Abstract
Recent clinical studies indicate an excess of angiostatic factors such as soluble endoglin (sEng) is related to the occurrence of preeclampsia. Although recent clinical studies report that sEng is increased in preeclamptic women, the mechanisms underlying its over-expression remain unclear. Evidence suggests that hypoxia and induction of heme oxygenase-1 (HO-1) have opposing effects on sEng expression, the former stimulatory and the latter inhibitory. Hence, we hypothesized that placental ischemia due to reduced uterine perfusion pressure (RUPP) in the pregnant rat would increase sEng expression, and decrease HO-1. Mean arterial pressure (MAP) was obtained via arterial catheter and serum and placental proteins were measured by western blot. MAP was increased (132±3 vs. 102±2 mm Hg; P<0.001) and fetal (2.35±0.05 vs. 1.76±0.08 g; P<0.001) and placental weight were decreased (0.47±0.04 vs. 0.58±0.03 g; P<0.01) in the RUPP compared to normal pregnant (NP) controls. Serum sEng (0.10±0.02 vs. 0.05±0.01 arbitrary pixel units (apu); P<0.05) and placental Eng (4.7±2.3 vs. 1.45±0.42 apu; P<0.05) were increased along with placental HIF1-α (1.42±0.25 vs. 0.68 ± 0.09 apu; P < 0.05) expression in the RUPP vs. the NP dams. Placental HO-1 (1.4±0.3 vs. 2.5±0.1 apu; P<0.05) expression decreased in the RUPP compared to NP dams. The present findings support our hypothesis that placental ischemia due to RUPP increases the expression of sEng and shifts the balance of angiogenic factors in the maternal circulation towards an angiostatic state. The present study provides further evidence that placental ischemia is a strong in vivo stimulus of angiostatic factors during pregnancy.
Keywords: preeclampsia, gestation, endoglin, blood pressure
INTRODUCTION
Preeclampsia is a major obstetric problem and a significant source of maternal and neonatal morbidity and mortality in contemporary pregnancies1, 2. In recent years the incidence of preeclampsia has increased approximately 40% 2. Despite the thorough characterization of the markers that constitute the preeclamptic syndrome, such as marked proteinuria, endothelial cell dysfunction and insufficient placentation3, 4, the mechanisms underlying the genesis and progression of the hypertension associated with preeclampsia remain unclear.
Recent clinical studies indicate that angiostatic factors such as soluble fms-like tyrosine kinase and soluble endoglin (sEng) may play an important role in the development and progression of preeclampsia5, 6. Endoglin (Eng) is a component of the transforming growth factor-β (TGF-β) receptor complex and is a hypoxia inducible protein associated with cellular proliferation and nitric oxide (NO) signaling 7, 8. sEng, on the other hand, has been shown to be anti-angiogenic as it is thought to impair TGF-β binding to cell surface receptors6, 7. In the pregnant rat, elevations of circulating sEng potentiates the effects of increased plasma sFlt-1 to produce a preeclampsia-like syndrome including development of hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome), reduced fetal growth, severe hypertension and nephritic range proteinuria6. In addition, Venkatesha et al. have shown that sEng inhibits in vitro endothelial cell tube formation to a similar extent as sFlt-1. Thus, there is compelling experimental evidence that compliments clinical observations that sEng is an important factor in the pathogenesis of preeclampsia.
Although recent data suggest that circulating sEng concentrations may presage the clinical onset of preeclamptic symptoms9, 10, the mechanisms underlying the increased expression of sEng remain unclear. Whether impaired placental perfusion initiates increased sEng which in turn causes endothelial dysfunction resulting in preeclamptic signs such as hypertension; or if a pathological rise in sEng production occurs independently of placental ischemia remains unknown.
Recent studies have reported that the endoglin gene is regulated by hypoxia inducible factor -1α (HIF1-α) and that both are increased in the hypoxic and preeclamptic placenta 11, 12. Interestingly, the inducible isoform of heme oxygenase (HO), HO-1 is reportedly decreased in the preeclamptic placenta 13. HO-1 is an important enzyme with a variety of roles, perhaps most importantly in the context of the present study is its role as an anti-oxidant and in protecting against vasoconstriction 14. Moreover, a recent study has shown that induction of HO-1 in vitro results in a decrease of sEng and sFlt-1 expression from trophoblast cells 15. While these reports are intriguing, it remains to be determined if the altered expression of HO-1, HIF1-α, Eng and sEng are sequelae of placental ischemia or possibly due to another genetic or environmental factor associated with preeclampsia.
