Abstract
Risperidone induces significant weight gain in female mice; however, the underlying mechanisms related to this effect are unknown. We investigated the effects of risperidone on locomotor activity, core body temperature, and uncoupling protein (UCP) and hypothalamic orexin mRNA expression. Female C57BL/6J mice were acclimated to individual housing and randomly assigned to either risperidone (4 mg/kg BW*day) or placebo (PLA). Activity and body temperature were measured over 48-hour periods twice a week for 3 weeks. Food intake and body weights were measured weekly. UCP1 (BAT), UCP3 (gastrocnemius), and orexin (hypothalamus) mRNA expressions were measured using RT-PCR. Risperidone-treated mice consumed more food (p=0.050) and gained more weight (p=0.0001) than PLA-treated mice after 3 weeks. During the initial 2-days of treatment, there was an acute effect of treatment on activity (p=0.046), but not body temperature (p=0.290). During 3 weeks of treatment, average core body temperatures were higher in risperidone-treated mice compared to controls during the light phase (p=0.0001), and tended to be higher during the dark phase (p=0.057). Risperidone-treated mice exhibited lower activity levels than controls during the dark phase (p=0.006); there were no differences in activity during the light phase (p=0.47). UCP1 (p<0.01) and UCP3 (p<0.05) mRNA expressions were greater in risperidone-treated mice compared to controls, whereas, orexin mRNA expression was lower in risperidone-treated mice (p<0.01). These results suggest that risperidone-induced weight gain in mice is a consequence of increased energy intake and reduced activity, while the elevation in body temperature may be a result of thermogenic effect of food intake and elevated UCP1, UCP3, and a reduced hypothalamic orexin expression.
Keywords: Antipsychotic, Weight Gain, Mice, Body Temperature, Locomotor Activity
1. Introduction
Risperidone, an atypical-antipsychotic drug (AAD) with lower incidence of extrapyramidal side effects compared to typical antipsychotic drugs, is commonly used to treat schizophrenia and bipolar disorder [1]. Unfortunately, AADs, including risperidone, cause weight gain and may exacerbate risk factors for obesity-related metabolic disorders such as type 2 diabetes [2–5]. Although reported less frequently, other side effects related to risperidone treatment include fatigue/tiredness, hyperprolactinemia, akathisia (motor restlessness), and body temperature dysregulation [1;2]. The underlying mechanisms responsible for risperidone-induced weight gain are not clearly defined. A better understanding of the pathways involved in these metabolic related side effects would provide groundwork for clinical application in patients treated with risperidone and other AADs.
Patients with schizophrenia are as overweight and obese as the general population [6] and body mass index increased dramatically and significantly among young women with schizophrenia between 1987 and 1996 [7]. Due to this underlying risk for obesity among people with mental illness, it is difficult to distinguish between the disease effect and the drug effect on weight gain in patients treated with AADs. To investigate the underlying drug effect without the presence of disease, we developed a mouse model in which normal weight female C57BL/6J mice treated with AADs gain significant body weight relative to PLA-treated mice [4]. Previous reports investigating the effects of AAD-induced weight gain in animals suggest that AAD-induced weight gain is due primarily to an increase in energy intake [3;4;8]; however, few reports have monitored core body temperature and activity during AAD treatment.
Body temperature regulation (thermoregulation) is important for maintaining body mass homeostasis, especially in small rodents [9]. Rodents [10] and humans [11] possess various abilities to engage in adaptive thermogenesis, suggesting a genetic component for defending body weight. In rats given risperidone, body temperature decreased significantly compared to controls and this effect was seen over a 21-day period without weight gain [12]. In humans, risperidone treatment has been associated with both hyperthermia [13] and hypothermia [14] and in a few cases neuroleptic malignant syndrome with extremely high fever and muscle rigidity [15]. The effect of risperidone on thermoregulation is possibly related to interactions with dopaminergic and serotoninergic receptors or other receptors in the CNS, but the specific mechanisms of how these temperature modifications affect body weight are not known.
Uncoupling proteins (UCPs), including UCP1 and UCP3, have been investigated for their possible roles in body temperature regulation and energy balance. UCP1, found predominately in brown adipose tissue (BAT), dissipates the pH-gradient generated by oxidative phosphorylation, releasing chemical energy as heat [16]. UCP1 expression in BAT is decreased in most genetic and hypothalamic obese animals [16;17] and overexpression of UCP1 in skeletal muscle decreases the percentage of fat in transgenic mice [18]. In contrast, mice lacking UCP1 in BAT are not obese on standard chow or high-fat diets [19;20]. UCP3, mainly expressed in skeletal muscle, is up-regulated during fasting [21] and may be involved with the facilitation of fatty acid oxidation in muscle [22]. UCP3 knockout mice do not become obese on high fat diets [23]. Most reports do not associate UCP3 with thermogenesis and the exact biological function of UCP3 remains unknown. UCPs may be involved with body temperature alterations and energy balance in risperidone-treated mice. However, there are no previous reports investigating UCP mRNA expressions in mice administered risperidone.
Weight gain results from an increase in food intake and/or a decrease in energy expenditure, including resting energy expenditure and activity related energy expenditure [24]. Risperidone’s effects on locomotor activity are not well documented. Amphetamine-induced locomotor activity in hippocampal-lesioned Sprague-Dawley rats was prevented with risperidone treatment [25]. Conversely, BALB/c mice treated with amphetamine to produce hyperactivity and self-injurious behavior do not show reduced activity when treated with risperidone, but the treatment does alleviate self-injurious behavior [26]. Risperidone treatment reduced hyperactivity in children with bipolar disorder, autism, and attention-deficit/hyperactivity disorder [27;28]. Risperidone’s activity-related effects may be associated with increased risk for weight gain in patients treated with risperidone, particularly if the reduced activity decreases total energy expenditure.
