Abstract
Alcohol use by pregnant women is a significant public health issue despite well-described risks to the fetus including physical and intellectual growth retardation and malformations. While clinical studies are limited, they suggest that in utero alcohol exposure also results in significant immune deficiencies in naïve neonates. However, little is known about fetal alcohol exposure (FAE) effects on adult infections. Therefore, to determine the long-term effects of FAE on disease susceptibility and the adult immune system, we infected FAE adult mice with influenza virus. Herein, we demonstrate that mice exposed to ethanol during gestation and nursing exhibit enhanced disease severity as well as increased and sustained pulmonary viral titers following influenza virus infection. Secondary exposure to alcohol as an adult further exacerbates these effects. Moreover, we demonstrate that FAE mice have impaired adaptive immune responses, including decreased numbers of virus-specific pulmonary CD8 T cells, a decreased size and frequency of pulmonary B cell foci, and reduced production of influenza-specific antibody following influenza infection. Together, our results suggest that FAE induces significant and long-term defects in immunity and susceptibility to influenza virus infection and that FAE individuals could be at increased risk for severe and fatal respiratory infections.
Keywords: Lung; Influenza Virus; Fetal Alcohol Exposure; T cells, Cytotoxic; B cells
Introduction
Despite the well-described risks of alcohol use during pregnancy(1-5), alcohol abuse and binge drinking by pregnant women continues to be a significant public health problem, with an estimated 35% of pregnant women consuming alcohol each month and 1 in 9 women binge drinking during the first trimester of pregnancy(6-8). Prenatal ethanol exposure is known to cause a variety of physical and mental abnormalities in the exposed fetus including growth retardation, muscular and skeletal abnormalities, and intellectual and behavioral impairments(1-5). In addition to these developmental lesions, in utero and prenatal ethanol exposure has been shown to cause a variety of immune deficits both in humans and animal models.
Although limited, clinical research has demonstrated that in utero alcohol exposure results in an increased incidence of both minor and life-threatening bacterial infections as well as decreased white cell counts in the cord blood(9). Further, even limited alcohol use during gestation has been shown to increase a newborn’s risk for neonatal infections by 2.5-fold, while excessive alcohol abuse rendered newborns 3-4 times more likely to acquire severe neonatal infections(10). Animal models have demonstrated that in utero ethanol (EtOH) exposure can result in delayed T and B cell development, reduced lymphocyte numbers in the blood and spleen and decreased cellular responses to mitogen stimulation up to early post weaning age(4, 9, 11-18).
The results of several studies have suggested that lymphocyte numbers and proliferation in responses to mitogen stimulation are reduced also in adult rats exposed to EtOH in utero (4, 12, 13, 19). Further, young adult mice exposed to EtOH in utero also have decreased contact hypersensitivity and graft versus host responses (14), suggesting the effects of ethanol exposure on immunity can last into adulthood. Similarly, fetal alcohol exposure in humans appears to have long-term effects as in utero EtOH exposed adolescents exhibit impaired immune responses and increased susceptibility to infection (20, 21). Together these results suggest that offspring exposed to EtOH in utero may be at increased risk for infections even into adulthood.
Whereas the effects of in utero EtOH exposure are well documented in the setting of an immunologically naïve animal, little has been done to examine the effects of fetal alcohol exposure on immunity following pathogen infection, and more specifically, viral infection. It is known that adult alcoholics are at increased risk for acquiring community-acquired pneumonia and are more prone to severe disease outcomes (22-26). We have recently demonstrated that chronic consumption of EtOH as an adult likewise results in an increased severity of influenza virus infection and impaired antiviral immune responses (27). Given the current threat of potential pandemic influenza (i.e. H5N1 Avian Influenza, etc) and the constant threat of seasonal and epidemic influenza(28-30), we have determined the effects of fetal alcohol exposure on the severity and outcome of influenza virus infection. Here, we demonstrate that adult mice exposed to alcohol only during gestation and nursing have increased influenza-associated morbidity and mortality, increased pulmonary viral titers, and decreased numbers of both B cells and influenza-specific CD8 T cells in the lungs following influenza infection. Secondary exposure to alcohol as an adult significantly exacerbated these effects. Finally, we demonstrate that mice from a different inbred background that were exposed to ethanol as a fetus and during nursing 6 months previously exhibit similarly increased susceptibility to a second strain of influenza virus. These results demonstrate that the severe immune deficiencies resulting from alcohol exposure in utero and during nursing are long-lived even in the absence of further ethanol consumption, and are independent of the host and viral background.
Materials and Methods
Mice
6-7 wk old C57BL/6 and BALB/c mice were obtained from The NCI (Frederick, MD). All mice were housed and maintained in the specific pathogen free animal care facility at the University of Iowa. All experiments were performed in accordance with regulatory standards and guidelines and were approved by the Institutional Animal Care and Use Committee.
