Abstract
Using an event-related functional MRI design, we explored the relative roles of dorsal and ventral prefrontal cortex (PFC) regions during specific components (Encoding, Delay, Response) of a working memory task under different memory-load conditions. In a group analysis, effects of increased memory load were observed only in dorsal PFC in the encoding period. Activity was lateralized to the right hemisphere in the high but not the low memory-load condition. Individual analyses revealed variability in activation patterns across subjects. Regression analyses indicated that one source of variability was subjects’ memory retrieval rate. It was observed that dorsal PFC plays a differentially greater role in information retrieval for slower subjects, possibly because of inefficient retrieval processes or a reduced quality of mnemonic representations. This study supports the idea that dorsal and ventral PFC play different roles in component processes of working memory.
Working memory (WM), the cognitive system that allows individuals to maintain and manipulate information held temporarily in mind, may be divided into separate processes such as those required for the retention of information and those required for allocating attention and coordinating information that is being temporarily maintained (1, 2). Evidence has accumulated to support the notion that PFC may be organized to support different WM processes. Imaging studies have observed that ventral PFC is engaged by simple rehearsal (or maintenance) processes (3, 4). Studies that use more complex WM tasks have shown PFC activations that often occur bilaterally and in regions dorsal to those found in studies of simple retention (Brodmann’s areas 9 and 46) (5). One theory has proposed that PFC is functionally divided in a dorsal/ventral fashion according to the type of cognitive operation that must be performed on information held in WM (6). Empirical studies have supported the notion that ventral PFC mediates maintenance processes whereas dorsal PFC is recruited when additional processing of information, such as manipulation or monitoring, is necessary (7, 8).
Studies that use more complex tasks, such as n-back and dual-tasks (e.g., refs. 9–11, 5), have not clearly distinguished the role of PFC in these different WM processes because information processing demands increase concurrently with maintenance demands. In fact, one study that used a task with no overt requirements to manipulate information held in WM suggested a role for dorsal PFC in WM maintenance. Rypma et al. (12) observed dorsal PFC activation in a WM task in which subjects were required to maintain one, three, or six letters for 5 seconds. When subjects were required to maintain three letters in WM, relative to one letter, activation in frontal regions was limited to left ventral PFC (Brodmann’s area 44). When subjects were required to maintain six letters, relative to one letter, additional activation of dorsal PFC was observed, similar to that observed during tasks in which successful performance required the manipulation of information held in WM (e.g., refs. 7 and 8).
WM tasks like the one used by Rypma et al. (12) involve several components for encoding, retention, and retrieval of information. The blocked design used in the Rypma et al. study did not permit observation of the temporal dynamics of brain activity associated with these different components. Thus, the recruitment of dorsal PFC they observed in the high memory-load condition (compared with the low memory-load condition) may have occurred during any or all of these task periods. The aim of the current study was to explore the relative roles of dorsal and ventral PFC regions during specific components of a WM task under different memory-load conditions. We accomplished this by using an event-related functional MRI (fMRI) (13) design that permits discrimination among functional changes associated with temporally separated trial components. Specifically, this method allowed us to isolate the task periods (i.e., Encoding, Delay, or Retrieval) in which dorsal PFC and ventral PFC exhibit load-dependent effects, that is, where cortical activity is greater with higher memory loads.
Another limitation of blocked neuroimaging studies is that inferences are usually based mainly on aggregate data in task performance and fMRI activation. This approach has been useful for understanding the role of various cortical regions in WM, but does not consider the possibility that inferences may be drawn about the neural correlates of individual differences in WM task performance. The present study also examined possible relationships between individual differences in performance and individual differences in cortical activity.
METHODS
Subjects.
Six right-handed subjects (age = 21–30 yr; 3 men) were recruited from the medical and undergraduate campuses of the University of Pennsylvania. Subjects were excluded if they had any medical, neurological, or psychiatric illness or if they were taking any type of prescription medication. All subjects gave informed consent.
MRI Technique.
Imaging was carried out on a 1.5T SIGNA scanner (GE Medical Systems) equipped with a fast gradient system for echoplanar imaging. A standard radiofrequency head coil was used with foam padding to comfortably restrict head motion. High-resolution sagittal and axial T1-weighted images were obtained from every subject. A gradient echo, echoplanar sequence (repetition time = 2,000 ms, echo time = 50 ms) was used to acquire data sensitive to the blood-oxygen-level-dependent (BOLD) signal. Resolution was 3.75 mm × 3.75 mm in-plane and 5 mm between planes (21 axial slices were acquired). Twenty seconds of gradient and radiofrequency pulses preceded the actual data acquisition to allow tissue to reach steady-state magnetization.