Orexins, neuropeptides located in the hypothalamus, have been associated with regulation of the sleep/wake cycle, feeding behavior, spontaneous physical activity, and thermogenesis [29]. Orexin knockout (KO) mice are unable to reduce their body temperature during inactivity (light phase) and have a phenotype of narcolepsy [30]. This inability of orexin KO mice to reduce body temperature may provide an explanation for fragmented sleep during narcolepsy, because heat loss is an essential aspect of sleep. Body temperatures were not different between orexin KO mice and wild-type (WT) littermates during sustained wakefulness (dark phase); however, locomotor activity was lower in orexin KO mice compared to wildtypes during wakefulness [30]. Rats administered orexin A along with risperidone experienced elevated colonic temperature and heart rate [31]. Risperidone may cause alterations in the gene expression of orexin in the hypothalamus; however, there are no published data showing this relationship.
In this study, we use our mouse model of risperidone-induced weight gain to determine the effect of risperidone on food intake, core body temperature, and locomotor activity. we hypothesized that risperidone-induced weight gain would be associated with increased food intake, decreased body temperature, and decreased activity in mice housed at 22°C (ambient temperature) during three weeks of treatment.
2. Materials and methods
2.1 Animals and experimental overview
Eight-week-old female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) were group housed for one week and then housed individually in a temperature (22.0 ± 1.0 °C) USA) with a 12/12 hour light/dark cycle throughout the experiment. Mice were fed a low-fat condensed milk diet (Research Diets, New Brunswick, NJ, USA; D12489B) and given water ad libitum. At ten weeks of age, mice had transmitters surgically implanted to measure activity and body temperature (see below). Following surgery, mice were acclimated to plain peanut butter pill administration (placebo; twice a day; 0900 and 1500 hours) for two weeks. After the first week of placebo administration, mice were weighed and randomly assigned to placebo or risperidone-treated groups. All experiments were conducted on a single set of 12 mice (6/group). Risperidone treatment started when mice were 12-weeks-old and ended after three weeks. All procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
2.2 Surgery, activity and body temperature measurements
In order to measure activity counts and body temperatures, 10-week-old mice had transmitters (Respironics, Bend, Oregon, USA; PDT-4000 E-mitters,) implanted into their abdominal cavity. Mice were anesthetized using isoflurane (2%), the abdomen shaved and wiped with alcohol and betadine and a small incision (1–2 cm) was made, the transmitter was inserted into the abdominal cavity and the abdominal wall and skin were sutured. Following surgery, mice were monitored for two weeks to insure complete recovery before starting the activity experiment [32]: recovery was defined as a return to pre-surgery weight.
Activity and body temperature were measured using Mini-mitter telemetry set-ups (Respironics, Bend, Oregon, USA; ER-4000 telemetry system) at baseline (two days prior to treatment) and during the three weeks of treatment. Mice remained in their home cage and were placed on individual monitoring bases for two 48-hour periods weekly (six measurement periods over the 3 weeks). Each measurement started at 1500 hours and ended 48 hours later. Data was tabulated using Labview (National Instruments, Austin, TX, LabVIEW Version 5.0) and collected into 60-second bins throughout the 48 hours. Software errors were corrected by eliminating activity counts that exceeded 12 counts within a 1-second interval as suggested by the manufacturer. Body temperature was averaged into 1-hour periods for each animal, and then was averaged for the two groups. Activity counts were averaged into counts/minute for each animal, and then were averaged for the two groups.
2.3 Food intake and body weights
Baseline food intake and body weights were measured during a one-week period before treatment initiation. During the three-week experiment, food intake and body weights were measured weekly (weights were measured at 1000 hrs).
2.4 Drug treatment
Mice were treated twice a day orally with peanut butter pills at 0900 and 1500 hrs with risperidone-treated mice receiving 4 mg/kg˙BW˙ day (each pill contained ½ the daily dose). This dose was based on a concentration that induced the greatest weight gain in an optimal dosing experiment [4]. As previously described, risperidone (Toronto Research Chemicals; North York, Ontario, Canada) was dissolved in heated (37 °C) peanut butter (Jif, Cincinnati, OH, USA) to achieve the target dosage, mixed for approximately 15 min, placed into plastic molds (Corticosterone pellet molds, Ted Pella, Inc., Redding, CA, USA) to form individual pills (each weighing approximately 100 mg), and stored at −80 °C until used for treatment.
2.5 Real time RT-PCR
Real time RT-PCR was conducted as previously described [33]. Mice were euthanized by decapitation and trunk blood collected. BAT, gastrocnemius muscle, and hypothalamus tissue “blocks” (1.5mm wide across the midline and 1.5mm thick bordered anteriorly by the optic chiasm and posteriorly by the mammillary bodies) were rapidly collected, frozen in liquid nitrogen, and stored at −80°C. Total RNA was prepared using TRIzol reagent (Life Technologies, Gathiersburg, MD) according to the manufacture’s directions after which cDNA was synthesized using SuperScript III kit (Invitrogen). Multiplex Real Time RT-PCR was carried out using LUX-labeled oligonucleotide primers (Invitrogen) analyzed on a Chromo4 Instrument (MJ Research) for each gene of interest with Opticon MonitorTM software version 3.0. Primers for mouse UCP1, UCP3, and orexin were as follows: UCP1 sense 5’-GGT CAG AAT GCA AGC CCA GAG-3’; UCP1 antisense 5’-CGG GTG TAA GCA TTG TAG GTC CCFAMG-3’; UCP3 sense 5’- CGC CAA CTC CAT CAT TAA CTG CGGG FAM G -3’; and UCP3 antisense 5’-GGG ACG TTG TCT GTC ATC AGC-3’; orexin sense primer 5’-GCT CCG TGC AAC AGT TCG TA-3’; orexin antisense primer: 5’-CAG CAA GCC TCT GCC CGA CTG CFAM G-3’. PCR conditions were 50°C for 2min, 95°C for 2 min and then 45 cycles of 95°C for 15 sec, 60°C for 45 sec and 72°C for 30 sec. Certified housekeeping primer sets were purchased for the housekeeping genes (Invitrogen). The threshold cycle (CT value) corresponding to exponential growth of PCR product during the log-linear phase for both the target gene (FAM labeled) and internal reference gene (b-actin or GAPDH, JOE labeled) were calculated and analyzed for each sample in triplicate using the deltaCT comparative method to determine relative expression levels. Amplification efficiencies and assay validations were determined from control standard curves for both the gene of interest and the reference RNA generated for each 96-well assay. Coefficient of variation was kept at ≤3%.