Fetal Alcohol Exposure
After 1 wk of acclimation, female mice were placed on 5% (wt/vol) EtOH (pharmaceutical grade) in the drinking water, as the only source of drinking water, for 2 wks (see Fig 1 for model). Mice were provided with solid mouse chow ab libitum at all times during the studies. After 2 wks, EtOH was increased to 10% and male mice were added for 5-7 d. Following breeding, males were removed and the females remained on 10% EtOH until parturition. At birth, litters were standardized to 6 pups/dam and EtOH was increased to 12%. After weaning, mice were maintained on water (without EtOH) until their body weight reached 17 g (∼5-7 wks). Half of the FAE mice were then conditioned onto 20% EtOH over the course of a wk (10% for 2 d, 15% for 5 d, 20% for the duration of the study) (EtOH:EtOH) while the other half remained on water (EtOH:Water). After 6 wks of chronic adult EtOH exposure (∼14-16 wks of age), mice were used for experiments. Pups from water conditioned mothers (Water:Water) as well as adult only EtOH exposed(6 wks EtOH; Water: EtOH), similar to our previous studies (27), mice were used as controls.
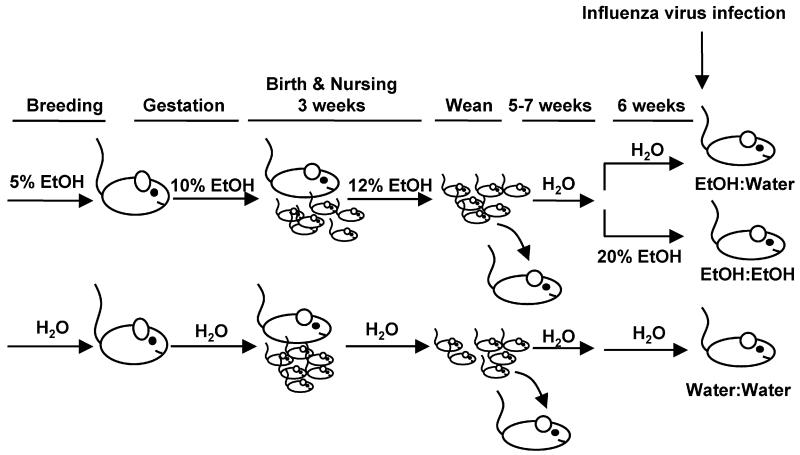
FIGURE 1.
Model of fetal alcohol exposure. Female mice were placed on 5% EtOH prior to breeding, then 10% for the duration of breeding and gestation. Following birth, the EtOH concentration was increased to 12% until weaning. After weaning, pups were placed on water until their weight reached a minimum of 17 grams (∼8-10 weeks old), then they were divided into two groups one of which was returned to EtOH (a final concentration of 20%) (EtOH:EtOH). The other group remained on water (EtOH:Water). After 6 weeks at 20% EtOH, mice were then influenza virus infected. Pups from water fed dams (Water:Water) were used as controls.
Influenza virus infection
Mouse-adapted influenza A viruses A/PR/8/34 (H1N1) and A/JAPAN/305/57 (H2N2) stocks were prepared as described(27). Mice were anesthetized with Isofluorane and C57BL/6 mice and BALB/c mice infected i.n. with a 3.2×104 pfu dose of A/PR/8/34 or a 2.0×105 pfu dose of A/JAPAN/305/57, respectively. Morbidity and mortality were monitored daily.
Pulmonary virus titer
Lungs from infected mice were rapidly homogenized and viral titers determined as previously described(33) using an endpoint dilution assay and expressed as Tissue Culture Infectious Dose50 (TCID50). Briefly, 10-fold dilutions of homogenized and clarified lung from influenza virus infected mice were mixed with 105 MDCK cells in DMEM. After 24 h incubation at 37°C, the inoculum was removed and DMEM media containing 0.0002% L-1-(tosylamido-2-phenyl)ethyl chloromethyl ketone (TPCK)-treated trypsin (Worthington Diagnostics, Freehold, NJ) and penicillin (100U/ml)/streptomycin (100mg/ml) was added to each well. After 3 d incubation at 37°C in a humidified atmosphere of 5% CO2, supernatants were mixed with an equal volume of 0.5% chicken RBC, the agglutination pattern read, and the TCID50 values calculated.
T cell analysis
Single-cell suspensions of lungs were stained with MHC tetramers or assayed for intracellular IFNγ as previously described(27). Responses to peptides PA224 and NP366 for C57BL/6 mice were measured. Tetramers were obtained from The NAIAD Tetramer Facility (Germantown, MD).
Immunohistochemistry
Lungs were inflated and fixed in PBS-Formalin on d 0, 4, 8, 10 and 14 p.i, then sectioned. Sections were deparaffinized in xylenes, rehydrated in graded alcohols. Ag unmasking was performed for B220 (citrate buffer, pH 6.0; microwave, 3 minutes x 1000 watts) and NP Ag (Proteinase K, Dako, 5 minutes). Endogenous peroxidase activity and background staining were respectively blocked with hydrogen peroxide and Fab blocker (Jackson ImmunoResearch) with background buster reagent (Innovex Biosciences). Sections were immersed with anti-mouse B220 monoclonal (Serotec, Co) or anti-NP H16 mAb. Vector Company biotinylated anti-rat, mouse absorbed reagent (B220 staining) or DAKO Mouse Envision HRP System reagent (NP staining) was applied to slides and allowed to incubate. Vector Company Vectastain Elite ABC reagent was then applied to the slides, followed by DAKO DAB Plus and then DAB Enhancer. Slides were counterstained with hematoxylin, routinely dehydrated and cover slipped.