Behavioral Task.
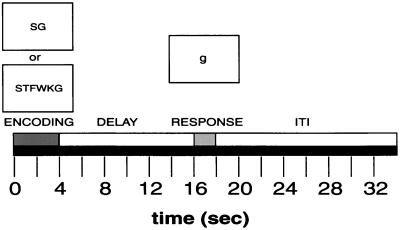
To start each trial, two or six letters were presented simultaneously in pseudo-random order for 4 seconds followed by a 12-second unfilled delay. A probe letter then appeared for 2 seconds during which the subject pressed a button with their right thumb if the probe item was part of the memory set or with their left thumb if the probe item was not part of the memory set (Fig. 1). After these behavioral events there was a 16-second intertrial interval (ITI). The total time from trial onset to trial offset was 34 seconds. This design allowed us to examine neural activity associated with stimulus-encoding, 4 seconds and 8 seconds into the delay period, and response. Subjects viewed a backlit projection screen from within the magnet bore through a mirror mounted on the head coil. Stimulus presentation and reaction time (RT) recording were handled by a Power Macintosh computer.
Figure 1.
Trial sequence.
All subjects completed 10 runs of 8 trials each. A total of 136 gradient-echo echoplanar images in time were obtained per slice in each 272-second run. Thus, a total of 1,360 observations were obtained for each voxel in the brain for each subject, giving us considerable power to detect effects within subjects.
Data Analysis.
Off-line data processing was performed on Sun Microsystems (Mountain View, CA) Ultra workstations. After image reconstruction and before motion correction, data were sinc interpolated in time to correct for the fMRI acquisition sequence because hemodynamic responses were to be compared across slices that were obtained at different points in the acquisition sequence. If left uncorrected, this would have introduced considerable variability and bias (a phase advance) into the hemodynamic responses. The data were motion-corrected by using a slicewise motion-compensation method to remove spatially coherent signal changes with a partial correlation method (13) and by applying a six-parameter, rigid-body, least squares realignment routine (14).
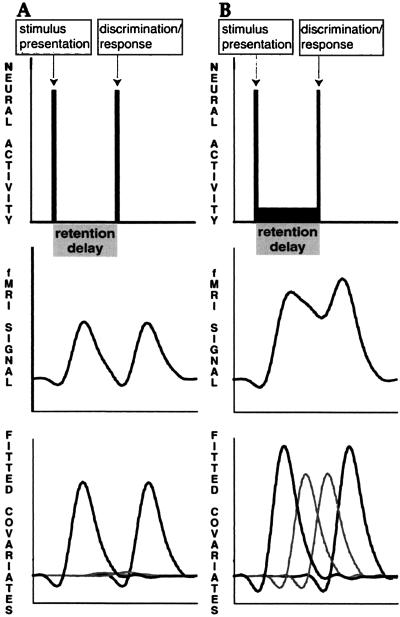
The details of the event-related fMRI analysis used in this study are presented elsewhere (13). Briefly, fMRI signal changes that occurred during particular temporal periods of the behavioral trials were modeled with covariates comprised of shifted, BOLD impulse-response functions, the fMRI response resulting from a brief pulse of neural activity (15). Changes in BOLD signal associated with the Encoding, Delay, and Response periods of the behavioral task were tested with covariates that modeled the expected BOLD signal response in the event of an increase in neural activity (relative to the ITI) occurring in each of the task periods (Fig. 2).
Figure 2.
Individual analysis logic. A depicts a brief period of neural activity (Top) associated with both encoding and the response components, with no increase above baseline during the delay. Such neural activity change leads to a profile of fMRI signal change (Middle). The model covariates (shifted impulse-response functions) scaled by their resulting least-squares coefficients are shown at Bottom (gray lines, delay covariates; black lines, encoding and response covariates). The covariates modeling the delay make only a small contribution to variance explanation in A. In B, there is some neural activity increase relative to baseline during the delay. It can be seen that in B, the delay covariates explain more variance in the fMRI signal than in A.
