Abstract
Expression of conditioned fear has been reported to be independent of perceptual awareness of conditioned stimuli (CSs). Previous studies have been criticized, however, for not adequately assessing perceptual awareness. We fear-conditioned participants to one of two symbols and measured skin conductance responses to dichoptically masked and unmasked CSs. Participants also performed a target detection task and sensitivity (d′) to the masked conditioned stimuli (CS+, CS−) was measured. Results showed that sensitivity under masking conditions was related to conditioned responses to masked CSs but not unmasked CSs. Thus, a strong relationship between expression of conditioned fear and awareness of the CS+ emerges when the latter is assessed by signal detection methods. Without consensus on how awareness should be defined, these findings bring balance to previous studies that have typically used less sensitive assessments of awareness.
Descriptors: Awareness, Fear conditioning, Masking, Signal detection, Skin conductance
Pavolvian fear-conditioning is the acquisition of a conditioned response (CR) to a neutral stimulus (CS+) after being paired with an aversive, unconditioned stimulus (US, electric shock). Differential fear-conditioning studies with negatively valenced pictures (e.g., angry faces, snakes) as conditioned stimuli have reported that autonomic CRs can be elicited by subliminal presentation of the CS+ (Esteves, Dimberg, & Öhman, 1994; Öhman & Soares, 1993; Olsson & Phelps, 2004). Neuroimaging data show activation of fear-related structures such as the amygdala by subliminal CS1 presentations (Critchley, Mathias, & Dolan, 2002; Morris, Öhman, & Dolan, 1998). Lovibond and Shanks (2002) challenged these and other related findings that suggest expression of a learned CS-US contingency can be mediated by processes that are dissociable from conscious awareness. In studies using subliminal CSs, for instance, Lovibond and Shanks argued that measures assessing perceptual awareness may not have been sufficiently sensitive to identify participants with residual awareness of stimulus features (also see Pessoa, 2005).
Subliminal conditions have been created by backward masking in which a target stimulus is followed by a masking stimulus with stimulus onset asynchronies (SOAs) of 17–50 ms. In studies putatively demonstrating CRs to subliminally presented CS+s, participants classified as unaware of the CS+ and CS− may have been able to discriminate features of these stimuli. Any discernible differences such as luminance or stimulus size (e.g., Morris et al., 1998, Figure 1) could drive differential responding independently of forming representations necessary for stimulus identification, being how perceptual awareness is typically defined (Lovibond & Shanks, 2002; Pessoa, 2005). Indeed, signal detection studies show variable effectiveness of backward masking emotional faces; many individuals can discriminate masked target faces with extremely short SOAs of 17–33 ms (Maxwell & Davidson, 2004; Pessoa, Japee, & Ungerleider, 2005). These findings support Lovibond and Shanks’ hypothesis that individuals unable to recognize backwardly masked CSs may be able to discriminate their features and gain partial awareness. It should be noted that objective thresholds of awareness obtained by signal detection analyses have been criticized for stripping the definition of awareness of all subjective aspects (Merikle & Daneman, 2000). However, subjective threshold tests of awareness may not exhaust, in practice, all possible ways in which participants could have conscious access to distinguishing stimulus features.