2.6 Statistical analyses
All statistical analyses were performed using SAS (Version 8; SAS Institute Cary, NC). Repeated measures analysis of variance (ANOVA) was used to determine if there were significant differences in food intake, body weight, body temperature, and activity levels between PLA- and risperidone-treated mice over time, and post-hoc Bonferoni correction was applied for the multiple comparisons in body temperature and activity. One-way ANOVA was used to determine differences between risperidone- and PLA-treatment on the relative expression of UCP1, UCP3, and orexin in BAT, gastrocnemius muscle, and hypothalamus, respectively. Data are reported as mean ± standard error of the mean (SEM). The criterion for statistical significance was p≤0.05 or adjusted by Bonferoni correction if necessary and exact p values are reported.
3. Results
3.1 Food intake and body weight
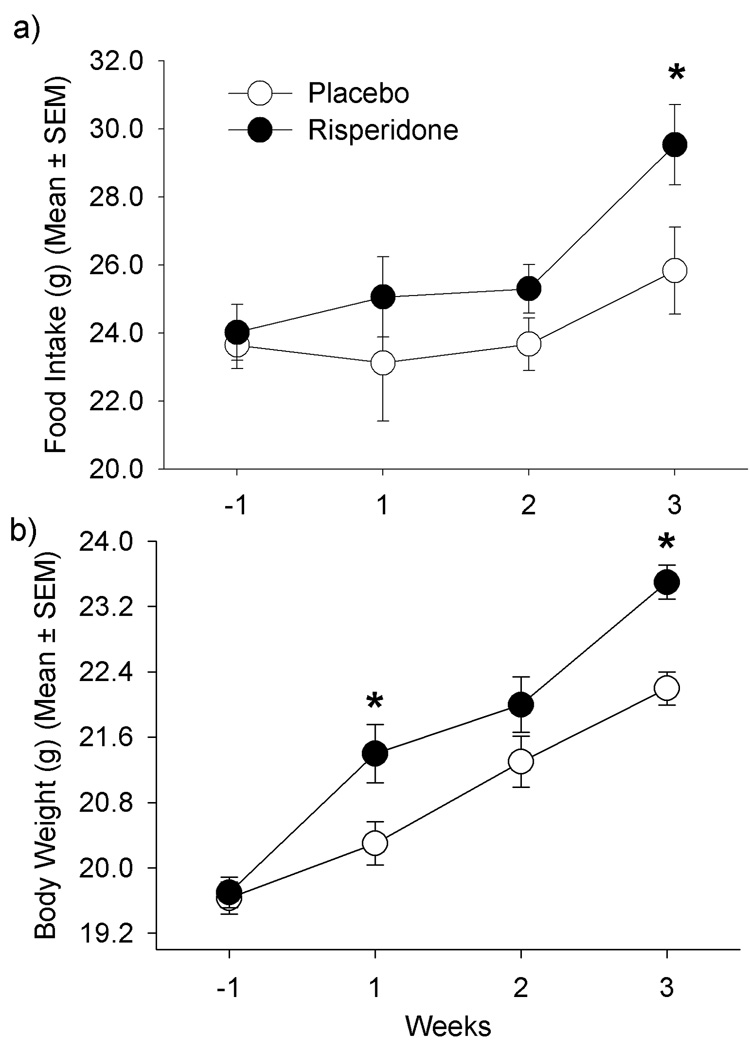
There was a significant effect of treatment on food intake, and risperidone-treated mice presented significantly higher food intake than placebo-treated mice after 3 weeks (Figure 1a; p=0.050). Both groups increased food intake over time (p=0.003), but there was no significant time by treatment interaction (p=0.49).
Figure 1.
a) Weekly food intake from one week prior to treatment initiation and during 3 weeks of treatment with placebo or risperidone. Treatment effect (p=0.050); time effect (p=0.003); time x treatment interaction (p=0.49); b) Body weights at baseline and during 3 weeks of treatment with placebo or risperidone. Treatment effect (p=0.029); time effect (p=0.0001); time x treatment interaction (p=0.004)
Both placebo and risperidone-treated mice gained significant weight over time (p=0.0001). There was a significant effect of treatment on body weight (Figure 1b; p=0.029), and risperidone-treated mice were heavier than PLA-treated mice at the end of the experiment (23.5±0.21 vs. 22.2±0.20g, respectively; p=0.001). A significant time by treatment interaction was also found (p=0.004).