Hemagglutination Inhibition Assay
The hemagglutination inhibition antibody titer in serum was determined by first heat inactivating 50 ul of serum at 56°C for 30 minutes. The sera were then absorbed by adding 200 μl 1% chicken red blood cell suspension (1:5 dilution of original serum sample) for an additional 30 minutes at room temperature. After incubation, the red blood cells were pelleted for 5 seconds at 14,000 rpm. Sera were then transferred to 96 well plates and diluted serially in duplicate in PBS/0.05% BSA. Influenza virus was then added to wells containing serially diluted sera, mixed, and incubated at room temperature. After 30 min, 1% chicken red blood cells were added to each well and plates were incubated at room temperature for 30-40 minutes allowing hemagglutination to occur. The HAI titers are defined as the highest serum dilution capable of preventing hemagglutination.
Results
Establishing a model of Fetal Alcohol Exposure
In humans, the majority of alcohol-exposed newborns do not exhibit the physical and behavioral changes that are often associated with Fetal Alcohol Syndrome, resulting in an underestimation of the adverse consequences of fetal alcohol exposure(10). Here, we examined the effects of ethanol (EtOH) exposure in a model that mimics fetal alcohol exposure but does not cause external physical defects. To this end, we have established a model of Fetal Alcohol Exposure (FAE) using an adaptation of the EtOH-in-drinking water mouse model of chronic EtOH exposure (27, 32). Using this EtOH feeding model, adult mice exhibit a range of immune and tissue lesions normally observed in chronic alcoholics without the overt stress response often associated with other models of EtOH exposure(32).
For the FAE model (See Fig 1), female C57BL/6 mice were exposed to 5% (w/v) alcohol-in-drinking water and mouse chow ab libitum as previously described(27, 32) for 2 wks prior to breeding. For breeding, female and male mice were placed together and alcohol was increased to 10% EtOH for 5-7 d. After a wk, the male was removed and the pregnant female remained on 10% EtOH for the length of gestation. At birth, EtOH litter sizes were culled to 6 pups to avoid competition for milk and the dam was given 12% EtOH until weaning. At these concentrations of EtOH, we did not observe any evidence of the physical malformations often associated with Fetal Alcohol Syndrome, suggesting this is an appropriate model for human Fetal Alcohol Exposure (FAE). Control pups were obtained from females provided only water (without EtOH) during breeding, pregnancy, and nursing. Litters from control dams were likewise culled to 6 pups. At weaning, all pups were placed on water and chow for approximately 5-7 wks until they were a minimum of 17 grams. Upon reaching sufficient weight, the pups were subdivided and half remained on water (EtOH:Water, also referred to as FAE mice) while the other half were conditioned as adults to EtOH over the course of a wk, reaching a final concentration of 20% EtOH(27, 32), to model FAE children who go on to chronically consume EtOH as adults (EtOH:EtOH). These mice were maintained on 20% EtOH for 6 wks prior to the initiation of the influenza experiments. Pups from water exposed mothers were age and weight matched to serve as non-EtOH exposed controls (Water:Water).
FAE results in increased influenza-associated morbidity, mortality and pulmonary viral titers
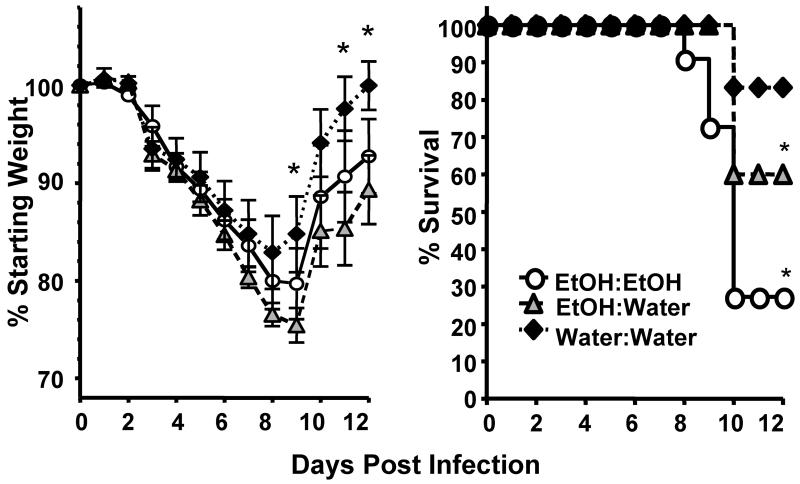
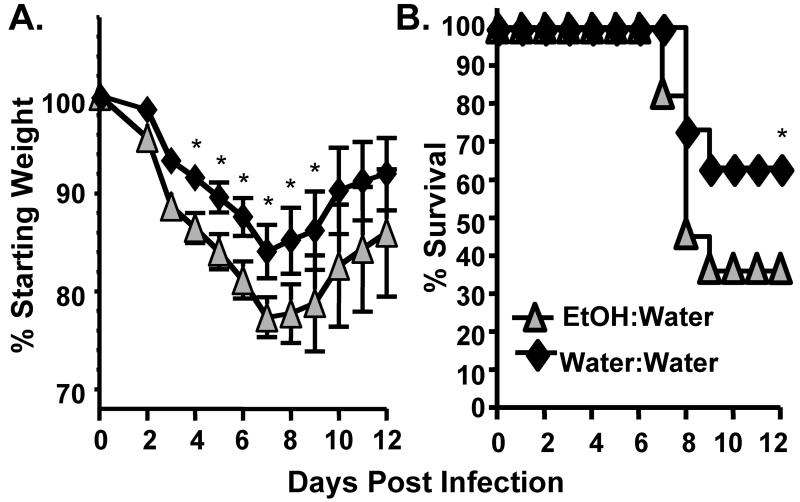