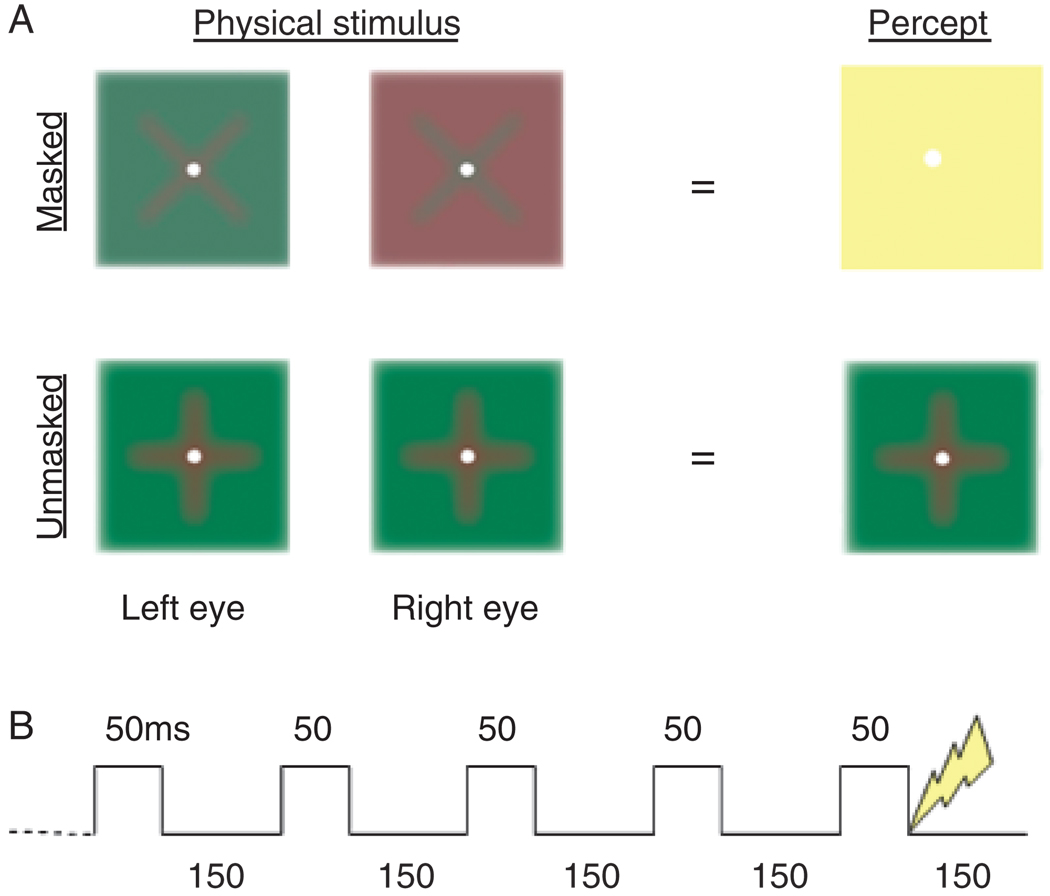
Figure 1.
A: Two symbols (“+” and “ × ”) used for target detection and fear conditioning. During fear conditioning, CSs were masked (top) and unmasked (bottom), with opponent-color stimuli or same-color stimuli presented to each eye, respectively. In the former case, masking produces a percept of uniform yellow. B: Schematic of stimulus presentation, with a stimulus pair being flashed five times (ON for 50 ms, OFF for 150 ms). During fear conditioning, the shock was delivered at the final termination of the unmasked CS+.
This conceptual problem notwithstanding, we determined whether objective discriminative ability under masking conditions predicts autonomic responses to conditioned stimuli under the same masking conditions. We used dichoptic chromatic masking that exploits the opponent-process structure of color perception by presenting, separately and simultaneously to each eye, the same stimulus with opponent colors (e.g., red “ × ” to the left eye and green “ × ” to the right eye). When opponent-color stimuli are flashed briefly, observers typically report a uniformly yellow percept (Moutoussis & Zeki, 2002). This form of masking was used in fMRI showing that masked faces and houses activate similar structures as unmasked ones (Moutoussis & Zeki, 2002). Because dichoptic masking does not require a masking stimulus, problems associated with assessing conditioned responding under masked and unmasked conditions during fear acquisition, such as the masking stimulus potentially becoming an inhibiting stimulus (Esteves et al., 1994), may be avoided.