3.2 Core body temperature
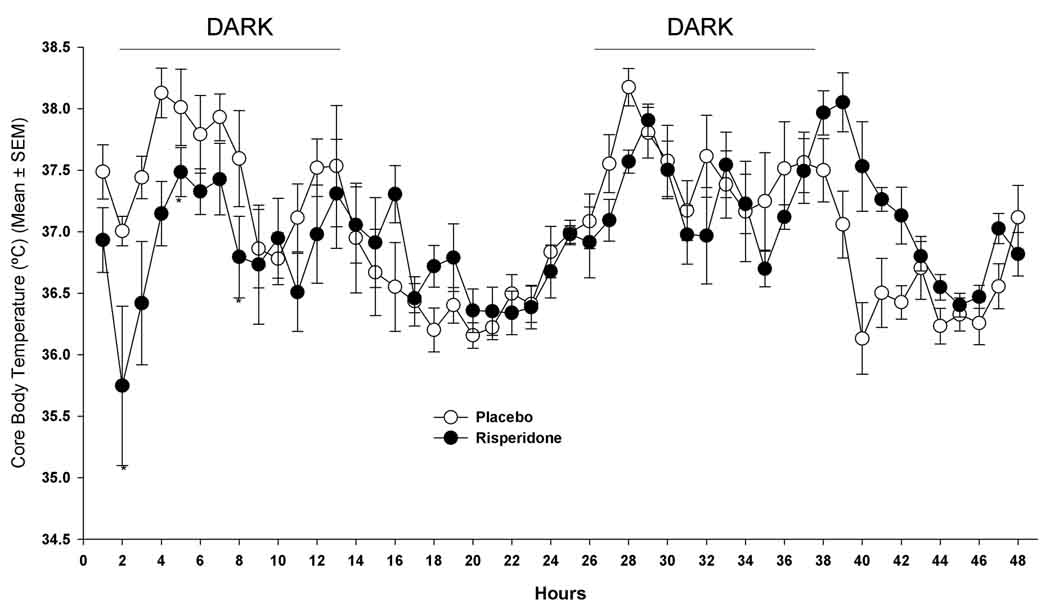
Baseline body temperatures were not different between placebo and risperidone-treated mice during a 22-hour pre-treatment measurement (p=0.52; placebo, 37.1±0.2°C and risperidone, 36.9±0.2°C). There was no overall effect of treatment on body temperature during the initial 2-days of treatment (p=0.55). Following initial drug administration, risperidone-treated mice had decreased body temperature at 1-hour (36.9±0.2 °C at baseline and 35.7±06 °C at 1-hour) while PLA-treated mice had a slight increase (37.1±0.2°C at baseline and 37.5±0.1°C at 1-hour). During the initial 47 hours following the first treatment there was a significant time effect (p=0.0001) and a significant time by treatment interaction (p=0.0001). Compared with PLA-treated mice, risperidone-treated mice had significantly lower body temperatures at 4 (p=0.009), 18 (p=0.044), and 28 hours (p=0.004) (Figure 2), but had higher body temperatures at 39 (p=0.013), 40 (p=0.008), 41 (p=0.018), 42 (p=0.016) 46 (p=0.003) and 47 hours (p=0.04) after initial treatment (Figure 2). However, after applying the Bonferoni correction there were no differences in body temperature between PLA- and risperidone-treated mice at any single time point.
Figure 2.
Core body temperatures for days 1 and 2 of treatment (arrows indicate treatment time; initial treatment given at 0 hours; * p<0.05). Treatment effect (p=0.55); time effect (p=0.0001); time x treatment interaction (p=0.0001).
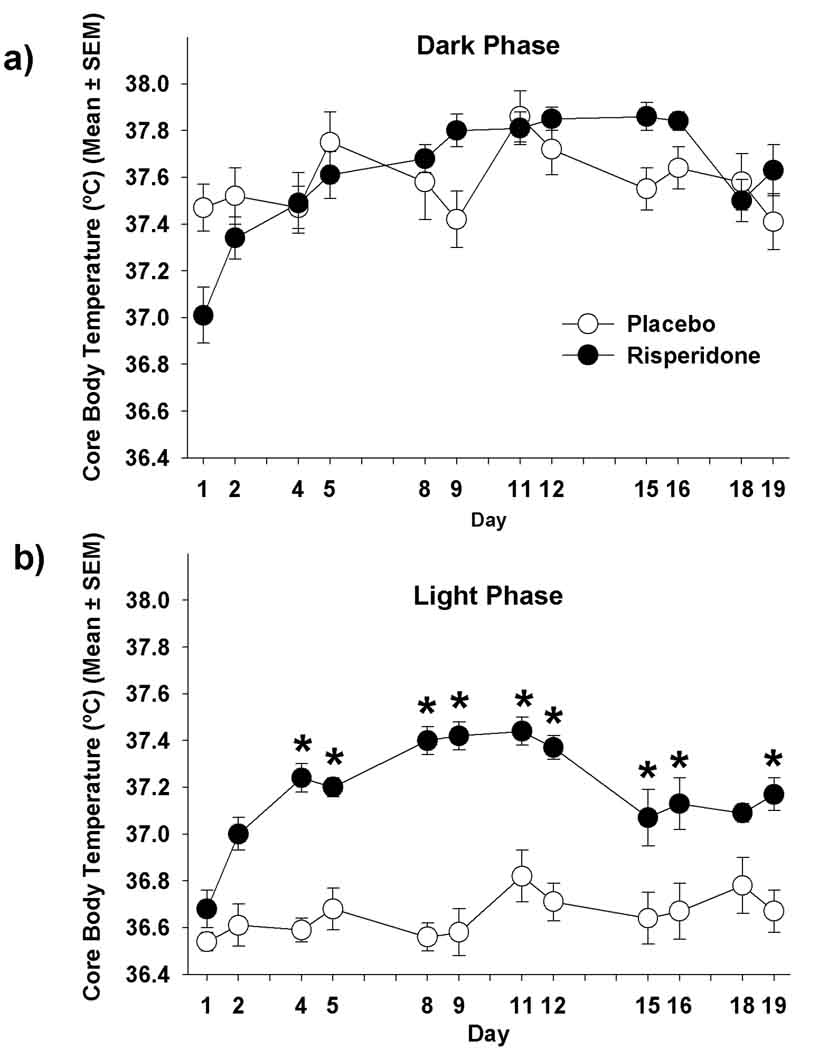
Examined over the 3-week experimental period, there was no significant effect of treatment on body temperature during the dark phase (p=0.057). However, a significant time effect (p=0.0001) and time by treatment interaction (p=0.0005, Figure 3a) were observed. On day 1 of treatment, risperidone-treated mice had lower body temperatures than PLA-treated mice during the dark phase (p=0.034). In contrast, on days 9 (p=0.011) and 15 (p=0.006), risperidone-treated mice had higher body temperatures than the placebo mice during the dark phase. Following Bonferoni correction there was no difference in body temperature at any single time point during the dark phase on any particular day.
Figure 3.