Because fMRI data are temporally autocorrelated under the null hypothesis (13), the data were analyzed by using the modified general linear model for serially correlated error terms (16). A time-domain representation of the expected 1/f power structure (13) and a filter that removes frequencies above 0.244 were placed within the K matrix. This filter was also applied to the fMRI time series to remove artifacts at the Nyquist frequency (0.25 Hz). Low-frequency (sine and cosine) confounds up to 0.025 Hz and trial-effect covariates were included in our model to account for frequency components and mean signal change, respectively, that were associated with each trial. To examine activity in specific regions of PFC, dorsal PFC regions of interest (ROIs) were drawn to include middle and superior frontal gyri (corresponding to Brodmann’s areas 9 and 46) according to the Talairach and Tournoux (17) atlas on standard T1 axial slices in the axial plane. A similar procedure was used to draw ventral PFC ROIs to include inferior frontal gyrus corresponding to Brodmann’s areas 44, 45, and 47. These ROIs were then normalized to each subject’s T1 axial images by using a 12-parameter affine transformation (14) with a nonlinear deformation routine (18).
Relationships with each task period and the ITI were assessed by contrasts (yielding t statistics with ≈1,195 df) involving the parameter estimates that corresponded to the covariates that modeled each task period (see Fig. 1). Note that three covariates modeled each 4 seconds of the Delay period. Given estimates of the temporal smoothness of the hemodynamic response (13), the covariate modeling the first 4 seconds of the Delay period would be contaminated by hemodynamic activity from the Encoding period. Thus, only the second 4-second interval (designated as Delay period 1) and third 4-second interval of the Delay period (designated as Delay period 2) are considered in the analyses. Delay periods 1 and 2 were analyzed both separately and together. The mapwise-corrected false positive rate was controlled at α = 0.05 by Bonferroni correction for the number of voxels per map (≈15,000 voxels; t ≈ 4.5) or per ROI (≈400 voxels; t ≈ 3.7).
We assessed cortical activation in each subject’s dorsal and ventral PFC in the Encoding, Delay, and Response periods for the two memory-load conditions (i.e., two vs. six letters) by using two measures. The first was the spatial extent of activation as measured by the numbers of suprathreshold voxels that occurred within each ROI during each task period in each memory-load condition. The second measure was activation magnitude, as measured by the “regional mean t,” i.e., the t value across voxels (nonthresholded) for the spatially averaged time series, within each ROI, during each task period, in each memory-load condition. Laterality was measured by assessing differences in extent and magnitude of activation between each hemispheric ROI during each task period and memory-load condition. We tested differential hemispheric involvement in dorsal and ventral PFC to investigate Rypma et al.’s (12) report of greater right- than left-hemisphere dorsal involvement in high memory-load conditions. The hypothesis of greater dorsal and ventral PFC involvement with increased memory load across subjects was tested by using Fisher’s sign tests.
Derivation of an Impulse-Response Function.
Our rationale for deriving an impulse-response function is explained elsewhere (15). An impulse-response function was derived from primary sensorimotor cortex in each subject in the following manner. Before performing the WM task described above, each subject performed a simple RT task in which a central white fixation cross changed briefly (500 msec) to a circle every 16 seconds, cueing subjects to make a bilateral button press. Twenty such events occurred during the 320-second scan (160 images). All scanning parameters were identical to those used for the WM experiment.
RESULTS
Behavioral Performance
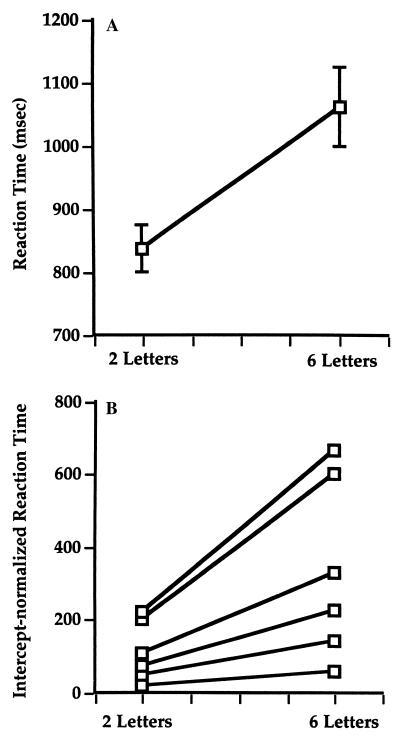
Performance accuracy was not different between the two-letter (90.2%, SD = 5.0) and the six-letter conditions, (89.0%, SD = 10.4), t(5) < 1. Mean median RTs, shown in Fig. 3A, were significantly faster in the two-item condition (837.2 ms, SD = 222.3) as compared with the six-item condition (1061.7 ms, SD = 383.0), t(5) = 3.3, P < 0.02. Examination of individual subjects’ RT slopes (the least-squares estimated coefficient of a linear regression of RT on memory load interpolated between the two- and six-letter memory-load conditions; see Fig. 3B) varied considerably between subjects. The RT slopes ranged from 9.8 msec (subject DK) to 111.0 msec (subject EH).