Before and after fear-conditioning, participants completed a target detection task involving yes–no responses of whether a symbol (“ × ” and “+”) was presented or not. Sensitivity (d′) under dichoptic masking was measured (MacMillan & Creelman, 1991). Participants were fear-conditioned to one of these symbols (CS+) by pairing unmasked presentations of the CS+ with a mildly aversive shock (UCS). Skin conductance responses (SCRs), the predominant way of assessing CR expression in masking studies, were measured to both masked and unmasked CS+ and CS− presentations. Although phylogenetically based fear-relevant stimuli are especially powerful in eliciting CRs under masking conditions (Öhman & Mineka, 2001), some work purportedly shows similar effects with ontogenetically based fear-relevant stimuli such as guns (Flykt, 1999) and threatening words (Van Den Hout, De Jong, & Kindt, 2000), as well as neutral tonal stimuli (Knight, Nguyen, & Bandettini, 2003). Insofar as past literature presents no clear boundary between those stimuli that can and those that cannot elicit a fear response without awareness, we used neutral symbols that could be easily masked. We predicted that sensitivity under masking conditions would be differentially associated with CRs to masked CSs but not to unmasked CSs.
Method
Participants
Thirty-six healthy participants (22 women) were recruited (mean age = 25 years, range 18–48) and screened by physical exams and structured clinical interviews for the DSM-IV (First, Spitzer, Williams, & Gibbon, 1995). All participants had normal visual acuity (20/30) and color vision and gave written informed consent. This study was approved by the Institutional Review Board of the National Institute of Mental Health.
Stimuli and Apparatus
Dichoptic conditions were created with a custom-designed mirror stereoscope (http://www.optosigma.com). Red and green symbols were shown against square backgrounds. Stimuli were isoluminant and blurred to remove high contrast edges (Figure 1A). Presentation 9.7 software (http://www.neurobehavioralsystems.com) controlled stimulus presentation.
USs were electric shocks, administered through two Ag/AgCl electrodes attached to the right wrist by a constant current stimulator (Contact Precision Instruments, London, UK). Electrodes were attached just prior to conditioning and a shock work-up was used to determine a moderately uncomfortable level for each participant (3–5 mA). Two Ag/AgCl electrodes filled with 0.5% saline/neutral base electrode gel (Biopac Systems, Inc.) and attached to the medial phalanges (volar surface) of the second and third fingers of the left hand measured skin conductance by constant voltage excitation (lowpass filter = 10 Hz). Psylab 7 software was used to record and analyze SCR.
Design and Procedure
The target detection task, administered before and after conditioning, was comprised of 200 trials, divided into four blocks with 1-min breaks between blocks. Each block consisted of 25 trials with an empty square (target-absent) and 25 with one of the symbols (target-present) ordered randomly. Each trial began with presentation of a pair of square frames with a fixation dot for 3 s. The opponent-color stimulus pair was then flashed for 850 ms: on for 50 ms, off for 150 ms, five times in succession (Figure 1B). Flashing the stimuli produces a more sustained masking effect (Moutoussis & Zeki, 2002). During off intervals, the square frames remained on screen. Opponent-color stimulus pairs were switched between eyes across trials to expose them equally to each single stimulus. Participants were instructed to look for an “ × ” or “+” and respond whether anything was presented within the square background. No special instructions were given to control response bias. The next trial began immediately after responding.
For fear conditioning, participants were presented 44 stimulus pairs with the same timing (850 ms) as during target detection, with 10 s interstimulus intervals. Of the 44 pairs, 12 were presented in masked format (opponent-color pairs) and 32 in unmasked format (same-color pairs, Figure 1a). Six types of masked stimulus pairs were presented twice: “+,” “ ×,” and blank square in two possible dichoptic configurations. The masked blank square served as a filler stimulus. Four types of unmasked stimulus pairs were presented eight times: “+” and “ × ” in the same color (red or green) to both eyes. Shock followed the offset of 10 of 16 unmasked presentations of either the “+” or “ ×,” counterbalanced across participants.
Each type of stimulus pair, masked and unmasked, was presented once before shocks were administered. Habituation was followed by continuous reinforcement in which six unmasked presentations of the CS+ were paired with shock and six unmasked presentations of the CS− were not. Next, partial reinforcement consisted of eight unmasked presentations of the CS+ and CS−, with four of the CS+s paired with shock. Masked presentations occurred only during partial reinforcement. The order of presentation was pseudorandom, with the same stimulus (CS+ and CS−) presented no more than three times consecutively regardless of being masked or unmasked. Participants were required to focus on stimulus presentation and identify any relationship between the visual stimuli and shock. After conditioning, participants were asked to report this relationship. No extinction phase was administered.