We infected adult EtOH:Water, EtOH:EtOH and water control mice with a sublethal dose of Influenza A (mouse adapted A/PR/8/34) and monitored the mice daily for morbidity (as measured by weight loss) and mortality. Alcohol exposure in utero and during nursing (EtOH:Water/FAE) resulted in significantly increased morbidity and mortality following influenza virus infection as compared to adult Water:Water controls (Fig 2). This increase in mortality was similar to that which we have previously described (27) and is observed in mice consuming ethanol for 6 wks only as adults (Water:EtOH, Supplemental Fig 1). It is important to note however, that unlike the chronic adult drinking Water:EtOH mice, the EtOH:Water FAE mice had not been exposed to EtOH in the previous 11-13 wks prior to influenza virus infection. This suggests that exposure to EtOH in utero and during nursing may cause long-term changes in pulmonary virus susceptibility and underlying immune responses. Interestingly, the increase in virus-induced mortality seen in FAE mice is exacerbated if the FAE mice are further exposed to chronic ethanol again as an adult (EtOH:EtOH, Figure 2).
FIGURE 2.
FAE results in increased influenza associated morbidity and mortality. Water:Water, EtOH:Water and EtOH:EtOH mice were infected with a sublethal dose of mouse-adapted influenza A virus and monitored daily for morbidity (left) and mortality (right). Data are pooled from 2 separate experiments, n=13-17 mice/group. *p<0.05 morbidity: EtOH:Water mice compared to Water:Water controls; mortality, EtOH:water and EtOH:EtOH groups vs. Water:Water mice.
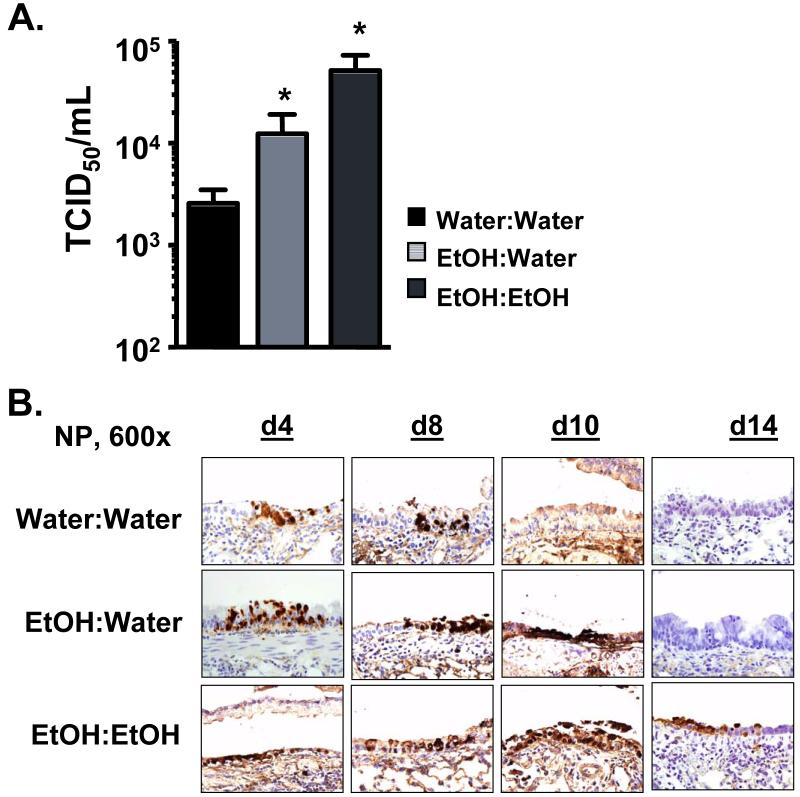
Increased morbidity and mortality following influenza virus infection is often associated with increased viral loads. In our previous studies examining the effects of adult chronic EtOH consumption on influenza immunity, we observed significantly increased pulmonary viral titers in adult EtOH mice compared to water controls(27). To determine if EtOH exposure in utero results in a similar increase in pulmonary viral titers, we again infected C57BL/6 EtOH:Water, EtOH:EtOH and Water:Water control mice with a sublethal dose of influenza virus. As shown in Figure 3, we observed significantly increased pulmonary viral titers in the lungs of both EtOH:Water and EtOH:EtOH mice as measured by end-point dilution assay in MDCK cells on d 8 p.i. (Fig 3A) or by immunohistochemistry for influenza nucleocapsid protein (NP) antigen (Fig 3B) as compared to controls on d 4-14 p.i. While Water:Water control mice cleared the majority of the influenza NP protein from the pulmonary epithelium between d 8-10 p.i. (Fig 3B), EtOH:Water mice continued to have significant levels of NP protein through d 10, suggesting a delay in the ability of the EtOH:Water mice to clear the virus. A similar increase in NP protein load on day 10 p.i. was observed in control adult only chronic EtOH consuming mice (Water: EtOH, Supplemental Fig 2). Correlated with the increased mortality demonstrated in Fig 2, EtOH:EtOH mice had significant levels of NP protein even through d 14 p.i., a time when both controls and surviving EtOH:Water mice have cleared the infection. Together, these results show that FAE EtOH exposure has a profoundly negative effect on influenza virus clearance and that additional EtOH exposure as an adult further exacerbates the length of time before viral clearance in FAE mice. Additionally, the results suggest that the enhanced mortality of EtOH:Water and EtOH:EtOH mice may relate to higher and sustained levels of virus within the lungs.
