3Hg+ reference materials. Instead, quality control and accuracy is assessed by spiking blank (deionized) water and Lake water samples at ca. 0.025 -0.030 ng l-1 natural abundance CH3Hg+ and 0.25 ng l-1 Hg2+. Concentrations were then calculated by ID methods. In general, excellent recovery for both the laboratory fortified blank and the sample spikes have been obtained for both CH3Hg+ and Hg2+ (Table 2).
As described earlier, instrument detection limits for this methodology are ca. 0.04 pg l-1 based on signal:noise of the data. However, a more meaningful metric for assessing the appropriate detection limits for the samples is the method detection limit (MDL) determined on the standard deviation of repeated analyses of blanks or low level samples. Method detection limit was determined by repeated analysis of blanks over a five month sampling duration. The blank was deionized water treated identically to the samples, i.e. filtered through pre-ashed quartz filters, spiked with the enriched species specific isotope spikes and equilibrated overnight. The blanks are then taken through the same analytical process as the samples. The average blank values and standard deviation for 23 blank analyses run over the course of five months by two different analysts are given in Table 2. For CH3Hg+ the mean blank concentration is 0.002 ng l-1 and a standard deviation of 0.001 ng l-1, so based on the 3σ approach this would give an MDL of 0.005 ng l-1. Our repeated analysis of Lake Champlain waters from two different sites over 5 months yielded a pooled standard deviation of 0.002 ng l-1 for 18 sampling events (2 sites × 9 samplings, n=3 for each event) calculating 3σ for the standard error of the mean in this case yields an MDL of 0.003 ng l-1. So, based either on a laboratory blank that is otherwise taken through all steps of the method, or the pooled standard deviation of replicate analysis of low level samples the MDL for CH3Hg+ is 0.003-0.005 ng l-1. For Hg2+ the mean blank value is 0.024 ng l-1 with a standard deviation of 0.018 ng l-1 resulting in an MDL of 0.078 ng L-1. The pooled standard deviation for the samples is 0.034 ng l-1 which gives a detection limit (based on standard error of the mean at 99% confidence) 0.05 ng L-1. The limit of quantification (LOQ) is often defined at 10σ, so our LOQ for CH3Hg+ is 0.01 ng L-1, while Hg2+ LOQ is considerably higher at 0.340 ng l-1. Indeed, our Lake Champlain water samples are around the LOQ for Hg2+. From this data, and the results of the kinetic study reported above, it is clear that the general uncertainty of Hg2+ determination by this method is much greater than for CH3Hg2+. However, the detection limits reported in Table 2 compare favorably with other studies. Lambertsson and Björn(24) used similar methodology (species specific isotope dilution, purge and trap GC coupled to quadrupole ICP-MS) for CH3Hg+ determination with a detection limit 0.004 ng l-1; however, the blank values for CH3Hg+ reported for the direct derivitization in that study are 0.02 ng l-1 which are 10× greater than in our method. Logar et al.(25) presented a solvent extraction method with CV-AFS detection for quantifying both CH3Hg+ and Hg2+ in natural waters with detection limits of 0.006 and 0.06 ng l-1, respectively for a 300 ml sample. The USGS Wisconsin Water Science Center using the EPA1630 methodology routinely achieve detection limits of 0.04 ng l-1 for CH3Hg+(26). The recently reported liquid chromatographic method for CH3Hg+ determination has detection limits of 0.007 ng l-1 for a 40 ml water sample(5,27). A recent comprehensive review of Hg speciation methods for water, sediments and biological tissues reports that LODs for Hg speciation in waters range from 0.004 – 5.6 ng l-1 for CH3Hg+ and 0.07 -5.2 ng l-1 for Hg2+ with results complied from 21 cited studies(28).
A number of studies have shown that the ethylation, purge and trap procedure can lead to methylation of Hg2+ as an artifact of the procedure(29). This effect is of particular importance where Hg2+ ≫ CH3Hg+ as in these cases even a small (< 1%) methylation of Hg2+ can lead to a significant over-estimation of CH3Hg+. Using species-specific enriched stable isotopes the isotope dilution calculation can be corrected for methylation and demethylation artefacts(10,30). Methylation was <1% by our methodology, however, this still had a significant effect (ca. 5%) on the final isotope dilution calculated values. Consequently all final reported concentrations were based on these double spike species specific methodologies.