Figure 3.
(A) Behavioral results in two-letter and six-letter memory-load conditions. (B) Intercept-normalized RTs show individual differences in slopes.
Imaging Data
Mapwise analysis of voxels that were suprathreshold for a contrast of the coefficients for each task period, across memory-load conditions (the Encoding period, each of the two Delay periods separately and combined, and the Response period) vs. the ITI, revealed activity across a number of brain regions. In all six subjects, activity correlated with the Encoding period was observed in bilateral dorsal and ventral PFC, bilateral premotor cortex, anterior and posterior cingulate, bilateral middle temporal gyrus, bilateral inferior parietal lobule, and left superior occipital gyrus. In the Delay periods, activity was observed in bilateral dorsal and ventral PFC, lateral premotor, and parietal cortex. In the Response period, activity was observed in bilateral dorsal and ventral PFC, lateral premotor cortex, anterior and posterior cingulate, bilateral middle temporal, and bilateral inferior parietal lobules.
Comparison of Activation Between Memory-Load Conditions During Task Periods in PFC ROIs.
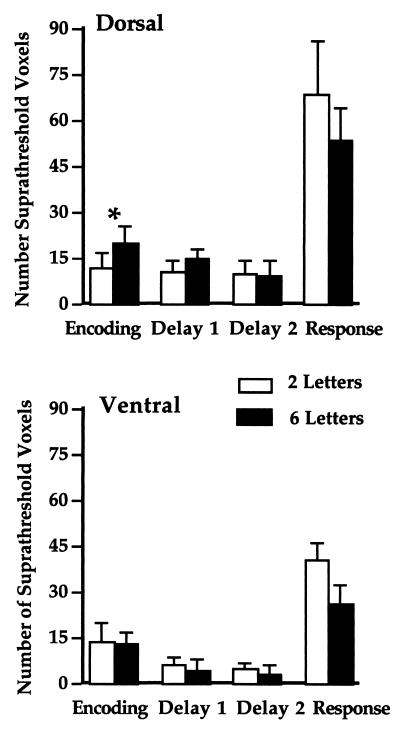
Dorsal PFC. In the Encoding period, all six subjects showed a greater extent of activation in the six-letter than two-letter memory-load condition across the two hemispheres (Fisher’s sign test P = 0.02; Fig. 4). There was a trend toward a difference in signal magnitude between memory-load conditions only in the right hemisphere (five of the six subjects, P = 0.09). All six subjects showed a more right-lateralized extent of activation in the six-letter condition compared with the ITI (P = 0.02). No such effect occurred in the two-letter memory-load condition.
Figure 4.
Regional mean number of suprathreshold voxels and standard errors in two-letter and six-letter conditions; ∗ indicates significant load-dependent effect (i.e., greater activity in the six-letter than in the two-letter condition).
In the Delay periods, activation was quite variable across subjects. In the first and second Delay periods analyzed individually, no significant difference in the extent or magnitude of activation was found between memory-load conditions. A similar finding was observed when the two Delay periods were analyzed together. Tests of laterality were also not significant.
In the Response period, there were trends toward greater extent (P = 0.09) and magnitude of activation (P = 0.09) in the two-letter than the six-letter condition across hemispheres. There was a trend toward more right-lateralized extent and magnitude of activation in the six-letter condition compared with the ITI (P = 0.09). A similar effect occurred in the two-letter load condition (P = 0.09).
Ventral PFC.
In the Encoding period, no significant difference in the extent or magnitude of activation was found between memory-load conditions. In the two-letter condition compared with the ITI, there were trends toward greater activation extent (P = 0.09) and magnitude (P = 0.09) in the left hemisphere. No reliable differences between hemispheres were observed in the six-letter condition.
In the Delay periods, activation was quite variable across subjects. In the first and second Delay periods analyzed individually, no significant difference in the extent or magnitude of activation was found between memory-load conditions. A similar finding was observed when the two Delay periods were analyzed together. In the first Delay period, there was a trend toward greater extent of left-hemisphere activation in the six-letter condition compared with the ITI (P = 0.09) which was significant in the second Delay period (P = 0.02). No laterality effect was found in signal magnitude. No reliable differences between hemispheres were observed in the two-letter condition.
In the Response period, all six subjects showed a greater extent of activation in the two-letter than in the six-letter memory-load condition across the two hemispheres (P = 0.02). There was a trend toward greater signal magnitude in the two-letter than in the six-letter condition (P = 0.09). There was no evidence of lateralized activity during the Response period.