Analysis
To measure discriminative ability or sensitivity before and after conditioning, d′ was computed over 100 target-present trials and 100 target-absent trials, combining the CS+ and CS− presentations. Based on simulations by Kadlec (1999), d′ estimates show minimal bias from extremely conservative responding with at least 100 target-present and 100 target-absent trials.
For fear-conditioning, SCR amplitude was scored as the maximal deflection beginning 500–5000 ms after onset of pulsed stimuli. Peak changes less than 0.03 µS were considered noise, converted to 0, and included to compute SCR magnitudes. For unmasked presentations, SCRs were averaged for trials during continuous and partial reinforcement with no shock delivered (4 CS+, 14 CS−). For masked presentations, SCRs were averaged for trials during partial reinforcement (2 CS+, 2 CS−).
Three participants showed no SCRs to the shock and were classified as nonresponders. Five participants were also excluded from analyses for failing to correctly report the unmasked CS-US relationship, leaving 28 participants (17 female, mean age = 24) who showed conditioned and unconditioned SCRs. Preliminary analyses did not reveal any gender differences.
Results
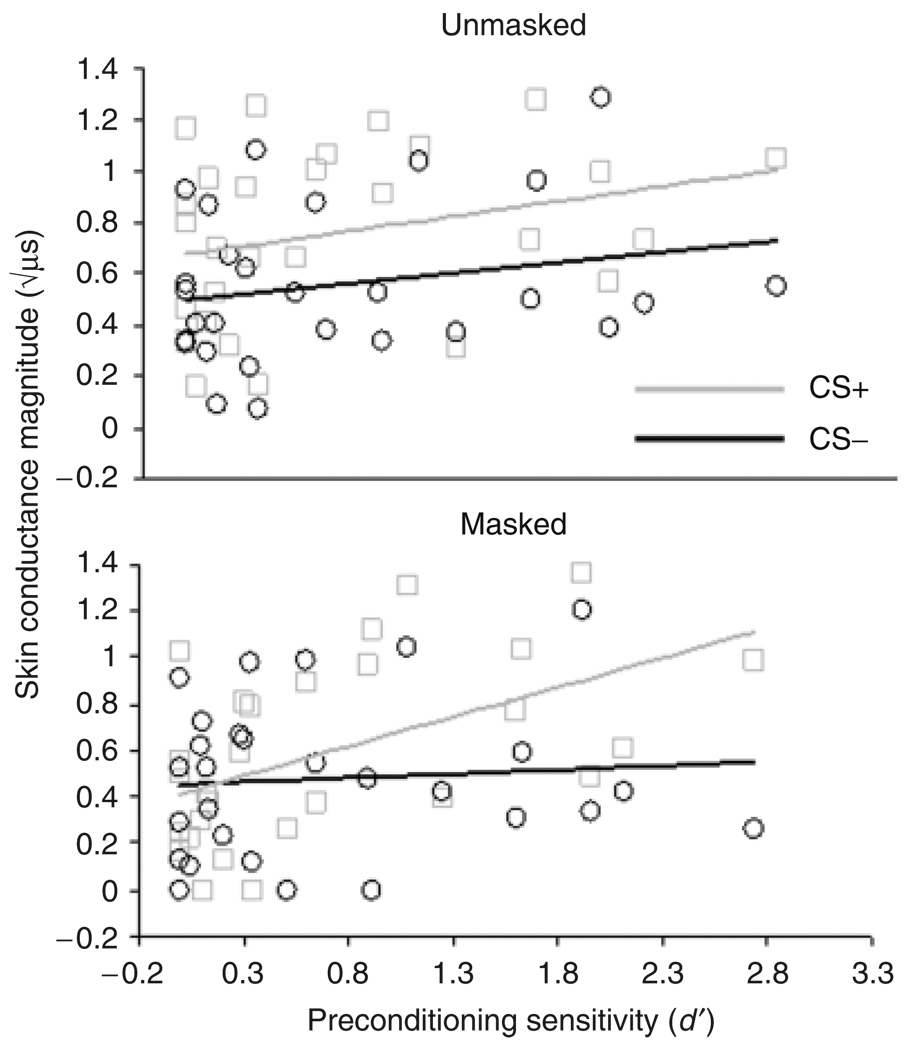
To determine whether d′ was differentially related to CRs under masked relative to unmasked conditions, we performed a 2 (CS+ vs. CS−) × 2 (masked vs. unmasked) repeated-measures ANOVA with preconditioning d′ as a continuous between-subjects predictor variable. Results showed a significant Stimulus × Condition × d′ interaction on SCR, F(1,26) = 5.13, p<.05, partial η2 = .17 (Figure 2). In the masked condition, there was a Stimulus × d′ interaction, F(1,26) = 8.37, p<.01, partial η2 = .24, but no main effect of stimulus on SCR, F<1. In the unmasked condition, there was a main effect of stimulus on SCR, F(1,26) = 6.87, p<.03, partial η2 = .21, but no interaction with d′, F<1. Visual inspection of the regression lines shows considerable evidence of heterogeneity of slopes in the masked condition but not in the unmasked condition (Figure 2).