Hence, the purpose of the present study was to test the hypothesis that placental ischemia produced by reduced uterine perfusion pressure (RUPP) in the pregnant rat leads to increased plasma and placental expression of sEng, along with increased placental HIF1-α expression and decreased HO-1 expression in the placenta. To this end, we employed our previously characterized and established model of RUPP hypertension, in which chronic reductions of uterine perfusion pressure lead to endothelial dysfunction and hypertension in the pregnant rat.
METHODS AND APPARATUS
Animals
Studies were performed in timed pregnant Sprague-Dawleyrats purchased from Harlan Inc. (Indianapolis Ind). Animals were housed in a temperature-controlled room (23°C) with a 12:12light:dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center. On day 14 of gestation, rat dams were randomly assigned to either RUPP (n = 12) or normal pregnant (NP; n = 10) control groups.
Reduced Uterine Perfusion Pressure (RUPP) Procedure
The RUPP procedure is a well established model for studying the link between placental ischemia and hypertension in the pregnant rat and has been described in detail previously 16–21.
Measurement of Mean Arterial Pressure (MAP) in Chronically Instrumented Conscious Rats
Animals were instrumented on day 17 of gestation and arterial pressure was determined in both groups of rats at day19 of gestation as described previously 16–21.
Conceptus Measurements & Serum Collection
After the measurement of MAP, the dams were placed under isoflourane anesthesia and a midline ventral incision was made to isolate the abdominal aorta for plasma and serum collection as reported previously 16–21. Blood was collected for subsequent assays into CorvacR sterile serum separator tubes (Sherwood Davis, St. Louis, MO). Pups and placentas were excised and weighed.
Protein Extraction and Quantitation
As described previously 21, total soluble protein was extracted in radioimmunoprecipitation assay (RIPA) lysis buffer containing phenylmethanesulphonylfluoride (PMSF) in dimethyl sulfoxide (DMSO), sodium orthovanadate and a protease inhibitor cocktail (SantaCruz Biotechnology, Inc.). Total soluble cellular protein concentration was determined using the bicinchoninic acid (BCA) method (Pierce Biotechnology).
Western Immunoblot
Western immunoblotting was performed on protein extracts from placental tissue as described previously by Gilbert et al. 21. As previously published21, the 67 kDa band corresponding to albumin observed on Ponceau-S stained membranes was used as a loading control. Western immunoblotting was performed on serum samples in the same manner with the following exceptions. Briefly, serum samples were processed using the Proteoseek spin column kit (Pierce Biotechnology, Rockford, IL) according to manufacturer’s directions. Protein (100 μg for placenta samples, 20 μl for serum samples) was separated by electrophoresis on 4–12% sodium dodecyl sulfate (SDS) polyacrylamide separating gels (NuPAGE, Invitrogen).
The membranes were incubated 1 hr in Li-COR blocking solution (Li-COR, Inc. Lincoln, NE). To measure endoglin and soluble endoglin in the placenta and serum, membranes were incubated in Li-COR blocking solution containing a commercially available antibody for Eng (1:500; Santa Cruz Biotechnology, M-20) that identified bands at ~65 kDa and ~ 100 kDa corresponding to the truncated sEng and full-length Eng, respectively. HIF-1α antibody (1:500, Novus Biologicals, Littleton, CO) or HO-1 (StressGen, Vancouver, Canada 1:2,000) overnight on a rocker at 4 C. Membranes were washed and incubated with the appropriate secondary antibodies that fluoresced at 700 and 800 nm, respectively (IRDye conjugated affinity purified antibodies, Rockland Inc. Gilbertsville, PA).
Statistical Analysis and Calculations
All data are presented as mean ± SEM. Western immunoblot data are presented as the ratio of target protein to Ponceau stained membrane and reported as arbitrary pixel units (apu). Conceptus data were calculated as mean per pregnancy. Comparisons between two groups were made with a t-test for independent samples and a Welch’s correction for unequal variances was applied when indicated and statistical significance was accepted when P < 0.05. Statistical calculations were made with GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA USA).