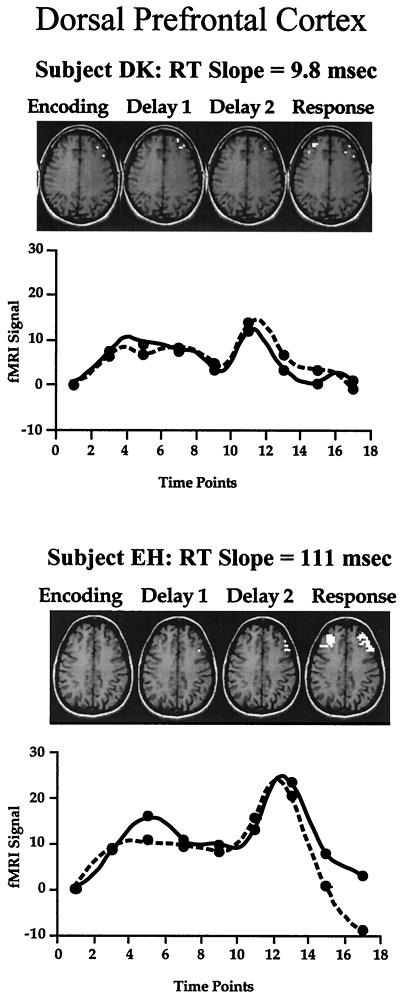
In the analyses of task components above, we observed considerable intersubject variability in fMRI signal magnitude and activation extent. Individual analyses of subjects’ activation patterns and trial-averaged time series suggested a relationship between the patterns of PFC activity observed during the different task periods and task performance. Fig. 5 shows PFC regions of cortical activity and the associated zero-anchored trial-averaged time-series of the subject with the smallest RT slope and high accuracy (subject DK) and the subject with the highest RT slope and low accuracy (subject EH). EH showed a greater spatial extent, greater change in signal, and slower increase in signal than DK across task components. We used regression analyses to test the relationship between memory retrieval rate (independent of memory-load condition) and dorsal and ventral PFC activation in each task period across all subjects.
Figure 5.
Examples of activation in dorsal PFC regions (across memory load conditions) for the subjects with highest RT rate and high accuracy and lowest RT rate and low accuracy and associated time series in the six-letter (solid lines) and two-letter (dashed lines) conditions.
Individual Differences in PFC Activity.
To test the relationship between PFC activation and task performance in the group, we determined, for each subject, the extent of activation in each task period, independent of memory-load, by determining the number of voxels that were suprathreshold for a contrast involving the sum of coefficients of the task period covariates in the two-letter and the six-letter memory-load conditions in each PFC ROI. Next, we performed a linear regression of subjects’ RT slope and number of suprathreshold voxels at each task period. The logic of these analyses was that the relationship between memory-retrieval rate and regional cortical activity could best be characterized by comparing RT slope to voxels showing a net positive effect in each task period, that is, voxels that were responsive in each task period across memory-load conditions.
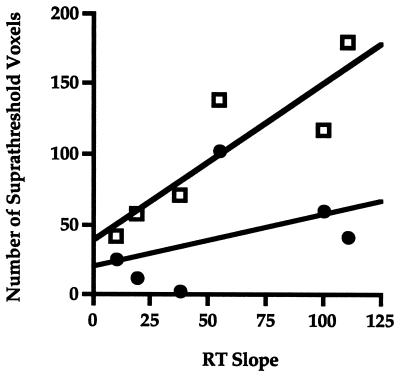
Table 1 shows the nonnormalized regression coefficients that characterize the relationship between RT slope and spatial extent of activation (i.e., number of suprathreshold voxels) in each task period. The most important finding was in the Response period, where coefficients for the dorsal PFC ROI showed a positive relationship between RT slope and spatial extent of activation. This relationship was not observed in ventral PFC (for a scatterplot, see Fig. 6). In dorsal PFC, there was an increase of 1.34 activated voxels per millisecond of increase in RT slope that accounted for 76% of the variance. Similar analyses involving the intercept were nonsignificant. Because the small number of subjects limited power in this study, we performed nonparametric tests (which do not require assumptions of underlying normal distributions) on these data and obtained the same pattern of significant and nonsignificant results. We also tested the relationship between activation and performance without a statistical threshold by measuring each subject’s regional mean t value in dorsal and ventral PFC ROIs and then performed a regression of subjects’ RT slope and regional mean t at each task period. The results of this analysis are similar to those we performed with suprathreshold voxels (see Table 2). In the Response period, a strong positive correlation was observed between RT slope and the mean t value of the dorsal PFC ROI that accounted for 84% of the variance. Again, this pattern of results also occurred with nonparametric tests.