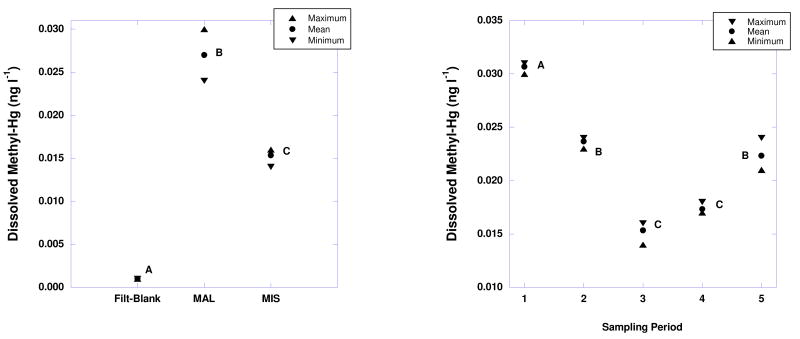
We employed the isotope dilution method to determine the temporal change in concentration of CH3Hg+ and Hg2+ in Lake Champlain for both eutrophic and oligotrophic bays. The comprehensive results of that monitoring study will be reported elsewhere; however, to illustrate the utility of this analytical method for quantifying low level CH3Hg+ in Lake Champlain the replicate speciation analysis of the eutrophic and oligotrophic bays from the May 2008 sampling are presented in Figure 3. The replicates represent true field-replicate samples (replicate 2 l sample collections) rather than repeated analysis of the same sample. Thus, the variance exhibited in Figure 3 includes both sampling and analytical sources. The high precision of ID-GC-ICP-MS readily permitted detection of statistically significant differences between the oligotrophic Mallets Bay and the eutrophic Missisquoi Bay even at concentrations well below 0.04 ng l-1, the typical detection limit of CVAFS. In all cases the sample concentrations were statistically significantly different from blank samples representing the laboratory filtration step. Figure 3 also illustrates how ID-GC-ICP-MS permitted the detection of statistically significant temporal variation in the Missisquoi Bay system during 2008, with all of the bi-weekly samplings exhibiting concentrations < 0.04 ngl-1. This highly sensitive analytical method allows the characterization of the spatial and temporal variation in mercury speciation in fresh-water systems that were not possible to investigate using previous methods.
Figure 3.
(Left) Maximum, mean, and minimum methylHg concentrations of 3 field-replicate samples from Malletts Bay (MAL) and Missisquoi Bay (MIS) of Lake Champlain June 25th, 2008. The maximum, mean, and minimum concentrations of the laboratory filtration-step blank are included for comparison. ANOVA indicated significant differences between the blanks and samples (Prob > F, <0.0001). Different letters indicate significant differences between the means based on all pairs using Tukey-Kramer HSD (p < 0.01). (Right) Maximum, mean, and minimum methylHg concentrations of 3 field-replicate samples from Missisquoi Bay, Lake Champlain over the course of 5 bi-weekly samplings in 2008. ANOVA indicated significant differences between the sampling dates (Prob > F, <0.0001). Different letters indicate significant differences between the means based on all pairs using Tukey-Kramer HSD (p < 0.01).
Acknowledgments
This research was supported by NIH Grant Number P42 ESO7373 (to BJ, VT and RAB) from the National Institute of Environmental Health Sciences.