RESULTS
Blood pressure during late gestation
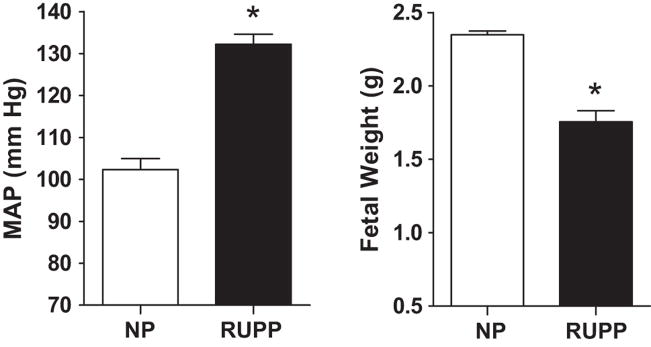
MAP (132±3 vs. 102±2 mm Hg; P<0.001; Figure 1A) was increased in the RUPP dams when contrasted to the NP dams.
Figure 1. Blood pressure (A) and fetal weight (B) during late gestation.
Resting mean arterial pressure (MAP) was increased (P < 0.001; panel A) in the reduced uterine perfusion pressure (RUPP, n = 12) dams when contrasted to the normal pregnant (NP, n = 10) dams. RUPP (n = 12) fetuses were 25% lighter (P < 0.05) than fetuses from NP (n = 10) dams fetal (panel B).
Conceptus morphometrics
RUPP fetuses were 25% lighter than fetuses from NP dams fetal (2.35±0.05 vs. 1.76±0.08 g; P<0.001; Figure 1B). Placental weight was decreased 19% (0.47±0.04 vs. 0.58±0.03 g; P<0.01) in the RUPP compared to normal pregnant (NP) controls. The number of implantation sites in the uteri was not different between the RUPP and NP groups (15 ± 1 vs. 15 ± 1). The number of viable fetuses at dGA 19 was lower in the RUPP pregnancies compared to the NP pregnancies (6 ± 1 vs. 15 ± 1; P < 0.05).
Effect of placental ischemia on expression of sEng and Eng in serum and placenta
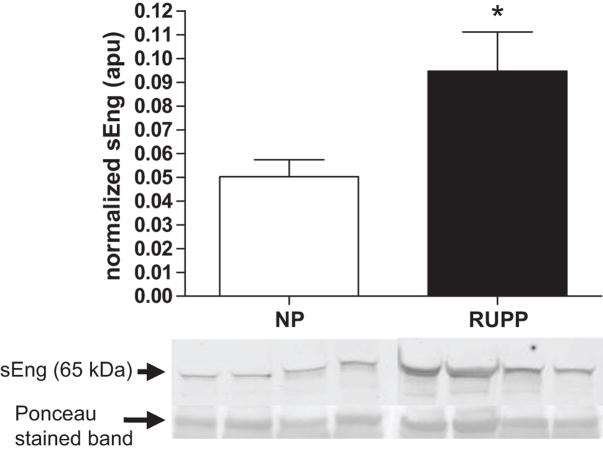
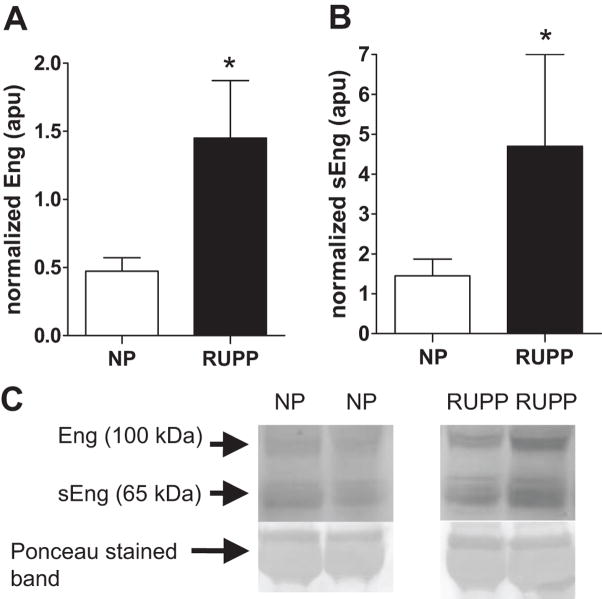
Figure 2 illustrates that the truncated form of Eng, sEng (P<0.05) was increased in the serum of RUPP compared to the NP dams. Figure 3, panels A and C, show that full-length placental Eng (P<0.05) was increased in the RUPP compared to the NP dams. Figure 3, panels B and C, illustrate that sEng was increased in the RUPP compared to the NP control placentas.
Figure 2. Effect of placental ischemia on expression of serum sEng.
Circulating sEng was increased in the serum of reduced uterine perfusion pressure (RUPP, n = 12) compared to the normal pregnant (NP, n = 10) dams. sEng blot data is expressed relative to the 67 kDa band corresponding to albumin on Ponceau stained membranes.