Core body temperatures of mice treated with placebo or risperidone during the (a) dark phase [Treatment effect (p=0.057); time effect (p=0.0001); time x treatment interaction (p=0.0005)]; (b) light phase [Treatment effect (p=0.0001); time effect (p=0.0001); time x treatment interaction (p=0.0001)] * p<0.05 for individual time points after applying the Bonferoni correction.
There was a significant effect of treatment (p=0.0001), time (p=0.0001), and time by treatment interaction (p=0.0001) on body temperature during the light phase (Figure 3b). Risperidone-treated mice had higher body temperatures during the light phase than PLA-treated mice (p<0.05) on all treatment days except for days 1 (p=0.45) and 18 (p=0.08). After Bonferoni correction, risperidone-treated mice had higher body temperatures during the light phase than PLA-treated mice on days 4 (p=0.0.0001), 5 (p=0.0002), 8 (p<0.0001), 9 (p<0.0001), 11 (p=0.0010), 12 (p<0.0001) and 19 (p=0.0037).
3.3 Locomotor activity
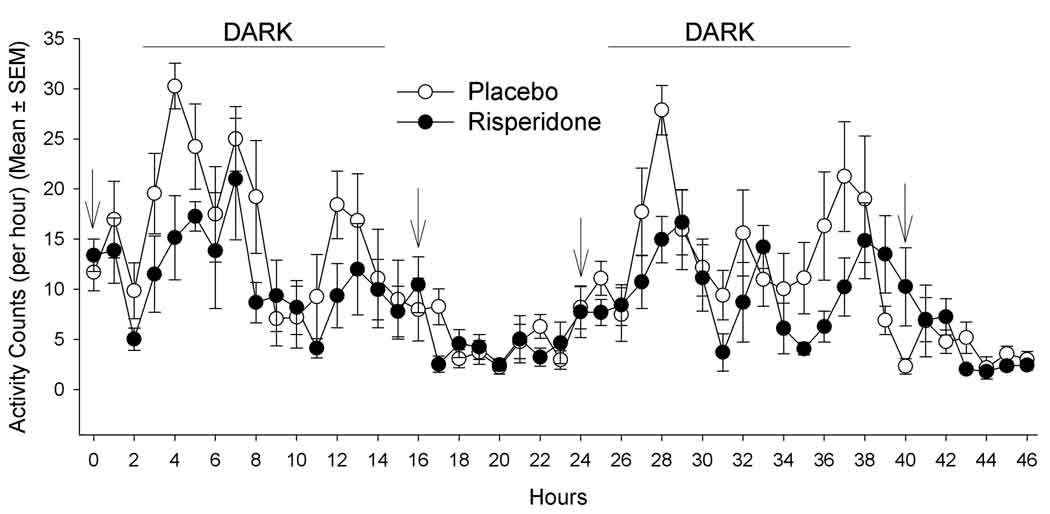
At baseline, activity counts were not different between PLA- and risperidone-treated mice during a 22-hour pre-treatment measure (p=0.51; 11.7±1.9 and 13.4±1.6 counts/min, respectively). During the initial 2-days of treatment, there was a significant effect of treatment (p=0.049) and time (p=0.0001) on activity (Figure 4). On day 1 of treatment, risperidone-treated mice had significantly less activity counts at 4-hours post initial treatment (two hours into the dark phase) compared to placebo mice (p=0.009; Figure 4), risperidone-treated mice had significantly less activity counts at 17 (p=0.016) and 28 (p=0.003) hours after initial treatment and showed a trend to have less activity counts at 12 (p=0.08), 22 (p=0.07), 31 (p=0.09), 35 (p=0.08), and 43 (p=0.07) hours after initial treatment. However, there were no differences between groups at any single time point after applying the Bonferoni correction.
Figure 4.
Activity counts for days 1 and 2 of treatment. Arrows indicate treatment time; initial treatment given at 0 hours. Treatment effect (p=0.049); time effect (p=0.0001); time x treatment interaction (p=0.20).
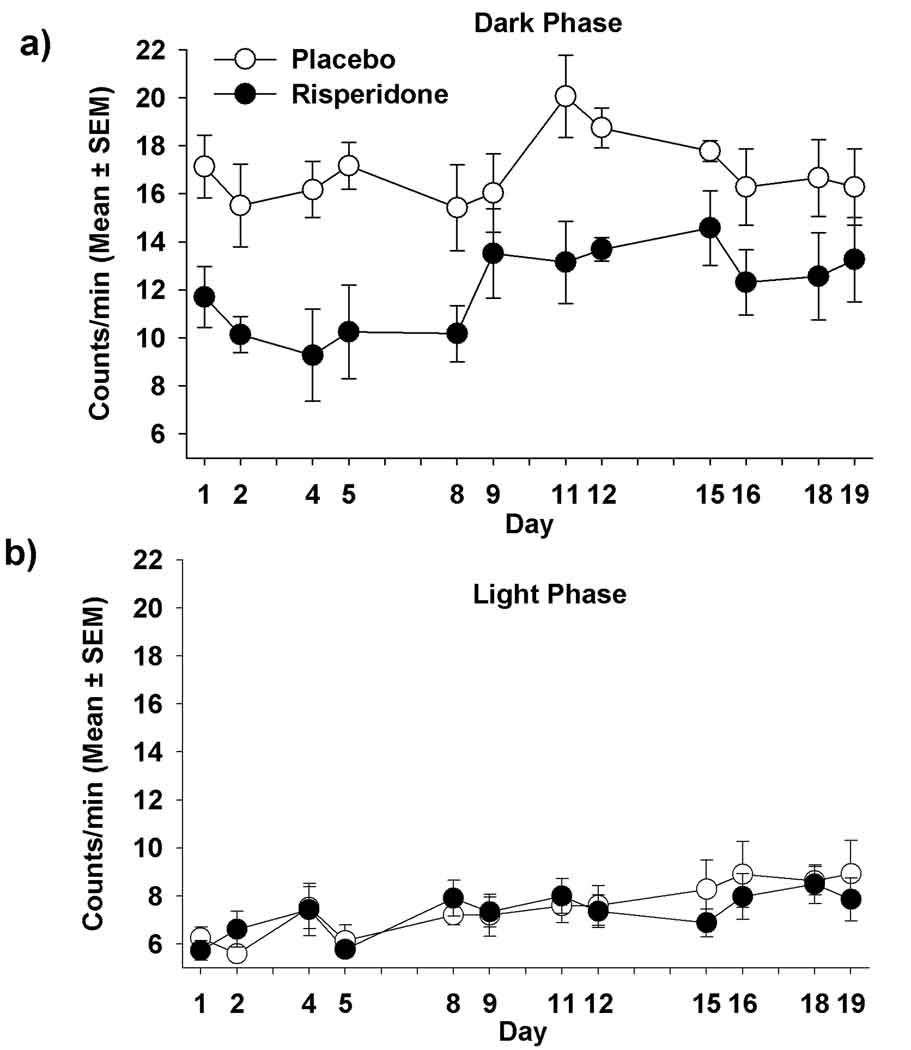
There was a significant effect of treatment (p=0.006), time (p=0.0001), but not a significant time by treatment interaction (p=0.470) during the dark phase (Figure 5a). Risperidone-treated mice had lower activity counts than PLA-treated mice (significantly lower on days 1, 2, 4, 5, 11, 12 and 16; p=0.006, 0.025, 0.030, 0.010, 0.018, 0.012 and 0.005, respectively), but no differences were found after Bonferoni correction (p>0.05). However, there was no significant effect of treatment (p=0.47) nor time by treatment interaction (p=0.51) on activity counts during the light phase (Figure 5b), even though both groups increased activity over time during the light phase (p=0.011).