References
- 1.Liang L, Horvat M, Bloom NS. An improved speciation method for mercury by GC CVAFS after aqueous-phase ethylation and room-temperature precollection. Talanta. 1994;41:371–379. doi: 10.1016/0039-9140(94)80141-x. [DOI] [PubMed] [Google Scholar]
- 2.Monperrus M, Tessier E, Veschambre S, Amouroux D, Donard O. Simultaneous speciation of mercury and butyltin compounds in natural waters and snow by propylation and species-specific isotope dilution mass spectrometry analysis. Anal Bioanal Chem. 2005;381:854–862. doi: 10.1007/s00216-004-2973-7. [DOI] [PubMed] [Google Scholar]
- 3.Emteborg H, Hadgu N, Baxter DC. Quality-control of a recently developed analytical method for the simultaneous determination of methylmercury and inorganic mercury in environmental and biological samples. J Anal At Spectrom. 1994;9:297–302. [Google Scholar]
- 4.Horvat M, Liang L, Bloom NS. Comparison of distillation with other current isolation methods for the determination of methylmercury compounds in low-level environmental samples. 2. Water. Anal Chimica Acta. 1993;282:153–168. [Google Scholar]
- 5.Vermillion BR, Hudson RJM. Thiourea catalysis of MeHg ligand exchange between natural dissolved organic matter and a thiol-functionalized resin: a novel method of matrix removal and MeHg preconcentration for ultratrace Hg speciation analysis in freshwaters. Anal Chimica Acta. 2007;388:341–352. doi: 10.1007/s00216-007-1207-1. [DOI] [PubMed] [Google Scholar]
- 6.Gao N, Armatas NG, Shanley JB, Kamman NC, Miller EK, Keeler GJ, Scherbatskoy T, Holsen TM, Young T, McIlroy L, Drake S, Olsen B, Cady C. Mass balance assessment for mercury in Lake Champlain. Environ Sci Technol. 2006;40:82–89. doi: 10.1021/es050513b. [DOI] [PubMed] [Google Scholar]
- 7.Christopher SJ, Long SE, Rearick MS, Fassett JD. Development of isotope dilution cold vapor inductively coupled plasma mass spectrometry and its application to the certification of mercury in NIST standard reference materials. Anal Chem. 2001;73:2190–2199. doi: 10.1021/ac0013002. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Gonzalez P, Marchante-Gayon JM, Alonso JIG, Sanz-Medel A. Isotope dilution analysis for elemental speciation: A tutorial review. Spectrochim Acta Part B-At Spectroscopy. 2005;60:151–207. [Google Scholar]
- 9.Heumann KG. Isotope-dilution ICP-MS for trace element determination and speciation: from a reference method to a routine method? Anal Bioanal Chem. 2004;378:318–329. doi: 10.1007/s00216-003-2325-z. [DOI] [PubMed] [Google Scholar]
- 10.Monperrus M, Gonzalez PR, Amouroux D, Alonso JIG, Donard OFX. Evaluating the potential and limitations of double-spiking species-specific isotope dilution analysis for the accurate quantification of mercury species in different environmental matrices. Anal Bioanal Chem. 2008;390:655–666. doi: 10.1007/s00216-007-1598-z. [DOI] [PubMed] [Google Scholar]
- 11.Nelms SM, Quetel CR, Prohaska T, Vogl J, Taylor PDP. Evaluation of detector dead time calculation models for ICP-MS. J Anal At Spectrom. 2001;16:333–338. [Google Scholar]
- 12.Perna L, LaCroix-Fralish A, Sturup S. Determination of inorganic mercury and methylmercury in zooplankton and fish samples by speciated isotopic dilution GC-ICP-MS after alkaline digestion. J Anal At Spectrom. 2005;20:236–238. [Google Scholar]
- 13.Sturup S, Chen C, Jukosky J, Folt C. Isotope dilution quantification of Hg-200(2+) and (CH3Hg+)-Hg-201 enriched species-specific tracers in aquatic systems by cold vapor ICPMS and algebraic de-convoluting. Int J Mass Spectrom. 2005;242:225–231. [Google Scholar]
- 14.Horvat M, Bloom NS, Liang L. Comparison of distillation with other current isolation methods for the determination of methylmercury compounds in low-level environmental samples. 1. Sediments. Anal Chimica Acta. 1993;281:135–152. [Google Scholar]