Figure 3. Effect of placental ischemia on expression of sEng and Eng in placenta.
Figure 3A shows that placental Eng and Figure 3B illustrates that immunoreactive sEng relative to the 67 kDa band corresponding to albumin on Ponceau stained membranes was increased in the reduced uterine perfusion pressure (RUPP, n = 12) compared to the normal pregnant (NP, n = 10) control placentas.
Effect of placental ischemia on expression of placental HIF-1α
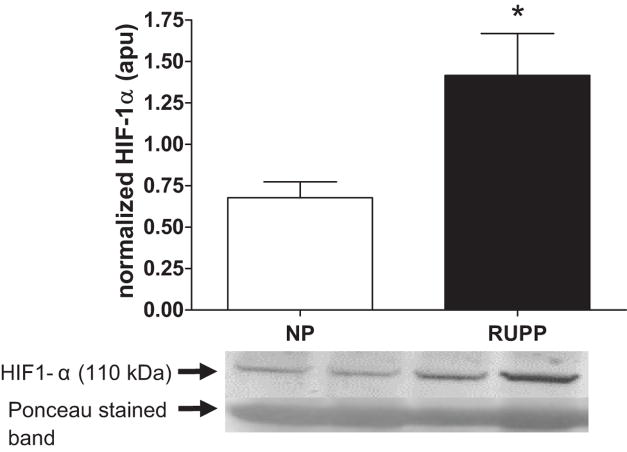
Figure 4 shows that placental HIF1-α expression was increased (P < 0.05) in the RUPP vs. the NP dams.
Figure 4. Effect of placental ischemia on expression of placental HIF-1α.
Figure 4 shows that immunoreactive placental HIF1-α relative to the 67 kDa band corresponding to albumin on Ponceau stained membranes were increased in the reduced uterine perfusion pressure (RUPP, n = 12) vs. the normal pregnant (NP, n = 10) dams.
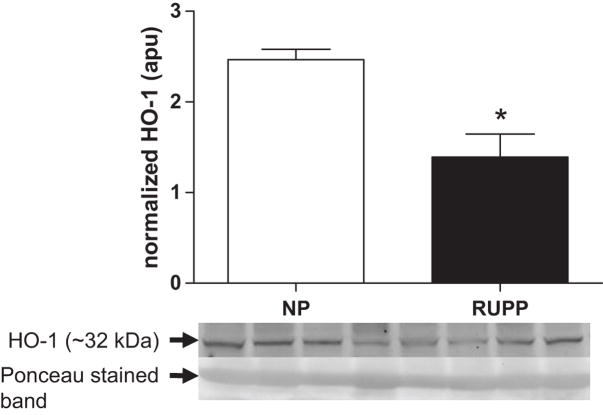
Effect of placental ischemia on expression of placental HO-1
Figure 5 illustrates the differences observed between RUPP and NP rats with respect to the expression of HO-1 in the placenta. Placental HO-1 expression was decreased (P<0.05) in the RUPP compared to NP dams.
Figure 5. Effect of placental ischemia on expression of placental HO-1.
Reduced uterine perfusion pressure (RUPP, n = 12) dams had reduced (P<0.05) immunoreactive HO-1 relative to the 67 kDa band corresponding to albumin on Ponceau stained membranes in placental lysates than did normal pregnant (NP, n = 10) control rats with respect to the expression of HO-1 in the placenta.
DISCUSSION
The present study reveals several interesting and novel findings regarding the relationship between placental ischemia, hypertension and alterations in angiogenic factors in the late gestation pregnant rat. Foremost, we report that circulating sEng expression is increased in the serum and placenta of pregnant rats with ischemic placentas when compared to pregnant control rats with non-ischemic placentas. We also demonstrate that immunoreactive HIF-1α is increased while immunoreactive HO-1 is decreased in the placenta of the RUPP dams with placental ischemia when contrasted with the NP control dams. Hence, the present study is the first to report elevated serum sEng concentrations and increased placental HIF-1α expression in a reproducible and well characterized animal model of hypertension that spontaneously arises following placental ischemia.
Our results show that maternal serum levels of sEng were increased along with increased levels of immunoreactive sEng in the placenta. While the fold increase in circulating levels of sEng we observed between the RUPP rat model of preeclampsia and normal pregnant rats (2 fold increase) are not as high as those reported in severely preeclamptic humans (2.5 fold increase), it is similar to the increase reported previously between normal pregnant and moderate preeclamptic (2 fold increase) women 6. Further, the present findings are in agreement with our previous observations regarding sFlt-1 expression that suggest the RUPP model has robust similarities to moderate preeclampsia21.