FIGURE 3.
FAE results in increased pulmonary viral titers. (A) Water:Water, EtOH:Water and EtOH:EtOH mice were infected with influenza virus and their lungs examined on d 8 p.i. for pulmonary viral titers by an MDCK cell endpoint dilution infection assay. Data are pooled from two separate experiments and represented as mean ± SEM; n=8-15/group, *p<0.05 compared to Water:Water controls. (B) Groups of mice were infected with influenza virus as in A and their lungs inflated and fixed on d 4, 8, 10 and 14 p.i. Lungs were then sectioned and stained for influenza NP antigen as described in the Material and Methods. Data are representative of one to two separate experiments, n=4-7/group
FAE results in impaired adaptive immunity following influenza virus infection
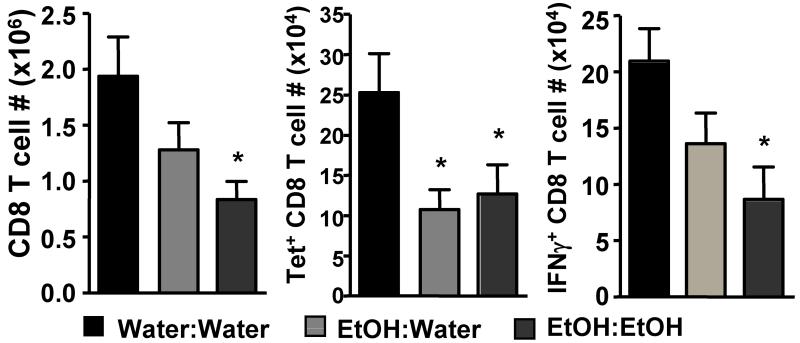
Clearance of a primary influenza virus infection requires killing of infected epithelial cells by influenza-specific cytotoxic T cells(33, 34). A range of studies have shown that in utero exposure to EtOH can reduce T cell responses (4, 12), and we have previously demonstrated that chronic adult alcohol exposure results in a significant reduction in the number of influenza-specific CD8 T cells in the lungs following influenza infection(27 and Supplemental Figure 3). Given these previous findings and the increased pulmonary viral loads observed in the lungs of the EtOH:Water and EtOH:EtOH FAE groups, we hypothesized that these mice would be similarly impaired in their ability to mount a primary influenza virus-specific CD8 T cell response. To test this hypothesis we again infected EtOH:Water, EtOH:EtOH and Water:Water control mice with a sublethal dose of influenza virus then examined the lungs on d 8 p.i. for numbers of total and influenza-specific CD8 T cells by MHC I tetramer staining and intracellular staining for IFNγ production. As shown in Fig 4, we observed reduced numbers of both total and influenza-specific CD8 T cells in the lungs of EtOH:Water mice on d 8 p.i.. EtOH:EtOH mice were even further reduced in numbers of IFNγ+ influenza-specific CD8 T cells, indicating that secondary EtOH exposure as an adult can further exacerbate immune defects that result from in utero EtOH exposure. These findings are in contrast with those reported by Jerrells and Weinberg(12). They observed a decrease in total lymphocyte counts and a decreased proliferative response to mitogen stimulation in adult rats exposed to EtOH in utero, but they did not find an exacerbation of this effect following secondary alcohol exposure as an adult(12). These contrasting results could be due to differences in the exposure model (liquid ethanol diet vs. ethanol-in-drinking water), the host used (rats vs. mice), or the mitogen versus antigen-specific responses measured. Regardless, it will be important to further examine secondary chronic EtOH exposure in additional infection models, as humans exposed to EtOH in utero are known to be at increased risk for alcohol and drug abuse later in life (3), a risk that our data suggest could result in even greater susceptibility to infections.
FIGURE 4.