Table 1.
Parameters and statistical tests from regression of RT slope and regional suprathreshold voxels across memory-load conditions
| Task period | Regression parameter
|
|||
|---|---|---|---|---|
| Slope | r2 | t | P | |
| Encoding | ||||
| Dorsal | 0.06 | 0.002 | 0.08 | 0.94 |
| Ventral | 0.07 | 0.002 | 0.09 | 0.93 |
| Delay 1 | ||||
| Dorsal | 0.24 | 0.33 | 1.40 | 0.24 |
| Ventral | −0.41 | 0.17 | −0.89 | 0.42 |
| Delay 2 | ||||
| Dorsal | 0.30 | 0.30 | 1.30 | 0.26 |
| Ventral | 0.10 | 0.89 | 4.93 | 0.02* |
| Response | ||||
| Dorsal | 1.34 | 0.76 | 3.53 | 0.02* |
| Ventral | 0.36 | 0.18 | 0.93 | 0.41 |
Indicates statistical significance.
Figure 6.
A scatterplot of the numbers of suprathreshold voxels during the response period in dorsal PFC (upper line, squares; slope = 1.34, r2 = .76) and ventral PFC (lower line, circles; slope = 0.36, r2 = .18) plotted against reaction time slopes.
Table 2.
Parameters and statistical tests from regression of RT slope and regional mean t value across memory-load conditions
| Task period | Regression parameter
|
|||
|---|---|---|---|---|
| Slope | r2 | t | P | |
| Encoding | ||||
| Dorsal | −0.36 | 0.13 | −0.77 | 0.48 |
| Ventral | 0.11 | 0.01 | 0.23 | 0.83 |
| Delay 1 | ||||
| Dorsal | −0.23 | 0.05 | −0.47 | 0.66 |
| Ventral | −0.33 | 0.11 | −0.71 | 0.52 |
| Delay 2 | ||||
| Dorsal | 0.64 | 0.42 | 1.68 | 0.16 |
| Ventral | 0.50 | 0.25 | 1.15 | 0.31 |
| Response | ||||
| Dorsal | 0.91 | 0.84 | 4.50 | 0.01* |
| Ventral | 0.06 | 0.004 | 0.13 | 0.91 |
Indicates statistical significance.
DISCUSSION
In this experiment, we identified brain activity that correlated with each of the components of the task (Encoding, Delay, and Response). In dorsal PFC, we observed load-dependent memory effects only during the Encoding period, where greater right-hemisphere activation than left-hemisphere activation was observed in the high, but not the low, memory-load condition. In ventral PFC we observed load-equivalent activation.
Rypma et al. (12) observed activation in ventral PFC with both low (three-letter) and high (six-letter) memory loads and additional bilateral dorsal PFC only with high memory loads. They argued that ventral PFC is involved in maintenance of a subcapacity WM load. When WM load exceeds short-term memory capacity (19), dorsal PFC may be additionally recruited to mediate strategic processes necessary for maintenance of a high WM load. Rypma et al.’s block-design study could not determine when such processes may have been engaged. Our results suggest that dorsal PFC may play its principal role during encoding of information with high memory loads and not necessarily during the maintenance of information.
We did not observe delay-period load-dependent effects in PFC as other studies have (20). This variance in results may be because of differences in the paradigms used in these studies and the present study. For example, Cohen et al. (20) used an n-back task in which higher memory-load requirements correlated with requirements to hold information for longer periods of time and with more distracting stimuli (i.e., more intervening items) during these periods, whereas our task varied memory load (i.e., amount of information) alone across a constant time interval. Another possible explanation for our failure to detect delay-period load effects is a lack of statistical sensitivity in this task period. Because load-dependent effects with verbal information have also been observed in left posterior parietal cortex (Brodmann’s area 40) (20, 21) and deficits in span performance have been observed following left parietal lesions (22), we performed analyses of our data in this region similar to those we performed in PFC. Those analyses indicated load-dependent effects in signal magnitude during the delay in each subject, consistent with these prior studies. This further result ameliorates concerns that the failure to detect load-dependent effects was related to issues of statistical power.