- 15.Lamborg CH, Tseng CM, Fitzgerald WF, Balcom PH, Hammerschmidt CR. Determination of the mercury momplexation characteristics of dissolved organic matter in natural waters with “reducible Hg” titrations. Environ Sci Technol. 2003;37:3316–3322. doi: 10.1021/es0264394. [DOI] [PubMed] [Google Scholar]
- 16.Miller CL, Mason RP, Gilmour CC, Heyes A. Influence of dissolved organic matter on the complexation of mercury under sulfidic conditions. Environ Toxicol Chem. 2007;26:624–633. doi: 10.1897/06-375r.1. [DOI] [PubMed] [Google Scholar]
- 17.Qian J, Skyllberg U, Frech W, Bleam WF, Bloom PR, Petit PE. Bonding of methyl mercury to reduced sulfur groups in soil and stream organic matter as determined by X-ray absorption spectroscopy and binding affinity studies. Geochim Cosmochim Acta. 2002;66:3873–3885. [Google Scholar]
- 18.Karlsson T, Skyllberg U. Bonding of ppb levels of methyl mercury to reduced sulfur groups in soil organic matter. Environ Sci Technol. 2003;37:4912–4918. doi: 10.1021/es034302n. [DOI] [PubMed] [Google Scholar]
- 19.Hintelmann H, Welbourn PM, Evans RD. Measurement of complexation of methylmercury(II) compounds by freshwater humic substances using equilibrium dialysis. Environ Sci Technol. 1997;31:489–495. [Google Scholar]
- 20.Khwaja AR, Bloom PR, Brezonik PL. Binding constants of divalent mercury (Hg2+) in soil humic acids and soil organic matter. Environ Sci Technol. 2006;40:844–849. doi: 10.1021/es051085c. [DOI] [PubMed] [Google Scholar]
- 21.Gasper JD, Aiken GR, Ryan JN. A critical review of three methods used for the measurement of mercury (Hg2+)-dissolved organic matter stability constants. Appl Geochem. 2007;22:1583–1597. [Google Scholar]
- 22.Hesterberg D, Chou JW, Hutchison KJ, Sayers DE. Bonding of Hg(II) to reduced organic, sulfur in humic acid as affected by S/Hg ratio. Environ Sci Technol. 2001;35:2741–2745. doi: 10.1021/es001960o. [DOI] [PubMed] [Google Scholar]
- 23.Parker JL, Bloom NS. Preservation and storage techniques for low-level aqueous mercury speciation. 2005;337:253–263. doi: 10.1016/j.scitotenv.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Lambertsson L, Bjorn E. Validation of a simplified field-adapted procedure for routine determinations of methyl mercury at trace levels in natural water samples using species-specific isotope dilution mass spectrometry. Anal Bioanal Chem. 2004;380:871–875. doi: 10.1007/s00216-004-2863-z. [DOI] [PubMed] [Google Scholar]
- 25.Logar M, Horvat M, Akagi H, Pihlar B. Simultaneous determination of inorganic mercury and methylmercury compounds in natural waters. Anal Bioanal Chem. 2002;374:1015–1021. doi: 10.1007/s00216-002-1501-x. [DOI] [PubMed] [Google Scholar]
- 26.deWild JF, Olsen M, Olund SD. Determination of methyl mercury by aqueous phase ethylation, followed by gas chromatographic separation with cold vapor atomic fluorescence detection. USGS. 2002 [Google Scholar]
- 27.Shade CW, Hudson RJM. Determination of MeHg in environmental sample matrices using Hg-thiourea complex ion chromatography with on-line cold vapor generation and atomic fluorescence spectrometric detection. Environ Sci Technol. 2005;39:4974–4982. doi: 10.1021/es0483645. [DOI] [PubMed] [Google Scholar]
- 28.Stoichev T, Amouroux D, Martin-Doimeadios RCR, Monperrus M, Donard OFX, Tsalev DL. Speciation analysis of mercury in aquatic environment. Appl Spectrosc Rev. 2006;41:591–619. [Google Scholar]
- 29.Bloom NS, Colman JA, Barber L. Artifact formation of methyl mercury during aqueous distillation and alternative techniques for the extraction of methyl mercury from environmental samples. Fres J Anal Chem. 1997;358:371–377. [Google Scholar]
- 30.Point D, Alonso JIG, Davis WC, Christopher SJ, Guichard A, Donard OFX, Becker PR, Turk GC, Wise SA. Consideration and influence of complexed forms of mercury species on the reactivity patterns determined by speciated isotope dilution model approaches: A case for natural biological reference materials. J Anal At Spectrom. 2008;23:385–396. [Google Scholar]