Figure 2.
Scatterplots, with least-squares lines, showing relationships between preconditioning sensitivity (d′) and skin conductance responses (square root transformed) to the CS+ and CS− on unreinforced acquisition trials, under unmasked (top) and masked conditions (bottom). The d′ variable did not significantly deviate from a normal distribution, ZK−S = .71, p>.10. Note that increased divergence of the least-squares lines as d′ increases reflects a positive relationship between conditioned responding and sensitivity.
A significant three-way interaction on SCR was also obtained with postconditioning d′ as a continuous between-subjects predictor variable, F(1,26) = 4.29, p<.05, partial η2 = .14 (data not shown). In the masked condition, there was a Stimulus × d′ interaction, F(1,26) = 11.04, p<.01, partial η2 = .30, but no main effect of stimulus, F(1,26) = 2.40, n.s., partial η2 = .08. In the unmasked condition, there was no main effect of stimulus nor a Stimulus × d′ interaction on SCR, F(1,26) = 2.04, n.s., partial η2 = .07, and F(1,26) = 1.23, n.s., partial η2 = .05, respectively.
Discussion
We investigated whether perceptual sensitivity to conditioned stimuli is related to CR performance under masking conditions. Dichoptic chromatic masking was used to mask two symbols. As predicted, performance on pre- and postconditioning tasks to assess discriminative ability of these masked symbols revealed a strong positive relationship between sensitivity and CR magnitude to the masked stimuli. Participants with relatively high sensitivity to the masked stimuli before and after conditioning showed greater SCRs to the masked CS+ relative to the masked CS−. Those with relatively low sensitivity lacked differential SCRs to the masked stimuli. Sensitivity to masked stimuli was not related to CRs to unmasked presentations, suggesting that the relationship that emerged for masked presentations was not mediated by CR performance per se or differences in CS-US associative strength. Instead, these findings reflect a specific relationship between discriminative ability and expression of learning under masking conditions (Figure 2).
These results are inconsistent with previous reports of CR expression in participants who lack perceptual awareness. One potential explanation for this inconsistency is that because autonomic responses do not necessarily reflect increases in fear but can also be driven by the general signal value of a stimulus (Siddle, 1991), our results could be demonstrating the necessity of perceptual awareness for expression of relational learning and not fear-conditioning. Hamm and Vaitl (1996) reported differential startle reflex potentiation but not SCRs in participants lacking contingency awareness of a supraliminal CS+ and shock US. Although the SCR is an ambiguous index of fear, we find it very unlikely that the shock US used here, which our laboratory has used extensively to study fear and anxiety (e.g., Grillon, 2002), did not elicit a fear response and promote fear conditioning. Moreover, others have failed to replicate the finding of differential startle responses in unaware participants (Grillon, 2002; Purkis & Lipp, 2001). Finally, it is not clear that startle modulation would be a less ambiguous measure of fear-conditioning than SCR with the current procedure. Brief, pulsed CS presentations could introduce effects of visual prepulse inhibition on startle, which could interfere with measurement of conditioned fear-potentiated startle (cf. Grillon & Davis, 1997).