Figure 5.
Activity counts of mice treated with placebo or risperidone during the (a) dark phase [treatment effect (p=0.006); time effect (p=0.0001); time x treatment interaction (p=0.47)]; (b) light phase [treatment effect (p=0.472); time effect (p=0.011); time x treatment interaction (p=0.51)].
3.4 mRNA expression of UCPs and orexin
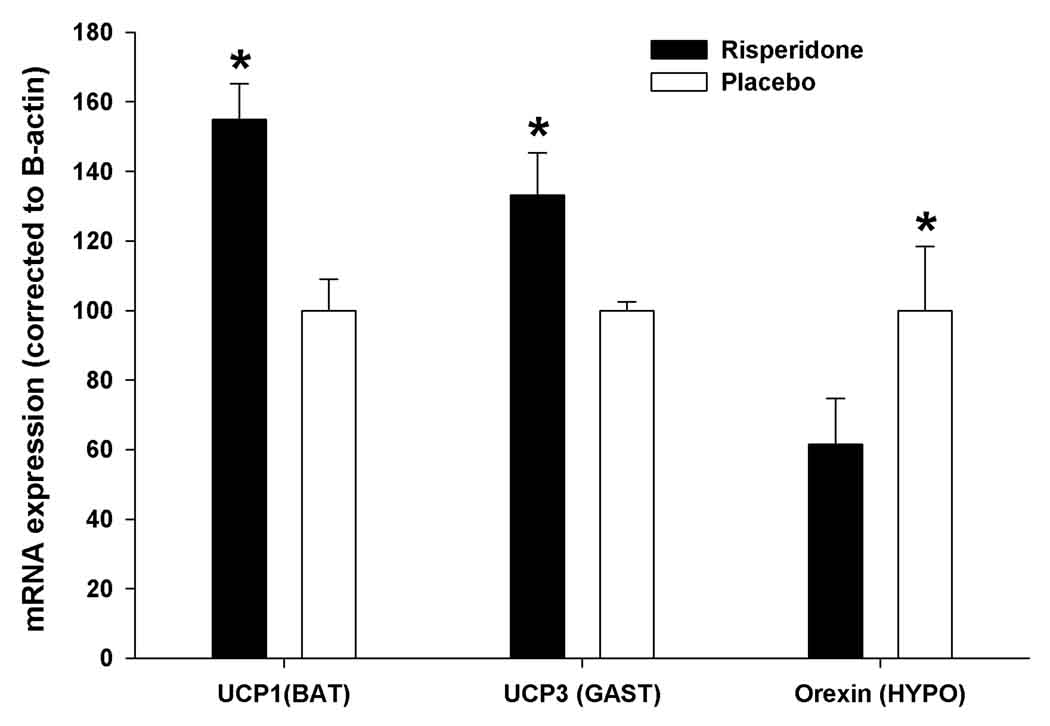
After 3 weeks of treatment, mRNA expressions of UCP1 in BAT and UCP3 in gastrocnemius muscles were significantly higher in mice treated with risperidone compared to placebo (p<0.01 and p<0.05, respectively, Figure 6). Relative expression of orexin in hypothalamus was significantly lower (040% less) in risperidone-treated mice than PLA-treated mice (p<0.01, Figure 6).
Figure 6.
Relative mRNA expressions of UCP1 in BAT, UCP3 in gastrocnemius muscle (GAST), and Orexin in hypothalamus (HYPO) from mice following three weeks of placebo or risperidone treatment. *p<0.05.
4. Discussion
Our previous findings indicate that risperidone-treated mice gain significant body weight compared to controls [4]; however, the mechanism(s) for this weight inducing effect are unknown. In the present experiment, we report for the first time that risperidone-treated mice, compared to PLA-treated mice, experienced the following: 1) consumed more food and gained significantly more weight; 2) had higher body temperatures during the light phase; 3) had lower activity during the dark phase; 4) had higher UCP1 and UCP3 mRNA expressions in BAT and gastrocnemius muscle, respectively; and 5) had lower orexin mRNA expression in hypothalamus.