Previous studies have reported that the increase in sEng and sFlt-1 presage the onset of preeclamptic symptoms but the mechanism underlying the increased serum levels remained unclear 10, 22. The present data suggest that RUPP and placental ischemia during pregnancy is a stimulus for placental production and secretion of sEng. Indeed, previous authors have suggested that the primary source of circulating sEng in preeclamptic humans derives from the uteroplacental unit 6. While the study performed by Venkatesha et al. demonstrated that sEng plays a role in the pathogenesis of several manifestations of the preeclamptic syndrome (e.g. HELLP syndrome, hypertension and proteinuria), their study did not reveal any potential mechanisms for the pathological increase of sEng in preeclamptic pregnancies. Taken together, these studies suggest that the increase in circulating sEng observed in preeclampsia may be a consequence of aberrant placental perfusion and contribute to the pathogenesis of several characteristics of the preeclamptic syndrome.
In accord with our previous findings, we found that MAP was increased and fetal weight was decreased in the present cohort of RUPP dams. These findings reiterate the robust nature of this model of hypertension associated with placental ischemia. Moreover, these data agree with previous findings that adenoviral overexpression of sEng and sFlt-1 results in fetal growth restriction in the pregnant rat 6.
In the present study we show that HIF1-α is increased along with sEng in the placenta of pregnant rats with reduced uterine perfusion. While the preeclamptic placenta has been shown to express increased levels of sEng, HIF1-α and sFlt-1, under in vitro culture conditions and using trophoblast cells there appears to be a disconnect between HIF1-α and sEng 23. This may indicate that factors elaborated by other parts of the placenta or elsewhere in the mother play an important role in the regulation of sEng and in vivo. Viewed together with our previous findings that a reduction in utero-placental blood flow24 results in increased placental sFlt-1 21, the present data suggest that an untimely and excessive decrease in placental PO2 may underlie the pathological over-expression HIF-1α and placental and serum sEng.
We also report that HO-1 expression is reduced in the RUPP placenta. Previous studies have reported conflicting data suggesting that HO-1 expression may be decreased 13 or increased 25 in the placenta of preeclamptic women and decreased 26 or unchanged 27 due to hypoxia in vitro. One study has also reported that while HO-1 may not be induced by hypoxia, enzyme activity may be decreased 27. Interestingly, preivous work has indicated that HO-1 induction may be suppressed by factors such as interferon γ(IFNγ)28, 29. Moreover, Cudmore et al. have recently reported that induction of HO-1 negatively regulates sEng and sFlt-1 expression in vitro 15. Viewed in concert, it appears there may be enough evidence to hypothesize a role for decreased expression or activity of HO-1 in the pathologic over expression of anti-angiogenic molecules in the setting of placental ischemia during preeclampsia. Further studies are planned to investigate this possibility.
PERSPECTIVES
While there is increasing evidence supporting a role for anti-angiogenic factors in the pathogenesis of preeclampsia, the sequence of events leading to the increase of these factors has remained unclear. The present study which relies on data gathered by employing a well characterized and robust animal model of preeclampsia provides further evidence that placental ischemia is a primary factor in the pathogenesis of preeclampsia by initiating the generation and secretion of sEng from the placenta.
While the present study does not elucidate the mechanism underlying the production and secretion of sEng as a result of placental ischemia, our data does reveal several intriguing possibilities for mediation of this pathway. Clearly, it appears that HIF-1α is involved and is a likely candidate to be a molecule playing a central regulatory role. Suppression of HO-1 expression, possibly due to elevated cytokines such as IFNγ may play a permissive role in the over expression of sEng and sFlt-1 as it is a central mediator of responses to hypoxia. Alternatively, the present work suggests that stimulation of TGF-β signaling may hold potential as a possible intervention in preeclamptic pregnancies. In summary, the present work provides a platform from which other studies may be launched to elucidate underlying mechanisms and develop efficacious interventions for preeclampsia.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by National Institutes of Health grants HL51971 and HL90269.
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S) STATEMENT
None.