We employed a more sensitive measure of perceptual awareness than used previously, which could also explain our disparate findings. Although signal detection methods of measuring awareness may sacrifice exclusivity for exhaustiveness in that they could be measuring both conscious and unconscious perceptual processes (Merikle & Daneman, 2000), the current results nevertheless bring balance to previous studies that may have not exhaustively measured perceptual awareness. Without an objective assessment of awareness, there seems to be no guarantee that participants are not using differences in luminance, spatial frequency, and so forth to discriminate CSs and show expression of fear conditioning. Such a state of affairs may reflect unconscious expression of fear learning depending on one’s definition of awareness, but may also reflect successful adaptation to extremely noisy perceptual conditions by tuning into stimulus features that remain consciously accessible. The present findings should engender some caution in how perceptual awareness is to be measured in future studies and how previous findings based on one method of defining awareness are interpreted.
Acknowledgments
This study was supported by NIMH’s Intramural Research program.
REFERENCES
- Critchley H, Mathias C, Dolan R. Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Esteves F, Dimberg U, Öhman A. Automatically elicited fear: Conditioned skin conductance responses to masked facial expressions. Cognition & Emotion. 1994;8:393–413. [Google Scholar]
- First MB, Spitzer RI, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Flykt A. A threat imminence approach to human fear responding: Direction of threat, aversive contexts & electrodermal responses . Stockholm, Sweden: Uppsala Universitet; 1999. (Acta Universitatis Upsaliensis Studia Psychologica Upsaliensia, 18) [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology. 1997;34:511–517. doi: 10.1111/j.1469-8986.1997.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D. Affective learning: Awareness and aversion. Psychophysiology. 1996;33:698–710. doi: 10.1111/j.1469-8986.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Kadlec H. Statistical properties of d′ and β estimates of signal detection theory. Psychological Methods. 1999;4:22–43. [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences, USA. 2003;100:15280–15283. doi: 10.1073/pnas.2535780100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- MacMillan NA, Creelman CD. Detection theory: A user’s guide. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- Maxwell JS, Davidson RJ. Unequally masked: Indexing differences in perceptual salience of ‘unseen’ facial expressions. Cognition and Emotion. 2004;18:1009–1026. [Google Scholar]
- Merikle PM, Daneman M. Conscious vs. unconscious perception. In: Gazzaniga MS, editor. The new cognitive neurosciences. 2nd ed. Cambridge, MA: MIT Press; 2000. pp. 1295–1303. [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. The relationship between cortical activation and perception investigated with invisible stimuli. Proceedings of the National Academy of Sciences, USA. 2002;99:9527–9532. doi: 10.1073/pnas.142305699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJ. On the automatic nature of phobic fear: Conditioned electrodermal responses to masked fear-relevant stimuli. Journal of Abnormal Psychology. 1993;102:121–132. doi: 10.1037//0021-843x.102.1.121. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Learned fear of ‘unseen’ faces after Pavlovian, observational, and instructed fear. Psychological Science. 2004;15:822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology. 2005;15:188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Japee S, Ungerleider LG. Visual awareness and the detection of fearful faces. Emotion. 2005;5:243–247. doi: 10.1037/1528-3542.5.2.243. [DOI] [PubMed] [Google Scholar]
- Purkis HM, Lipp OV. Does affective learning exist in the absence of contingency awareness? Learning and Motivation. 2001;32:84–99. [Google Scholar]
- Siddle DAT. Orienting, habituation, and resource allocation: An associative analysis. Psychophysiology. 1991;28:245–259. doi: 10.1111/j.1469-8986.1991.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Van Den Hout MA, De Jong P, Kindt M. Masked fear words produce increased SCRs. An anomaly for Öhman’s theory of pre-attentive processing in anxiety. Psychophysiology. 2000;37:283–288. doi: 10.1017/s0048577200980673. [DOI] [PubMed] [Google Scholar]