Reduced pulmonary influenza-specific CD8 T cell responses in FAE mice. Water:Water, EtOH:Water and EtOH:EtOH mice were infected with influenza virus and their lungs were examined on d 8 p.i. for numbers of total (i.e. CD8a+ cells, left) and influenza-specific CD8 T cells by influenza-PA224 and NP366 peptide:MHC I tetramer binding (center) ex vivo or intracellular staining for IFNγ production (right) following a 6 hr incubation with PA224 and NP366 peptides as described in the Materials and Methods. Data are pooled from three separate experiments and represented as mean ± SEM; n=12-13/group; *p<0.05 compared to Water:Water controls
FAE is reported to impair B cell responses in the neonate(4, 11, 12), so we determined what effects in utero EtOH exposure had on B cell responses in adult lungs following influenza virus infection. FAE mice and controls were infected with influenza virus at the times shown in Fig 1, and their lungs inflated and fixed on d 10 and 14 p.i. As seen in Fig 5A, B cell foci (B220+ cells) had accumulated in the lungs of control mice on d 10 p.i. and reached significant size and number by d 14 p.i. In contrast, EtOH:Water mice had very few foci on d 10 and these continued to be reduced in both size and number on d 14 p.i. Strikingly, EtOH:EtOH mice displayed few detectable B cell foci even on d 14 p.i.. Consistent with the reduced number of B cells in the lungs, we likewise observed reduced influenza-specific antibody in the sera of FAE and EtOH:EtOH mice when compared to Water:Water controls (Fig 5B). While the level of influenza-specific antibodies in the blood did increase in the FAE mice (∼10x) from day 8 to 14 p.i., the total amount of influenza-specific antibody present was significantly reduced and represented only ∼20% of the control response. Further, similar to the paucity of B cells in the lungs of EtOH:EtOH mice, little increase in influenza-specific antibody was observed in their sera from day 8 to 14 p.i.. Control mice chronically exposed to EtOH only as adults (6wks) likewise showed a decrease in size and number of B cell foci as well as influenza-specific antibody production (Supplemental Fig 4).
FIGURE 5.
Reduced B cell immunity in FAE mice. (A) Lungs from influenza-infected Water:Water, EtOH:Water and EtOH:EtOH mice were inflated, fixed and stained for B220+ cells on d 10 and 14 p.i. Data are representative of one to two separate experiments, n=4-7/group (B) Water:Water, EtOH: Water and EtOH:EtOH mice were infected with influenza and the titer of influenza-specific antibody in the serum was determined by hemagglutination inhibition assay as described in the Materials and Methods on days 8 and 14 p.i. *p<0.05 compared to Water:Water controls. The HAI dilution represents the highest serum dilution capable of preventing influenza-virus hemagglutination of red blood cells.
Together, these results suggest that in utero EtOH exposure results in significant and long-term impairment of primary adaptive immunity (i.e. T cell and B cell responses). Protection from secondary homologous influenza virus infection is primarily mediated by antibody responses, while recovery from heterologous challenge relies on the presence of cross-reactive cytotoxic T cells. Since EtOH:Water and EtOH:EtOH mice are impaired in both primary T and B cell immunity, it will be important to determine in future studies what effects this adaptive immune cell deficiency has on the development of immune memory and the ability of the FAE mice to repel secondary homologous and heterologous influenza virus challenges in the few FAE animals which survive primary influenza virus challenge.
Long-term defects in antiviral immunity following FAE
The above experiments demonstrate that FAE results in significant impairment of adaptive immunity following influenza virus infection in young adult mice (14-16 wks of age). These results correspond well to previous work describing impaired responses to mitogen stimulation and decreased graft vs. host responses in young adult mice exposed to ethanol in utero(4, 12-14). To our knowledge, no studies have been performed in older adults to examine if the FAE-induced immune defect is sustained. Further, we have previously shown that the effects of chronic adult alcohol exposure on immunity to influenza virus are observed in multiple strains of mice following infection by multiple influenza virus strains (27). Therefore we determined if older FAE mice exhibit increased disease severity after influenza infection compared to their age-matched control counterparts, and if the effects of FAE are globally observed on multiple genetic backgrounds and during infections with differing subtypes of influenza virus. We addressed both concepts by infecting 6 month old BALB/c Water:Water and EtOH:Water mice with a 0.1 LD50 dose of Influenza A (mouse adapted A/JAPAN/305/57) and monitored the mice daily for morbidity and mortality. Strikingly, EtOH:Water mice exhibited significantly increased morbidity and mortality as compared to their age and weight matched Water:Water counterparts (Fig 6) despite the nearly 6 months since the mice were last exposed to EtOH. These results suggest that the effects of EtOH exposure in utero and during nursing last well into later stages of life, perhaps for the full life of the animal, and are not mouse or viral strain specific. It is unclear from the available literature if FAE adult humans have the same increased susceptibility to viral infections observed in our murine model, but these results underscore the need for longitudinal clinical studies to assess the effects of in utero EtOH exposure on long-term immunity.
FIGURE 6.