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 5.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Correlation between Soluble Endoglin, Vascular Endothelial Growth Factor Receptor-1, and Adipocytokines in Preeclampsia. Journal of Clinical Endocrinology Metabolism. 2007;92:2672–2679. doi: 10.1210/jc.2006-2349. [DOI] [PubMed] [Google Scholar]
- 6.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 7.Jerkic M, Rivas-Elena JV, Prieto M, Carron R, Sanz-Rodriguez F, Perez-Barriocanal F, Rodriguez-Barbero A, Bernabeu C, Lopez-Novoa JM. Endoglin regulates nitric oxide-dependent vasodilatation. The FASEB Journal. 2004:03–0197fje. doi: 10.1096/fj.03-0197fje. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Issa R, Kumar P, HAMPSON IN, Lopez-Novoa JM, Bernabeu C, Kumar S. CD105 prevents apoptosis in hypoxic endothelial cells. Journal of Cell Science. 2003;116:2677–2685. doi: 10.1242/jcs.00470. [DOI] [PubMed] [Google Scholar]
- 9.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 10.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential Changes in Antiangiogenic Factors in Early Pregnancy and Risk of Developing Preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin Expression Is Regulated by Transcriptional Cooperation between the Hypoxia and Transforming Growth Factor-beta Pathways. Journal of Biological Chemistry. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Lewis DF, Wang Y. Placental Productions and Expressions of Soluble Endoglin, Soluble fms-Like Tyrosine Kinase Receptor-1, and Placental Growth Factor in Normal and Preeclamptic Pregnancies. Journal of Clinical Endocrinology Metabolism. 2008;93:260–266. doi: 10.1210/jc.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed A, Rahman M, Zhang X, Acevedo CH, Nijjar S, Rushton I, Bussolati B, St JJ. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol Med. 2000;6:391–409. [PMC free article] [PubMed] [Google Scholar]
- 14.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 15.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative Regulation of Soluble Flt-1 and Soluble Endoglin Release by Heme Oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 16.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 17.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 18.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2001;38:742–745. doi: 10.1161/01.hyp.38.3.742. [DOI] [PubMed] [Google Scholar]
- 19.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43:832–836. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert JS, Dukes M, LaMarca BB, Cockrell K, Babcock SA, Granger JP. Effects of Reduced Uterine Perfusion Pressure on Blood Pressure and Metabolic Factors in Pregnant Rats. American Journal of Hypertension. 2007;20:686–691. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 22.Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. American Journal of Obstetrics and Gynecology. 2008;199:266. doi: 10.1016/j.ajog.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 23.Jeyabalan A, McGonigal S, Gilmour C, Hubel CA, Rajakumar A. Circulating and Placental Endoglin Concentrations in Pregnancies Complicated by Intrauterine Growth Restriction and Preeclampsia. Placenta. 2008;29:555–563. doi: 10.1016/j.placenta.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol. 2007;293:H2080–H2084. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
- 25.Eide IP, Isaksen CV, Salvesen K+, Langaas M, Sch++nberg SA, Austgulen R. Decidual expression and maternal serum levels of heme oxygenase are increased in pre-eclampsia. Acta Obstetricia et Gynecologica Scandinavica. 2008;87:272–279. doi: 10.1080/00016340701763015. [DOI] [PubMed] [Google Scholar]
- 26.Newby D, Cousins F, Myatt L, Lyall F. Heme oxygenase expression in cultured human trophoblast cells during in vitro differentiation: effects of hypoxia. Placenta. 2005;26:201–209. doi: 10.1016/j.placenta.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Appleton SD, Marks GS, Nakatsu K, Brien JF, Smith GN, Graham CH. Heme oxygenase activity in placenta: direct dependence on oxygen availability. Am J Physiol Heart Circ Physiol. 2002;282:H2055–H2059. doi: 10.1152/ajpheart.01084.2001. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Nakayama M, Takeda K, Fujita H, Shibahara S. Suppression of Heme Oxygenase-1 mRNA Expression by Interferon-+| in Human Glioblastoma Cells. Journal of Neurochemistry. 1999;72:2356–2361. doi: 10.1046/j.1471-4159.1999.0722356.x. [DOI] [PubMed] [Google Scholar]
- 29.Udono-Fujimori R, Takahashi K, Takeda K, Furuyama K, Kaneko K, Takahashi S, Tamai M, Shibahara S. Expression of heme oxygenase-1 is repressed by interferon-+| and induced by hypoxia in human retinal pigment epithelial cells. European Journal of Biochemistry. 2004;271:3076–3084. doi: 10.1111/j.1432-1033.2004.04241.x. [DOI] [PubMed] [Google Scholar]