Our finding of greater dorsal PFC activation during high-load information encoding may be interpreted as the result of differences in stimulus displays between the two conditions. This result, however, replicates Rypma et al.’s (12) finding of increased dorsal PFC activity with high memory loads with stimulus displays that were equivalent between the two memory-load conditions. Moreover, the result of greater right-hemisphere dorsal PFC activation is consistent with prior WM imaging studies showing that right-dorsal PFC is more commonly recruited during complex working memory tasks (5). It may be that initial encoding of information in a simple maintenance task requires cognitive operations (e.g., monitoring the contents of WM, updating and coordination of multiple memory buffers) similar to those required in more complex tasks.
In the present study, considerable variability occurred both in subjects’ performance and in their fMRI signal. Regression analyses of subjects’ rate of response and extent and amount of activation in the dorsal and ventral PFC regions indicated that one source of variability in subjects’ fMRI data may be their processing speed. Memory retrieval rate, as indexed by RT slope, accounted for most of the variability in dorsal PFC regions during the response period of the task but minimally in other task periods and in ventral PFC regions.
These results suggest that reductions in information retrieval rate, possibly reflecting the rate of memory scanning (e.g., ref. 23), are related to increases in dorsal PFC activation. This result is important for several reasons. First, it suggests one possible model for the neural correlates of processing efficiency. It may be that increases in processing speed are related to increases in the efficiency of neural processes. Such a model may be contrasted with models that link performance improvements to increases in WM activation capacity (e.g., ref. 24) to the extent that they predict cortical activation increases associated with improvements in performance. Second, it suggests that individual differences in cortical efficiency exert effects in a specific cortical region (dorsal PFC). Third, these findings, together with the fact that RT slope did not correlate with ventral PFC activity, suggest different roles for dorsal and ventral PFC in retrieval of information held in working memory. Such a model of PFC organization may be consistent with behavioral results (e.g., ref. 23) showing independence of memory-scanning retrieval processes and rehearsal-maintenance processes. Specifically, the memory-scanning rate observed by Sternberg and others (e.g., ref. 23) was considerably faster than the observed rate of subvocal rehearsal (e.g., refs. 24 and 25). It may be that dorsal PFC mediates memory retrieval whereas ventral PFC mediates memory maintenance.
Other neuroimaging studies using different tasks and different populations are consistent with the findings in this study regarding the relationship between neural activity and task performance. In support of the neural efficiency explanation of processing speed, Haier et al. (27) have observed reduced glucose metabolic rate (measured by using positron emission tomography while subjects performed a visual manipulation task) in subjects with higher, compared with those with lower, Raven Progressive Matrices scores. Similarly, Kosslyn et al. (28) have observed smaller increases in parietal-lobe regional cerebral blood flow in subjects with faster RTs compared with those with slower RTs during a mental imagery task. Similar to the present study, this relationship was region-specific; opposite effects were observed in primary sensory regions (Brodmann’s areas 17 and 19). In studies comparing younger and older adults, Grady et al. (29), observed less functionally distinct “what” and “where” visual processing streams in older adults who performed more slowly on a face-matching task than younger adults. One other study (30) found the opposite result: increased glucose metabolic rate in high-scoring Raven matrices subjects as opposed to low scorers. The authors note, however, that indications of strategy differences between the two groups, as well as the extreme-groups nature of the design, render a clear interpretation of these results difficult.
The proposed relationship between processing speed and neural efficiency is consistent with models of the slowing of information processing. Cerella (31), for instance, proposed a “disconnection hypothesis” in which cognitive task performance requires information transmission across a vast array of interconnected nodes. The smaller the number of nodes across which information must be transmitted, the more direct the processing paths and the quicker the information processing. Reductions in the integrity of direct processing links lead to slowing in information processing rate because more links are required to propagate signal between interconnected nodes. Increases in the number of connections required to traverse nodes may lead to increases in neural activity. Slowing in the rate at which information can be maintained in working memory could also lead to early degradation in the quality of mnemonic information available for later retrieval (e.g., refs. 32 and 33). One consequence of low-quality information maintenance could be an increase in neural activity at retrieval.
The results of the present study suggest a number of conclusions regarding the roles of PFC regions in WM. In general, both dorsal and ventral PFC appear to play roles in encoding, maintenance, and retrieval of verbal information. These finding are consistent with monkey electrophysiological (34) and human neuroimaging studies (e.g., ref. 35) that have found PFC activity during both mnemonic and nonmnemonic components of working memory tasks. Dorsal PFC may play a greater role in encoding of information for subsequent retrieval than in other processes engaged by WM tasks, especially under high memory-load conditions. The role of dorsal PFC in retrieval appears to increase for subjects who show slower memory retrieval, possibly because of reductions in processing efficiency or the quality of mnemonic representations.