Effects of FAE on influenza infection in older mice. 6 month old BALB/c EtOH:Water and Water:Water mice were infected with mouse-adapted influenza (A/JAPAN/305/57) and monitored daily for morbidity (A) and mortality (B). n=11-13/group; *p<0.05 compared to Water:Water controls
Discussion
Our above results suggest that there are long-term changes in the immune response in FAE mice. It is important to note that in the latter experiments shown in Fig 6 the mice have not been exposed to ethanol in the previous 6 months. Therefore it is unlikely that the effects observed are due to direct effects of ethanol on T cells, B cells, or other immune cells such as DC and macrophages (Mϕ), as the natural turnover of these cells or seeding of substantial numbers of new naïve cells would have occurred after the removal of ethanol. Therefore our results suggest fetal and neonatal EtOH exposure alters the development of the peripheral immune system in a manner independent of lymphocyte and accessory cell turnover. Two potential explanations are epigenetic changes in immune cell genes or loss of immune organ integrity. Consistent with these theories current studies suggest that in utero exposure to EtOH results in long-term changes in the hypothalamic-pituitary-adrenal (HPA) axis through epigenetic modifications(4). Like the HPA axis, the presence and development of lymph nodes and spleens with normal architectures is dependent upon programming early during development and in the absence of key signals these organs fail to develop correctly (35-37). Consistent with this idea, studies have demonstrated that in utero exposure to ethanol inhibits the development of the thymic epithelium (4). In addition, our preliminary histologic assessment of spleens from 12 wk FAE mice indicates altered development of white pulp units, with smaller follicles and T cells zones (data not shown).
While adaptive immunity is essential for clearance of a primary influenza challenge, innate immunity also plays an important role in controlling the viral load prior to establishment of an adaptive immune response. Recently, in utero EtOH exposure has been shown to have adverse effects on the function of newborn alveolar Mϕ (aMϕ)(38, 39). Oxidative stress is increased in the fetal alcohol exposed lung, resulting in decreased aMϕ phagocytosis and increased apoptosis. In a model of Staphylococcus aureus infection, the decreased function of fetal alcohol exposed alveolar macrophages resulted in increased susceptibility to neonatal infection (39). While the role of aMϕ in influenza virus infection is not as well understood, these cells are part of the first line of defense in the lungs and are known to have an important role in cytokine production and recruitment of innate and adaptive immune cells following respiratory challenge. To date while we have not observed a decrease in aMϕ or pulmonary DC numbers in EtOH:Water FAE mice, we have observed alterations in splenic DC function (data not shown). Therefore it currently remains unknown if EtOH exposure in utero and during nursing has similar adverse effects on adult aMϕ and pulmonary DC function (38-40) and if such potential functional changes contribute to the altered adaptive immune response observed in FAE mice.
In conclusion, we demonstrate that FAE results in serious long-term impairment of both B and T cell adaptive immune responses and that this impairment results in increased risk for severe disease and death. Given the distinct requirement for adaptive immunity following primary influenza virus infections, our studies underscore the need to develop potent strategies to boost immunity and immune-mediated protection to this and potentially other important respiratory pathogens in these at risk individuals (9, 20).
Supplementary Material
Acknowledgements
We thank Dr. Steve Varga for critical reading of this manuscript.
Footnotes
This work was supported by NIH AA-014405 to R.T.C. and Department of Pathology Start-Up Funds to K.L.L.
- EtOH
- ethanol
- p.i.
- post infection
- NP
- influenza nucleocapsid protein
- HA
- influenza hemagglutinin protein
- PA
- influenza acid polymerase protein
- d
- day
- FAE
- fetal alcohol exposure
- Mϕ
- macrophage
- DC
- dendritic cell
- HPA
- hypothalamic-pituitary-adrenal
- HAI
- hemagglutinin inhibition
- aMϕ
- alveolar macrophage
Disclosures
The authors have no financial conflict of interest.
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Caruso K, ten Bensel R, The University of Minnesota experience Fetal alcohol syndrome and fetal alcohol effects. Minn. Med. 1993;76:25–29. [PubMed] [Google Scholar]
- 2.Clarren SK, Smith DW. The fetal alcohol syndrome. Lamp. 1978;35:4–7. [PubMed] [Google Scholar]
- 3.Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J. Dev. Behav. Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp. Biol. Med. (Maywood) 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- 5.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahim SH, Diekman ST, Floyd RL, Decoufle P. Comparison of binge drinking among pregnant and nonpregnant women, United States, 1991-1995. Am. J. Obstet. Gynecol. 1999;180:1–7. doi: 10.1016/s0002-9378(99)70139-0. [DOI] [PubMed] [Google Scholar]
- 7.Ebrahim SH, Luman ET, Floyd RL, Murphy CC, Bennett EM, Boyle CA. Alcohol consumption by pregnant women in the United States during 1988-1995. Obstet. Gynecol. 1998;92:187–192. doi: 10.1016/s0029-7844(98)00205-1. [DOI] [PubMed] [Google Scholar]