Weight gain during three weeks of risperidone treatment was associated with increased food intake and a significant reduction in activity levels during the active phase. The reduced activity during the active phase may cause risperidone-treated mice to have reduced total energy expenditure, and combined with significant elevation in food intake, place the mice in greater positive energy balance than the controls. Others have shown that animals treated with risperidone have reduced locomotor activity [34]. It has been speculated that AADs reduce activity and energy expenditure, and thus promote excessive energy balance in human [24;35] However, this contention has not been explored systematically in rodents. Female Wistar rats receiving risperidone (0.5 mg/kg s.c.) for twelve days did not have lower energy expenditure compared to controls [3]. Coccurello et al. [36] found no changes in energy expenditure and locomotor activity in female CD-1 mice after chronic administration of olanzapine, however, a decreased respiratory quotient was observed. A limitation of our current study is that we did not measure energy expenditure during the three weeks of risperidone treatment.
All mice in the current experiment were acclimated for two weeks to individual housing at 22°C and body temperatures were not different at the beginning of the experiment. During the three weeks of treatment, risperidone-treated mice failed to reduce core body temperature during the light phase as control animals did. There are a number of possible explanations for this failure to reduce body temperature in risperidone-treated mice. Elevated food intake can cause elevated body temperature due to the thermogenic effect of food [37] and elevated UCP1 in BAT can lead to elevated body temperature [38]. Similar to our findings, UCP1 expression in BAT was enhanced following twenty-one days of risperidone treatment (0.5 mg/kg BW*day) in rats [39]. In addition, UCP3 expression was higher in the gastrocnemius muscle of risperidone-treated mice. The thermogenic effect of food and the up-regulation of UCPs may be involved with the inability of risperidone-treated mice to reduce body temperature during the light phase.
Body temperature regulation may also involve the hypothalamic neuropeptide orexin, which has been shown to be important in the regulation of food intake and locomotor activity [40]. Similar to risperidone-treated mice, orexin KO mice show a pattern of elevated core body temperature during the light phase and reduced locomotor activity during the dark phase [30]. Furthermore, risperidone can enhance sympathetic firing rate and colonic temperature induced by orexin in rats [41]. These findings suggest that orexin may be involved with risperidone’s affect on body temperature and locomotor activity. Further evaluation of risperidone’s interactions with specific neuroreceptors and neuropeptides will be needed to determine specific pathways that cause core body temperature to remain elevated during the light phase and locomotor activity to be reduced during the dark phase when mice are treated with risperidone.
In summary, the risperidone-induced weight gain in our mouse model appears to be related to elevated food intake as well as reduced activity. The thermogenic effect of increasing food intake, the increased expression of UCPs, and a reduction in hypothalamic orexin may result in the inability of risperidone-treated mice to lower their body temperature during the light phase of the day. Follow-up experiments investigating the mechanisms in this novel mouse model are needed and could potentially lead to clinical interventions for risperidone-treated patients experiencing weight gain.
Acknowledgements
This work was supported by the following: RO1DK068261 (TRN), the Clinical Nutrition Research Unit (P30DK56336), the Neuroscience Blueprint Center Grant (P30NS057098), and the Diabetes Research and Training Center (P60DK079626). MBC was supported by F32DK064532 and CAD by T32DK062710.
References
- 1.Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Hoschl C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Haupt DW. Differential metabolic effects of antipsychotic treatments. Eur Neuropsychopharmacol. 2006;16(Suppl 3):S149–S155. doi: 10.1016/j.euroneuro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Baptista T, de Baptista EA, Lalonde J, Plamondon J, Kin NM, Beaulieu S, Joober R, Richard D. Comparative effects of the antipsychotics sulpiride and risperidone in female rats on energy balance, body composition, fat morphology and macronutrient selection. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1305–1311. doi: 10.1016/j.pnpbp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes (Lond) 2005;29:607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- 5.Fell MJ, Marshall KM, Williams J, Neill JC. Effects of the atypical antipsychotic olanzapine on reproductive function and weight gain in female rats. J Psychopharmacol. 2004;18:149–155. doi: 10.1177/0269881104042613. [DOI] [PubMed] [Google Scholar]
- 6.Allison DB, Fontaine KR, Heo M, Mentore JL, Cappelleri JC, Chandler LP, Weiden PJ, Cheskin LJ. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60:215–220. doi: 10.4088/jcp.v60n0402. [DOI] [PubMed] [Google Scholar]
- 7.Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987–1996. Schizophr Res. 2002;55:277–284. doi: 10.1016/s0920-9964(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 8.Fell MJ, Marshall KM, Williams J, Neill JC. Effects of the atypical antipsychotic olanzapine on reproductive function and weight gain in female rats. J Psychopharmacol. 2004;18:149–155. doi: 10.1177/0269881104042613. [DOI] [PubMed] [Google Scholar]
- 9.Batt RA, Tyler DD, Sutton CM. Influence of restricted food intake on brown adipose tissue function in genetically obese mice (genotype, ob/ob) Biochim Biophys Acta. 1985;838:229–235. doi: 10.1016/0304-4165(85)90083-2. [DOI] [PubMed] [Google Scholar]
- 10.DeRuisseau LR, Parsons AD, Overton JM. Adaptive thermogenesis is intact in B6 and A/J mice studied at thermoneutrality. Metabolism. 2004;53:1417–1423. doi: 10.1016/j.metabol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 12.Ota M, Mori K, Nakashima A, Kaneko YS, Takahashi H, Ota A. Resistance to excessive bodyweight gain in risperidone-injected rats. Clin Exp Pharmacol Physiol. 2005;32:279–287. doi: 10.1111/j.1440-1681.2005.04184.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Trappler B, Ng YK, Leeman CP. Risperidone-induced neuroleptic malignant syndrome. Ann Pharmacother. 1996;30:775–778. doi: 10.1177/106002809603000713. [DOI] [PubMed] [Google Scholar]