Acknowledgments
We thank Eric Zarahn and Geoff Aguirre for their support on this project. This work was supported by American Federation for Aging Research and National Institutes of Health Grants NS01762 and AG13483.
ABBREVIATIONS
- PFC
prefrontal cortex
- WM
working memory
- fMRI
functional magnetic resonance imaging
- ITI
intertrial interval
- ROI
region of interest
- RT
reaction time
- BOLD
blood-oxygen-level dependent
References
- 1.Norman D, Shallice T. CHIP Report 99. Berkeley, CA: University of California; 1980. [Google Scholar]
- 2.Baddeley A. Working Memory. New York: Oxford Univ. Press; 1986. [Google Scholar]
- 3.Awh E, Jonides J, Smith E, Schumacher E, Koeppe R, Katz S. Psychol Sci. 1996;7:25–31. [Google Scholar]
- 4.Paulesu E, Frith C, Frackowiak R. Nature (London) 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 5.D’Esposito M, Aguirre G K, Zarahn E, Ballard D, Shin R K. Cog Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 6.Petrides M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Amsterdam: Elsevier; 1994. [Google Scholar]
- 7.Owen A. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 8.D’Esposito, M., Postle, B. R., Ballard, D. & Lease, J. (1998) Brain Cognit., in press. [DOI] [PubMed]
- 9.D’Esposito M, Detre J, Alsop D, Shin R, Atlas S, Grossman M. Nature (London) 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J D, Forman S D, Braver T S, Casey B J, Servan-Schreiber D, Noll D C. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 11.Smith E E, Jonides J, Koeppe R A. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Rypma B, Prabhakaran V, Desmond J E, Glover G H, Gabrieli J D E. NeuroImage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- 13.Zarahn E, Aguirre G, D’Esposito M. NeuroImage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- 14.Friston K J, Ashburner J, Frith C, Poline J-B, Heather J, Frackowiak R. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- 15.Aguirre, G. K., Zarahn, E. & D’Esposito, M. (1998) NeuroImage, in press.
- 16.Worsley K J, Friston K J. NeuroImage. 1995;2:173–182. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 17.Talairach J, Tournoux P. A Co-Planar Stereotaxic Atlas of the Human Brain: An Approach to Medical Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- 18.Ashburner J, Friston K. NeuroImage. 1996;3:S111. [Google Scholar]
- 19.Waugh N C, Norman D A. Psychol Rev. 1965;72:89–104. doi: 10.1037/h0021797. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J D, Perlstein W M, Braver T S, Nystrom L E, Noll D C, Jonides J, Smith E E. Nature (London) 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 21.Jonides J, Schumacher E H, Smith E E, Lauber E J, Awh E, Minoshima S, Koeppe R A. J Cog Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 22.Warrington E K, Logue V, Pratt R T C. Neuropsychologia. 1971;9:377–387. doi: 10.1016/0028-3932(71)90002-9. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg S. Acta Psychol (Amst) 1969;30:276–315. [Google Scholar]
- 24.Just M A, Carpenter P A. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- 25.Landauer T K. Percept Mot Skills. 1962;15:646. doi: 10.2466/pms.1962.15.3.646. [DOI] [PubMed] [Google Scholar]
- 26.Broadbent D E. Perception and Communication. New York: Pergamon; 1958. [Google Scholar]
- 27.Haier R J, Siegel B, Tang C, Abel L, Buchsbaum M S. Intelligence. 1992;16:415–426. [Google Scholar]
- 28.Kosslyn S M, Thompson W L, Kim I J, Rauch S L, Alpert N M. J Cogn Neurosci. 1996;8:78–82. doi: 10.1162/jocn.1996.8.1.78. [DOI] [PubMed] [Google Scholar]
- 29.Grady C L, Maisog J M, Horwitz B, Ungerleider L G, Mentis M J, Salerno J A, Pietrini P, Wagner E, Haxby J V. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson G E, Haier R J, LaCasse L, Hazen K. Intelligence. 1995;21:267–278. [Google Scholar]
- 31.Cerella J. In: Handbook of the psychology of aging. Birren J E, Schaie K W, editors. New York: Academic; 1990. [Google Scholar]
- 32.Salthouse T A. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 33.Myerson J, Hale S, Wagstaff D, Poon L W, Smith G A. Psychol Rev. 1990;97:475–487. doi: 10.1037/0033-295x.97.4.475. [DOI] [PubMed] [Google Scholar]
- 34.Funahashi S, Bruce C J, Goldman-Rakic P S. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Esposito M, Ballard D, Aguirre G K, Zarahn E. NeuroImage. 1998;8:274–282. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]