- 8.Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, Shankaran S, Bada HS, Walls HH, Huestis MA, Finnegan LP, Maza PL. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 9.Johnson S, Knight R, Marmer DJ, Steele RW. Immune deficiency in fetal alcohol syndrome. Pediatr. Res. 1981;15:908–911. doi: 10.1203/00006450-198106000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier TW, Drews-Botsch C, Falek A, Coles C, Brown LA. Maternal alcohol abuse and neonatal infection. Alcohol Clin. Exp. Res. 2005;29:1035–1043. doi: 10.1097/01.alc.0000167956.28160.5e. [DOI] [PubMed] [Google Scholar]
- 11.Wolcott RM, Jennings SR, Chervenak R. In utero exposure to ethanol affects postnatal development of T- and B-lymphocytes, but not natural killer cells. Alcohol Clin. Exp. Res. 1995;19:170–176. doi: 10.1111/j.1530-0277.1995.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 12.Jerrells TR, Weinberg J. Influence of ethanol consumption on immune competence of adult animals exposed to ethanol in utero. Alcohol Clin. Exp. Res. 1998;22:391–400. [PubMed] [Google Scholar]
- 13.Norman DC, Chang MP, Castle SC, Van Zuylen JE, Taylor AN. Diminished proliferative response of con A-blast cells to interleukin 2 in adult rats exposed to ethanol in utero. Alcohol Clin. Exp. Res. 1989;13:69–72. doi: 10.1111/j.1530-0277.1989.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 14.Gottesfeld Z, Christie R, Felten DL, LeGrue SJ. Prenatal ethanol exposure alters immune capacity and noradrenergic synaptic transmission in lymphoid organs of the adult mouse. Neuroscience. 1990;35:185–194. doi: 10.1016/0306-4522(90)90133-o. [DOI] [PubMed] [Google Scholar]
- 15.Basham KB, Whitmore SP, Adcock AF, Basta PV. Chronic and acute prenatal and postnatal ethanol exposure on lymphocyte subsets from offspring thymic, splenic, and intestinal intraepithelial sources. Alcohol Clin. Exp. Res. 1998;22:1501–1508. [PubMed] [Google Scholar]
- 16.Biber KL, Moscatello KM, Dempsey DC, Chervenak R, Wolcott RM. Effects of in utero alcohol exposure on B-cell development in the murine fetal liver. Alcohol Clin. Exp. Res. 1998;22:1706–1712. [PubMed] [Google Scholar]
- 17.Ewald SJ. T lymphocyte populations in fetal alcohol syndrome. Alcohol Clin. Exp. Res. 1989;13:485–489. doi: 10.1111/j.1530-0277.1989.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 18.Ewald SJ, Frost WW. Effect of prenatal exposure to ethanol on development of the thymus. Thymus. 1987;9:211–215. [PubMed] [Google Scholar]
- 19.Giberson PK, Weinberg J. Effects of prenatal ethanol exposure and stress in adulthood on lymphocyte populations in rats. Alcohol Clin. Exp. Res. 1995;19:1286–1294. doi: 10.1111/j.1530-0277.1995.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 20.Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- 21.Oleson DR, Magee RM, Donahoe RM, Falek A, Coles CD. Immunity and prenatal alcohol exposure. A pilot study in human adolescents. Adv. Exp. Med. Biol. 1998;437:255–264. [PubMed] [Google Scholar]
- 22.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch. Intern. Med. 1995;155:1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 23.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc. Am. Thorac. Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Bagby GJ, Happel KI, Summer WR, Nelson S. Pulmonary host defenses and alcohol. Front. Biosci. 2002;7:d1314–1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]
- 25.MacGregor RR, Louria DB. Alcohol and infection. Curr. Clin. Top. Infect. Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- 26.Schmidt W, De Lint J. Causes of death of alcoholics. Q. J. Stud. Alcohol. 1972;33:171–185. [PubMed] [Google Scholar]
- 27.Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. J. Immunol. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauci AS. Race against time. Nature. 2005;435:423–424. doi: 10.1038/435423a. [DOI] [PubMed] [Google Scholar]
- 29.Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 30.LaForce FM, Nichol KL, Cox NJ. Influenza: virology, epidemiology, disease, and prevention. Am. J. Prev. Med. 1994;10(Suppl):31–44. [PubMed] [Google Scholar]
- 31.Abdallah RM, Starkey JR, Meadows GG. Toxicity of chronic high alcohol intake on mouse natural killer cell activity. Res. Commun. Chem. Pathol. Pharmacol. 1988;59:245–258. [PubMed] [Google Scholar]
- 32.Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin. Exp. Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 35.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 37.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 38.Gauthier TW, Ping XD, Harris FL, Wong M, Elbahesh H, Brown LA. Fetal alcohol exposure impairs alveolar macrophage function via decreased glutathione availability. Pediatr. Res. 2005;57:76–81. doi: 10.1203/01.PDR.0000149108.44152.D3. [DOI] [PubMed] [Google Scholar]
- 39.Ping XD, Harris FL, Brown LA, Gauthier TW. In vivo dysfunction of the term alveolar macrophage after in utero ethanol exposure. Alcohol Clin. Exp. Res. 2007;31:308–316. doi: 10.1111/j.1530-0277.2006.00306.x. [DOI] [PubMed] [Google Scholar]
- 40.Wierzba-Bobrowicz T, Lewandowska E, Kosno-Kruszewska E, Lechowicz W, Skorzewska A, Gwiazda E, Pasennik E. Dendritic and microglial cells in pups of alcohol-treated female rats. Folia. Neuropathol. 2003;41:131–137. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.