- 14.Razaq M, Samma M. A case of risperidone-induced hypothermia. Am J Ther. 2004;11:229–230. doi: 10.1097/00045391-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Bajjoka I, Patel T, O'Sullivan T. Risperidone-induced neuroleptic malignant syndrome. Ann Emerg Med. 1997;30:698–700. doi: 10.1016/s0196-0644(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 16.Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000;345:161–179. [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, Minokoshi Y, Shimazu T. Brown adipose tissue after ventromedial hypothalamic lesions in rats. Am J Physiol. 1985;248:E20–E25. doi: 10.1152/ajpendo.1985.248.1.E20. [DOI] [PubMed] [Google Scholar]
- 18.Couplan E, Gelly C, Goubern M, Fleury C, Quesson B, Silberberg M, Thiaudiere E, Mateo P, Lonchampt M, Levens N, De MC, Ortmann S, Klaus S, Gonzalez-Barroso MD, Cassard-Doulcier AM, Ricquier D, Bigard AX, Diolez P, Bouillaud F. High level of uncoupling protein 1 expression in muscle of transgenic mice selectively affects muscles at rest and decreases their IIb fiber content. J Biol Chem. 2002;277:43079–43088. doi: 10.1074/jbc.M206726200. [DOI] [PubMed] [Google Scholar]
- 19.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong DW, He Y, Karas M, Reitman M. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, beta3-adrenergic agonists, and leptin. J Biol Chem. 1997;272:24129–24132. doi: 10.1074/jbc.272.39.24129. [DOI] [PubMed] [Google Scholar]
- 22.Bezaire V, Spriet L, Campbell S, Sabet N, Gerrits M, Bonen A, Harper ME. UCP3 overexpression increases mouse skeletal muscle capacity for fatty acid transport and oxidation. Obesity Research. 2004;12:A2. doi: 10.1096/fj.04-2765fje. [DOI] [PubMed] [Google Scholar]
- 23.Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- 24.Gothelf D, Falk B, Singer P, Kairi M, Phillip M, Zigel L, Poraz I, Frishman S, Constantini N, Zalsman G, Weizman A, Apter A. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry. 2002;159:1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- 25.Richtand NM, Taylor B, Welge JA, Ahlbrand R, Ostrander MM, Burr J, Hayes S, Coolen LM, Pritchard LM, Logue A, Herman JP, McNamara RK. Risperidone pretreatment prevents elevated locomotor activity following neonatal hippocampal lesions. Neuropsychopharmacology. 2006;31:77–89. doi: 10.1038/sj.npp.1300791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner GC, Avena N, Kita T, Nakashima T, Fisher H, Halladay AK. Risperidone reduction of amphetamine-induced self-injurious behavior in mice. Neuropharmacology. 2004;46:700–708. doi: 10.1016/j.neuropharm.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Malone RP, Gratz SS, Delaney MA, Hyman SB. Advances in drug treatments for children and adolescents with autism and other pervasive developmental disorders. CNS Drugs. 2005;19:923–934. doi: 10.2165/00023210-200519110-00003. [DOI] [PubMed] [Google Scholar]
- 28.Frazier JA, Meyer MC, Biederman J, Wozniak J, Wilens TE, Spencer TJ, Kim GS, Shapiro S. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry. 1999;38:960–965. doi: 10.1097/00004583-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Kotz CM. Integration of feeding and spontaneous physical activity: Role for orexin. Physiology & Behavior. 2006;88:294–301. doi: 10.1016/j.physbeh.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Mochizuki T, Klerman EB, Sakurai T, Scammell TE. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R533–R540. doi: 10.1152/ajpregu.00887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monda M, Viggiano A, Viggiano A, Viggiano E, De LV. Risperidone potentiates the sympathetic and hyperthermic reactions induced by orexin A in the rat. Physiol Res. 2006;55:73–78. doi: 10.33549/physiolres.930906. [DOI] [PubMed] [Google Scholar]
- 32.Harkin A, O'Donnell JM, Kelly JP. A study of VitalView for behavioural and physiological monitoring in laboratory rats. Physiol Behav. 2002;77:65–77. doi: 10.1016/s0031-9384(02)00810-7. [DOI] [PubMed] [Google Scholar]
- 33.He X, Schoeb TR, Panoskaltsis-Mortari A, Zinn KR, Kesterson RA, Zhang J, Samuel S, Hicks MJ, Hickey MJ, Bullard DC. Deficiency of P-selectin or P-selectin glycoprotein ligand-1 leads to accelerated development of glomerulonephritis and increased expression of CC chemokine ligand 2 in lupus-prone mice. J Immunol. 2006;177:8748–8756. doi: 10.4049/jimmunol.177.12.8748. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Palit G, Dhawan BN. Comparative behavioural effects of typical and atypical antipsychotic drugs in rhesus monkey. Eur J Pharmacol. 2003;462:133–138. doi: 10.1016/s0014-2999(02)02957-6. [DOI] [PubMed] [Google Scholar]
- 35.Virkkunen M, Wahlbeck K, Rissanen A, Naukkarinen H, Franssila-Kallunki A. Decrease of energy expenditure causes weight increase in olanzapine treatment - a case study. Pharmacopsychiatry. 2002;35:124–126. doi: 10.1055/s-2002-31521. [DOI] [PubMed] [Google Scholar]
- 36.Coccurello R, Caprioli A, Ghirardi O, Conti R, Ciani B, Daniele S, Bartolomucci A, Moles A. Chronic administration of olanzapine induces metabolic and food intake alterations: a mouse model of the atypical antipsychotic-associated adverse effects. Psychopharmacology (Berl) 2006;186:561–571. doi: 10.1007/s00213-006-0368-5. [DOI] [PubMed] [Google Scholar]
- 37.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 38.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 39.Ota M, Mori K, Nakashima A, Kaneko YS, Fujiwara K, Itoh M, Nagasaka A, Ota A. Peripheral injection of risperidone, an atypical antipsychotic, alters the bodyweight gain of rats. Clin Exp Pharmacol Physiol. 2002;29:980–989. doi: 10.1046/j.1440-1681.2002.t01-1-03755.x. [DOI] [PubMed] [Google Scholar]
- 40.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–162. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 41.Monda M, Viggiano A, Viggiano A, Viggiano E, Messina G, Tafuri D, De LV. Quetiapine lowers sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Neuropeptides. 2006;40:357–363. doi: 10.1016/j.npep.2006.07.003. [DOI] [PubMed] [Google Scholar]